Abstract
Background
Metal-on-metal (MoM) THAs have reduced wear rates compared with metal-on-polyethylene. However, elevated serum metal ion levels and pseudotumors have been reported in large MoM articulations.
Questions/purposes
We therefore determined (1) if corrosion occurred at the cone/taper interface leading to instability in patients with large-diameter THAs; (2) how patients presented clinically and radiographically; (3) if adverse periprosthetic tissue reactions occurred; (4) whether metal was released from the implants into the periprosthetic tissues; and (5) if head size correlated with metal release.
Methods
We reviewed 114 patients who had revisions of large-diameter head MoM articulations. Mean time of implantation was 46 months. To identify adverse reactions and particle load, tissues were stained by hematoxylin and eosin and CD3/CD20/CD68 antibodies. Periprosthetic tissues were analyzed for metal content and distribution in different regions. Electrochemical reactions between the stem and adapter were investigated by a minicell electrode.
Results
Electrochemical studies on the stem and the head adapter showed a risk for galvanic corrosion. Ninety-four percent of patients had instability at the cone/taper interface. All patients presented with early clinical symptoms; 59 patients had radiographic signs of loosening. One hundred four patients had foreign body reactions and necrosis. The largest amounts of metal released were titanium or iron. We found no correlation between head size and metal ion release.
Conclusions
These findings suggest that in modular cone/taper connections, friction of the MoM articulations may cause failure of the cone/taper interface leading to galvanic corrosion and loosening. It is unclear whether the design of this MoM system provides sufficient stability at the taper.
Introduction
Metal-on-metal (MoM) articulations in THAs have been described with 28-mm modular heads [41]. These THAs made from cobalt-chromium alloy were introduced to overcome the problem of polyethylene (PE) wear-induced osteolysis [4]. Surface replacements of the hip are based on MoM as well. In case of a failed femoral component, the revision on the femoral side usually is not difficult and the patients can be revised using a standard implant. The cups are generally well fixed and revision of those cups may lead to major bone loss [16]. Therefore, large-diameter MoM modular ball heads (eg, 36-mm heads and larger) and/or thinner necks were developed as salvage implants that are compatible with standard stems to avoid cup revision [12]. Many surgeons use larger heads in primary THA in MoM articulations as well [32]. The modularity of these large-diameter heads can provide correct reconstruction of leg length and offset.
The MoM technology has been associated with low volumetric wear using 28-mm heads [6, 9]. However, in failed cases of MoM THAs with 28-mm heads, lymphocytic infiltrations have been observed in patients with cobalt accumulation in the periprosthetic tissues [29]. Various adverse biologic reactions in the periprosthetic tissue have been observed [28, 31–34]. Concern has also been expressed that surfaces from implants may enhance corrosion, promoting release of metal ions into surrounding tissues and possibly causing systemic toxic, carcinogenic, or allergic reactions [2, 18, 19, 22, 42]. Metallic debris accumulates in tissues adjacent to the implant [35], in the regional [14] and in distant lymph nodes, the liver, and spleen [24]. Increased levels of trace metal have been reported in serum and urine [7], especially if there is loosening of the prosthesis [40].
Metallic wear debris is not biologically inert: metal accumulation in local synovial tissue is associated with an inflammatory reaction [17, 28, 44], necrosis of the joint capsule [1], and bone resorption [20] leading to implant loosening [21]. Additionally, pseudotumors resulting from severe wear [31, 44] or aseptic loosening [31, 45] with large MoM articulations (eg, hip resurfacings) have been recognized [5]. We wondered whether loosening could be associated with insufficient stability at the cone/taper interface and corrosion leading to metallic debris into the surrounding tissues.
We therefore determined: (1) if corrosion occurred at the cone/taper interface leading to instability in patients with revised large-diameter THAs; (2) how patients presented clinically and radiographically; (3) if an adverse reactions to the metallic debris occurred; (4) if the metal released from the implant components was retained in periprosthetic tissues; and (5) if head size correlated with metal release from the unstable cone/taper interfaces.
Materials and Methods
We retrospectively reviewed all 110 patients who had 114 revisions of large-diameter head MoM THAs between 2009 and 2010. Between 2004 and 2008 we performed large-diameter head MoM THAs in 650 patients with 805 hips. The indications for surgery were groin pain and limping. Septic loosening was excluded by aspiration before and microbiological sampling at the time of surgery. There were 63 male and 51 female patients. The age ranged from 26 to 82 years (mean age, 62.7 years). The time of implantation was 26 to 68 months (average, 46 months). All patients had previous implantation of a MoM articulation with an LDH® head (Zimmer Inc, Warsaw, IN, USA) and a DUROM® hip cup (Zimmer Inc). Eight patients had bilateral implants, four of which were revised bilaterally. Four patients had unilateral revision and are under close surveillance of the contralateral implant.
Radiographs were obtained preoperatively to document loosening or osteolysis. Fifty-nine patients had radiographic signs of osteolysis or radiolucent lines [3, 15]. Nine patients had osteolysis/radiolucent lines of the cup only; in 39 cases, there were radiographic signs of osteolysis/radiolucencies on the stem side and in 11 cases around both the cup and stem.
