Abstract
Background
Polyethylene (PE) wear particles are believed to cause aseptic loosening and thereby impair function in hip arthroplasty. Highly crosslinked polyethylene (XLPE) has low short- and medium-term wear rates. However, the long-term wear characteristics are unknown and it is unclear whether reduced wear particle burden improves function and survival of cemented hip arthroplasty.
Questions/purposes
We asked whether XLPE wear rates remain low up to 10 years and whether this leads to improved implant fixation, periprosthetic bone quality, and clinical function compared to conventional PE.
Methods
We randomized 60 patients (61 hips) to receive either PE or XLPE cemented cups combined with a cemented stem. At 10 years postoperatively, 51 patients (52 hips) were evaluated for polyethylene wear and component migration estimation by radiostereometry, for radiolucent lines, bone densitometry, and Harris hip and pain scores. Revisions were recorded.
Results
XLPE cups had a lower mean three-dimensional wear rate between 2 and 10 years compared to conventional PE hips: 0.005 mm/year versus 0.056 mm/year. We found no differences in cup migration, bone mineral density, radiolucencies, functional scores, and revision rate. There was a trend toward improved stem fixation in the XLPE group. The overall stem failure rate was comparably high, without influencing wear rate in XLPE hips.
Conclusions
XLPE displayed a low wear rate up to 10 years when used in cemented THA, but we found no clear benefits in any other parameters. Further research is needed to determine whether cemented THA designs with XLPE are less prone to stem loosening.
Level of Evidence
Level I, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-012-2400-x) contains supplementary material, which is available to authorized users.
Introduction
Aseptic loosening is the most common reason for failure and revision of cemented THA with a metal-on-polyethylene articulation [22]. Extensive efforts have been made to reveal the etiology of aseptic loosening and the pathophysiologic mechanisms involved. Histology [1, 3] and laboratory [25, 27, 37] studies have led to the conclusion that polyethylene (PE) wear particles play an important role in the loosening process [6, 26], initiating an intensive search for articulations less prone to wear.
Highly crosslinked polyethylene (XLPE) has been used in clinical practice for more than 10 years [30]. In a variety of studies with mean followup ranging from 5 to 8.6 years, wear rates have been lower than for conventional PE [5, 10, 34, 38, 46, 52]. For uncemented THA, the low wear rates of XLPE have been accompanied by absent or low occurrence of periprosthetic osteolysis [4, 30], supporting the PE particle theory.
However, it is less clear whether XLPE influences the durability of cemented THA. In one autopsy study [49], the authors suggested aseptic cemented cup loosening is a process starting at the periphery of the acetabular cement-bone interface proceeding toward the dome. PE particle-laden macrophages and free PE particles are abundant, especially at the osteolysis leading edge [49], leading to the conclusion that loosening of cemented cups is mainly a biologic process. This theory has however been questioned in part, with an alternative suggestion that early micromotion stimulates the development of a fibrous and more accessible interface [36]. Metal and cement particles may play a part in aseptic loosening in cemented stems along with mechanical debonding [28, 56]. If PE particles play a major and unique role in osteolysis and loosening development, cemented THA with XLPE cups should have less migration, osteolysis, and loosening and also less bone demineralization around the cup and stem compared to THA with conventional PE. In previous studies of the cohort described here, we found no differences regarding cup fixation, radiolucencies, or bone mineral density loss at 2 years and low XLPE wear rates at 5 years [10–12]. However, it is unclear whether these findings would persist at longer followup.
We therefore evaluated (1) whether XLPE wear resistance persisted in long-term followup and whether these improved wear characteristics led to (2) improved fixation, (3) fewer radiolucencies, (4) less loss of bone mineral density, and (5) improved functional scores and lower revision rate in cemented THA compared to conventional PE at 10 years’ followup.
Patients and Methods
We prospectively followed 59 patients who underwent unilateral cemented THA and one patient who had sequential bilateral procedures recruited between 1998 and 2001 from the THA waiting list in our hospital and were selected according to our inclusion and exclusion criteria (Table 1). During that same period, we performed a total of 337 THAs with similar diagnoses. Each hip was randomized by closed envelopes to receive all-PE cups with either XLPE (Durasul®; Zimmer, Inc, Warsaw, IN, USA) or conventional PE (Sulene®; Zimmer, Inc). The patient with bilateral procedures was randomized at the first operation and then received the opposite kind of PE on the other side. Originally, 31 hips received XLPE and 30 received PE cups. Neither patients nor surgeons were blinded. The mean age was 55 years (range, 41–70 years) and the mean weight 82 kg (range, 47–120 kg). Four patients were deceased (two in each group) and two declined to participate (both in XLPE group), all unrevised. Three had been revised (one stem revision in the PE group; one stem and one total revision in the XLPE group), leaving 52 patients (27 PE, 25 XLPE) attending the 10-year followup (Table 2). All patients gave informed consent to participate in the study. The study was approved by the local ethics committee (Number R312-98) and conforms to the Helsinki declaration.
Table 1.
Inclusion and exclusion criteria
| Criteria |
| Inclusion |
| Age 35 to 75 years |
| Primary or secondary osteoarthritis |
| Arthrosis secondary to idiopathic femoral head necrosis, dysplasia, metabolism |
| Legg-Calvé-Perthes and epiphysiolysis |
| Exclusion |
| Inflammatory arthritis |
| Earlier hip fracture or infectious arthritis |
| Corticosteroid or cytostatic treatment |
| Known osteomalacia, osteoporosis |
Table 2.
Patient demographics and implant data at surgery
| Variable | PE | XLPE |
|---|---|---|
| Number of hips | 27 | 25 |
| Age (years)* | 56 (41–70) | 55 (42–68) |
| Male/female | 15/12 | 12/13 |
| Primary/secondary arthrosis | 22/5 | 19/6 |
| Weight (kg)* | 83 (58–120) | 82 (47–116) |
| Cup size (mm)† | 54 (48–60) | 54 (48–60) |
| Stem Size 1/2/3 | 5/15/7 | 1/14/10 |
| Standard/high-offset | 17/10 | 19/6 |
| Charnley Group A/B/C | 16/7/4 | 16/2/7 |
| Preoperative Harris hip score (points)† | 46 (18–68) | 44 (18–78) |
| Preoperative Harris pain score (points)† | 10 (0–30) | 10 (0–30) |
For all variables, p > 0.1 (Mann-Whitney U test); *values are expressed as mean, with range in parentheses; †values are expressed as median, with range in parentheses; PE = polyethylene; XLPE = highly crosslinked polyethylene.
