Abstract
Background
The relative risk of revision of the Titan® femoral stem due to aseptic loosening increased after 2000; however, the reasons for this have not been established. A retrieval analysis was initiated with the aim of delineating the failure mechanism.
Questions/Purposes
We asked whether aseptic loosening in stems after 2000 was associated with (1) appearance of osteolytic lesions, (2) wear particle exposure, (3) stem damage, or (4) changes to the implant or surgical instrumentation.
Methods
Femoral stems, cement, tissue, and radiographs were collected from 28 patients. We assessed the development of osteolytic lesions in 17 patients. Exposure to wear particles was quantified in 18 patients. Stem damage was assessed in 15 patients. We observed differences in the implants by examination of 24 retrieved stems. Information concerning changes to instrumentation was requested from the manufacturer.
Results
We found osteolysis in all patients receiving implants after 2000, which was associated with a median dose of cement and stem particles of 14,726/mm2. Abrasion covered 59% of the surface of stems implanted from 1999. We identified geometric changes to the stem, the percent weight of aluminum in the stem’s oxide layer decreased from 25% to 14% after 1997 and the rasp used to prepare the femoral cavity changed to a broach in 1999.
Conclusions
Stems implanted from 2000 failed through osteolysis induced by particles released from the cement and implant. Changes to implant geometry, surface oxide layer, and surgical tools occurred in the same time frame as the reduction in survivorship.
Introduction
The Norwegian Arthroplasty Register (NAR) has recorded data on primary and revision hip arthroplasty procedures since September 15, 1987. Its main purpose is to function as a surveillance tool to identify inferior implants and techniques as early as possible to avoid their use in large numbers of patients. The resulting system has led to the identification and subsequent withdrawal of a number of inferior products, which have been documented in the literature [6, 9, 10]. Many countries have now adopted national arthroplasty registers to assess the efficacy of procedures. However, they are designed for treatment surveillance and therefore the underlying failure mechanisms are not always understood. A logical progression of a national register is to incorporate the ability to perform detailed analysis of failure mechanisms. One method of achieving this is through collection and analysis of retrieved implants on a national scale. To demonstrate this, we present the results of our investigation of the increased revision rates observed for the Titan® femoral stem.
The Titan® stem was produced by Landos-Landanger (Chaumont, France) until February 1997 and by DePuy (in the same factory) from February 1997 until 2009 when it was discontinued. It is a straight, double-tapered Müller-type stem with a modular head and with standard and lateralized (+7.7 mm) offset options. It was manufactured from titanium alloy (Ti6Al4V) by forging, machining, and sand blasting to achieve a satin finish and electrochemically anodized, giving it a thickened surface oxide layer. Despite reports of other cemented titanium femoral stems performing unsatisfactorily due to problems such as galvanic corrosion [23] or stem abrasion induced by low stiffness [17, 24] leading to loosening secondary to osteolysis, the Titan® performed well. The NAR reported a success rate of 97% at 8 years for stems implanted between 1987 and 1997; however, the performance deteriorated from about 2000 [5]. A detailed investigation of survivorship revealed the relative risk of revision (RR) from 2001 to 2008 with aseptic loosening as the end point was 4.7 times higher than in the period 1996 to 2000 [8]; however, it could not explain the reasons for the deterioration in results. A retrieval analysis was initiated with the aim of delineating the mechanisms involved in the failures.
We therefore asked whether aseptic loosening in stems after 2000 was associated with (1) appearance of osteolytic lesions, (2) wear particle exposure, (3) stem damage, or (4) changes to the implant or surgical instrumentation.
Patients and Methods
Femoral stems, cement, tissue, and AP radiographs were collected from 28 patients whose Titan® femoral stems had been revised (Table 1). Seventeen stems had failed due to aseptic loosening and two due to periprosthetic fracture. A further nine stems were removed without record before the initiation of the study. Patient information could not be traced for these samples as no identification was recorded at the time of collection; however, they were included in the study to increase the chances of capturing design changes (Question 4).
