Abstract
Background
Reconstruction rings and bone allografts have been proposed to manage severe acetabular bone loss. However, a high early failure rate of the Graft Augmentation Prosthesis (GAP) II reinforcement ring (Stryker Orthopaedics, Mahwah, NJ, USA) has been reported in one small series.
Questions/Purposes
We therefore determined (1) the survival of this device in combination with impacted morselized allograft bone in patients with severe defects and (2) the complication rate.
Methods
We retrospectively reviewed 24 patients (21 aseptic and three septic) with severe acetabular bone loss (10 hips with Type III defects and 14 with Type IV defects according to the American Academy of Orthopaedic Surgeons classification). We determined function and numbers of failures. The minimum followup was 24 months (mean, 34 months; range, 24–72 months).
Results
At latest followup, the reconstruction had failed in nine of the 24 patients: six with aseptic loosening, three with infection. The average postoperative Merle d’Aubigné-Postel score of the patients whose reconstructions had not failed was 16.6 points; at latest followup, these patients had radiographic evidence of incorporation and consolidation of bone allografts. Seven of the nine patients whose reconstructions had failed underwent reoperation. Fatigue fracture of the ring at the plate-cup union occurred in five patients at an average of 45 months postoperatively. All patients with failed reconstructions who underwent reoperation were treated with Trabecular Metal™ (Zimmer Inc, Warsaw, IN, USA) cups and were functioning well at latest followup.
Conclusions
We observed a high rate (37%) of early catastrophic failures of the GAP II reconstruction ring, particularly in patients with Type IV defects. Due to this high failure rate, we have abandoned its use.
Level of Evidence
Level IV, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Substantial bone loss has been a major concern in revision THAs [1, 14, 23, 24]. Patients with more than one failed revision surgery frequently have severe cavitary and segmented defects. Kurtz et al. [15] projected, between 2005 and 2030, the number of THAs would increase 137%.
Impaction grafting has been a well-established method, with a reported survivorship for revision THAs at 51.7 months of 95.8% (95% CI, 92.3%–99.1%) [6]. In cases presenting with severe bone loss, the technique has required additional devices [11, 18], such as reconstruction rings or cages with iliac and ischial fixation, to transform these complex, uncontained defects in contained deficiencies [4].
The Graft Augmentation Prosthesis (GAP) II (Stryker Orthopaedics, Mahwah, NJ, USA) reconstruction ring is a grit-blasted titanium hydroxyapatite-coated reconstruction ring with an inferior hook fixed to the teardrop, multiple dome screws through the cup, and two superior plates with screw fixation to the pelvis. One previous study [9] using this device reported failures in five of 12 patients at 5 to 8 years with Type II and III defects according to the system of the American Academy of Orthopaedic Surgeons (AAOS) [7].
To confirm whether we had a similarly high rate of failure, we determined (1) the survival of the GAP II reinforcement ring and impacted morselized allograft bone used in patients with severe acetabular defects and (2) the complication rate.
Patients and Methods
From 2003 to 2007, we implanted 24 GAP II reinforcement rings in combination with impacted bone allografts for acetabular reconstruction. The specific indications for use of this cage were AAOS Type III or IV bone loss. We performed 51 revisions in 2003 (two GAPs, 4%), 67 in 2004 (0 GAPs), 74 in 2005 (six GAPs, 8%), 80 in 2006 (six GAPs, 7.5%), and 81 in 2007 (10 GAPs, 12%). During the study time, we also implanted the Burch-Schneider cage (Zimmer Inc, Warsaw, IN, USA) (five patients) in AAOS Type III or IV defects and the Kerboull ring (Implantes Fico SRL, Buenos Aires, Argentina) (three patients) in AAOS Type III defects, always in combination with impacted bone allografts. The right hip was affected in 14 patients and the left hip in 10. There were 20 women and four men with an average age of 69 years (range, 41–84 years). Initial diagnosis was osteoarthritis in 15 patients, developmental dysplasia of the hip in five, and rheumatoid arthritis in four. The indication for revision surgery was aseptic loosening in 19 patients, septic failures in three, and periprosthetic fractures in two.