Power calculations were performed for radiostereometry analysis (RSA) of wear. Twenty patients in each group allowed us to detect an expected 0.2-mm proximal wear difference with 87% probability at 0.05 significance assuming an SD of 0.2 mm. Conventional PE was expected to wear 0.2 to 0.3 mm linearly in 2 years with an SD of 0.2 mm based on previous observations [48], while the XLPE wear rate in hip simulators was close to zero [35], giving the estimated effect size. The number of participants was then chosen to compensate for dropouts.
Acetabular implants were flanged Weber® cups (Centerpulse Orthopedics Ltd, Winterthur, Switzerland). Durasul® XLPE was manufactured from compression-molded GUR® 1050 PE bars. PE disks were heated to 125°C and irradiated with an electron beam at an absorbed dose of 95 kGy. The irradiated PE was subsequently remelted for 2 to 3 hours at 150°C. Cups were machined from these XLPE disks and finally sterilized using ethylene oxide. The corresponding conventional PE cups were manufactured from compression-molded GUR® 1050 PE sheets and sterilized by gamma irradiation (25–40 kGy) in nitrogen. The manufacturer marked all cups with 10 1.0-mm tantalum beads along the rim and in the inferior part of the dome.
All patients received Spectron EF Primary® stems (Smith & Nephew, Memphis, TN, USA), which is a straight cobalt-chromium stem, proximally grit-blasted with an average surface roughness of 2.8 μm and distally smoother with an average roughness of 0.7 μm. Stems came with three preattached pegs, two proximally and one distally, each with a 1.0-mm tantalum marker on the top. All femoral heads had a diameter of 28 mm and were made of cobalt-chromium. The components were fixed with Palacos® with gentamicin cement (Schering Plough, Heraeus Kultzer, Wehrhrim, Germany) using a third-generation cementing technique. During surgery, performed through a modified Hardinge approach, six to nine 0.8-mm tantalum markers were inserted into the acetabular and femoral bone, respectively. This procedure did not influence overall surgical technique, including cementing.
The patients followed routine postoperative treatment, including full postoperative weightbearing from the day after surgery.
Patients were seen after 3 and 6 months and then after 1, 2, 3, 5, 7, and 10 years. Preoperatively and at each followup, we obtained a Harris hip score (HHS) [21] including its subscale Harris pain score (HPS) [21]. Plain and RSA radiographs were taken within 7 days postoperatively and then at the same intervals as clinical followup. Bone density was measured within 7 days postoperatively and then at 1, 2, 3, 5, 7, and 10 years. Here we report data from the 10-year followup only.
We performed supine uniplanar RSA postoperatively (within 7 days), after 3 and 6 months, and then after 1, 2, 3, 5, 7, and 10 years using UmRSA® 6.0 software (RSA Biomedical, Umeå, Sweden). In RSA, each implant component and bony structure is represented by three-dimensional (3D) segments (four segments/hip) with at least three stable tantalum markers. We excluded segments with fewer than three stable markers or a condition number of more than 150 indicating an inadequate dispersion of markers [54]. Upper limit of marker stability (mean error of rigid body fitting) was 0.35 mm in each segment [54]. We estimated wear by measuring proximal femoral head penetration (along the longitudinal y-axis) and the total (3D) penetration. According to previously published data regarding this and other studies on XLPE, there is a substantial proximal penetration over the first 1 to 2 years due to creep [10, 18]. We therefore calculated wear rates based on penetration difference between 2 and 10 years. Cup motions were analyzed as translation and rotation along and around the x- (transverse), y- (longitudinal), and z-(sagittal) axes, whereas stem movements are presented solely as rotations around the same axes and migration along the y-axis, as this is the main direction of stem displacement. Cup translations were measured at the center of gravity of cup markers and the proximal/distal stem translation at the center of gravity of the rigid body defined by stem markers and the femoral head center. In four cases, some of the stem markers could not be visualized or had poor fixation, leaving insufficient number of markers or too high condition number in the stem segment. In these cases, the translation of the femoral head center was used [29]. Precision estimates of cup migration and proximal femoral head penetration in this study have been presented previously [11]. Precision of the stem subsidence was estimated by 51 double examinations separated by 15 to 30 minutes and repositioning of the patient and x-ray tubes [54]. Based on an expected difference of zero between the measurements, the 99% precision calculated as SD of the differences × 2.7 (SD × t99%, two-tailed, n = 51) [44] was 0.15 mm using the whole stem segment (three stem markers and the femoral head center) and 0.20 mm using the femoral head center only. In summary, eight segments in seven patients were excluded. In addition, wear data in one hip were aberrant, probably due to interposed soft tissue. Thus, proximal and total (3D) femoral head penetration could be analyzed in 23 XLPE and 27 control hips. Corresponding figures were 23 and 27 hips for stem migration and 24 and 26 hips for cup migration, respectively.
One of us (PEJ) determined osteolysis using conventional digital radiographs and the Mdesk™ suite (RSA Biomedical). Only 10-year examinations were analyzed since most postoperative films were lost during a large-scale reorganization of the radiology department at the hospital. Images were deidentified before analysis and then read in random order. We assessed linear and focal osteolysis around the cup and stem and also signs of debonding between the stem and cement. Linear osteolysis of more than 0.2 mm was classified using modified Charnley-DeLee zone definitions for frontal and lateral cup projections, with no osteolysis coded as 0, up to 50% of the zone coded 1, 50% to 99% of zone coded 2, and osteolysis in the entire zone of interest coded 3 [7, 11]. We also measured maximum osteolysis width in each zone and percentage of linear osteolysis in relation to the total length of visible cement-bone interface in each projection. The femur was analyzed likewise in separate Gruen zones on the AP and lateral views [20]. Any focal osteolysis was roughly classified as small (< 1 cm) or large (≥ 1 cm) and registered by location. We also measured the distance between the uppermost lateral part of the stem and the inner cement mantle contour. With small distances (≤ 0.2 mm), it was difficult to distinguish debonding from digital radiographic edge effects and those distances were judged as zero. The number of observations in different regions and projections varied depending on exposure quality, generally worse on lateral than frontal projections.