Table 1.
Summary of retrieved stems
| Stem | Group* | Manufacturer | Reason for failure | Samples | Bearing couple (head/cup) | Head diameter (mm) |
|---|---|---|---|---|---|---|
| 1 | A | Landos | Periprosthetic fracture | Patient notes, radiographs, stem | SS/polyethylene | 32 |
| 2 | A | Landos | Periprosthetic fracture | Patient notes, radiographs, stem, tissue | SS/polyethylene | 32 |
| 3 | A | Landos | Retrieved before study | Stem | ||
| 4 | A | Landos | Retrieved before study | Stem | ||
| 5 | B | Landos | Retrieved before study | Stem | SS/polyethylene | 32 |
| 6 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 7 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 8 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 9 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 10 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 11 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 12 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 13 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 14 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | Biolox® forte/polyethylene | 28 |
| 15 | C | DePuy | Aseptic loosening | Patient notes, radiographs, tissue | CoCr/polyethylene | 28 |
| 16 | C | DePuy | Aseptic loosening | Patient notes, radiographs, tissue | CoCr/polyethylene | 28 |
| 17 | C | DePuy | Aseptic loosening | Patient notes, radiographs, tissue | CoCr/polyethylene | 28 |
| 18 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | Biolox® forte/polyethylene | 28 |
| 19 | C | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 20 | C | DePuy | Aseptic loosening | Patient notes, radiographs, tissue | Biolox® forte/polyethylene | 28 |
| 21 | C | DePuy | Retrieved before study | Stem | ||
| 22 | C | DePuy | Retrieved before study | Stem | CoCr/polyethylene | 28 |
| 23 | C | DePuy | Retrieved before study | Stem | CoCr/polyethylene | 28 |
| 24 | C | DePuy | Retrieved before study | Stem | CoCr/polyethylene | 28 |
| 25 | C | DePuy | Retrieved before study | Stem | ||
| 26 | C | DePuy | Retrieved before study | Stem | ||
| 27 | D | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
| 28 | D | DePuy | Aseptic loosening | Patient notes, radiographs, stem, tissue | CoCr/polyethylene | 28 |
* For a description of groups, see Table 2; SS = stainless steel; CoCr = cobalt-chromium alloy.
We examined prerevision radiographs for appearance of osteolytic lesions or radiolucent lines that had not been visible on earlier images. In each case, the earlier image was taken immediately after the primary operation or no less than 7 years before revision. The necessary images were available for analysis in 17 patients. In the remaining two patients, only images immediately before revision were available and therefore no assessment could be made. Images were analyzed using standard measurement functions in ImageJ (NIH, Bethesda, MD, USA). We defined osteolysis as any newly developing cystic lesion with endosteal scalloping [13] greater than 3 mm and assessed its appearance on a three-point scale of endosteal scalloping, no scalloping, and unreadable. The appearance of radiolucency at the prosthesis-cement and cement-bone interfaces was assessed on a three-point scale of gap, no gap, and unreadable. Inter- and intraobserver repeatability of both assessment methods was tested. Two observers (PE, IOM) rated all pairs of images individually. The rater reliability was compared using the intraclass correlation coefficient (ICC), which was greater than 0.99 in all cases.
Samples of periprosthetic tissue from the trochanteric region or the membrane around the femoral stem were collected from 18 patients at revision surgery (Table 1). One of us (PJH) observed cell type and tissue morphology in the tissue using optical microscopy. Particle load and size were analyzed in the same sections by a high-resolution optical darkfield microscope (CytoViva, Inc, Auburn, AL, USA), and the size and dose (number of particles/mm2) of foreign body particles were measured using image analysis software (NIS-Elements; Nikon, Tokyo, Japan). The composition of particles was determined by energy dispersive x-ray spectroscopy (EDS; ThermoFisher Scientific, Waltham, MA, USA). Polarized light microscopy (Eclipse E200 POL; Nikon) was used to screen for birefringent structures indicative of polyethylene particles.