Patients with infection underwent a two-staged protocol [8]. Three of the 24 patients underwent their index THA at our institution. The preoperative Merle d’Aubigné-Postel score [8] averaged 4.1 points. The average number of previous surgeries was two for patients without infection and three for those with infection. In 23 of the 24 patients, acetabular revision was combined with a femoral revision. None of the patients died or were lost to followup. The minimum followup was 24 months (mean, 34 months; range, 24–72 months). We had prior approval of our local ethics review board.
We classified acetabular deficiencies radiographically before surgery and confirmed the classification after removal of components during the procedure using the system reported by the AAOS classification [7] (Table 1). Fourteen patients had a Type IV defect and 10 had a Type IIIB defect. All patients underwent the procedure using a posterolateral approach under hypotensive epidural anesthesia in a laminar flow operating room.
Table 1.
Epidemiology and results
| Patient | Age (years) | Sex | Primary diagnosis | Number of previous surgeries | Revision diagnosis | AAOS defect | Followup (months) | Preoperative MDA | Postoperative MDA | Number of screws | Migration (mm) | Plate fracture | Allograft incorporation | Clinical failure | Radiographic failure | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | Female | OA | 1 | AL | III | 30 | 6 | 18 | 5 | < 5 | No | Yes | No | No | |
| 2 | 84 | Female | OA | 2 | AL | IV | 81 | 8 | 16 | 5 | < 5 | No | Yes | No | No | |
| 3 | 70 | Female | OA | 3 | AL | III | 45 | 8 | 16 | 5 | < 5 | No | Yes | No | No | PPFx |
| 4 | 71 | Female | OA | 2 | AL | IV | 58 | 5 | 15 | 5 | < 5 | No | Yes | No | No | |
| 5 | 56 | Male | ON | 1 | AL | IV | 54 | 6 | 18 | 4 | < 5 | No | Yes | No | No | |
| 6 | 56 | Female | OA | 1 | AL | III | 31 | 7 | 16 | 5 | < 5 | No | Yes | No | No | |
| 7 | 72 | Female | OA | 1 | AL | III | 58 | 8 | 18 | 4 | < 5 | No | Yes | No | No | |
| 8 | 84 | Female | OA | 2 | SL | IV | 51 | 4 | 16 | 4 | < 5 | No | Yes | No | No | |
| 9 | 77 | Female | OA | 1 | AL | III | 78 | 7 | 16 | 4 | < 5 | No | Yes | No | No | |
| 10 | 83 | Female | OA | 1 | AL | III | 22 | 6 | 16 | 4 | < 5 | No | Yes | No | No | |
| 11 | 62 | Female | OA | 3 | AL | IV | 32 | 6 | 18 | 6 | 5 | No | Yes | No | No | |
| 12 | 79 | Male | OA | 2 | AL | III | 48 | 6 | 16 | 4 | < 5 | No | Yes | No | No | |
| 13 | 74 | Female | OA | 1 | AL | IV | 22 | 6 | 16 | 5 | < 5 | No | Yes | No | No | |
| 14 | 73 | Female | OA | 1 | AL | IV | 41 | 8 | 16 | 8 | < 5 | No | Yes | No | No | D |
| 15 | 61 | Female | ON | 1 | PPFx | III | 36 | 3 | 18 | 4 | < 5 | No | Yes | No | No | |
| 16 | 77 | Male | OA | 2 | AL | III | 26 | 8 | 16 | 6 | 6 | No | No | Yes | Yes | |
| 17 | 41 | Female | DDH | 1 | PPFx | IV | 34 | 6 | 18 | 4 | > 5 | No | Yes | No | Yes | |
| 18 | 75 | Female | OA | 1 | AL | IV | 32 | 3 | 16 | 6 | > 5 | No | Yes | No | Yes | |
| 19 | 69 | Female | OA | 4 | SL | IV | 53 | 6 | F | 4 | > 5 | Both | No | Yes | Yes | I |
| 20 | 69 | Female | OA | 4 | SL | III | 54 | 8 | F | 4 | < 5 | No | No | Yes | Yes | D + I |
| 21 | 78 | Female | OA | 2 | AL | IV | 55 | 5 | F | 4 | > 5 | Both | Yes | Yes | Yes | |
| 22 | 67 | Female | DDH | 4 | AL | IV | 50 | 7 | F | 5 | > 5 | 1 plate | Yes | Yes | Yes | D |
| 23 | 57 | Female | DDH | 5 | AL | IV | 27 | 6 | F | 5 | > 5 | Both | No | Yes | Yes | I |
| 24 | 74 | Male | OA | 2 | AL | IV | 43 | 6 | F | 7 | > 5 | 1 plate | No | Yes | Yes |
AAOS = American Academy of Orthopaedic Surgeons; MDA = functional Merle d’Aubigné-Postel score; OA = osteoarthritis; ON = osteonecrosis; DDH = developmental dysplasia of the hip; AL = aseptic loosening; SL = septic loosening; PPFx = periprosthetic fracture; D = dislocation; I = infection.
