Abstract
Background
Modern metal-on-metal hip resurfacing arthroplasty designs have been used for over a decade. Risk factors for short-term failure include small component size, large femoral head defects, low body mass index, older age, high level of sporting activity, and component design, and it is established there is a surgeon learning curve. Owing to failures with early surgical techniques, we developed a second-generation technique to address those failures. However, it is unclear whether the techniques affected the long-term risk factors.
Questions/purposes
We (1) determined survivorship for hips implanted with the second-generation cementing technique; (2) identified the risk factors for failure in these patients; and (3) determined the effect of the dominant risk factors on the observed modes of failure.
Methods
We retrospectively reviewed the first 200 hips (178 patients) implanted using our second-generation surgical technique, which consisted of improvements in cleaning and drying the femoral head before and during cement application. There were 129 men and 49 women. Component orientation and contact patch to rim distance were measured. We recorded the following modes of failure: femoral neck fracture, femoral component loosening, acetabular component loosening, wear, dislocation, and sepsis. The minimum followup was 25 months (mean, 106.5 months; range, 25–138 months).
Results
Twelve hips were revised. Kaplan-Meier survivorship was 98.0% at 5 years and 94.3% at 10 years. The only variable associated with revision was acetabular component position. Contact patch to rim distance was lower in hips that dislocated, were revised for wear, or were revised for acetabular loosening. The dominant modes of failure were related to component wear or acetabular component loosening.
Conclusions
Acetabular component orientation, a factor within the surgeon’s control, determines the long-term success of our current hip resurfacing techniques. Current techniques have changed the modes of failure from aseptic femoral failure to wear or loosening of the acetabular component.
Level of Evidence
Level III, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Modern metal-on-metal hip resurfacing arthroplasty designs have been used for more than 10 years as an alternative prosthetic solution to conventional THA for patients with end-stage osteoarthritis [1, 10, 15, 18, 30, 33, 37, 38]. Identified risk factors for failure include female gender as a surrogate variable for component size [8, 29, 34], large femoral head defects [1], low BMI [27], older age at surgery [9], and component design [22, 34]. Recently, high levels of sporting activities have also been associated with revision of hip resurfacing [28]. In addition, several centers have shown a surgeon learning curve associated with this procedure [31, 32, 40].
We previously demonstrated a 63% reduction in the relative risk of femoral component aseptic failure with a 5.6-year mean followup [5] subsequent to modifications in surgical technique [3]. However, our previously reported 10-year study applied to the early cohort implanted with the first-generation technique [6]. Briefly, the modifications of the second-generation technique included an increase in the number of drilled holes in the dome and the chamfer area, the use of a high-speed burr for cleaning of the femoral defects, and the use of dome suction during the application of the cement [6].
The purposes of the present study were to (1) determine survivorship in the first 200 hips implanted with the second-generation cementing technique; (2) identify the risk factors for failure in these patients; and (3) determine the effect of the dominant risk factors on the observed modes of failure of this group of patients.
Patients and Methods
We retrospectively reviewed all 178 patients (200 hips) who received a hip resurfacing device (Conserve® Plus; Wright Medical Technology, Inc, Arlington, TN, USA) using a second-generation surgical technique [5] between March 2000 and January 2002. During that same time, we also treated 83 hips with other forms of primary arthroplasty. The Conserve® Plus device features a one-piece acetabular component made of cobalt and chromium, double heat-treated, and solution annealed. The porous coating consists of sintered beads 75 to 150 μm in size. The coverage of the head by the socket ranges from 158.9° to 163.3° for femoral head sizes ranging from 36 mm to 60 mm in 2-mm increments. The use of the Conserve® Plus prosthesis was approved by the Food and Drug Administration in December 2009. The contraindications for this implant were: (1) patients with renal dysfunction; (2) patients with a leg length discrepancy greater than 2 cm; and (3) patients with severe osteoporosis. We included patients regardless of etiology, gender, size, or bone quality. The average age of the cohort was 48.6 years (range, 15–78 years). There were 129 males (72.5%) and 49 females (27.5%). Primary osteoarthritis was the dominant diagnosis (Table 1). Ninety-three hips (46.5%) had femoral head defects greater than 1 cm. The minimum followup was 25 months (mean, 106.5 months; range, 25–138 months). Three patients were lost after 4, 9, and 15 months of followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. We obtained Institutional Review Board approval for the study. As part of the Conserve® Plus multicenter investigational device exemption study, the patients were followed annually until the device was approved by the Food and Drug Administration and then every 2 years if the status of the reconstruction did not require more regular followup visits.