One of us (PE) quantified the level of abrasion on the stems. So that comparison based on date of implantation could be made, only the 15 stems for which patient notes were available were analyzed. Anterior, posterior, medial, and lateral photographs were taken, and the size of the worn area was calculated using image analysis software (NIS-Elements) and expressed as a percentage of the total area. Before analysis, inter- and intraobserver repeatability of the method was assessed. Two observers (PE, IOM) made 16 measurements of four abraded areas. The rater reliability was compared using the ICC, which was greater than 0.98 in both cases. The damage on each stem was assessed using a semiquantitative grading system [23] consisting of three abrasion levels (1A = small rub marks; 2A = larger areas with intensive polishing; 3A = abraded surface areas with scratches) and three corrosion levels (1C = violet-black-colored oxide film; 2C = voluminous white corrosion products; 3C = macroscopically visible shallow pits). Before analysis, inter- and intraobserver repeatability of the method was assessed. Two of us (PE, PJH) assessed five stems individually. The rater reliability was compared using the ICC, which was greater than 0.99 in both cases.
Stem design changes were identified by inspecting 24 femoral stems (Table 1). Component catalog, serial, and lot numbers were identified from the implant or obtained from the registry. This allowed for identification of the manufacturer, model, and size of the femoral components. The date of sterilization of the retrieved components was determined from the lot numbers by the current manufacturer for a sample of 21 stems. One of us (PE) visually observed the appearance of all stems. Two Size 11 standard and two Size 14 lateralized stems were reverse engineered through laser scanning, and three-dimensional CAD models were created. Differences in geometry were quantified using standard measurement functions available in CAD software (Creo® Elements/Pro; Parametric Technology Corp, Needham, MA, USA). The chemical composition of the stems undamaged surface oxide layer was quantified using EDS. Four measurements were taken on each stem and the mean element composition determined. The surface roughness of the stems was characterized using a profilometer (Mahr GmbH, Göttingen, Germany). Measurements were taken on undamaged surfaces to quantify the original surface finish. The dates of any changes to the tool used to prepare the femoral cavity were requested from the manufacturer and the national distributor, along with samples of components.
All data are shown as mean ± SD unless otherwise stated. Comparison of aluminum contained in the stem’s oxide layer was made using single-way ANOVA, after which minimum significant differences between individual means were calculated according to the T method. Both methods were implemented manually in a computer spreadsheet. We compared the surface roughness measurements using ANOVA. To account for the time difference between product sterilization and usage, a time frame of introduction was estimated by adding 2 and 34 months to the earliest date of sterilization. This range was chosen based on the time between sterilization and implantation of stems analyzed in the retrieval study.
Results
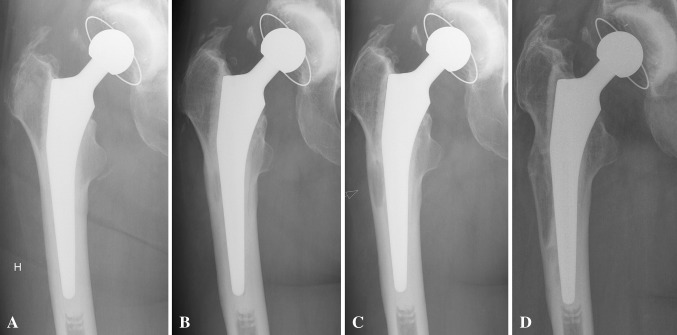
All 17 hips that failed due to aseptic loosening were implanted from 1999 onwards. Sixteen assessed radiographically had developed osteolysis before revision (Fig. 1). No such lesions were observed in hips implanted before 1999 that failed due to periprosthetic fracture.
Fig. 1A–D.