We ruled out the presence of active infection during single-stage surgeries or second-stage reimplantations in all patients using intraoperative frozen-section biopsy [17], bacteriologic studies, and permanent histology of removed tissues. For all implants, we completely removed the polymethylmethacrylate, granulation tissue, and interface and identified the remaining acetabulum to determine the type of bone defect.
We obtained bone allografts from frozen femoral heads from our own bank following the protocol of the American Association of Tissue Banks for the harvesting and processing of grafts [10]. We made 7- to 10-mm cancellous chips with a grinder from the unwashed bone allografts and mixed for 15 minutes with 1 g dry powdered vancomycin/femoral head, according to the method in previous investigations [2, 5]. Grafts were impacted according to the technique described by the Nijmegen group [20, 22] using specific instruments (Exchange Revision Instruments System; Stryker). In cases with a pelvic discontinuity, we impacted the bone allograft until the fracture was stabilized. Once we obtained stability, we fixed the ring to the iliac bone and to the teardrop. All patients had a GAP II implant used in their reconstruction, with a 28-mm polyethylene liner cemented into place. The outer diameter of the liners we used ranged from 48 to 54 mm, with an average of 50 mm. We used an average of five screws/ring (range, four to eight screws). The average operative time was 146 minutes (range, 90–190 minutes).
In patients without evidence of preoperative infection, we prescribed antibiotic prophylaxis with 1 g cefazolin every 8 hours for 48 hours. All patients with a previous infection received the same intravenous antibiotic treatment used after the first stage; antibiotics were stopped when the infection was controlled. Routine prophylaxis for thromboembolic disease was continued for the first postoperative month; this consisted of intravenous heparin during surgery, early postoperative mobilization, 0.4 mg enoxaparin in patients with a high clinical risk of thromboembolic disease (ie, malignancy, particularly if associated with chemotherapy; antiphospholipid syndrome, immobility, or a history of venous thromboembolism; administration of tamoxifen, raloxifene, oral contraceptives, or estrogen; morbid obesity; stroke; atherosclerosis; an American Society of Anesthesiologists physical status classification of 3 or greater), and 325 mg aspirin in patients with a low clinical risk [19]. We did not routinely prescribe prophylaxis against heterotopic calcification. The mean transfusion requirements were 2.5 concentrated red blood cell units/patient (range, one to five units).
The rehabilitation protocol included early mobilization 48 hours after surgery, ambulation with a walker, and toe-touch weightbearing on the operated side for 90 days. After that, we encouraged patients to progressively increase weightbearing as tolerated with the use of a cane for at least 1 month.