The LDH® head as the modular head combined with a cobalt-chromium sleeve and the DUROM® hip cup are described by Lavigne et al. [27]. As stems, 72 CLS®, 18 Muller® straight stems, or 24 Weber-Stuehmer® stems had been implanted. Except the cemented Muller® straight stems, all stems were uncemented. The modular sleeve of the LDH® head also consists of a cobalt-chromium alloy. The tapers are made from titanium alloy in case of CLS® and Weber-Stuehmer® stems and from iron-based alloy (Protasul®) in case of the Muller® straight stems. The head diameters ranged from 48 to 58 mm with an average of 46 mm.
Intraoperatively we recorded presence of pseudotumors [33], macroscopic metallosis, loose cone/taper connection, and stability of the stems (tested using a sliding hammer connected to the neck of the stem following a standard protocol provided by the manufacturer). All cups were removed and the articulations were exchanged to ceramic-on-polyethylene bearings. During the revisions surgeries, 107 of the 114 heads (94%) heads were loose on the taper. However, the sleeve/head interface appeared well fixed in all cases. Four to five tissue samples were obtained from the bursa/pseudotumor, capsule, tissue from behind the acetabular cup after cup removal, fibrous tissue in the proximal femur, and from the revised stem and were fixed in 5% formalin.
The histological samples were processed by routine methods stained with hematoxylin and eosin. Ten sections of 3 to 4 μm were made and were reviewed by three independent investigators (CHL, HM, TM) for signs of ALVAL (aseptic lymphocyte-dominated vasculitis associated lesion [42]) and scored as described by Willert et al. [42] with no or only isolated phagocytized particles without major macrophage reaction (−), a few particles phagocytized in some spots and/or accumulated perivascularly in the lymphatics (+, few), evident accumulation of particles phagocytized in macrophages also perivascular in the lymphatics (++, many), tissue loaded with particles, including foreign body granulomas (+++, abundant) and tissue overstuffed with particles, and foreign body granulomas dominating the structures everywhere (++++ , excessive). A similar grading was developed for lymphocytic infiltration with differentiation diffuse/perivascular infiltration (per field of view). All three observers agreed on both scoring schemes in all cases.
Three to four sections of each sample had immunohistochemical staining with monoclonal anti-CD3 antibody for detection of T-lymphocytes and with macrophage-specific monoclonal anti-CD68 antibody performed to differentiate between a foreign body (macrophage-dominated) and an ALVAL (lymphocyte-dominated reaction).
The metal content was measured in different areas of the periprosthetic tissues. The tissues were sampled at the time of surgery from the bursa, the capsule, from the proximal femur, and behind the acetabular cup after implant removal. Tissues were then fixed in 5% formalin. All tissues were dried at 55°C to constant weight followed by chemical digestion with 65% nitric acid and 30% hydrogen peroxide in a microwave oven (EN 13805:2002). This treatment does not modulate the amount of metal content of the tissue. The dissolved tissues were analyzed by inductively coupled plasma optical emission spectrometry (EN ISO 11885:2009) for cobalt (Co), chromium (Cr), nickel (Ni), molybdenum (Mo), titanium (Ti), and iron (Fe). The detection limit for Co is 25 ng/g, Ni 25 ng/g, Mo 20 ng/g, Ti 15 ng/g, Cr 5 ng/g, and Fe 2.5 ng/g. The results were then normalized per grams of tissue. Additionally, results of Ti determination were audited by Zeemann graphite-furnace AAS (Varian Z880).
To confirm the metallurgy of the implant materials and to prepare for the electrochemical inspection, the CLS-Spotorno stem and LDH Metasul adapter were analyzed by x-ray fluorescence. For evidence of electrochemical reaction between the stem and adapter, a minicell electrode was made by using platinum wire. The open circuit potential was measured against the reference system SE 11 (Ag/AgCl sat. reference system, Meinsberg).
Metal analyses were conducted two times per sample to ensure the validity of the results. Experiments yielded comparable observations. Data were first analyzed by analysis of variance and by using Spearman’s rank correlation coefficient.
Results
Alloy constituents were confirmed, eg, as a Ti alloy (Ti6Al7Nb, PROTASUL®-100; Zimmer Inc), with different roving textured surfaces and a higher percentage of aluminum in the distal section; the sleeve of the LDH head adapter was confirmed to consist of a CoCrMo alloy (HS21, ZimaloyTM; Zimmer Inc). Electrochemical studies on the CLS stem and the LDH head adapter showed an open circuit potential in normal saline suggesting galvanic corrosion.
One hundred six (93%) of the 114 hips had joint effusions and tissues with a grayish necrotic appearance were found around the implants, respectively (Fig. 1). Intraoperatively, in 94% (n = 107), the cones and the tapers were unstable and showed a black color resembling corrosion (Fig. 2). Regions of osteolysis in the proximal femur were curetted and cleared of the granulation tissue (Fig. 3). The cups were explanted from all 114 THAs. Nine stems were unstable and were revised. During revision surgery in 90 hips (79%), an extended bursa formation was observed resembling pseudotumors. All bearing surfaces were revised to ceramic-PE articulations.