AP radiographs show the progression of osteolysis common between incidences of aseptic loosening in Stem 18. (A) At 0.2 years postoperatively, no osteolysis is visible. (B) At 2.5 years postoperatively, osteolysis and a radiolucent line at the stem-cement interface appear. (C) At 4.3 years postoperatively, growth of osteolysis and radiolucency can be seen. (D) At 6.4 years postoperatively, the state of osteolysis and radiolucency can be seen immediately before revision.
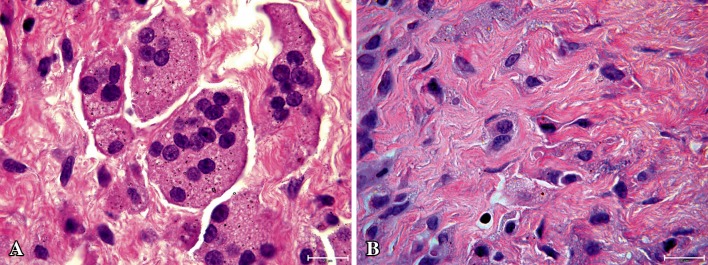
Microscopically, tissue samples from patients with aseptic loosening showed connective tissue with macrophage infiltration and giant cell formation engulfing particles (Fig. 2A). The median dose of particles observed was 14,726 particles/mm2. In a sample of six patients with osteolysis, the dose of polyethylene particles was 0.6 to 4.9 particles/mm2 and therefore no further measurements were recorded. The sample from a patient revised for periprosthetic fracture appeared healthy (Fig. 2B), with a dose of 83 particles/mm2. EDS analysis of particles indicated they included a mixture of zirconium, titanium, aluminum, and vanadium.
Fig. 2A–B.
Tissue morphology of patients (A) with and (B) without osteolysis is compared. (A) Connective tissue from a patient with osteolysis shows macrophage infiltration and giant cell formation loaded with wear particles indicative of wear particle-induced osteolysis (stain, hematoxylin and eosin; original magnification, ×100). (B) Tissue from a patient with a well-fixed prosthesis without osteolysis appears healthy (stain, hematoxylin and eosin; original magnification, ×100).
Grade 3A abrasion was observed on all stems implanted from 1999, covering a mean of 59% of the surface. These stems had a characteristic pattern of abrasion on the posteromedial and anterolateral regions. The highest grade of corrosion was 1C or less on 11 stems. On the remaining four stems, we observed localized areas of Grade 3C, no larger than 2 mm2.
We identified three changes to the stem and one to the tool used to prepare the femoral cavity. Two stem geometries were observed having one or two introducer holes (Fig. 3), an increased depth of cement key (Table 2), and minor changes in the detailed form of the proximal region (Fig. 4). Further, we observed a higher (p < 0.001) percentage of aluminum in the oxide layer of stems sterilized before 1998 (Table 2), noticeable by a variation in color (Fig. 4). Finally, three manufacturer’s markings were observed. These differences allowed the stems to be separated into four groups, A to D (Table 1), which were produced over separate time periods (Table 2): Group A, up to 1996; Group B, 1998 to 2000; Group C, 1999 to 2001; and Group D, 2007 to 2009. The tool used to cut the femoral cavity changed from a rasp to a broach. The national distributor and current manufacturer reported the broach was introduced into the market in 1999.
Fig. 3.
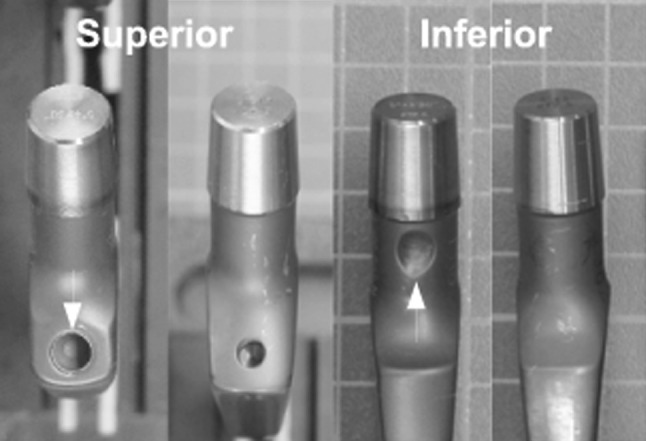
Retrieved Titan® stems show variation in the design of the introducer holes. Examples of two different geometries (Group A had one geometry and Groups B–D another geometry) are viewed from two angles (first two superior, second two inferior). The two stem geometries shown represent the following groups from left to right: Group A, Groups B to D, Group A, and Group B to D. White arrows indicate the larger superior and additional inferior holes observed on Group A compared to the other groups. Similar differences were observed on standard (shown) and lateralized stems.