We collected data prospectively using an information sheet that had individual data for each patient. Two authors (MAB, DMD) evaluated patients clinically and radiographically at 15, 45, 90, and 180 days postoperatively and then yearly using the Merle d’Aubigné-Postel scoring system [8]. We routinely obtained pelvic AP and frog leg lateral views. We evaluated allograft incorporation by the radiographic criteria described by Slooff et al. [22] and evaluated graft consolidation of the acetabular graft. Consolidation was defined by the presence of trabecular bone crossing the graft-host junction in both views. We defined clinical failure as having had further acetabular revision regardless of the reason. We considered a hip functional when pain was absent or slight during walking (with or without the use of a cane) and when there were no radiographic findings indicating the possibility of future surgery. These radiographic findings were a fractured hook and/or screws, a fracture of any of both plates, and progressive radiolucencies or more than 5 mm of migration [21]. We defined a stable GAP ring when the device had an intact hook, screws and both plates, and less than 5 mm of migration in the horizontal or vertical plane at latest followup.
We determined survival using the Kaplan-Meier survival method [12] using acetabular clinical or radiographic failure as the end point with SPSS® for Windows® 17 (IBM Corp, Armonk, NY, USA).
Results
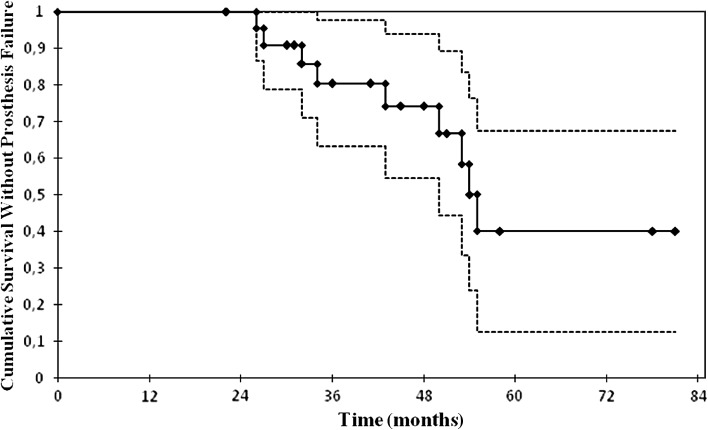
The survival at 34 months with acetabular clinical or radiographic failure as the end point was 67% (95% CI, 63%–98%) (Fig. 1). With clinical or radiographic acetabular failure as the end point, we observed failure in nine of 24 patients at last followup (Table 1). These failures included six patients with aseptic loosening, five with fracture of the ring at an average of 45 months (range, 27–55 months), and three with deep infection. The fractures started at the plate-cup union in all patients, and one patient had an intrapelvic protrusio with an iliac artery pseudoaneurysm treated at another institution by a vascular surgeon and then sent to ours with an active infection that we treated with a two-stage protocol (Fig. 2). We considered three patients with more than 5 mm of migration and allograft resorption to be radiographic failures, although they were not reoperated on (Fig. 3). Fifteen patients had a stable ring, eight of whom had AAOS Type III defects and seven Type IV defects; 14 had a previous diagnosis of aseptic loosening and one had an infection. These 15 patients had an average postoperative Merle d’Aubigné-Postel score [8] of 16.6 points, an average horizontal migration of 1.4 mm medially, and an average vertical migration of 2.2 mm proximally. Radiographic incorporation and consolidation of bone allografts occurred in all 15 patients.
Fig. 1.
Kaplan-Meier femoral survivorship analysis with clinical or radiographic failure as the end point is shown. Each diamond symbol represents a censored case. Dotted lines = 95% CIs. The survival at the mean followup of 34 months was 67% (95% CI, 63%–98%).