Table 1.
Demographic characteristics of the study group
| Patient characteristics | Mean values (range) or count (percent) |
|---|---|
| Age at surgery (years) | 48.6 (15–78) |
| Weight (kg) | 80.6 (42–135) |
| Height (cm) | 174.8 (140–193) |
| Body mass index (kg/m2) | 26.2 (16–41) |
| Male/female ratio | 129 (72.5%)/49 (27.5%) |
| Etiology | |
| Osteoarthritis | 114 (57%) |
| DDH | 30 (15%) |
| Trauma | 19 (9.5%) |
| Osteonecrosis | 15 (7.5%) |
| LCP and SCFE | 12 (6%) |
| Inflammatory | 10 (5%) |
DDH = developmental dysplasia of the hip; LCP = Legg-Calvé-Perthes disease; SCFE = slipped capital femoral epiphysis.
All procedures were performed through a posterior approach. The surgical technique used for implantation of the devices has been described elsewhere [2]. The modifications made between the first 300 hips (first generation) and the next 371 (second generation) all aimed to reduce the rate of femoral aseptic failure (femoral component loosening or femoral neck fracture). A 3.2-mm drill bit was used to increase the bone-cement interface area by drilling numerous holes not only in the dome portion of the head, but also in the chamfered section. A high-speed burr was used to completely remove all cystic debris from the femoral head. A suction tip was inserted in the dome hole to keep the femoral head dry during the manual application of cement on the femoral head [5]. One hundred one hips (50.5%) were reconstructed with femoral components 46 mm or smaller in diameter.
All patients received prophylactic antibiotics for 2 days, adjusted low-dose warfarin for 3 weeks, and then aspirin for an additional 3 weeks. Walking was allowed on the first postoperative day, bearing weight as tolerated. Then crutches were used for 3 to 4 weeks. Physiotherapy was prescribed for 2 months, including hip abduction exercises, stretching of flexion contractures, hip flexion exercises, and active or active resistive muscular contractions.
The patients were followed 4 months after surgery and then annually. We used the UCLA hip scoring system [7] to assess pain, walking ability, function, and activity and the SF-12 [28] to evaluate the patient’s quality of life. In case a patient could not be followed in our clinic, a telephone consultation was arranged after the patient had forwarded to our office a set of recent radiographs performed at a local orthopaedist facility and following our protocol. One hundred thirty-one hips were last followed in a clinic setting and 69 by telephone consultation. Complications such as dislocations, nerve injuries, sepsis, masses or fluid collections, thromboembolic phenomena, or other blood management-related complications that were related to the implantation of the hip arthroplasty device were recorded. Fifty-seven hips did not have a 10-year minimum followup. This is reflected in our analysis by the width of the 95% confidence intervals.
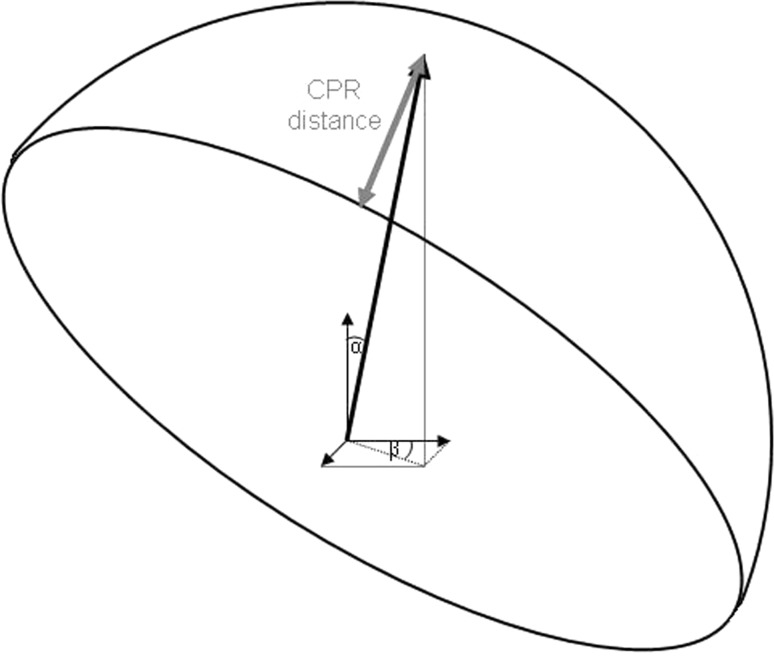
At each clinic visit, we obtained AP pelvis radiographs and crosstable lateral radiographs [23]. Two of us (KT, RW) measured acetabular component abduction and anteversion angles using Einzel-Bild-Röntgen-Analysis cup software, Version 2003 (University of Innsbruck, Innsbruck, Austria) [13, 26], a method for which the interobserver reliability is high (Cronbach α coefficient of 0.84) [12]. Contact patch to rim distance was computed as suggested by Langton et al. [25]. The contact patch to rim distance (Fig. 1) is the distance between the point of intersection of the hip reaction force and the closest point on the inner side of the cup rim. Four variables are needed for contact patch to rim distance calculation including femoral head coverage by the socket, femoral head size, acetabular component abduction angle, and acetabular component anteversion angle. In this calculation, the direction of the joint reaction force is a constant based on the results from Bergmann et al. [11] who found that, on average, the peak joint reaction force deviates medially 14° from the vertical axis and forward 16° from the transverse axis when the subject is in a standing position.