Table 2.
Differences between stem groups and dates of manufacture indicated by the sterilization date
| Group | Geometry and color | Oxide layer aluminium content (% weight)* | Surface roughness (Ra) (μm)* | Mark on stem | Sterilization date | Time frame of introduction |
|---|---|---|---|---|---|---|
| A | Shallow cement key (0.7 mm) Introducer holes on the superior and inferior faces of the neck Dark blue color |
24.0 ± 3.4 | 0.65 ± 0.06 | Landos | Up to 1994 | Up to 1996 |
| B | Deep cement key (1.1 mm) Single, small introducer hole on superior face of neck Dark blue color |
25.0 ± 2.6 | 0.69 ± 0.05 | Landos | November 1997 | 1998–2000 |
| C | Deep cement key (1.1 mm) Single, small introducer hole on superior face of neck Paler gray-blue color |
13.5 ± 2.1 | 0.68 ± 0.12 | DePuy | November 1998 to September 2004 | 1999–2001 |
| D | Deep cement key (1.1 mm) Single, small introducer hole on superior face of neck Paler gray-blue color |
13.7 ± 0.7 | 0.78 ± 0.07 | No mark | June 2007 to January 2008 | 2007–2009 |
* Values are expressed as mean ± SD.
Fig. 4.

Retrieved Titan® stems show variations in proximal stem geometry and color between groups. Stems shown from left to right are Group A lateralized, Group A standard, Group C lateralized, and Group C standard.
Discussion
Registers are designed in large part to identify treatment failures and therefore the underlying failure mechanisms are not always understood. One method of enhancing their ability to identify the root causes of failures is to coordinate survivorship and retrieval analyses. To demonstrate this concept, we present the results of our investigation of the increased RR observed for the Titan® femoral stem. Historically, the performance of cemented titanium alloy femoral stems has been mixed. They are generally considered to perform unsatisfactorily; however, some designs have survivorship ranging from 97.7% at 9 years to 95.4% at 13 years [3, 4, 11]. In 2008, surgeons identified a change in the performance of the Titan® stem, and the NAR later reported a drop in survivorship due to aseptic loosening from about 2000 [5]. As survivorship data alone could not identify the reasons for the deterioration in performance [8], a joint investigation between the NAR and a national retrieval center was initiated to determine whether aseptic loosening in stems after 2000 was associated with (1) appearance of osteolytic lesions, (2) wear particle exposure, (3) stem damage, or (4) changes to the implant or surgical instrumentation.
This investigation is subject to a number of limitations. First, we did not retrieve all failed stems and therefore it is possible our retrieval analysis is not representative of the larger population. However, aseptic loosening of cemented femoral stems due to wear particles released from abrasion of the stem on the cement is well documented [20] and commonly associated with cemented titanium alloy stems [17, 24]. From our findings, it is reasonable to conclude a similar mode of failure in this study. Second, the clinical relevance of the reported dates of product changes is dependent on the assumed delay between sterilization and clinical use. The length of delay was based on evidence for the stems collected in our retrieval study, which may not represent the larger population. Further, it is likely newer versions reached hospitals over time, depending on the popularity of implant size/option, size of hospitals stock, and patient volume. This prevents us from identifying a correlation between product changes and RR but not the approximate comparison of time frames we have made. Third, by not including well-functioning stems from Groups C and D in our retrieval analysis, it is possible osteolysis is common in well-functioning Titan® stems. However, based on previous reports of failure mechanisms in cemented titanium stems [17, 24], it is reasonable to assume our conclusion concerning our first question is valid.