Bone mineral density was measured with dual-energy x-ray absorptiometry (DEXA). On the acetabular side, we evaluated five regions of interest (ROIs) around the cup, two superior, one medial, and two inferior. On the femoral side, seven ROIs were analyzed according to Gruen Zones 1 to 7 in the frontal plane [20], aggregated by geometric mean into upper femur (ROI 1, 2, 6, 7) and lower femur (ROI 3 to 5) in the final analysis. For the postoperative examination, we used a Lunar DPX-L densitometer (GE/Lunar Co, Madison, WI, USA) for the first 10 patients and a Lunar DPX-IQ (GE/Lunar Co) for the remaining 51 patients. The correlation and conversion of measurement data between those two densitometers were described earlier [11]. For the 10-year DEXA evaluation, we used a Hologic Discovery™ densitometer (Hologic, Bedford, MA, USA) for all patients. The relationship between the Lunar and Hologic densitometer measurements was determined by doing 12 sequential double examinations for femur and seven for the pelvis. Because of the limited number of cases in each ROI, measurement pairs from the different ROIs were pooled into one series for femur and one for acetabulum. The correlation was analyzed by linear regression yielding one conversion equation for femur (BMDHologic = 0.19 + 0.80 × BMDLunar, r2 = 0.84) and one for pelvis (BMDHologic = 0.40 + 0.67 × BMDLunar, r2 = 0.80). These equations were used to convert postoperative Lunar measurements to Hologic equivalent scale. In all cases, we attempted to exclude the cement from the DEXA measurement and also to place and size ROIs on 10-year examinations according to the postoperative examination. Precision estimates for the pelvis have been reported previously [11]. DEXA results are expressed as difference in percentage between postoperative and 10-year measurements. Twenty XLPE and 24 control hips could be evaluated on the acetabular side and 23 XLPE and 27 control hips on the femoral side. Reasons for loss of observations were lack of or suboptimal postoperative or followup examinations.
Femoral head penetration, cup translation and rotation, femoral stem subsidence and varus-valgus tilt, osteolysis extent and width, median distance between upper lateral stem and inner cement contour, bone mineral density change, HHS, and HPS were compared pairwise between study and control group using the Mann-Whitney U test. The Fisher exact test was used to analyze differences in the incidence of focal osteolysis and revision rate. We used a Spearman correlation to determine the relationship between the distance between upper lateral stem and inner cement contour and stem subsidence measured with RSA. We made no formal correction for multiple statistical analyses but took them into account when interpreting results. We performed statistical analysis using IBM® SPSS® Statistics Version 20 (IBM Corp, Armonk, NY, USA).
Results
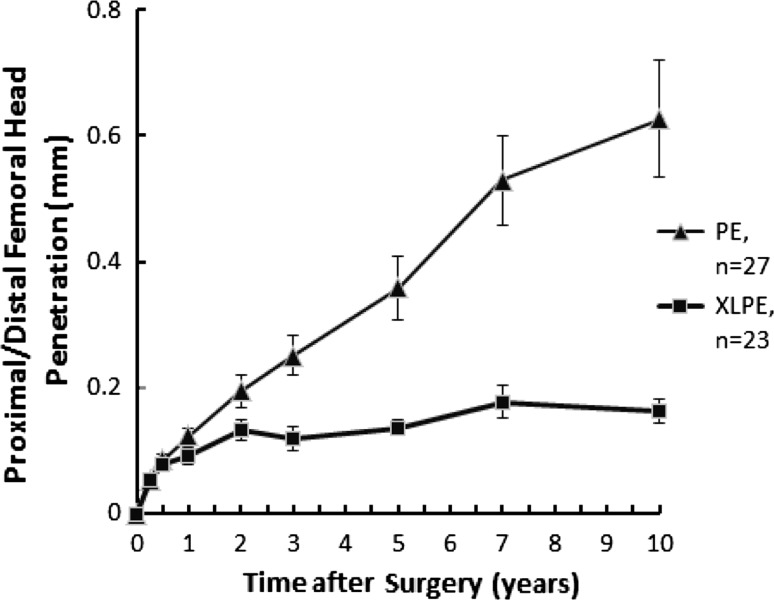
The mean proximal femoral head penetration rate between 2 and 10 years was lower (p < 0.001) in the XLPE group (0.005 mm/year; standard error [SE], 0.002 mm/year) than in the conventional PE group (0.055 mm/year; SE, 0.009 mm/year) (Fig. 1). The corresponding mean 3D wear rate was also lower (p < 0.001) in the XLPE group (0.005 mm/year; SE, 0.002 mm/year) than in the control group (0.056 mm/year; SE, 0.009 mm/year) (Fig. 2).
Fig. 1.
A graph shows the proximal (+)/distal (−) femoral head penetration up to 10 years for the XLPE and conventional PE groups. Mean proximal penetration rate between 2 and 10 years was lower (p < 0.001) in the XLPE group than in the conventional PE group. Values are expressed as mean ± SE.
Fig. 2.
A graph shows the 3D femoral head penetration up to 10 years for the XLPE and conventional PE groups. Mean 3D penetration rate between 2 and 10 years was lower (p < 0.001) in the XLPE group than in the control group. Values are expressed as mean ± SE.
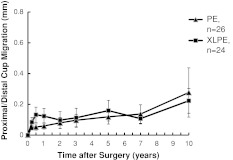
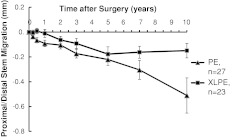
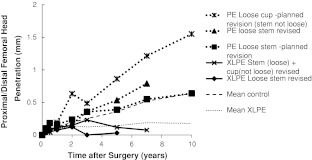
Cup translations and rotations at 10 years did not differ between groups (Table 3), which is also apparent graphically (Fig. 3). Femoral component subsidence and varus tilt at 10 years were slightly more pronounced in the PE group (Table 3), which is also apparent graphically (Fig. 4). Individual subsidence curves for revised and loose stems planned for revision in PE and XLPE groups are shown (Fig. 5).