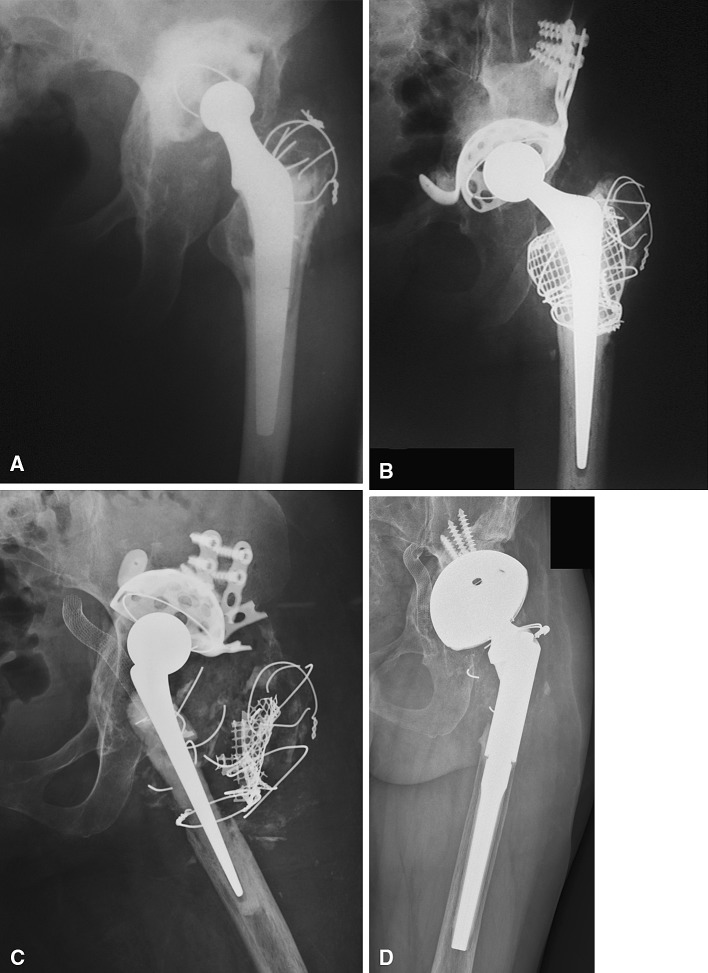
Fig. 2A–D.
AP radiographs show the left hip of Patient 23 (A) with an aseptic failed primary cemented Charnley THA (Thackray, Leeds, UK), (B) after revision surgery with acetabular reconstruction using a GAP reinforcement ring and impacted bone allografts, (C) with intrapelvic protrusio of the failed ring and a surgically treated iliac artery pseudoaneurysm, and (D) 2 years after the second revision (two-stage protocol) with a Trabecular Metal™ cup and an uncemented modular stem.
Fig. 3A–C.
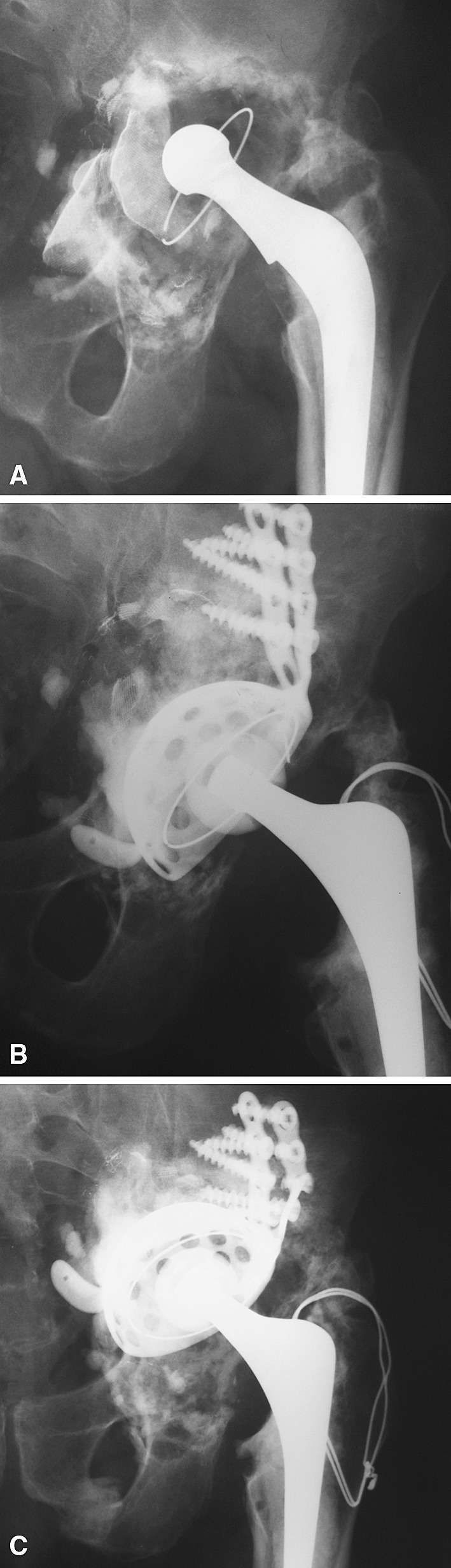
AP radiographs show the left hip of Patient 24 (A) with an aseptically loose primary cemented Charnley THA and massive bone loss, (B) with immediate postoperative reconstruction using impacted bone allografts and a GAP ring, and (C) with allograft resorption, intrapelvic migration, and plate fracture 2 years postoperatively.