All patients revised for aseptic loosening were implanted from 1999, and of the 16 assessed radiographically, all had developed osteolytic scallops larger than 3 mm in the proximal femur before revision. Radiolucency at the prosthesis-cement interface was evident in each case.
The accompanying periprosthetic tissue contained foreign body particles and associated tissue reaction. Periprosthetic tissue collected from a well-fixed stem was healthy and did not show a discernible level of foreign body debris. In contrast, samples from failures due to aseptic loosening showed connective tissue with macrophage infiltration, giant cell formation, and a considerably larger quantity of zirconium, titanium, and aluminum particles. The mean particle size from all cases was submicron, which has been implicated in the onset of wear particle-induced osteolysis [18]. Such clinical findings have previously been associated with debonding at the stem-cement interface, followed by micromovement, generation of bone-cement and titanium particulate wear debris, and ultimately loosening secondary to osteolysis [17]. There was no evidence to suggest polyethylene wear had contributed to osteolysis, with less than 0.1% of particles being polyethylene.
Severe abrasion was observed on the anterolateral and posteromedial regions of all stems that were revised for aseptic loosening. Such damage is due to retroversion torque as previously documented in cases of loosening in cemented stems [1, 17]. This further supports the hypothesis that wear particles generated by micromovement at the cement-stem interface resulted in osteolysis leading to loosening.
Four stages of design were observed in the retrieved components. The stem’s geometry changed after 1994. The chemical composition of the stem’s oxide layer changed from high to low aluminum content between November 1997 and November 1998. A characteristic of this change was that the color changed from a dark blue to a paler gray-blue, which was visible to the naked eye. This change was also apparent in marketing literature. The composition of oxide films on titanium alloy are known to vary with the method of preparation [2, 21] and alloy grain structure, which are two potential causes for the observed change. There was no difference (p = 0.32) between the mean surface roughness (Ra) of the undamaged sand-blasted surfaces of the groups. The national distributor and the current manufacturer confirmed the tool used to prepare the femoral cavity changed from a rasp to a broach in 1999. A detailed investigation of survivorship revealed the RR with aseptic loosening as the end point was 4.7 times higher from 2001 to 2008 than from 1996 to 2000 [8]. Considering the likely delay between the date of sterilization and the date of implantation, it is reasonable to conclude the change in the chemical composition of the stem’s oxide layer and introduction of the broach may have contributed to this drop in performance. Although of interest from the perspective of product continuity, the observed changes to the stem geometry were considered too early to have caused the drop in performance. In general, it is understood the strength of the cement-bone interface is dependent on the quality of interdigitation [15], which may be different for bone prepared with rasp or a broach. However, no subsidence of cement or cement-bone radiolucency was observed on the radiographs, indicating a weak bone-cement bond. On the other hand, the surface finish of a cemented femoral stem can have a substantial effect on its performance [7, 12, 14, 19, 22].
Our study demonstrates the benefits of integrating a retrieval center into the workflow of a national arthroplasty registry. Retrieval centers are powerful research tools in their own right; however, coordination with a register in a single system enhances the possibility to establish cause-and-effect relationships. We found Titan® stems implanted from 1999 commonly failed through osteolysis induced by particles realized from the cement and the implant. Such clinical findings have previously been associated with debonding at the stem-cement interface, followed by micromovement, generation of bone cement and titanium alloy particulate debris, and ultimately loosening secondary to wear particle-induced osteolysis [17, 24]. Therefore, it is reasonable to conclude similar events are responsible for the poor performance in the Titan® stem from approximately 2000. Changes to the implant geometry, surface oxide chemistry, and the tool used to prepare the femoral cavity were identified over the product’s lifetime. The latter two changes occurred in the same time frame as an increase in the RR. Others have found small changes to components can have devastating effects on clinical outcome [16, 20] and therefore we suggest it should be mandatory that surgeons, not only regulatory bodies, are made aware of all changes to implants and that similar legislation should apply to associated instrumentation.
