Abstract
Background
Dislocation after THA continues to be relatively common. Dual mobility sockets have been associated with low dislocation rates, but it remains unclear whether their use in primary THA would not introduce additional complications.
Questions/Purposes
We therefore asked whether a current cementless dual mobility socket (1) reduced the dislocation rate after primary THA, (2) provided a pain-free and mobile hip, and (3) provided durable radiographic fixation of the acetabular component without any unique modes of failure.
Methods
We retrospectively reviewed 168 patients who underwent primary THA using a dual mobility socket between January 2000 and June 2002. The average age at surgery was 67 years. We assessed the rate of dislocation, hip function, and acetabular fixation on serial radiographs. Of the 168 patients, 119 (71%) had clinical and radiographic evaluation at a minimum of 5 years (mean, 6 years; range, 5–8 years).
Results
A long-neck option left the base of the Morse taper uncovered in 53 hips. Four patients underwent revision for dislocation between the femoral head and the mobile insert (intraprosthetic dislocation) at a mean 6 years; all four revisions occurred among the 53 hips with an incompletely covered Morse taper.
Conclusions
A current cementless dual mobility socket was associated with a pain-free and mobile hip and durable acetabular fixation without dislocations if the long-neck option was not used. However, intraprosthetic dislocation related to contact at the femoral neck to mobile insert articulation required revision in four hips. Surgeons should be aware of this specific complication.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
THA has been associated with high levels of function, relatively low complication rates, and high survival. However, with an incidence ranging from 1% to higher than 10% [3], dislocation has continued to be a matter of concern with functional and financial consequences [6, 30]. As an alternative to standard sockets, Bousquet et al. [4] introduced, in the late 1970s, the tripolar device, a dual mobility socket, for patients at increased risk of postoperative instability. These included patients older than 75 years who had prior hip surgery, with neuromuscular diseases, cognitive dysfunction, and American Society of Anesthesiologists scores of 3 or more [3]. The concept of dual mobility with an increase in the effective femoral head diameter has reduced the dislocation rates in both primary and revision THAs to between 0% and 4% [2, 5, 9, 13, 16, 17, 26–29]. It has also been used to treat recurrent dislocation, with rates of subsequent dislocation of between 1.7% and 4% [12, 14, 19, 21]. The main cause of failure of the original Bousquet dual mobility socket (Novae-1®; Serf, Décines, France) was loosening of the cementless socket in 3% to 12% of patients at 10 to 22 years [5, 9, 17, 26–29]. Loosening of the socket has been attributed to the alumina coating of the metal shell providing limited bone on-growth to the socket [18, 31]. Since this early period, a number of cemented and cementless dual mobility designs have emerged in Europe [13, 14, 18, 21, 31], and some have been recently approved by the FDA. Dual mobility sockets have gained popularity in Europe since the early 2000s, currently representing 1/3 of the cementless sockets implanted in France [15]. Most of the existing published literature reported on the original Bousquet design, which has been abandoned due to an unacceptable high loosening rate of the socket [5, 9, 17, 26–29]. The original Bousquet socket was modified by changing the metal cup to a spherocylindrical design and adding a hydroxyapatite coating on the outer surface. Some surgeons now use these modified dual mobility sockets for the vast majority of the patients, including primary THA. While these designs have been associated with low dislocation rates, it is unclear whether there would be specific complications associated with these unique designs.
We therefore asked whether a current design of a cementless dual mobility socket (1) prevented dislocation after primary THA, (2) provided a pain-free and mobile hip, and (3) showed stable radiographic fixation without producing any unique modes of failure.