Table 3.
Cup and stem rotation and translation at 10 years
| Variable | PE | XLPE | p value* |
|---|---|---|---|
| Cup rotation (°) | n = 26 | n = 24 | |
| x-axis (forward tilt +) | 0.05 (0.10) | −0.06 (0.13) | 0.55 |
| y-axis (anteversion +) | −0.02 (0.14) | −0.17 (0.14) | 0.53 |
| z-axis (increased inclination +) | 0.60 (0.58) | 0.24 (0.10) | 0.51 |
| Cup translation (mm) | n = 26 | n = 24 | |
| x-axis (medial +) | −0.29 (0.22) | −0.15 (0.05) | 0.71 |
| y-axis (proximal +) | 0.29 (0.17) | 0.22 (0.08) | 0.61 |
| z-axis (forward +) | −0.003 (0.08) | −0.13 (0.07) | 0.32 |
| Stem rotation (°) | n = 26 | n = 20 | |
| x-axis (forward tilt +) | −0.06 (0.05) | −0.15 (0.07 | 0.81 |
| y-axis (anteversion +) | −0.80 (0.35) | −0.55 (0.24) | 0.65 |
| z-axis (valgus +) | −0.15 (0.05) | 0.03 (0.04) | 0.005 |
| Stem migration (mm) | n = 27 | n = 23 | |
| y-axis (proximal +) | −0.51 (0.14) | −0.15 (0.06) | 0.05 |
Values are expressed as mean, with standard error in parentheses; * Mann-Whitney U test; PE = polyethylene; XLPE = highly crosslinked polyethylene.
Fig. 3.
A graph shows the proximal (+)/distal (−) migration of the cup up to 10 years for the XLPE and conventional PE groups. Cup translations and rotations at 10 years did not differ between groups. Values are expressed as mean ± SE.
Fig. 4.
A graph shows the proximal (+)/distal (−) migration of the stem up to 10 years for the XLPE and conventional PE groups. Femoral component subsidence and varus tilt at 10 years were slightly more pronounced in the PE group. Values are expressed as mean ± SE.
Fig. 5.
A graph shows the proximal (+)/distal (−) femoral head penetration of five hips revised or planned to be revised at 10-year followup. The two loose stems were not accompanied by accelerated wear in the XLPE group, and only one of three cups revised or waiting to be revised in the control group showed higher wear rates than expected.
There were slight differences in acetabular radiolucency extent and width, partly in opposite directions (Table 4). The extent and width of radiolucencies around the femoral components did not differ (Appendix 1; supplemental materials are available with the online version of CORR). The total extent of linear osteolysis along visible acetabular and femoral cement-bone interfaces shows a weak difference on lateral femoral radiographs (Table 5). We observed no focal osteolysis in the pelvic bone. On the femoral side, focal osteolysis visible on frontal views was without exception located in the calcar region (PE: six of 27 hips; XLPE: five of 25 hips). One region of osteolysis was judged as large; all others in both groups were small. There was no difference between groups (p = 1.0). On lateral radiographs, there were two small regions of focal osteolysis in Gruen Zones 12 and 13 of an apparently stable stem where the XLPE cup had a complete linear osteolysis, indicating aseptic loosening. We observed no other focal osteolysis on the lateral radiographs in either group (PE: n = 24; XLPE: n = 25; p = 1.0). The median distance between upper lateral stem and inner cement contour was 0.3 mm (range, 0–3.1 mm) and correlated (p < 0.001) with stem subsidence. Twelve patients in the XLPE group had a distance of 0.2 mm or more, whereas the corresponding figure in the control group was 19 (p = 0.16).
Table 4.
Acetabular linear osteolysis and maximum osteolysis width using radiographic analysis (frontal projections)
| Variable | Charnley-DeLee Zone 1 | Charnley-DeLee Zone 2 | Charnley-DeLee Zone 3 | Charnley-DeLee Zone 4 | Charnley-DeLee Zone 5 | Charnley-DeLee Zone 6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE | XLPE | p value | PE | XLPE | p value | PE | XLPE | p value | PE | XLPE | p value | PE | XLPE | p value | PE | XLPE | p value | |
| Number of analyzable hips | 27 | 25 | 27 | 25 | 27 | 25 | 19 | 18 | 16 | 17 | 20 | 18 | ||||||
| Osteolysis (number of hips) | 0.22† | 0.02† | 0.7† | 0.3† | 0.1† | 0.3† | ||||||||||||
| 0% | 4 | 10 | 20 | 13 | 6 | 3 | 8 | 13 | 13 | 17 | 7 | 5 | ||||||
| 0%–49% | 8 | 4 | 1 | 9 | 12 | 10 | 4 | 3 | 1 | 0 | 5 | 8 | ||||||
| 50%–99% | 9 | 6 | 2 | 1 | 4 | 6 | 3 | 1 | 0 | 0 | 3 | 4 | ||||||
| 100% | 6 | 5 | 4 | 2 | 5 | 6 | 4 | 1 | 2 | 0 | 5 | 1 | ||||||
| Maximum linear osteolysis width (mm)* | 0.4 (0.3–0.5) | 0.2 (0–0.5) | 0.04‡ | 0 (0–0.3) | 0 (0–0.3) | 0.3‡ | 0.4 (0.3–0.9) | 0.3 (0.2–0.8) | 0.4‡ | 0.3 (0–1.0) | 0 (0–0.2) | 0.04‡ | 0 (0–0) | 0 (0–0) | 0.4‡ | 0.5 (0–1.1) | 0.5 (0–0.7) | 0.8‡ |
* Values are expressed as median, with 25 and 75 percentiles in parentheses; †exact test; ‡Mann-Whitney U test; PE = polyethylene; XLPE = highly crosslinked polyethylene.
Table 5.