Acknowledgments
We thank Dr Ove Furnes, Dr Tore Heier, and Dr Helge Wangen for their special commitment to the topic of this paper. We also thank Irene Ohlen Moldestad for her technical assistance in conducting implant analysis. Finally we would like to thank Dr Jean-Pierre Vidalain, DePuy France and Ortomedic AS for discussions regarding the design and manufacture of the implant.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at University of Bergen, Bergen, Norway, and Haukeland University Hospital, Bergen, Norway.
References
- 1.Anthony PP, Gie GA, Howie CR, Ling RS. Localised endosteal bone lysis in relation to the femoral components of cemented total hip arthroplasties. J Bone Joint Surg Br. 1990;72:971–979. doi: 10.1302/0301-620X.72B6.2246300. [DOI] [PubMed] [Google Scholar]
- 2.Ask M, Lausmaa J, Kasemo B. Preparation and surface spectroscopic characterization of oxide films on Ti6Al4 V. Appl Surf Sci. 1989;35:283–301. doi: 10.1016/0169-4332(89)90013-5. [DOI] [Google Scholar]
- 3.Baumann B, Hendrich C, Barthel T, Bockholt M, Walther M, Eulert J, Rader CP. 9-to 11-year results of cemented titanium Mueller straight stem in total hip arthroplasty. Orthopedics. 2007;30:551–557. doi: 10.3928/01477447-20070701-01. [DOI] [PubMed] [Google Scholar]
- 4.Boyer P, Lazennec JY, Poupon J, Rousseau MA, Ravaud P, Catonné Y. Clinical and biological assessment of cemented titanium femoral stems: an 11-year experience. Int Orthop. 2009;33:1209–1215. doi: 10.1007/s00264-008-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espehaug B, Furnes O, Engesaeter LB, Havelin LI. 18 years of results with cemented primary hip prostheses in the Norwegian Arthroplasty Register. Acta Orthop. 2009;80:402–412. doi: 10.3109/17453670903161124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espehaug B, Havelin LI, Engesaeter LB, Vollset SE, Langeland N. Early revision among 12,179 hip prostheses: a comparison of 10 different brands reported to the Norwegian Arthroplasty Register, 1987–1993. Acta Orthop Scand. 1995;66:487–493. doi: 10.3109/17453679509002300. [DOI] [PubMed] [Google Scholar]
- 7.Gravius S, Wirtz DC, Siebert CH, Andereya S, Mueller-Rath R, Maus U, Mumme T. In vitro interface and cement mantle analysis of different femur stem designs. J Biomech. 2008;41:2021–2028. doi: 10.1016/j.jbiomech.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 8.Hallan G, Espehaug B, Furnes O, Wangen H, Hol PJ, Ellison P, Havelin LI. Is there still a place for the cemented titanium femoral stem? A study of 10 108 cases from the Norwegian Arthroplasty Register. Acta Orthop. 2012;83:1–6. doi: 10.3109/17453674.2011.645194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havelin LI, Espehaug B, Vollset SE, Engesaeter LB. Early aseptic loosening of uncemented femoral components in primary total hip replacement. J Bone Joint Surg Br. 1995;77:11–17. [PubMed] [Google Scholar]
- 10.Havelin LI, Espehaug B, Vollset SE, Engesaeter LB. The effect of the type of cement on early revision of Charnley total hip prostheses: a review of eight thousand five hundred and seventy-nine primary arthroplasties from the Norwegian Arthroplasty Register. J Bone Joint Surg Am. 1995;77:1543–1550. doi: 10.2106/00004623-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Hinrichs F, Kuhl M, Boudriot U, Griss P. A comparative clinical outcome evaluation of smooth (10–13 year results) versus rough surface finish (5–8 year results) in an otherwise identically designed cemented titanium alloy stem. Arch Orthop Trauma Surg. 2003;123:268–272. doi: 10.1007/s00402-003-0515-y. [DOI] [PubMed] [Google Scholar]