Patients and Methods
We retrospectively reviewed 168 patients (168 hips) who had a primary THA using a cementless dual mobility socket between January 2000 and June 2002 at two tertiary centers. During this period, the operating surgeons used a dual mobility socket in all primary THAs. There were 92 women and 76 men with a mean ± SD age of 67 ± 12 years (range, 18–92 years) at the time of index procedure. Their mean BMI was 29 ± 5 kg/m2 (range, 15–42 kg/m2). The initial diagnosis was primary osteoarthritis in 125 hips, osteonecrosis of the femoral head in 25, femoral neck fracture in 11, posttraumatic osteoarthritis in three, congenital hip dysplasia in two, and inflammatory arthritis in two. Of the 168 patients, 22 died before the minimum 5-year followup, 23 were lost to followup, and four required revision. The remaining 119 patients had complete clinical and radiographic evaluation at the minimum 5-year followup (mean, 6 years; range, 5–8 years). All patients gave informed consent for participation in this study.
All surgeries were performed by one of two authors (HA, BB). All patients received the Tregor® (Aston® Medical, Saint Étienne, France) cementless dual mobility socket. This device (Fig. 1) consisted of a spherocylindrical CoCr outer shell, double coated with titanium and hydroxyapatite plasma spray, with a highly polished inner surface. This shell articulated with a compression-molded GUR® 1050 UHMWPE mobile component sterilized with 2.5 Mrad gamma radiation in a vacuum atmosphere. The opening diameter of the polyethylene mobile component was 6.7% smaller than the femoral head, and its minimal thickness ranged from 6.3 to 13 mm for 46- and 62-mm outer diameter shells, respectively. This principle created two articulations: an inner bearing between the femoral head and the insert and an outer bearing between the insert and the shell. The femoral head was captured in the polyethylene component using a snap-fit-type mechanism, which acted as a large unconstrained head inside the metal cup. The thickness of the metal shell was 1.7 mm at the apex and 5.5 mm at the equator. This created a medialization of about 5 mm of the center of rotation of the polyethylene insert when compared with the center of rotation of the metal shell, increasing the stability of the device. No locking ring or other means of constraint was associated. This device was available for use with either a 22.2- or 28-mm-diameter femoral head. We used only 22.2-mm femoral heads, and the mean size of the shell was 54 ± 3 mm (range, 48–62 mm). On the femoral side, all patients had a cementless anatomic stem (Respect®; Aston® Medical) made of titanium alloy (TiAl6V4) with a double plasma-sprayed coating of pure titanium and hydroxyapatite in the proximal 1/3.
Fig. 1.
The photograph shows the Tregor® tripolar unconstrained acetabular component (Aston® Medical, Saint Étienne, France) consisting of, from inside out, a spherical mobile polyethylene insert that accepts a 22.2- or 28-mm femoral head and is not constrained into a spherocylindrical metal shell made of CoCr. Photograph courtesy of Aston® Medical.
The operative approach for the index operation was posterolateral in all hips. The mobile polyethylene component was snapped on the femoral head using a specifically designed power press and placed into the metal shell. To accommodate for leg length, the surgeon intraoperatively decided on a long-neck option (> 4 mm) in 53 hips, leaving the base of the Morse taper uncovered, whereas the head completely covered the Morse taper in the remaining 115 hips.
Postoperatively, patients received anticoagulation therapy (enoxaparin, 40 mg/day) for 5 weeks, systemic antibiotics for 48 hours, and NSAIDs (ketoprofen, 100 mg/day) for 5 days to prevent heterotopic ossification. Immediately after the operation, patients began passive motion exercises of the involved joint with the assistance of a therapist and continued them until active motion of the hip was possible. Patients were free to walk with two supports after 3 days. For all patients, we allowed full weightbearing as tolerated. We took no specific measures to prevent dislocation, including restrictions to mobility.