Fig. 1.
Schematic representing the calculation of the contact patch to rim (CPR) distance. Angle α (14°) is the medial deviation of the joint reaction force from vertical in the frontal plane and angle β (16°) is the forward deviation of the joint reaction force from the transverse axis in the horizontal plane. These values represent average direction measurements made by Bergmann et al. [11] for the standing position.
The date the patient was last contacted (and expressed that the prosthetic joint had not been revised) was used for implant survivorship calculation. We generated Kaplan-Meier survivorship curves using the time to any revision surgery as the end point and the log-rank test was used to compare survivorship rates between genders. We computed the Cox proportional hazard ratio to determine the effect of selected variables on the survivorship of the procedure. The following typical modes of failures for the procedure were defined: femoral neck fracture, aseptic femoral component loosening, aseptic acetabular component loosening, wear (including adverse local tissue reactions [36]), dislocation, and sepsis. Independent t-tests were used to compare the contact patch to rim distance of hips that dislocated, were revised for wear, or were revised for acetabular component loosening with that of the rest of the cohort. All statistical analyses were performed using Stata™ Version 6.0 (College Station, TX, USA).
Results
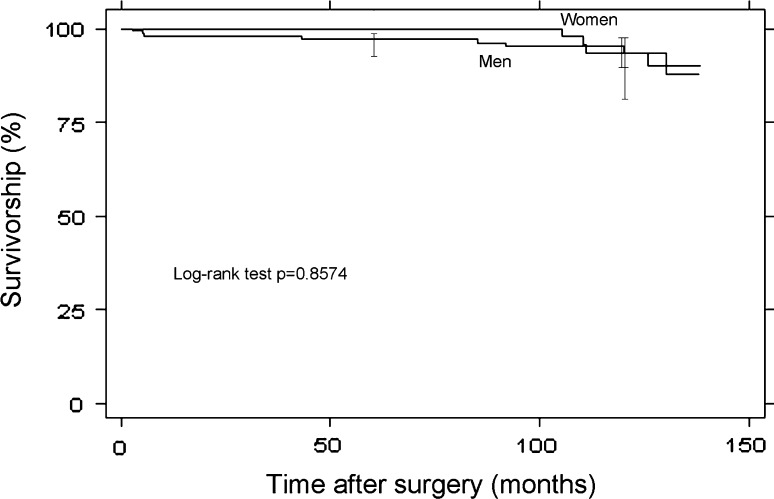
The 5-year Kaplan-Meier survivorship estimate was 98.0% (95% CI, 94.6%–99.2%). The 10-year Kaplan-Meier survivorship estimate was 94.3% (95% CI, 89.2%–97.0%). There was no difference (p = 0.857) in survivorship between male and female patients (Fig. 2). Twelve hips underwent revision surgery in this series. In one hip the acetabular component migrated through the acetabular wall and was reconstructed with a cemented crosslinked polyethylene acetabular component articulating with the original resurfacing femoral head [7]. Two hips sustained a femoral neck fracture 5 months after resurfacing and were converted to a conventional THA. Two hips had loosening of the femoral component 43 and 85 months after resurfacing and were converted to a conventional THA as well. Four hips had loosening of the acetabular component at an average of 108.5 months (range, 91–126 months). Three of these hips were maintained as metal-on metal resurfacing by inserting a new socket of larger thickness articulating with the original femoral component. The fourth one was converted to a conventional THA at an outside institution. Three hips were revised because of wear-related failures. The first of these patients had largely elevated ion levels (131 for cobalt serum level and 61 for chromium serum level) and an adverse local tissue reaction (fluid collection). The second had high ion levels (33 for cobalt serum level and 47 for chromium serum level) but no adverse local tissue reactions. The third one had a fluid collection identified by MRI but no ion studies were made before the revision, which was performed outside our facility. All three were converted to conventional THA, two with ceramic-on-crosslinked polyethylene bearings and one with a ceramic-on-metal bearing. The clinical outcome of these revision surgeries will be the subject of a future publication.