Of the six patients who underwent reoperation, five had AAOS Type IV defects and one had a Type III defect. All of these patients underwent reoperation with Trabecular Metal™ cups (Trabecular Metal™ Acetabular Revision System; Zimmer Inc) without allograft. We observed partial incorporation of the previously impacted bone allograft in the reoperated patients. Among these patients, we observed three dislocations, one of which had a femoral head dissociation with entrapment of the head into the ring [3]. We treated this patient with open reduction and changed the femoral head. Another patient with dislocation also underwent reoperation by cementing a constrained liner into the ring. We treated the third patient nonoperatively.
Discussion
Reconstruction rings and bone allografts have been proposed for the management of severe acetabular bone defects in revision hip surgery. A high early failure rate of the GAP II reinforcement ring was previously reported in one small series of patients with AAOS Type II and III acetabular defects [9]. To confirm whether we had a similarly high rate of failure, we determined the survival of the GAP II reinforcement ring and impacted morselized allograft bone used in patients with severe acetabular defects and the complication rate.
Limitations to this study included the following. First, we had a small patient cohort. These were not, however, common cases, and even in high-volume centers, the numbers were relatively small. Second, we had short-term followup, with a mean of 34 months. The mean time to failure of those patients whose rings fractured was 45 months. Thus, we might anticipate the number of failures will increase. However, the failure rate was high and we believed it important to confirm the previous report describing early failures [9]. Third, we lacked a control group of patients with similar age, weight, and acetabular defects in which alternate approaches were used. However, others using impaction grafting and supplementary devices have not reported such a large number of early failures [16, 21]. Fourth, we did not perform radiostereometric studies, which would likely detect earlier failures and perhaps predict some failures among those patients who were functioning well at the time of latest followup.
Due to limited information in the literature, we were prompted to report our short-term findings with the GAP II reinforcement ring because of the early catastrophic failures we observed in combination with impacted morselized bone allografts. Duffy et al. [9] observed seven failures at an average 5-year followup when using this device in a series of 12 patients undergoing primary and revision THAs. The authors revised five patients because of fatigue failure of the implant associated with allograft resorption and two patients for recurrent dislocations. Because of this high mechanical failure rate, they abandoned this device in favor of implants with more mechanical strength [9]. We believed graft resorption was a cause of stress shielding and no bearing led to micromotion or played a major role in these failures. The absence of structural bone may have also explained these fatigue failures. Kerboull et al. [13] reported a 92% survivorship at 13 years with loosening of the acetabular component as the end point using the Kerboull ring and structural bone in patients with AAOS Type III and IV defects (Table 2).
Table 2.