We evaluated all patients at 6 weeks, 6 months, 1 year, and annually thereafter. The operative surgeon rated hip function using the Merle d’Aubigné [23] grading system. An independent observer (MH) analyzed serial AP radiographs of the pelvis, noting the inclination angle of the cup and the presence and progression of radiolucent lines according to the zones described by DeLee and Charnley [7]. Fixation of the acetabular component was evaluated according to the criteria of Massin et al. [22]. They defined loosening of the socket as cup migration exceeding 3 mm, angular rotation exceeding 3°, or a continuous radiolucent line wider than 2 mm (reflecting the observer’s confidence in his or her ability to detect radiolucent lines on plain radiographs). On the femoral side, parameters investigated included the presence and evolution of radiolucent lines in any of the seven zones described by Gruen et al. [10], reactive lines, cancellous bone densification, and pedestal formation around the tip of the stem. Loosening of the femoral component was evaluated according to the criteria of Engh et al. [8], whereas subsidence (> 3 mm) was determined by comparison of two measurements between serial radiographs, as described by Pellegrini et al. [25]. Periprosthetic cystic or scalloped lesions exceeding 2 mm in diameter that were not noted on the immediate postoperative radiographs were defined as osteolysis (we were unaware of studies of interobserver variability to make this assessment).
We performed a survivorship analysis according to the actuarial method on the entire cohort using revision for any reason as the end point. The survival curve was derived from the actuarial life table [24]. We calculated the SD, given as a percentage, and the 95% CI from the data in the life table [24]. We determined differences in proportions of patients having revisions with normal and long necks using a Fisher’s exact test. Statistical analysis was performed with StatView® statistical software (Version 5.0; SAS Institute, Inc, Cary, NC, USA).
Results
There were no dislocations between the polyethylene insert and the metal. Four dislocations (2%) occurred between the femoral head and the mobile insert (intraprosthetic dislocation) after a mean of 6 ± 2 years (range, 3–7 years) in three women and one man with a mean age of 65 years (range, 50–79 years) (Fig. 2). The initial diagnosis was primary osteoarthritis in three patients and femoral neck fracture in one patient. These patients experienced slight discomfort in the groin with a mean Merle d’Aubigné score of 15.5. In the four hips with intraprosthetic dislocation, the Morse taper was partially uncovered (Fig. 3), whereas no case of intraprosthetic dislocation was recorded in the 115 hips with completely covered Morse taper; thus, a higher proportion (p = 0.009) of dislocations occurred in patients with the long neck: four of 53 versus 0 of 115. The capturing area of the revised mobile inserts was grossly deformed and had macroscopic signs of wear (Fig. 4). These four hips underwent revision requiring femoral head and mobile insert exchange in three hips; the remaining hip underwent revision of the socket, femoral head, and mobile insert because macroscopic scratches were visible on the inner surface of the metal shell. In all four hips, the surgeons used a 28-mm head to fully cover the Morse taper to avoid recurrence of the same complication. The leg length discrepancy was less than 1 cm. At 3-year followup, these hips were well functioning, with no episode of dislocation at the small or large bearing.
Fig. 2A–B.
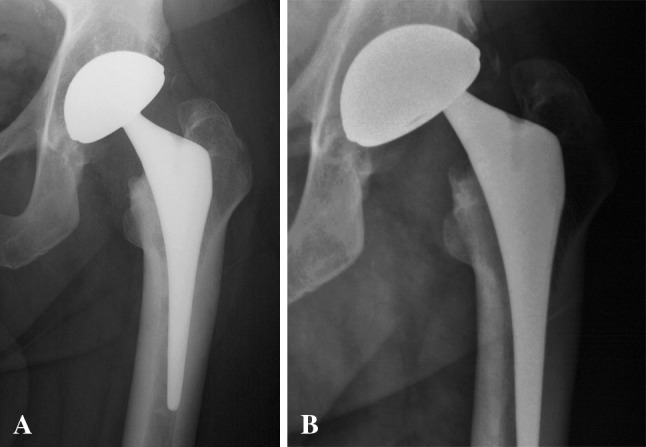
(A) An AP radiograph shows the left hip a 50-year-old man with primary osteoarthritis treated with uncemented THA using a cementless dual mobility socket. (B) At 6.9-year followup, he experienced discomfort in the groin. The AP view of the hip shows a typical aspect of intraprosthetic dislocation with an eccentric femoral neck when compared to the center of the metal shell.