Fig. 2.
Kaplan-Meier survivorship curves of the first 200 hips operated on with the second-generation surgical technique and separated by gender. The time to revision surgery for any reason was used as the end point.
Of the variables studied, only acetabular component position increased the risk of revision (Table 2). The mean acetabular component abduction angle was 43.9° (range, 16°–71°). The mean acetabular component anteversion angle was 18.9° (range, 4°–51°). The mean contact patch to rim distance was 13.7 mm (range, 0.9–22.1 mm). Three subgroups showed lower contact patch to rim distances than the rest of the cohort: (1) hips that sustained dislocation episodes (p < 0.001) (mean contact patch to rim distance of 4.7 mm; range, 3.2–7.1 mm); (2) hips that were revised for high wear (p < 0.001) (mean contact patch to rim distance of 4.1 mm; range, 3.2–4.7 mm); and (3) hips that were revised for acetabular component loosening (p = 0.030) (mean contact patch to rim distance of 10.0 mm; range, 7.6–12.1 mm).
Table 2.
Cox proportional hazard ratios and significance levels for the risk factors commonly associated with failure of resurfacing
| Risk factor | Hazard ratio | p value | 95% CI |
|---|---|---|---|
| Female gender | 0.895 | 0.857 | 0.266–3.008 |
| Age at surgery | 0.973 | 0.223 | 0.930–1.017 |
| Component size | 0.950 | 0.442 | 0.835–1.082 |
| Femoral head defects > 1 cm | 1.143 | 0.817 | 0.368–3.550 |
| Body mass index | 0.986 | 0.840 | 0.8575–1.133 |
| Bilateral status | 0.1785 | 0.099 | 0.0230–1.383 |
| UCLA activity score | 0.948 | 0.823 | 0.593–1.515 |
| Stem-shaft angle | 0.958 | 0.399 | 0.866–1.059 |
| Cup abduction angle | 1.110 | 0.001 | 1.043–1.181 |
| Cup anteversion angle | 1.056 | 0.057 | 0.998–1.117 |
| Contact patch to rim distance | 0.830 | 0.003 | 0.7326–0.940 |
There were a total of 13 complications among the 200 hips (6.5%). These included three dislocations, two of which were related to excessive cup abduction angles and were successfully treated with closed reduction, whereas the third one was associated with excessive anteversion of the cup and underwent reorientation of the acetabular component 4 days after surgery. All three patients recovered and their hips were stable at last followup. Four patients had femoral nerve palsies that resolved without any treatment and were attributed to the use of a particular anterior pelvic stabilizer. Two patients had hematogenous sepsis determined by blood cultures, both controlled by débridement of the infected tissues surrounding the prosthesis and a course of antibiotics. Four patients had blood-related complications including one with thrombophlebitis for which warfarin prophylactic treatment was maintained, two with hematomas, one of which was evacuated, and one with a bleed for which warfarin was held for 2 days.
All UCLA hip scores and SF-12 scores improved from preoperative to last followup (Table 3). The mean postoperative physical score of the SF-12 did not differ from the general US population mean, which is 50.12 ± 9.45. However, the mean postoperative mental score of the SF-12 was greater (p < 0.001) than that of the general US population (50.04 ± 9.59).
Table 3.
Summary of mean preoperative and postoperative UCLA and SF-12 scores
| Score | Preoperative score | Last followup score |
|---|---|---|
| UCLA | ||
| Pain | 3.3 (1–7) | 9.4 (6–10) |
| Walking | 6.1 (2–9) | 9.7 (6–10) |
| Function | 5.5 (2–10) | 9.6 (4–10) |
| Activity | 4.4 (1–8) | 7.5 (4–10) |
| SF-12 | ||
| Physical | 31.9 (9.0–56.8) | 51.1 (26.9–61.5) |
| Mental | 47.4 (15.2–67.0) | 53.4 (14.7–64.7) |
Ranges shown in parentheses.