Results of acetabular reconstructions with bone loss using different methods
| Study | Reconstructive method | Number of hips | Success rate (%) | Mean followup (years) | Complications related to the reconstruction |
|---|---|---|---|---|---|
| Kerboull et al. [13] | Kerboull ring and structural bone | 53 | 92 | 10 | 1 PNP |
| Udomkiat et al. [24] | 3 different cages | 62 | 83 | 4.6 | 23% dislocation rate |
| Lunn et al. [16] | Kerboull ring and impaction grafting | 35 | 82 | 5 | 5 hook fractures, 2 broken screws, 5 graft resorptions, 3 dislocations |
| Sporer and Paprosky [23] | Trabecular Metal™ cup with or without augments | 13 | 92 | 2.6 | 1 possible radiographic loosening |
| Duffy et al. [9] | GAP ring and impaction grafting | 17 | 58 | 5 | 5 ring fractures |
| Sembrano and Cheng [21] | 5 different cages, with and without bone allograft or DBM | 72 | 80 | 5.1 | 3 loosenings, 3 infections |
| Kosashvili et al. [14] | Antiprotrusio cage and Trabecular Metal™ | 26 | 88 | 3.5 | 2 dislocations, 1 infection, 1 PNP |
| Ballester Alfaro and Sueiro Fernandez [1] | Trabecular Metal™ augments and antiprotrusio cage | 19 | 100 | 2.2 | 1 PNP, 1 acute infection |
| Current study | GAP ring and impaction grafting | 24 | 62 | 3 | 5 ring fractures, 6 aseptic failures, 3 deep infections |
DBM = demineralized bone matrix; PNP = peroneal nerve palsy.
Two of the three patients with infection (Patients 19 and 20) came to our hospital with a chronic infection and were treated with a two-stage protocol. Both patients had a reinfection. The fatigue failure of the ring in these particular cases could have been related to bone allograft resorption as a consequence of the sepsis.
All patients who underwent reoperation in our series were treated with a Trabecular Metal™ cup and did not require new operations, with a minimum followup of 14 months and a maximum of 32 months. After this initial experience with this type of ring, we decided to start using Trabecular Metal™ cups, most of the time in combination with bone allograft. Modular Trabecular Metal™ revision systems have not been used long enough to provide definitive recommendations; however, Trabecular Metal™ cups have presented high survival rates both for our short-term followup series of patients and for other institutions (Table 2). We particularly believe these types of cages were created for defects that were common in the past, but currently bone loss has been a much more serious complication, and newer and more resistant constructions are needed, such as the Trabecular Metal™ cup-cage constructs.
One of the main proposed advantages of this implant was plate malleability that allowed these plates to adapt to the host bone. However, this feature could have been related to fatigue fracture, as the broken rings in this series were associated with one or two fractured plates. We observed early catastrophic failures of the GAP II reconstruction ring (37% at an average of 34 months’ followup), particularly in Type IV defects. Of the six reoperations, five had AAOS [7] Type IV defects and one had a Type III defect. Due to this high failure rate at short-term followup, we abandoned its use and strongly recommend avoiding its indication in Type IV defects.
Acknowledgment
The authors thank Juan Manuel Lopez Ovenza, MD, for help with the statistics.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ballester Alfaro JJ, Sueiro Fernandez J. Trabecular metal buttress augment and the trabecular metal cup-cage construct in revision hip arthroplasty for severe acetabular bone loss and pelvic discontinuity. Hip Int. 2010;27(suppl 7)(S7):119–127. [DOI] [PubMed]
- 2.Buttaro M, Comba F, Piccaluga F. Vancomycin-supplemented cancellous bone allografts in hip revision surgery. Clin Orthop Relat Res. 2007;461:74–80. doi: 10.1097/BLO.0b013e318073c290. [DOI] [PubMed] [Google Scholar]
- 3.Buttaro M, Comba F, Piccaluga F. Modular femoral head dissociation after dislocation and entrapment in reconstruction ring: a case report. Hip Int. 2007;17:49–51. doi: 10.1177/112070000701700110. [DOI] [PubMed] [Google Scholar]