Fig. 1.
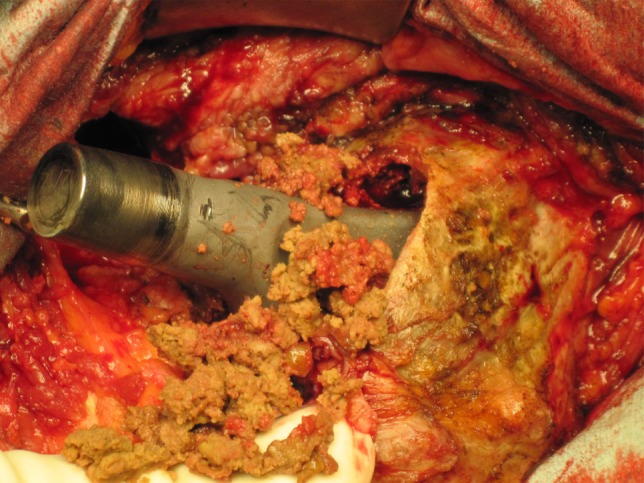
The intraoperative photograph shows the implant components after disassembling of the loose cone/taper connection with surrounding granulation tissue.
Fig. 2.
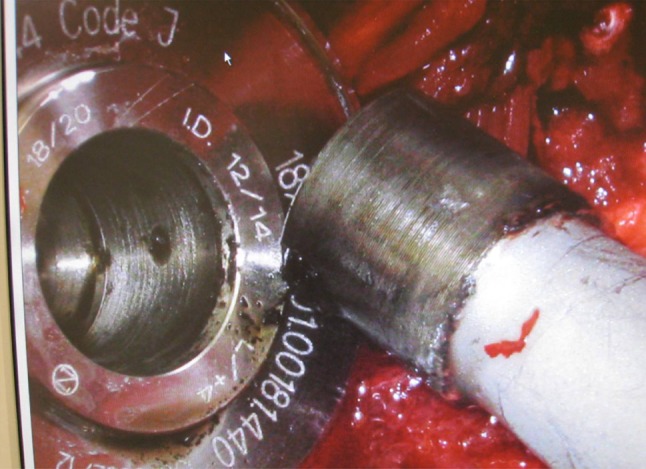
Higher magnification photograph shows corrosive changes at the cone and granulation tissues curetted from the osteolysis of the proximal femur.
Fig. 3.
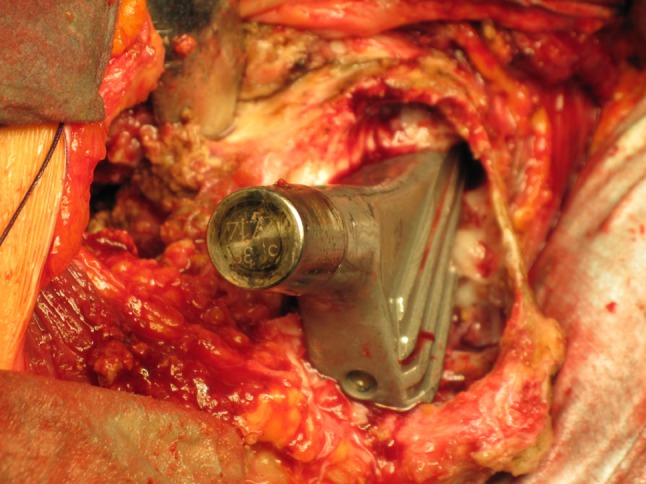
The intraoperative photograph demonstrates the osteolysis cleared of granulation tissue and its extension into the proximal femur.
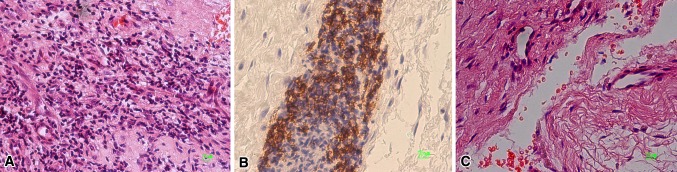
The histological classification revealed an excessive load with particles in 14 tissues, an abundant particle load in 18, many in 25, and few particles in 46 (Table 1). In 11 tissues, no particles were found and seven of those showed no macrophage infiltration. Four tissues exhibited T-lymphocyte infiltration as the dominant histomorphology pattern with positive staining for anti-CD3 monoclonal antibody. In 104 of the 114 (91%) revisions (Table 2), a macrophage-dominated reaction was shown by monoclonal anti-CD68 antibody (Fig. 4A). In 95 cases, vasculitis and necrotic tissues were observed and in nine cases, a lymphocyte-dominated histomorphology resembling an ALVAL reaction (Fig. 4B). Necrosis and fibrin exudation were additionally seen (Fig. 4C). Black metal particles were microscopically observed only in sections stained positive for monocytes or macrophages.
Table 1.