Discussion
Ten-year clinical and survivorship results of metal-on-metal hip resurfacing have been reported [6, 14, 16, 39] but are still not available from a majority of centers performing this type of surgery. Also, of the three reports with 10-year data, only two used currently available devices [6, 39]. Both studies showed the results of the early cases but further data are needed to account for the learning curve experienced by most surgeons taking on hip resurfacing and the surgical technique modifications they made over time. The purposes of the present study were to (1) determine survivorship in the first 200 hips implanted with the second-generation cementing technique; (2) identify the risk factors for failure in these patients; and (3) determine the effect of the dominant risk factors on the observed modes of failure of this group of patients.
Readers should note the limitations of our study. First, the contact patch to rim distance uses a constant direction for the joint reaction force, which is based on a static standing position, therefore not taking into consideration the interindividual differences in the orientation of the femoral neck nor the large variations associated with the diverse ambulatory activities [11]. However, we believe its computation still constitutes a useful assessment of cup positioning with possible applications beyond the simple prediction of wear issues. Second, the results from this study were obtained using a single hip resurfacing design and may not be applicable to other designs because large differences in performance have been reported between devices [19], which are most likely related to the design and amount of head coverage by the acetabular component, but may also be the result of manufacturing issues such as tolerances in roundness and clearance. Nevertheless, improvements in femoral head preparation and precision of the cup placement can be achieved with any design. Third, this report focuses on a series of hips implanted more than 10 years ago, long before the literature had any mention of cup positioning being related to excessive wear of metal-on-metal prostheses. At the time cup position was not a real concern, the size of the head being sufficient to avoid most dislocation problems. This explains why a number of hips presented a cup orientation outside of our recommended range. However, hip resurfacing is a complex surgical procedure that has been associated with a learning curve [31, 32, 40] and a small number of such outliers may still occur even with experienced surgeons. The senior author has been implanting hip resurfacing devices since 1975 and it is unlikely that identical results can be achieved immediately by a surgeon taking on resurfacing without sufficient prior training.
The 94.3% survivorship at 10 years from this series demonstrates the validity of the concept of hip resurfacing because it compares favorably with the survivorship of all conventional THAs in patients 50 to 59 years old (90% for men and less than 93% for women at 10 years) reported by the Swedish hip registry [35]. The patients in our study were included regardless of etiology, gender, size, or bone quality. No changes were made in our indications for resurfacing between the first- and second-generations series, and the percent of hips with femoral head defects or small components was comparable between the hips operated on with the first- and second-generation surgical techniques. However, femoral aseptic failure only occurred in 2% of the hips in this study. The cementation of the stem in all hips presenting large femoral head defects in this series is likely to have contributed to the observed improvement [4].
The most important result from this study is the shift in risk factors for the procedure in comparison with our series implanted with the first-generation technique. In our 10-year minimum report of the first 100 hips implanted with Conserve® Plus, large femoral defects, low BMI, and small component size were independent risk factors [6] (Table 4). In this study, none of these three variables showed any association with revision surgery and this demonstrates the efficiency of the surgical changes implemented between the two series, which aimed to reduce the rate of femoral aseptic failure.
Table 4.
Literature review of the studies presenting 10-year data for metal-on-metal hip resurfacing devices
| Study | Journal | Year | Design used | Survivorship |
|---|---|---|---|---|
| Bohm et al. [14] | Hip International | 2006 | Wagner | 17 revisions in 54 hips (69% survival) with 12- to 15-year followup |
| Daniel et al. [16] | JBJS Br | 2010 | McMinn | 93% in the 1994-1995 group; 84% in the 1996 group |
| Amstutz et al. [6] | JBJS Am | 2010 | Conserve Plus | 88.5% at 10 years |
| Treacy et al. [39] | JBJS Br | 2011 | BHR | 93.5% at 10 years |
| Current study | CORR | 2012 | Conserve Plus | 94.3% at 10 years |
JBJS Am = Journal of Bone and Joint Surgery, American volume; JBJS Br = Journal of Bone and Joint Surgery, British volume; CORR = Clinical Orthopaedics and Related Research; BHR = Birmingham hip resurfacing (Smith & Nephew, Warwick, UK); McMinn = McMinn Hybrid Resurfacing (Corin Medical Ltd, Cirencester, UK); Wagner = Wagner metal-on-metal resurfacing (Zimmer GmbH, Winterthur, Switzerland).