Linear osteolysis extent (percentage of visible bone-cement interface)
| Location/Radiographic view | Percentage | p value* | |
|---|---|---|---|
| PE | XLPE | ||
| Acetabulum† | |||
| Frontal | 34 (17–52), n = 27 | 34 (18–54), n = 25 | 0.9 |
| Lateral | 14 (5–30), n = 16 | 14 (0–24), n = 17 | 0.7 |
| Femur‡ | |||
| Frontal | 0 (0–25), n = 27 | 0 (0–3), n = 25 | 0.4 |
| Lateral | 3 (0–15), n = 25 | 0 (0–21), n = 25 | 0.05 |
* Mann-Whitney U test; †values are expressed as median, with 25 and 75 percentiles in parentheses; ‡values are expressed as median, with range in parentheses; PE = polyethylene; XLPE = highly crosslinked polyethylene.
We detected no major differences in bone mineral density reduction but a tendency toward pronounced reduction in acetabular ROI 4 for the XLPE group (Table 6).
Table 6.
Change in DEXA cup and stem at 10 years compared to postoperative examination.
| ROI | PE | XLPE | p value* |
|---|---|---|---|
| Cup change (%) | n = 24 | n = 20 | |
| ROI 1 | −4 (−38 to 33) | −3 (−39 to 29) | 0.41 |
| ROI 2 | −3 (−22 to 49) | −1 (−32 to 82) | 0.64 |
| ROI 3 | 15 (−23 to 96) | 5 (−28 to 56) | 0.22 |
| ROI 4 | −4 (−56 to 74) | −22 (−53 to 22) | 0.04 |
| ROI 5 | −5 (−38 to 47) | −11 (−39 to 33) | 0.53 |
| Stem change (%) | n = 27 | n = 23 | |
| Upper femur (mean of ROIs 1, 2, 6, 7) | −14 (−33 to 2) | −9 (−40 to 4) | 0.69 |
| Lower femur (mean of ROIs 3–5) | 0 (−34 to 22) | −1 (−32 to 13) | 0.30 |
Values are expressed as median, with range in parentheses; * Mann-Whitney U test; DEXA = dual-energy x-ray analysis; ROI = region of interest; PE = polyethylene; XLPE = highly crosslinked polyethylene.
The median HHS was 95 (range, 67–100; n = 27) in the PE group and 95 (range, 47–100; n = 24) in the XLPE group (p = 0.83). The median HPS was 44 (range, 30–44; n = 27) in the PE group and 44 (range, 20–44; n = 24) in the XLPE group (p = 0.23). The two groups had equal revision rates: one of 30 in the PE group and two of 31 in the XLPE group (p = 1.0).
Discussion
Aseptic loosening is the most common reason for THA failure. PE wear particles are believed to be one of the major factors in this process. Reduced production of particles could therefore be expected to improve fixation. We evaluated (1) whether XLPE wear resistance persisted in long-term followup and whether these improved wear characteristics led to (2) improved fixation, (3) fewer radiolucencies, (4) less loss of bone mineral density, and (5) improved functional scores and lower revision rates in cemented THA compared to conventional PE at 10 years’ followup.
Our study is subject to certain limitations. First, the power calculations were performed for RSA wear only and the study may be underpowered in all other assessed variables. However, major effects of clinical relevance would probably be detected. Second, there is also a certain selection bias. The study patients were somewhat younger with a greater proportion of males and primary osteoarthritis compared to the total group of eligible patients during the recruitment period. This may limit generalizability, but comparative results are valid due to the randomization procedure. Third, the Spectron EF Primary® femoral component has an unusually high loosening rate [15, 19]. This is also the case in this study, with five of 61 (8%) implanted stems revised or planned to be revised. Bone cement particles and metal debris activate inflammatory cells in a manner quite similar to that of PE particles [42]. Both types of particles, besides PE, are present in a cemented THA [51]. Metal debris from a roughened femoral component loose in the cement mantle may reduce but would likely not eliminate important PE-related differences in all measured study variables.
We compared our results to those previously reported (Table 7). We found persisting low XLPE wear rate after the 2-year bedding-in period. Also, the conventional PE of this study has a comparably low wear rate, consistent with other reports [17, 53] and also associated with a lowered risk for osteolysis and aseptic loosening [14, 40]. There have been concerns that the remelting process of Durasul® XLPE manufacturing could diminish its long-term fatigue resistance [45], leading to increased wear rates. No such effect was observed by us. The low XLPE wear rate does not affect cup migration. There are no reported comparative studies, but in a 10-year case series, eight cemented XLPE cups were stable, except for a tendency to rotate from 5 years on [47]. There was a weak tendency toward increased stem subsidence and varus tilt in the PE group. If true, a possible explanation might be that abundant PE particles in the articulation prevented a slowly subsiding stem to restore stability within the cement mantle. Subsidence of a cemented stem with a rough surface will increase the amount of metallic and cement particles in the joint, which could be expected to increase the wear rate in these hips. The two revised loosed stems were not accompanied by accelerated wear in the XLPE group, and only one of three cups revised or waiting to be revised in the control group had higher wear rates than expected (Fig. 5). At revision, as observed by us and others [19], the synovial lining in these hips was occasionally grayish and all stems showed areas where the rough surface had become polished. Even if the observed cases are few, this observation speaks in favor of XLPE as resistant against third-body wear, at least when subjected to particulate bone cement and cobalt-chromium alloy.
Table 7.