Scoring of histological sections from each revision (n = 114) was conducted as described previously [40]
| Grading | Description | Cells | Number of | Particle load | |
|---|---|---|---|---|---|
| Lymphocytes | Macrophages | ||||
| − | None | < 10 | 101 | 18 | 11 |
| + | Few | 11–20 | 4 | 52 | 46 |
| ++ | Many | 21–30 | 38 | 25 | |
| +++ | Abundant | 31–50 | 9 | 6 | 18 |
| ++++ | Excessive | > 50 | – | 14 | |
Tissues were examined and graded according to the number of cells, lymphocytes, macrophages, and cells loaded with particles per power field view at 40× magnification.
Table 2.
Comparison of the literature reveals different responses to small- and large-diameter metal-on-metal and hip resurfacings suggesting adverse tissue reactions
| Author | Metal-on-metal design | Osteolysis | Tissue response | Head size | Metal content | Corrosion | Particle liberation |
|---|---|---|---|---|---|---|---|
| Lavigne et al. [27] | Biomet, DePuy (Ultamet), Smith & Nephew (Sikomet), Zimmer (Metasul) | NA | + | > 50 mm | Blood | + | − |
| Jacobs et al. [18] | Metasul, DePuy (Ultamet) Modular cemented and uncemented | + | Foreign body reaction | − | NA | + | − |
| Langton et al. [25] | Resurfacing (ASR [DePuy], BHR [Smith & Nephew], Conserve [Wright]) | − | + | NA | NA | + | + |
| Langton et al. [26] | Resurfacing (ASR BHR Conserve) | NA | ARMD | NA | NA | NA | + |
| Willert et al. [42] | Metasul | + | ALVAL | 28 mm | NA | NA | + |
| Lohmann et al. [29] | Sikomet | + | ALVAL | 28 mm | Tissue | − | + |
| Savarino et al. [36] | NA | NA | NA | NA | Blood | + | NA |
| Long et al. [30] | Metasul | 37,4% (1-year followup) | Inflammation | 44–50 mm | NA | NA | NA |
| Mahendra et al. [31] | BHR, Conserve Plus [Wright], Cormet [Corin] | NA | Macrophages 86% ALVAL 14% | NA | NA | NA | + |
| Current study | LDH [Zimmer] Metasul | 52% | Foreign body reaction and necrosis (91%), ALVAL (9%) | ≥ 40 mm | Tissue | + | + |
NA = not available; ARMD = adverse reaction to metal debris; ALVAL = aseptic lymphocyte-dominated vasculitis associated lesion.
Fig. 4A–C.
(A) This is a section of retrieval tissue dominated by monocytes (Stain, hematoxylin & eosin [H&E]; original magnification, ×20). (B) Perivascular infiltration by lymphocytes was observed as described by Willert et al. [42] (Stain, CD20; original magnification, ×20) (C) Necrosis is seen in the revision tissues (Stain, H&E; original magnification, ×20).
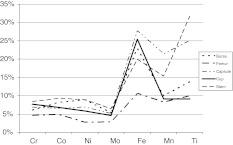
The amounts of the different metals in the periprosthetic tissues showed a broad range for Co, Cr, Ti, and Fe, whereas the amount of Ni release into the tissues did not differ from individual patients (Table 3). The highest levels of metal were found for Ti and Fe dependent on the stem used (Fig. 5). The measured levels for Co and Cr as well as Ni in the tissues were low. Ti and Fe were found highest in the capsule and in the bursa. Behind the acetabular cup the Ti levels remained low (Fig. 5).
Table 3.
Metal contents measured in the revision tissues are shown as minimum and maximum values (ng/g tissue)
| Metal | Minimum ng/g tissue | Maximum ng/g tissue |
|---|---|---|
| Cobalt | 49.8 | 831.9 |
| Chromium | 12.2 | 284.9 |
| Nickel | 56.6 | 87.6 |
| Titanium | 49.5 | 630.1 |
| Iron | 11.3 | 963.9 |
Fig. 5.
The released metals were determined in the different regions of the revision tissues from failed THAs. The highest metal content of Fe and Ti is found in the bursa and capsule.
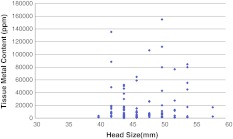
There was no correlation between head size and amount of metal released from the unstable cone/taper interfaces (Fig. 6).
Fig. 6.
Head size (mm) does not correlate with tissue metal content for each patient.
Discussion
Large-diameter heads are used to reconstruct the geometry of the hip during THA. However, concerns have been raised about large-diameter metal heads regarding potential corrosion at the cone/taper interface and increased failure rates [5, 8, 11, 13]. Early after implantation others also observed some patients presented with recurrent symptoms including groin pain and limping [10]. We therefore examined a group of 114 revisions of large-diameter heads to determine: (1) if corrosion occurred at the cone/taper interface leading to instability; (2) how the patients presented clinically and radiographically; (3) if an adverse reaction to the metallic debris had occurred; (4) whether the metal released from the implant components was retained in periprosthetic tissues; and (5) if head size correlated with metal release from the unstable cone/taper interfaces.