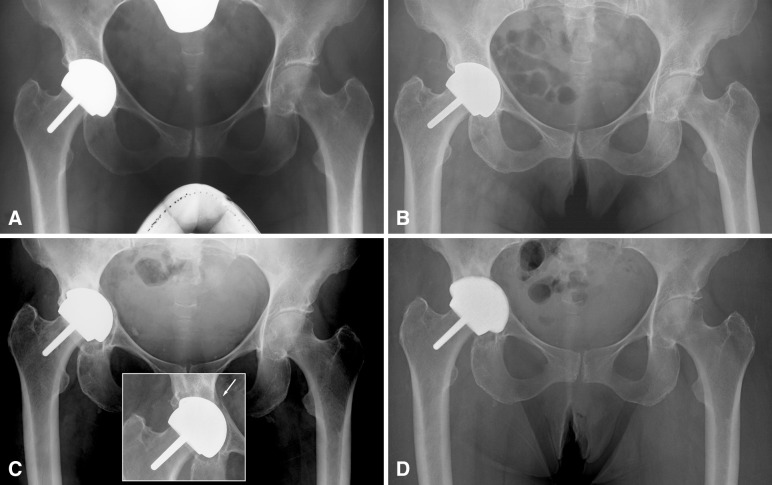
In addition, the dominant mode of failures also changed from short- to medium-term aseptic femoral failures to longer-term acetabular loosening or wear-related revisions. Concurrently, the only risk factor remaining for the procedure in patients operated on with the Conserve® Plus prosthesis and second-generation surgical technique is the positioning of the acetabular component, a variable that is well within the surgeon’s control. The effects of excessive abduction and anteversion angles of the cup on the wear properties of metal-on-metal bearings have now been well identified [17, 20, 24], and the measurement of the distance between the point of application of the joint reaction force and the rim of the acetabular component is a useful tool in predicting the risk of edge loading leading to increased wear rates [25]. In our series, this association was clear with all three hips revised for wear showing very low contact patch to rim distances. However, there may also be an association of low contact patch to rim distance with a mechanical mode of failure. It is possible that the repetitive application of the joint reaction force to a peripheral point of the cup would lead to its loosening, as suggested by Hulst et al. [21] (Fig. 3). Our recommendation for cup orientation with the Conserve® Plus components is to keep the abduction angle within ± 10° of 42° and the anteversion angle within ± 10° of 15°.
Fig. 3A–D.
(A) Five-month postoperative AP radiograph of a 63-year-old woman who received hip resurfacing for idiopathic osteoarthritis. The 38-mm inside diameter acetabular component was implanted with 56° of abduction and 22° of anteversion, which yielded a contact patch to rim distance of 7.6 mm. The bone-cup interface shows no radiolucencies, suggesting good fixation. (B) Two and a half years after surgery, there is radiolucency visible in DeLee and Charnley Zone III. (C) Nine years after surgery, the radiolucency has expanded around the cup indicating its loosening. The cup has also migrated into the acetabular wall, which showed an unexplained fracture but no complete loss of fixation 6 years after surgery (inset). (D) The reconstruction was maintained as a hip resurfacing and a cup of larger thickness with a titanium backing (Biofoam®; Wright Medical Technology Inc, Arlington, TN, USA) was inserted to articulate with the original resurfacing femoral component.
The results from this study suggest high clinical scores and survivorship can be achieved at 10 years with hip resurfacing in a young and highly active patient population, even with small components and in the presence of large femoral defects.
Acknowledgments
We thank Kohtaroh Takamura and Regina Woon for the measurements of acetabular component position using the EBRA software.
Footnotes
The institution of one or more of the authors (HCA) has received, in any one year, funding from St Vincent Medical Center, Los Angeles, CA, USA, and Wright Medical Technologies Inc, Arlington, TN, USA. Each author certifies that he (HCA), or a member of their immediate family, has or may receive payments or benefits, in any one year, an amount in excess of $100,000, from Wright Medical Technology Inc, Arlington, TN, USA.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that a waiver of informed consent for participation in the study was obtained from the institution.