- 12.Janssen D, Aquarius R, Stolk J, Verdonschot N. Finite-element analysis of failure of the Capital hip designs. J Bone Joint Surg Br. 2005;87:1561–1567. doi: 10.1302/0301-620X.87B11.16358. [DOI] [PubMed] [Google Scholar]
- 13.Joshi RP, Eftekhar NS, McMahon DJ, Nercessian OA. Osteolysis after Charnley primary low-friction arthroplasty: a comparison of two matched paired groups. J Bone Joint Surg Br. 1998;80:585–590. doi: 10.1302/0301-620X.80B4.7361. [DOI] [PubMed] [Google Scholar]
- 14.Leadley SR, Watts JF. The use of monochromated XPS to evaluate acid-base interactions at the PMMA/metal oxide interface. J Adhesion. 1997;60:175–196. doi: 10.1080/00218469708014418. [DOI] [Google Scholar]
- 15.Mann K, Ayers D, Werner F, Nicoletta R, Fortino M. Tensile strength of the cement-bone interface depends on the amount of bone interdigitated with PMMA cement. J Biomech. 1997;30:339–346. doi: 10.1016/S0021-9290(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 16.Massoud SN, Hunter JB, Holdsworth BJ, Wallace WA, Juliusson R. Early femoral loosening in one design of cemented hip replacement. J Bone Joint Surg Br. 1997;79:603–608. doi: 10.1302/0301-620X.79B4.7131. [DOI] [PubMed] [Google Scholar]
- 17.McGrath LR, Shardlow DL, Ingham E, Andrews M, Ivory J, Stone MH, Fisher J. A retrieval study of capital hip prostheses with titanium alloy femoral stems. J Bone Joint Surg Br. 2001;83:1195–1201. doi: 10.1302/0301-620X.83B8.9874. [DOI] [PubMed] [Google Scholar]
- 18.Revell PA, Al-Saffar N, Kobayashi A. Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng H. 1997;211:187–197. doi: 10.1243/0954411971534304. [DOI] [PubMed] [Google Scholar]
- 19.Scheerlinck T, Casteleyn PP. The design features of cemented femoral hip implants. J Bone Joint Surg Br. 2006;88:1409–1418. doi: 10.1302/0301-620X.88B11.17836. [DOI] [PubMed] [Google Scholar]
- 20.Shen G. Femoral stem fixation: an engineering interpretation of the long-term outcome of Charnley and Exeter stems. J Bone Joint Surg Br. 1998;80:754–756. doi: 10.1302/0301-620X.80B5.8621. [DOI] [PubMed] [Google Scholar]
- 21.Sittig C, Textor M, Spencer ND, Wieland M, Vallotton PH. Surface characterization of implant materials c.p. Ti, Ti-6Al-7Nb and Ti-6Al-4 V with different pretreatments. J Mater Sci Mater Med. 1999;10:35–46. doi: 10.1023/A:1008840026907. [DOI] [PubMed] [Google Scholar]
- 22.Verdonschot N. Philosophies of stem designs in cemented total hip replacement. Orthopedics. 2005;28:s833–s840. doi: 10.3928/0147-7447-20050802-07. [DOI] [PubMed] [Google Scholar]
- 23.Willert HG, Brobäck LG, Buchhorn GH, Jensen PH, Köster G, Lang I, Ochsner P, Schenk R. Crevice corrosion of cemented titanium alloy stems in total hip replacements. Clin Orthop Relat Res. 1996;333:51–75. [PubMed] [Google Scholar]
- 24.Witt JD, Swann M. Metal wear and tissue response in failed titanium alloy total hip replacements. J Bone Joint Surg Br. 1991;73:559–563. doi: 10.1302/0301-620X.73B4.2071635. [DOI] [PubMed] [Google Scholar]