We recognize that our study has limitations. First, we reviewed a single large MoM hip system, so our findings may not apply to other designs. Second, a clinical followup of all 805 hips has not yet been performed; therefore, a risk for failure cannot be clearly claimed. Third, three different stem designs are used in the present study; however, no differences were observed in clinical symptoms and tissue reactions depending on the used stem. Fourth, the serum metal contents were not determined, but most importantly, the local tissue reaction induces the loosening process. The current study shows an association of tissue metal content and tissue response in large-diameter MoMs. Various other studies investigated other designs and head diameters (Table 2). The strength of the study is that we have focused on radiographic signs of loosening, tissue response, corrosion, and particle release as well as metal accumulation in periprosthetic tissues.
Different materials can develop an open circuit potential (OCP) [8]. We found an OCP at the cone/taper interface by use of a minicell electrode. Although Ti and its alloys reportedly develop a protective layer by passivation from Ti to TiO2 [11], it is evident that a combination of different metals like Fe and CoCr or Ti and CoCr produce an electrochemical potential. Willert et al. [43] assumed that the passivation layer of the alloy safely protects the release of ions and may inhibit electrochemical conduction. However, micromotion damages the passivation layer, which can be electrochemically dissolved leading to galvanic corrosion [38]. Further fretting corrosion caused by oscillating micromotion with small amplitudes (< 100 μm) and micromotion abrade the surface developing small wear particles [15, 23]. If this process takes place in a joint, this fretting corrosion might be overlaid by pitting or crevice corrosion.
The stability of the cone/taper junction is influenced by the design of the components. The lengths of the tapers of the CLS® stems had been reduced from 15 mm to 12 mm before 2004. All of the CLS® stems used in the study had the shortened taper design. Shortening of the taper has the consequence that the inclination angle of the taper increases and thereby the length of the deadlock decreases. This may imply that the strength of the cone/taper connection is reduced. An optimal interface is necessary to avoid micromotion and thereby fretting and fretting corrosion. If micromotion and fretting corrosion occur, the release of wear particulate debris from the cone/taper connection increases. In a dynamic model of corrosion with high cyclic load stresses, others [11, 13] observed similar corrosive and electrochemical findings for mixed metal cases. These findings suggest mechanical loading plays a role in the corrosion process and leads to the conclusion that in low-force-connected cone tapers, joint friction of the artificial hip can cause rotation and thus loosening of the modular head of the implant neck during daily activities [42]. In our revisions, 94% of the cone/taper interfaces had failed and showed signs of corrosion. We suggest that a design provided for general use must be tested in various standard methods to ensure a stable taper lock. We found failures of large-diameter head MoM THAs are associated with instability of the cone/taper junction. One hundred ten of 650 patients who were followed in this series had early revision of their THA. In these patients, clinical symptoms like groin pain and limping occurred only 46 months after implantation leading to revision. More than 59 of the 110 patients had radiographic signs of osteolysis or radiolucencies. Similar observations were made by others [5, 25] who observed accelerating failures of the ASR hip replacement. This may imply that early failures in the large-diameter hip system are not design-specific but dependent on the large head. We observed large bursa formation in approximately 80% of the patients. Large bursa formation has been described in small-diameter MoM THA [35, 42] as well as in hip resurfacings [31, 34]. The data of the present failure series with low CoCr release but large amounts of Ti or Fe release may lead to the conclusion that bursa formation and large joint effusions are caused by metal release in general and are not specific for CoCr resurfacing THA. Potentially, the cytotoxicity of the released wear causes the large areas of necrosis in the tissues, which is also associated with inflammation and joint effusions.
We observed the different amounts of stored metals in the periprosthetic tissues depended on the alloy of the femoral stem. This suggests Ti and/or Fe rather than CoCr may contribute to the foreign body reaction. The histomorphology of the periprosthetic tissues did not only show a foreign body reaction to wear products, but also areas of necrosis representing their cytotoxic effects. Cell death by apoptosis has been described through the release metal ions by corrosion or wear [39]. We saw necrosis in the vast majority of the cases. This reaction is distinct from that in metal/PE articulations in which the foreign body reaction with multinucleated cells storing PE wear is the predominant tissue response. The immunohistologic observations support this failure mode with respect to tissue response. Particularly, CD68 staining, a marker of macrophage or monocytes that are capable of phagocytosis, was the dominant stain found in the tissues. Macrophages storing particles, CD68 positivity in the tissues, and the proof of high levels of Ti or Fe substantiate these conclusions. Only in nine cases, CD3 staining, as a marker for T-lymphocytes, showed a histological picture that resembles a hypersensitivity reaction or an ALVAL response. Several authors [29, 42] have reported reactions with lymphocytes rather ALVAL in failed 28-mm MoM. This periprosthetic tissue reaction is distinctly different from the histomorphologic picture seen in the present study because we clearly recognized a foreign body reaction and areas of necrosis rather than lymphocyte-dominated reaction and vasculitis. This may be attributed to the fact that in the studies with 28-mm heads, the cone/taper interfaces were stable resulting in a different profile of released particulate wear debris.