Studies with minimum 5-year followup comparing wear, osteolysis, and clinical function score between XLPE and conventional PE
| Study | Design | THA type | Femoral head | XLPE/PE type | Mean followup (years)* | Annual linear steady state wear (mm/year)† | Wear assessment method | Osteolysis | Function score |
|---|---|---|---|---|---|---|---|---|---|
| Capello et al. [5] (2011) | Retrospective matched control | Uncemented | CoCr 28 mm | Crossfire®/Stryker® UHMWPE | 7.5 (6–10.2) | XLPE: 0.031 (0.01) PE: 0.14 (0.08) |
Image analysis Livermore et al. [32] |
XLPE no osteolysis | NR |
| Garcia-Rey et al. [17] (2008) | Randomized | Uncemented | CoCr 28 mm | Durasul®/Sulene® | 5.5 (5–7.7) | XLPE: 0.006 (0.007‡) PE: 0.036 (0.01‡) |
Image analysis Dorr and Wan [13] |
No osteolysis | No difference |
| Fukoi et al. [16] (2011) | Consecutive series | Uncemented/hybrid | Zirconia ceramic 26 mm | Longevity®/Zimmer® UHMWPE | 5.4 (5–6.4) | XLPE: 0.0068 (NR) PE: 0.01 (NR) |
Image analysis Easy Scale for Orthopaedics v 3.1.2. (Nexis, Tokyo, Japan) |
XLPE no osteolysis | NR |
| Leung et al. [31] (2007) | Randomized | Uncemented | CoCr 28 mm | Marathon®/Enduron® | 5 | XLPE: 0.01 (NR) PE: 0.2 (NR) |
Image analysis Martell and Berdia [33] |
XLPE reduced osteolysis | NR |
| McCalden et al. [34] (2009) | Randomized | Hybrid | CoCr 28 mm | Longevity®/Zimmer® UHMWPE | 6.8 (5.6–7.6) | XLPE: 0.003 (0.05‡) PE: 0.051 (0.04‡) |
Image analysis Martell and Berdia [33] |
NR | No difference |
| Olyslaegers et al. [39] (2008) | Retrospective matched control | Hybrid | CoCr 28 mm | Longevity®/Zimmer® UHMWPE | 5 | XLPE: 0.05 (NR) PE: 0.1 (NR) |
Image analysis Martell and Berdia [33] |
No difference | No difference |
| Rayadhyaksha et al. [43] (2009) | Retrospective matched control | Uncemented/hybrid | CoCr 28 mm | Crossfire®/Stryker® UHMWPE | 6.1 (5–8.1) | XLPE: 0.054 (0.047) PE: 0.13 (0.076) |
Image analysis Martell and Berdia [33] |
NR | No difference |
| Thomas et al. [52] (2011) | Randomized | Hybrid | CoCr 28 mm | Longevity®/Zimmer® UHMWPE | NR (7–7.8) | XLPE: 0.005 (0.02‡) PE: 0.037 (0.03‡) |
RSA Selvik [50] |
No difference | No difference |
| Current study | Randomized | Cemented | CoCr 28 mm | Durasul®/Sulene® | 10 | XLPE: 0.005 (0.01) PE: 0.055 (0.05) |
RSA Selvik [50] |
No difference | No difference |
* Values are expressed as mean, with range in parentheses; †values are expressed as mean, with SD in parentheses; ‡SD was calculated by 0.5 x 95% CI x √N, where N = number of participants in group; XLPE = highly crosslinked polyethylene; PE = polyethylene; NR = not reported; RSA = radiostereometry.
We found a slight tendency toward reduction of radiolucencies all together. There are no XLPE case series or reports comparing PE and XLPE with regard to radiolucencies in intermediate- or long-term cemented THA. However, several studies on uncemented THA have reported decreased rates and sizes of osteolysis with XLPE liners [2, 31]. Therefore, the observed tendencies may become stronger with longer followup. There was a strong correlation between femoral component subsidence and the distance from upper lateral stem to inner cement contour, as well as practically no radiolucencies between cement and bone on the femoral side. This suggests Spectron EF Primary® stems subside mainly within cement mantles, which corresponds to previous findings [19].
XLPE did not have any substantial effect on bone mineral density reduction around cup and stem. Notably, overall bone density increased medially and decreased below the cup, indicating central load bearing. Patterns of bone loss around cemented cups vary in the literature probably due to design differences and short followup times [9, 41]. Femoral bone loss was more pronounced proximally than distally, as previously reported [8, 55].
HHS and HPS indicate good functional results. Neither these scores nor revision rate differed between groups, complying with previous intermediate-term studies [17, 34, 39, 43, 52].
In conclusion, XLPE cups showed low wear rates at up to 10-year followup but were not accompanied by any clearly beneficial effects on implant fixation, radiolucencies, bone mineral density loss, function, or implant survival. The lack of such advantages in this study may be due to metal and cement debris [19] concealing the effect of low XLPE wear. Moreover, XLPE wear particles, despite smaller wear volumes, could cause a marked tissue response because of high biologic activity [23, 24]. Finally, several mechanisms other than particle-induced bone resorption are reportedly important for aseptic loosening and osteolysis [36, 51] in cemented THA. Further studies of XLPE used with cemented fixation are needed. These should focus on performance of this material past 10 years and when used with stems with better long-term performance than the design used by us.
Electronic supplementary material
Acknowledgments
We thank Atifeh Hariri (biomedical scientist), Bita Shareghi (biomedical scientist), Elinor Gustavsson (radiographer), and Erna Andreasson (research technician) for kind and skilled help with DEXA and RSA examination and measuring.
Footnotes
The institution of one or more of the authors (PEJ, PH, JT, JK) has received funding from Sulzer (Geneva, Switzerland) and Zimmer, Inc (Warsaw, IN, USA). One or more of the authors has received funding from The Swedish Research Council (JK), IngaBritt and Arne Lundberg Research Foundation (JK), and Doctor Felix Neubergh Research Foundation (PEJ, JK).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Sahlgrenska University Hospital, Gothenburg, Sweden.