References
- 1.Amstutz H, Beaulé P, Dorey F, Duff M, Campbell P, Gruen T. Metal-on-metal hybrid surface arthroplasty: two to six year follow-up. J Bone Joint Surg Am. 2004;86:28–39. [PubMed] [Google Scholar]
- 2.Amstutz H, Beaulé P, Dorey F, Duff M, Campbell P, Gruen T. Metal-on-metal hybrid surface arthroplasty—surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1):234–249. doi: 10.2106/JBJS.F.00273. [DOI] [PubMed] [Google Scholar]
- 3.Amstutz H, Duff M. Eleven years of experience with metal-on-metal hybrid hip resurfacing: a review of 1000 Conserve Plus. J Arthroplasty. 2008;23(Suppl 1):36–43. doi: 10.1016/j.arth.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Amstutz H, Duff M. Cementing the metaphyseal stem in metal-on-metal resurfacing: when and why. Clin Orthop Relat Res. 2009;467:79–83. doi: 10.1007/s11999-008-0570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amstutz H, Duff M, Campbell P, Dorey F. The effects of technique changes on aseptic loosening of the femoral component in hip resurfacing. Results of 600 Conserve Plus with a 3-9 year follow-up. J Arthroplasty. 2007;22:481–489. doi: 10.1016/j.arth.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Amstutz H, Duff M, Campbell P, Gruen T, Wisk L. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92:2663–2671. doi: 10.2106/JBJS.I.01715. [DOI] [PubMed] [Google Scholar]
- 7.Amstutz H, Su E, Duff M, Fowble V. Are there benefits to one- versus two-stage procedures in bilateral hip resurfacing? Clin Orthop Relat Res. 2011;469:1627–1634. doi: 10.1007/s11999-010-1627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amstutz H, Wisk L, Duff M. Sex as a patient selection criterion for metal-on-metal hip resurfacing arthroplasty. J Arthroplasty. 2011;26:198–208. doi: 10.1016/j.arth.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Association AO. AOA National Joint Replacement Registry. Available at: http://www.dmac.adelaide.edu.au/aoanjrr/documents/AnnualReports2011/AnnualReport_2011_WebVersion.pdf; updated 2011. Accessed October 25, 2011.
- 10.Back D, Dalziel R, Young D, Shimmin A. Early results of primary Birmingham hip resurfacings. An independent prospective study of the first 230 hips. J Bone Joint Surg Br. 2005;87:324–329. doi: 10.1302/0301-620X.87B3.15556. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda G. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34:859–871. doi: 10.1016/S0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 12.Biedermann R, Krismer M, Stockl B, Mayrhofer P, Ornstein E, Franzen H. Accuracy of EBRA-FCA in the measurement of migration of femoral components of total hip replacement. Einzel-Bild-Rontgen-Analyse-femoral component analysis. J Bone Joint Surg Br. 1999;81:266–272. doi: 10.1302/0301-620X.81B2.8842. [DOI] [PubMed] [Google Scholar]
- 13.Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87:762–769. doi: 10.1302/0301-620X.87B6.14745. [DOI] [PubMed] [Google Scholar]
- 14.Bohm R, Schraml A, Schuh A. Long-term results with the Wagner metal-on-metal hip resurfacing prosthesis. Hip Int. 2006;16:58–64. doi: 10.1177/112070000601604S12. [DOI] [PubMed] [Google Scholar]
- 15.Daniel J, Pynsent PB, McMinn D. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–188. doi: 10.1302/0301-620X.86B2.14600. [DOI] [PubMed] [Google Scholar]
- 16.Daniel J, Ziaee H, Kamali A, Pradhan C, Band T, McMinn D. Ten-year results of a double-heat-treated metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2010;92:20–27. doi: 10.2106/JBJS.H.01821. [DOI] [PubMed] [Google Scholar]
- 17.Haan R, Pattyn C, Gill H, Murray D, Campbell P, Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 18.Smet K. Belgium experience with metal-on-metal surface arthroplasty. Orthop Clin North Am. 2005;36:203–213. doi: 10.1016/j.ocl.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Graves S, Rothwell A, Tucker K, Jacobs J, Sedrakian A. A multinational assessment of metal-on-metal bearings in hip replacement. J Bone Joint Surg Am. 2011;93(Suppl 3):43–47. doi: 10.2106/JBJS.K.01220. [DOI] [PubMed] [Google Scholar]
- 20.Hart A, Buddhdev P, Winship P, Faria N, Powell J, Skinner J. Cup inclination angle of greater than 50 degrees increases whole blood concentrations of cobalt and chromium ions after metal-on-metal hip resurfacing. Hip Int. 2008;18:212–219. doi: 10.1177/112070000801800304. [DOI] [PubMed] [Google Scholar]