Ti and Fe were detected at greater levels than Co and Cr. We therefore assume that this occurs from abrasive wear at the failed cone/taper connection. Analyzing retrievals from various hip systems others have reported a Ti-Cr-Mo interface may induce corrosion [11]. Crevice and cyclic stresses result in an unstable electrochemical environment. Furthermore, this will result in corrosion products. This corrosion and particulate accumulation could result in loss of mechanical integrity of the implants. These observations assume that galvanic corrosion at the cone/taper interface led to instability of the junction. It is not clear yet if additional strong torque forces or electrochemical reaction alone are responsible for instability of the cone/taper connection. In the revisions that we reviewed, all cups were explanted to convert the articulation. The DUROM® cup has been described by others as an implant that preferably fails in the acetabular side [9]. The tissues from acetabulum that were retrieved after cup removal did not show an increased load with Ti, which may have indicated abrasive wear at the bone/implant interface. The distribution of Ti and Fe in the tissues of other regions of the joint (bursas, capsule, and proximal femur) further supports the assumption that the wear is generated at the cone/taper interface. Shimmon et al. [37] suggested small femoral component size is a risk factor for early failure of the implant. However, we did not observe a correlation of the head size and the amount of metal constituents released from the implant components into the surrounding tissues. The most striking observation was that in the vast majority (107 hips), the cone/taper junction was unstable. The MoM articulation in the revised cases of this study had a modular cone/taper junction. The tapers were made from Ti or Fe alloy and are combined with CoCr-alloyed sleeves. Galvanic corrosion of the modular junction may release metal ions. Crevice and fretting can induce loosening of the cone/taper interface and then release wear particles from the different components [23]. We do not know the total amount of the released metal transported into the serum of the patients in the present study. Lavigne et al. [27] suggested that great amounts of Co are released to the serum from the modular cone/taper junction. It is not known yet if systemic effects may result from increased metal serum levels and cause a toxic effect.
Our findings suggest the need for better designed couplings of the modular components in THA. MoM technologies are under close surveillance because clearance, edge loading, and head size may have negative impacts on the lubrication of the articulating system resulting in potential toxic CoCr release. In the present series of revision, the reason for revision of 114 MoM THAs is mainly caused by the failure of the cone/taper interface that led to fretting corrosion and Ti and Fe release. This initiates clinical symptoms in the patients not only as a result of the loose cone/taper connection, but also as a result of the tissue response that induces osteolysis as well as a foreign body reaction to corrosive wear. The tissue reaction is not an ALVAL reaction as seen in other MoM failures [42]. It is not possible to predict failure of the cone/taper interface or to determine failure with radiographic methods. However, if osteolysis and/or joint effusions or pseudotumors occur in patients with modular large-diameter heads, this may suggest failure of the interface resulting in corrosion and wear release into the surrounding tissues.
Acknowledgments
We thank Dr. B. Feuerstein and Dr. G. Krause for technical support in the analysis of metal contents, Mrs. Carolin Hertzsch for help with the histological stainings, Dr. F. Awiszus for statistical analyses, and Dr. D. Brauers for providing demographic data of the patients.
Footnotes
This study was supported by a generous grant from the ENDO-Stiftung, Hamburg, Germany (HM, CHL).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Amstutz HC, Campbell P, Kossovsky N, Clarke IC. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop Relat Res. 1992;276:7–18. [PubMed] [Google Scholar]
- 2.Antoniou J, Zukor DJ, Mwale F, Minarik W, Petit A, Huk OL. Metal ion levels in the blood of patients after hip resurfacing: a comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J Bone Joint Surg Am. 2008;90(Suppl 3):142–148. doi: 10.2106/JBJS.H.00442. [DOI] [PubMed] [Google Scholar]
- 3.Bach CM, Biedermann R, Goebel G, Mayer E, Rachbauer F. Reproducible assessment of radiolucent lines in total knee arthroplasty. Clin Orthop Relat Res. 2005;434:183–188. doi: 10.1097/01.blo.0000153077.79573.a4. [DOI] [PubMed] [Google Scholar]
- 4.Boehler N. Experiences with metal on metal components in THR. Acta Orthop Belg. 1997;63(Suppl 1):96–97. [PubMed] [Google Scholar]