- 4.Buttaro MA, Comba F, Pusso R, Piccaluga F. Acetabular revision with metal mesh, impaction bone grafting, and a cemented cup. Clin Orthop Relat Res. 2008;466:2482–2490. doi: 10.1007/s11999-008-0442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttaro M, Pusso R, Piccaluga F. Vancomycin-supplemented impacted bone allografts in infected hip arthroplasty. J Bone Joint Surg Br. 2005;87:314–319. doi: 10.1302/0301-620X.87B3.14788. [DOI] [PubMed] [Google Scholar]
- 6.Comba F, Buttaro M, Pusso R, Piccaluga F. Acetabular reconstruction with impacted bone allografts and cemented cups: 2 to 13 years follow-up study of 142 aseptic revisions. J Bone Joint Surg Br. 2006;88:865–869. doi: 10.1302/0301-620X.88B7.17227. [DOI] [PubMed] [Google Scholar]
- 7.D’Antonio JA, Capello WN, Borden LS. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1989;243:126–134. [PubMed] [Google Scholar]
- 8.d’Aubigne RM, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed] [Google Scholar]
- 9.Duffy GP, O’Connor MI, Brodersen MP. Fatigue failure of the GAP ring. J Arthroplasty. 2007;22:711–714. doi: 10.1016/j.arth.2006.12.108. [DOI] [PubMed] [Google Scholar]
- 10.Fawcett K, Barr AR. Tissue Banking (American Association of Blood Banks) Basel, Switzerland: Karger; 1987. pp. 97–107. [Google Scholar]
- 11.Gie GA, Linder L, Ling RS, Simon JP, Slooff TJ, Timperley AJ. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg Br. 1993;75:14–21. doi: 10.1302/0301-620X.75B1.8421012. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 13.Kerboull M, Hamadouche M, Kerboull L. The Kerboull acetabular reinforcement device in major acetabular reconstructions. Clin Orthop Relat Res. 2000;378:155–168. doi: 10.1097/00003086-200009000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Kosashvili Y, Backstein D, Safir O, Lakstein D, Gross AE. Acetabular revision using an anti-protrusion (ilio-ischial) cage and trabecular metal acetabular component for severe acetabular bone loss associated with pelvic discontinuity. J Bone Joint Surg Br. 2009;91:870–876. doi: 10.1302/0301-620X.91B7.22181. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 16.Lunn JV, Kearns SS, Quinlan W, Murray P, O’Byrne J. Impaction allografting and the Kerboull acetabular reinforcement device 35 hips followed for 3–7 years. Acta Orthop. 2005;76:296–302. [PubMed] [Google Scholar]
- 17.Núñez L, Buttaro M, Morandi A, Pusso R, Piccaluga F. The value of intraoperative frozen section analysis in revision hip surgery. Acta Orthop. 2007;78:226–230. doi: 10.1080/17453670710013726. [DOI] [PubMed] [Google Scholar]
- 18.Piccaluga F. González Della Valle A, Encinas JC, Pusso R. Femoral stem revision with impaction grafting and a Charnley stem: a two- to twelve-year follow-up study. J Bone Joint Surg Br. 2002;84:544–549. doi: 10.1302/0301-620X.84B4.12484. [DOI] [PubMed] [Google Scholar]
- 19.Salvati EA, Sharrock NE, Westrich G, Potter HG, Valle AG, Sculco TP. The 2007 ABJS Nicolas Andry Award. Three decades of clinical, basic, and applied research on thromboembolic disease after THA: rationale and clinical results of a multimodal prophylaxis protocol. Clin Orthop Relat Res. 2007;459:246–254. doi: 10.1097/BLO.0b013e31805b7681. [DOI] [PubMed] [Google Scholar]
- 20.Schreurs BW, Slooff TJ, Gardeniers JW, Buma P. Acetabular reconstruction with bone impaction grafting and a cemented cup: 20 years’ experience. Clin Orthop Relat Res. 2001;393:202–215. doi: 10.1097/00003086-200112000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Sembrano JN, Cheng EY. Acetabular cage survival and analysis of factors related to failure. Clin Orthop Relat Res. 2008;466:1657–1665. doi: 10.1007/s11999-008-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slooff TJ, Schimmel JW, Buma P. Cemented fixation with bone grafts. Orthop Clin North Am. 1993;24:667–677. [PubMed] [Google Scholar]
- 23.Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty. 2006;21(6 suppl 2):87–90. doi: 10.1016/j.arth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Udomkiat P, Dorr LD, Won YY, Longjohn D, Wan Z. Technical factors for success with metal ring acetabular reconstruction. J Arthroplasty. 2001;16:961–969. doi: 10.1054/arth.2001.27669. [DOI] [PubMed] [Google Scholar]