References
- 1.Baxter RM, Ianuzzi A, Freeman TA, Kurtz SM, Steinbeck MJ. Distinct immunohistomorphologic changes in periprosthetic hip tissues from historical and highly crosslinked UHMWPE implant retrievals. J Biomed Mater Res A. 2010;95:68–78. doi: 10.1002/jbm.a.32813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitsch RG, Loidolt T, Heisel C, Ball S, Schmalzried TP. Reduction of osteolysis with use of Marathon cross-linked polyethylene: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2008;90:1487–1491. doi: 10.2106/JBJS.F.00991. [DOI] [PubMed] [Google Scholar]
- 3.Boss JH, Misselevich I, Behar J, Mendes DG. Histologic analysis of the periprosthetic tissues of long-term surviving cemented total hip arthroplasties. J Long Term Eff Med Implants. 1996;6:73–90. [PubMed] [Google Scholar]
- 4.Callaghan JJ, Cuckler JM, Huddleston JI, Galante JO. How have alternative bearings (such as metal-on-metal, highly cross-linked polyethylene, and ceramic-on-ceramic) affected the prevention and treatment of osteolysis? . J Am Acad Orthop Surg. 2008;16(suppl 1):S33–S38. doi: 10.5435/00124635-200800001-00008. [DOI] [PubMed] [Google Scholar]
- 5.Capello WN, D’Antonio JA, Ramakrishnan R, Naughton M. Continued improved wear with an annealed highly cross-linked polyethylene. Clin Orthop Relat Res. 2011;469:825–830. doi: 10.1007/s11999-010-1556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catelas I, Wimmer MA, Utzschneider S. Polyethylene and metal wear particles: characteristics and biological effects. Semin Immunopathol. 2011;33:257–271. doi: 10.1007/s00281-011-0242-3. [DOI] [PubMed] [Google Scholar]
- 7.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 8.Digas G, Karrholm J, Olofsson K. Five-year DEXA study of 88 hips with cemented femoral stem: influence of design variations on early migration of a cemented stem in THA. Int Orthop. 2009;33:1495–1500. doi: 10.1007/s00264-008-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Digas G, Karrholm J, Thanner J. Different loss of BMD using uncemented press-fit and whole polyethylene cups fixed with cement: repeated DXA studies in 96 hips randomized to 3 types of fixation. Acta Orthop. 2006;77:218–226. doi: 10.1080/17453670610045948. [DOI] [PubMed] [Google Scholar]
- 10.Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: two randomized studies using radiostereometric analysis. Acta Orthop. 2007;78:746–754. doi: 10.1080/17453670710014518. [DOI] [PubMed] [Google Scholar]
- 11.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. Highly cross-linked polyethylene in cemented THA: randomized study of 61 hips. Clin Orthop Relat Res. 2003;417:126–138. doi: 10.1097/01.blo.0000096802.78689.45. [DOI] [PubMed] [Google Scholar]
- 12.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. The Otto Aufranc Award. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res. 2004;429:6–16. doi: 10.1097/01.blo.0000150314.70919.e3. [DOI] [PubMed] [Google Scholar]
- 13.Dorr LD, Wan Z. Ten years of experience with porous acetabular components for revision surgery. Clin Orthop Relat Res. 1995;319:191–200. [PubMed] [Google Scholar]
- 14.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 15.Espehaug B, Furnes O, Engesaeter LB, Havelin LI. 18 years of results with cemented primary hip prostheses in the Norwegian Arthroplasty Register: concerns about some newer implants. Acta Orthop. 2009;80:402–412. doi: 10.3109/17453670903161124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukui K, Kaneuji A, Sugimori T, Ichiseki T, Kitamura K, Matsumoto T. Wear comparison between a highly cross-linked polyethylene and conventional polyethylene against a zirconia femoral head: minimum 5-year follow-up. J Arthroplasty. 2011;26:45–49. doi: 10.1016/j.arth.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A, Ortega-Chamarro J. New polyethylenes in total hip replacement: a prospective, comparative clinical study of two types of liner. J Bone Joint Surg Br. 2008;90:149–153. doi: 10.1302/0301-620X.90B2.19887. [DOI] [PubMed] [Google Scholar]
- 18.Glyn-Jones S, McLardy-Smith P, Gill HS, Murray DW. The creep and wear of highly cross-linked polyethylene: a three-year randomised, controlled trial using radiostereometric analysis. J Bone Joint Surg Br. 2008;90:556–561. doi: 10.1302/0301-620X.90B5.20545. [DOI] [PubMed] [Google Scholar]
- 19.Grose A, Gonzalez Della Valle A, Bullough P, Lyman S, Tomek I, Pellicci P. High failure rate of a modern, proximally roughened, cemented stem for total hip arthroplasty. Int Orthop. 2006;30:243–247. doi: 10.1007/s00264-005-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 21.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 22.Herberts P, Malchau H. Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthop Scand. 2000;71:111–121. doi: 10.1080/000164700317413067. [DOI] [PubMed] [Google Scholar]
- 23.Illgen RL, 2nd, Bauer LM, Hotujec BT, Kolpin SE, Bakhtiar A, Forsythe TM. Highly crosslinked vs conventional polyethylene particles: relative in vivo inflammatory response. J Arthroplasty. 2009;24:117–124. doi: 10.1016/j.arth.2008.01.134. [DOI] [PubMed] [Google Scholar]
- 24.Illgen RL, 2nd, Forsythe TM, Pike JW, Laurent MP, Blanchard CR. Highly crosslinked vs conventional polyethylene particles—an in vitro comparison of biologic activities. J Arthroplasty. 2008;23:721–731. doi: 10.1016/j.arth.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 25.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Ingham E, Fisher J, Rubash HE, Sinha RK, Shanbhag AS, Kim SY. Biological reactions to wear debris in total joint replacement: pathogenesis of bone loss after total hip arthroplasty. Proc Inst Mech Eng H. 2000;214:21–37. doi: 10.1243/0954411001535219. [DOI] [PubMed] [Google Scholar]
- 27.Ishiguro N, Kojima T, Ito T, Saga S, Anma H, Kurokouchi K, Iwahori Y, Iwase T, Iwata H. Macrophage activation and migration in interface tissue around loosening total hip arthroplasty components. J Biomed Mater Res. 1997;35:399–406. doi: 10.1002/(SICI)1097-4636(19970605)35:3<399::AID-JBM14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Jasty M, Maloney WJ, Bragdon CR, O’Connor DO, Haire T, Harris WH. The initiation of failure in cemented femoral components of hip arthroplasties. J Bone Joint Surg Br. 1991;73:551–558. doi: 10.1302/0301-620X.73B4.2071634. [DOI] [PubMed] [Google Scholar]
