Abstract
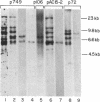
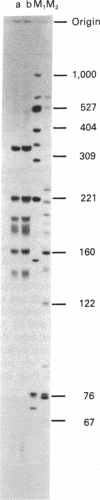
Southern blots of rat genomic DNA indicate the existence of at least 12 EcoRI DNA fragments containing actin gene sequences. By using specific probes and stringent conditions of hybridization, it was found that only one of these fragments contains sequences of the skeletal muscle alpha-actin gene. Recombinant bacteriophages originating from eight different actin genes were isolated from rat genomic DNA libraries. One of them, Act 15, contains the skeletal muscle actin gene. Another clone, Act I, contains a gene coding for a cytoplasmic actin, identified tentatively as the beta-actin gene. Both genes have a large intron very close to the 5' end of their transcribed region, followed by several small introns. DNA sequence analysis and comparison with the available data on actin genes in other organisms indicated an interesting relationship between the positions of introns and the evolutionary relatedness. Several intron sites are conserved from at least the echinoderms to the vertebrates; others appear to be present in some actin genes and not in others.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blake C. C. Exons and the structure, function and evolution of haemoglobin. Nature. 1981 Jun 25;291(5817):616–616. doi: 10.1038/291616a0. [DOI] [PubMed] [Google Scholar]
- Durica D. S., Schloss J. A., Crain W. R., Jr Organization of actin gene sequences in the sea urchin: molecular cloning of an intron-containing DNA sequence coding for a cytoplasmic actin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5683–5687. doi: 10.1073/pnas.77.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Timm R., Kimmel A. R., McKeown M. Unusual nucleotide sequences at the 5' end of actin genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6206–6210. doi: 10.1073/pnas.76.12.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 May;77(5):2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., de Baetselier A., Walker M. D., Behar L., Lehrach H., Frischauf A. M., Littauer U. Z. Brain tubulin and actin cDNA sequences: isolation of recombinant plasmids. Nucleic Acids Res. 1980 Aug 25;8(16):3553–3564. doi: 10.1093/nar/8.16.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Rosbash M., Davidson N. Determination of cellular RNA concentrations by electron microscopy of R loop-containing DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):2820–2824. doi: 10.1073/pnas.78.5.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K. L., Firtel R. A. Identification and analysis of Dictyostelium actin genes, a family of moderately repeated genes. Cell. 1978 Nov;15(3):763–778. doi: 10.1016/0092-8674(78)90262-3. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., Mertz J. E., Ross J. The precursor of mouse beta-globin messenger RNA contains two intervening RNA sequences. Cell. 1978 Jul;14(3):681–693. doi: 10.1016/0092-8674(78)90251-9. [DOI] [PubMed] [Google Scholar]
- Lu R. C., Elzinga M. Partial amino acid sequence of brain actin and its homology with muscle actin. Biochemistry. 1977 Dec 27;16(26):5801–5806. doi: 10.1021/bi00645a025. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Katcoff D., Carmon Y., Zevin-Sonkin D., Levi Z., Shaul Y., Shani M., Yaffe D. Identification of recombinant phages containing sequences from different rat myosin heavy chain genes. Nucleic Acids Res. 1980 May 24;8(10):2133–2146. doi: 10.1093/nar/8.10.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M. A., Keller E. B. The chromosomal arrangement of two linked actin genes in the sea urchin S. purpuratus. Nucleic Acids Res. 1981 Feb 11;9(3):591–604. doi: 10.1093/nar/9.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Nudel U., Zevin-Sonkin D., Zakut R., Givol D., Katcoff D., Carmon Y., Reiter J., Frischauf A. M., Yaffe D. Skeletal muscle actin mRNA. Characterization of the 3' untranslated region. Nucleic Acids Res. 1981 Feb 11;9(3):579–589. doi: 10.1093/nar/9.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Zevin-Sonkin D., Saxel O., Carmon Y., Katcoff D., Nudel U., Yaffe D. The correlation between the synthesis of skeletal muscle actin, myosin heavy chain, and myosin light chain and the accumulation of corresponding mRNA sequences during myogenesis. Dev Biol. 1981 Sep;86(2):483–492. doi: 10.1016/0012-1606(81)90206-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Rich A. Chick cytoplasmic actin and muscle actin have different structural genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2346–2350. doi: 10.1073/pnas.73.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The amino acid sequence of actin from chicken skeletal muscle actin and chicken gizzard smooth muscle actin. FEBS Lett. 1979 Jun 15;102(2):219–222. doi: 10.1016/0014-5793(79)80004-6. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]