- 5.Bolland BJ, Culliford DJ, Langton DJ, Millington JP, Arden NK, Latham JM. High failure rates with a large-diameter hybrid metal-on-metal total hip replacement: clinical, radiological and retrieval analysis. J Bone Joint Surg Br. 2011;93:608–615. doi: 10.1302/0301-620X.93B5.26309. [DOI] [PubMed] [Google Scholar]
- 6.Clarke IC, Good V, Williams P, Schroeder D, Anissian L, Stark A, Oonishi H, Schuldies J, Gustafson G. Ultra-low wear rates for rigid-on-rigid bearings in total hip replacements. Proc Inst Mech Eng H. 2000;214:331–347. doi: 10.1243/0954411001535381. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RF, Herrington J, Scales JT. Concentration of wear products in hair, blood and urine after total hip replacement. BMJ. 1973;1:527–529. doi: 10.1136/bmj.1.5852.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier JP, Surprenant VA, Jensen RE, Mayor MB, Surprenant HP. Corrosion between the components of modular femoral hip prostheses. J Bone Joint Surg Br. 1992;74:511–517. doi: 10.1302/0301-620X.74B4.1624507. [DOI] [PubMed] [Google Scholar]
- 9.Dorr LD, Long WT, Sirianni L, Campana M, Wan Z. The argument for the use of Metasul as an articulation surface in total hip replacement. Clin Orthop Relat Res. 2004;429:80–85. doi: 10.1097/01.blo.0000150343.66755.79. [DOI] [PubMed] [Google Scholar]
- 10.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. The John Charnley Award: Metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010;468:318–325. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27:1533–1544. doi: 10.1002/jbm.820271210. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert RE, Cheung G, Carrothers AD, Meyer C, Richardson JB. Functional results of isolated femoral revision of hip resurfacing arthroplasty. J Bone Joint Surg Am. 2010;92:1600–1604. doi: 10.2106/JBJS.I.00698. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg JR, Gilbert JL. In vitro corrosion testing of modular hip tapers. J Biomed Mater Res B Appl Biomater. 2003;64:78–93. doi: 10.1002/jbm.b.10526. [DOI] [PubMed] [Google Scholar]
- 14.Gray MH, Talbert ML, Talbert WM, Bansal M, Hsu A. Changes seen in lymph nodes draining the sites of large joint prostheses. Am J Surg Pathol. 1989;13:1050–1056. doi: 10.1097/00000478-198912000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Grupp TM, Weik T, Bloemer W, Knaebel HP. Modular titanium alloy neck adapter failures in hip replacement—failure mode analysis and influence of implant material. BMC Musculoskelet Disord. 2010;11:3. doi: 10.1186/1471-2474-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hing CB, Back DL, Bailey M, Young DA, Dalziel RE, Shimmin AJ. The results of primary Birmingham hip resurfacings at a mean of five years. An independent prospective review of the first 230 hips. J Bone Joint Surg Br. 2007;89:1431–1438. doi: 10.1302/0301-620X.89B11.19336. [DOI] [PubMed] [Google Scholar]
- 17.Howie DW. Tissue response in relation to type of wear particles around failed hip arthroplasties. J Arthroplasty. 1990;5:337–348. doi: 10.1016/S0883-5403(08)80093-9. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JJ, Skipor AK, Doorn PF, Campbell P, Schmalzried TP, Black J, Amstutz HC. Cobalt and chromium concentrations in patients with metal-on-metal total hip replacements. Clin Orthop Relat Res. 1996;329(Suppl):S256–S263. doi: 10.1097/00003086-199608001-00022. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JJ, Urban RM, Gilbert IL, Skipor AK, Black J, Jasty M, Galante JO. Local and distant products from modularity. Clin Orthop Relat Res. 1995;319:94–105. [PubMed] [Google Scholar]
- 20.Jasty M, Jiranek W, Harris WH. Acrylic fragmentation in total hip replacements and its biological consequences. Clin Orthop Relat Res. 1992;285:116–128. [PubMed] [Google Scholar]
- 21.Kim KJ, Rubash HE, Wilson SC, D’Antonio JA, McClain EJ. A histologic and biochemical comparison of the interface tissues in cementless and cemented hip prostheses. Clin Orthop Relat Res. 1993;287:142–152. [PubMed] [Google Scholar]
- 22.Kirkpatrick CJ, Alves A, Köhler H, Kriegsmann J, Bittinger F, Otto M, Williams DF, Eloy R. Biomaterial-induced sarcoma: a novel model to study preneoplastic change. Am J Pathol. 2000;156:1455–1467. doi: 10.1016/S0002-9440(10)65014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: a retrieval study of 16 cases. J Arthroplasty. 2009;24:1019–1023. doi: 10.1016/j.arth.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Langkamer VG, Case CP, Heap P, Taylor A, Collins C, Pearse M, Solomon L. Systemic distribution of wear debris after hip replacement. A cause for concern? J Bone Joint Surg Br. 1992;74:831–839. doi: 10.1302/0301-620X.74B6.1447243. [DOI] [PubMed] [Google Scholar]
- 25.Langton DJ, Jameson SS, Joyce TJ, Gandhi JN, Sidaginamale R, Mereddy P, Lord J, Nargol AV. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br. 2011;93:1011–1016. doi: 10.1302/0301-620X.93B8.26040. [DOI] [PubMed] [Google Scholar]