- 29.Kärrholm J, Razaznejad R. Fixation and bone remodeling around a low stiffness stem in revision surgery. Clin Orthop Relat Res. 2008;466:380–388. doi: 10.1007/s11999-007-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469:2262–2277. doi: 10.1007/s11999-011-1872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung SB, Egawa H, Stepniewski A, Beykirch S, Engh CA, Jr, Engh CA., Sr Incidence and volume of pelvic osteolysis at early follow-up with highly cross-linked and noncross-linked polyethylene. J Arthroplasty. 2007;22:134–139. doi: 10.1016/j.arth.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72:518–528. [PubMed] [Google Scholar]
- 33.Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs. J Bone Joint Surg Am. 1997;79:1635–1641. doi: 10.2106/00004623-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 34.McCalden RW, MacDonald SJ, Rorabeck CH, Bourne RB, Chess DG, Charron KD. Wear rate of highly cross-linked polyethylene in total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2009;91:773–782. doi: 10.2106/JBJS.H.00244. [DOI] [PubMed] [Google Scholar]
- 35.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17:157–167. doi: 10.1002/jor.1100170203. [DOI] [PubMed] [Google Scholar]
- 36.Mjoberg B. Theories of wear and loosening in hip prostheses: wear-induced loosening vs loosening-induced wear—a review. Acta Orthop Scand. 1994;65:361–371. doi: 10.3109/17453679408995473. [DOI] [PubMed] [Google Scholar]
- 37.Murray DW, Rushton N. Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg Br. 1990;72:988–992. doi: 10.1302/0301-620X.72B6.2246303. [DOI] [PubMed] [Google Scholar]
- 38.Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years. Clin Orthop Relat Res. 2010;468:3228–3233. doi: 10.1007/s11999-010-1379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olyslaegers C, Defoort K, Simon JP, Vandenberghe L. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty. 2008;23:489–494. doi: 10.1016/j.arth.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Oparaugo PC, Clarke IC, Malchau H, Herberts P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature. Acta Orthop Scand. 2001;72:22–28. doi: 10.1080/000164701753606644. [DOI] [PubMed] [Google Scholar]
- 41.Periasamy K, Watson WS, Mohammed A, Murray H, Walker B, Patil S, Meek RM. A randomised study of peri-prosthetic bone density after cemented versus trabecular fixation of a polyethylene acetabular component. J Bone Joint Surg Br. 2011;93:1033–1044. doi: 10.1302/0301-620X.93B8.26233. [DOI] [PubMed] [Google Scholar]
- 42.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2:102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajadhyaksha AD, Brotea C, Cheung Y, Kuhn C, Ramakrishnan R, Zelicof SB. Five-year comparative study of highly cross-linked (Crossfire) and traditional polyethylene. J Arthroplasty. 2009;24:161–167. doi: 10.1016/j.arth.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Ranstam J, Ryd L, Onsten I. Erratum: Accurate accuracy assessment: review of basic principles (Acta Orthopaedica Scandinavica (1999) 70 4 (319-321)) Acta Orthop Scand. 2000;71:106–108. doi: 10.1080/00016470052944017. [DOI] [PubMed] [Google Scholar]
- 45.Ries MD, Pruitt L. Effect of cross-linking on the microstructure and mechanical properties of ultra-high molecular weight polyethylene. Clin Orthop Relat Res. 2005;440:149–156. doi: 10.1097/01.blo.0000185310.59202.e5. [DOI] [PubMed] [Google Scholar]
- 46.Rohrl SM, Li MG, Nilsson KG, Nivbrant B. Very low wear of non-remelted highly cross-linked polyethylene cups: an RSA study lasting up to 6 years. Acta Orthop. 2007;78:739–745. doi: 10.1080/17453670710014509. [DOI] [PubMed] [Google Scholar]
- 47.Rohrl SM, Nivbrant B, Nilsson KG. No adverse effects of submelt-annealed highly crosslinked polyethylene in cemented cups. Acta Orthop. 2012;83:148–152. doi: 10.3109/17453674.2011.652889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmalzried TP, Dorey FJ, McKellop H. The multifactorial nature of polyethylene wear in vivo. J Bone Joint Surg Am. 1998;80:1234–1243. doi: 10.2106/00004623-199808000-00018. [DOI] [PubMed] [Google Scholar]
- 49.Schmalzried TP, Kwong LM, Jasty M, Sedlacek RC, Haire TC, O’Connor DO, Bragdon CR, Kabo JM, Malcolm AJ, Harris WH. The mechanism of loosening of cemented acetabular components in total hip arthroplasty: analysis of specimens retrieved at autopsy. Clin Orthop Relat Res. 1992;274:60–78. [PubMed] [Google Scholar]
- 50.Selvik G. Roentgen stereophotogrammetric analysis. Acta Radiol. 1990;31:113–126. doi: 10.3109/02841859009177472. [DOI] [PubMed] [Google Scholar]
- 51.Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–197. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 52.Thomas GE, Simpson DJ, Mehmood S, Taylor A, McLardy-Smith P, Gill HS, Murray DW, Glyn-Jones S. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93:716–722. doi: 10.2106/JBJS.J.00287. [DOI] [PubMed] [Google Scholar]
- 53.Triclot P, Grosjean G, Masri F, Courpied JP, Hamadouche M. A comparison of the penetration rate of two polyethylene acetabular liners of different levels of cross-linking: a prospective randomised trial. J Bone Joint Surg Br. 2007;89:1439–1445. doi: 10.1302/0301-620X.89B11.19543. [DOI] [PubMed] [Google Scholar]
- 54.Valstar ER, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop. 2005;76:563–572. doi: 10.1080/17453670510041574. [DOI] [PubMed] [Google Scholar]
- 55.Venesmaa PK, Kroger HP, Jurvelin JS, Miettinen HJ, Suomalainen OT, Alhava EM, Yli-Kyyny T, Tamminen I, Syri J, Venesmaa P, Kroger H. Periprosthetic bone loss after cemented total hip arthroplasty: a prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand. 2003;74:31–36. doi: 10.1080/00016470310013617. [DOI] [PubMed] [Google Scholar]
- 56.Zhang HY, Blunt L, Jiang XQ, Brown L, Barrans S, Zhao Y. Femoral stem wear in cemented total hip replacement. Proc Inst Mech Eng H. 2008;222:583–592. doi: 10.1243/09544119JEIM346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.