- 21.Hulst J, Ball S, Wu G, Duff M, Woon R, Amstutz H. Survivorship of Conserve® Plus monoblock metal-on-metal hip resurfacing sockets: radiographic midterm results of 580 patients. Orthop Clin North Am. 2011;42:153–159. doi: 10.1016/j.ocl.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Jameson S, Langton D, Nargol A. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92:28–37. doi: 10.1302/0301-620X.92B1.22769. [DOI] [PubMed] [Google Scholar]
- 23.Johnson C. A new method for roentgenographic examination of the upper end of the femur. J Bone Joint Surg Am. 1932;14:859–866. [Google Scholar]
- 24.Langton D, Jameson S, Joyce T, Hallab N, Natu S, Nargol A. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 25.Langton D, Sprowson A, Joyce T, Reed M, Carluke I, Partington P, Nargol A. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91:1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 26.Langton D, Sprowson A, Mahadeva D, Bhatnagar S, Holland J, Nargol A. Cup anteversion in hip resurfacing: validation of EBRA and the presentation of a simple clinical grading system. J Arthroplasty. 2009;25:607–613. doi: 10.1016/j.arth.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Duff M, Amstutz H, Dorey F. Metal-on-metal hip resurfacing for obese patients. J Bone Joint Surg Am. 2007;89:2705–2711. doi: 10.2106/JBJS.F.01563. [DOI] [PubMed] [Google Scholar]
- 28.Le Duff M, Amstutz HC. The relationship of sporting activity and survivorship after hip resurfacing. J Bone Joint Surg Am. 2012;94. [DOI] [PubMed]
- 29.McBryde C, Theivendran K, Thomas A, Treacy R, Pynsent P. The influence of head size and sex on the outcome of Birmingham hip resurfacing. J Bone Joint Surg Am. 2010;92:107–112. doi: 10.2106/JBJS.I.00197. [DOI] [PubMed] [Google Scholar]
- 30.Nishii T, Sugano N, Miki H, Takao M, Koyama T, Yoshikawa H. Five-year results of metal-on-metal resurfacing arthroplasty in Asian patients. J Arthroplasty. 2007;22:176–183. doi: 10.1016/j.arth.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Nunley R, Zhu J, Brooks P, Anderson Engh CJ, Raterman S, Rogerson J, Barrack R. The learning curve for adopting hip resurfacing among hip specialists. Clin Orthop Relat Res. 2010;468:382–391. doi: 10.1007/s11999-009-1106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill M, Beaule P, Bin Nasser A, Garbuz D, Lavigne M, Duncan C, Kim P, Schemitsch E. Canadian academic experience with metal-on-metal hip resurfacing. Bull NYU Hosp Jt Dis. 2009;67:128–131. [PubMed] [Google Scholar]
- 33.Pollard T, Baker R, Eastaugh-Waring S, Bannister G. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88:592–600. doi: 10.1302/0301-620X.88B5.17354. [DOI] [PubMed] [Google Scholar]
- 34.Prosser G, Yates P, Wood D, Graves S, Steiger R, Miller L. Outcome of primary resurfacing hip replacement: evaluation of risk factors for early revision. Acta Orthop. 2010;468:382–391. doi: 10.3109/17453671003685434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahlgrenska University Hospital DoO. The Swedish national hip arthroplasty register—annual report. Available at: www.shpr.se. 2009:1–107. Accessed October 25, 2011.
- 36.Schmalzried T. Metal-metal bearing surfaces in hip arthroplasty. Orthopedics. 2009;32. pii: orthosupersite.com/view.asp?rID = 42831. [DOI] [PubMed]
- 37.Silva M, Lee K, Heisel C, Dela Rosa M, Schmalzried T. The biomechanical results of total hip resurfacing arthroplasty. J Bone Joint Surg Am. 2004;86:40–46. doi: 10.2106/00004623-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Treacy R, McBryde C, Pynsent P. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87:167–170. doi: 10.1302/0301-620X.87B2.15030. [DOI] [PubMed] [Google Scholar]
- 39.Treacy R, McBryde C, Shears E, Pynsent P. Birmingham hip resurfacing: a minimum follow-up of ten years. J Bone Joint Surg Br. 2011;93:27–33. doi: 10.1302/0301-620X.93B1.24134. [DOI] [PubMed] [Google Scholar]
- 40.Witjes S, Smolders J, Beaulé P, Pasker P, Susante J. Learning from the learning curve in total hip resurfacing: a radiographic analysis. Arch Orthop Trauma Surg. 2009;129:1293–1299. doi: 10.1007/s00402-009-0875-z. [DOI] [PubMed] [Google Scholar]