- 26.Langton DJ, Joyce TJ, Jameson SS, Lord J, Orsouw M, Holland JP, Nargol AV, Smet KA. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93:164–171. doi: 10.1302/0301-620X.93B2.25099. [DOI] [PubMed] [Google Scholar]
- 27.Lavigne M, Belzile EL, Roy A, Morin F, Amzica T, Vendittoli PA. Comparison of whole-blood metal ion levels in four types of metal-on-metal large-diameter femoral head total hip arthroplasty: the potential influence of the adapter sleeve. J Bone Joint Surg Am. 2011;93(Suppl 2):128–136. doi: 10.2106/JBJS.J.01885. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Salvati EA, Betts F, DiCarlo EF, Doty SB, Bullough PG. Size of metallic and polyethylene debris particles in failed cemented total hip replacements. J Bone Joint Surg Br. 1992;74:380–384. doi: 10.1302/0301-620X.74B3.1587882. [DOI] [PubMed] [Google Scholar]
- 29.Lohmann CH, Nuechtern JV, Willert HG, Junk-Jantsch S, Ruether W, Pflueger G. Hypersensitivity reactions in total hip arthroplasty. Orthopedics. 2007;30:760–761. doi: 10.3928/01477447-20070901-12. [DOI] [PubMed] [Google Scholar]
- 30.Long WT, Dastane M, Harris MJ, Wan Z, Dorr LD. Failure of the Durom Metasul acetabular component. Clin Orthop Relat Res. 2010;468:400–405. doi: 10.1007/s11999-009-1071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahendra G, Pandit H, Kliskey K, Murray DW, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties—relation to implant failure and pseudotumor formation. Acta Orthop. 2009;80:653–659. doi: 10.3109/17453670903473016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertl P, Boughebri O, Havet E, Triclot P, Lardanchet JF, Gabrion A. Large diameter head metal-on-metal bearings total hip arthroplasty: preliminary results. Orthop Traumatol Surg Res. 2010;96:14–20. doi: 10.1016/j.otsr.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Pandit H, Glyn-Jomes S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CLM, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumors associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 34.Pandit H, Vlychou M, Whitwell D, Crook D, Luqmani R, Ostlere S, Murray DW, Athanasou N. Necrotic granulomatous pseudotumors in bilateral resurfacing hip arthroplasties: evidence for a type IV immune response. Virchows Arch. 2008;453:429–534. doi: 10.1007/s00428-008-0659-9. [DOI] [PubMed] [Google Scholar]
- 35.Revell PA. Tissue reactions to joint prostheses and the products of wear and corrosion. Curr Top Pathol. 1982;71:73–101. doi: 10.1007/978-3-642-68382-4_3. [DOI] [PubMed] [Google Scholar]
- 36.Savarino L, Granchi D, Ciapetti G, Cenni E. Nardi Pantoli A, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in patients with metal-on-metal hip bearings in total joint replacement: a comparison with metal-on-polyethylene bearings. J Biomed Mater Res. 2002;63:467–474. doi: 10.1002/jbm.10299. [DOI] [PubMed] [Google Scholar]
- 37.Shimmin AJ, Walter WL, Esposito C. The influence of the size of the component on the outcome of resurfacing arthroplasty of the hip: a review of the literature. J Bone Joint Surg Br. 2010;92:469–476. doi: 10.1302/0301-620X.92B4.22967. [DOI] [PubMed] [Google Scholar]
- 38.Spaehn H. Electrochemical corrosion in aqueous solutions without simultaneous load. In: Kunze E, editor. Corrosion and Corrosion Protection, Volume 1: Basics and Scientific Principles [in German] Berlin, Germany: Wiley-VCH; 2001. [Google Scholar]
- 39.Stea S, Visentin M, Granchi D, Cenni E, Ciapetti G, Sudanese A, Toni A. Apoptosis in peri-implant tissue. Biomaterials. 2000;21:1393–1398. doi: 10.1016/S0142-9612(00)00041-7. [DOI] [PubMed] [Google Scholar]
- 40.Sunderman FW, Jr, Hopfer SM, Swift T, Rezuke WN, Ziebka L, Highman P, Edwards B, Folcik M, Gossling HR. Cobalt, chromium, and nickel concentrations in body fluids of patients with porous-coated knee or hip prostheses. J Orthop Res. 1989;7:307–315. doi: 10.1002/jor.1100070302. [DOI] [PubMed] [Google Scholar]
- 41.Weber BG. Experience with the Metasul total hip bearing system. Clin Orthop Relat Res. 1996;329(Suppl):69–77. doi: 10.1097/00003086-199608001-00007. [DOI] [PubMed] [Google Scholar]
- 42.Willert HG, Buchhorn GH, Fayazzi A, Flury R, Windler M, Koester G, Lohmann CH. Metal/metal bearings and hypersensitivity in artifical hip joint—a clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 43.Willert HG, Buchhorn GH, Göbel D, Köster G, Schaffner S, Schenk R, Semlitsch M. Wear behavior and histopathology of classic cemented metal on metal hip endoprostheses. Clin Orthop Relat Res. 1996;329(Suppl):S160–S186. doi: 10.1097/00003086-199608001-00016. [DOI] [PubMed] [Google Scholar]
- 44.Willert HG, Semlitsch M. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res. 1977;11:157–164. doi: 10.1002/jbm.820110202. [DOI] [PubMed] [Google Scholar]
- 45.Witzleb WC, Ziegler J, Krummenauer F, Neumeister V, Guenther KP. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta Orthop. 2006;77:697–705. doi: 10.1080/17453670610012863. [DOI] [PubMed] [Google Scholar]