Fig. 3.

The diagram shows the contact between the rough Morse taper and the mobile polyethylene insert with a long-neck option that leaves the base of the Morse taper uncovered.
Fig. 4.
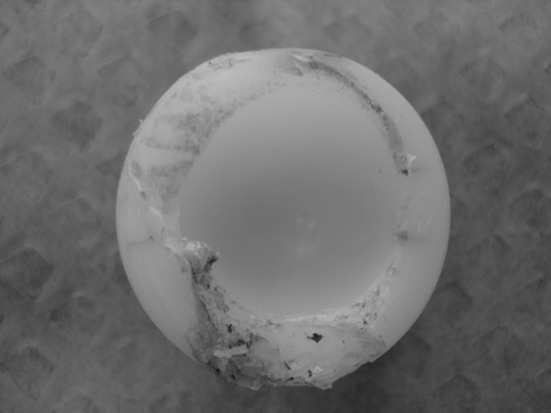
The photograph shows a revised mobile polyethylene insert for intraprosthetic dislocation. Gross wear and deformation at the capturing area are visible.
The mean Merle d’Aubigné functional hip score increased from 11 ± 3 (median, 11; range, 6–16) preoperatively to 17 ± 1 (median, 17; range, 9–18) at latest followup. The range of flexion was greater than 90° in 104 hips and 75° to 85° in 15 hips. At latest followup, the mean range of flexion of the series was 103° ± 16° (median, 100°; range, 70°–130°).
With revision for any reason as the end point, the cumulative survival rate at 7 years was 94.2% ± 5.8% (95% CI, 88.4%–100%). Of the 119 acetabular components, there were no cases of radiolucent lines, osteolysis, reactive lines around the cup, or cup migration. On the femoral side, there was cancellous bone densification in all hips in Zones 1 and 7 on AP radiographs. No case of radiolucent lines at the bone-implant interface was observed, and reactive lines were present in 15 of the 119 (13%) hips in Zones 3, 4, and 5. No femoral component had subsided. No acetabular or femoral component had loosened according to our criteria. Osteolysis was only present as calcar resorption of less than 2 mm in 45 of the 119 (38%) hips. The mean abduction angle of the socket was 45° ± 6° (median, 45°; range, 25°–55°).
Discussion
Since Gilles Bousquet and colleagues [4] introduced the dual mobility concept in the late 1970s, combining a low-friction torque arthroplasty and an ultralarge effective femoral head, this device has become a well-accepted treatment option in Europe for patients at high risk of postoperative instability after primary or revision THA and in the treatment of recurrent dislocation [2, 5, 9, 12–14, 16–19, 21, 26–29]. While the original Bousquet design was abandoned due to an unacceptably high loosening rate of the socket [5, 9, 17, 26–29], the concept of dual mobility has reduced the risk of dislocation, and modified designs have provided more reliable secondary cementless fixation of the socket using hydroxyapatite. Accordingly, its indication in France has expanded to currently reach 1/3 of the cementless sockets implanted [15]. Some surgeons, including two of us (HA, BB), even proposed the use of dual mobility sockets in all primary and revision THAs, irrespective of particular risk for instability [3]. This attitude is, of course, debatable because of potential polyethylene wear at the large bearing and specific modes of failure of dual mobility sockets. However, even in the absence of risk factors for dislocation, early or late dislocation does occur. It is anticipated the effective large-diameter femoral head would eliminate dislocation and might be more forgiving in cases of minor malposition. Our aim was to evaluate the ability of a current cementless dual mobility socket to (1) prevent dislocation after primary THA, (2) grant a pain-free and mobile hip, and (3) provide durable acetabular component radiographic fixation at a minimum 5-year followup, without producing specific modes of failure.
There were several limitations to our study. First, owing to the retrospective design, 23 of the initial 169 patients were lost to followup. However, this limitation is inherent to all retrospective series, and the status of more than 86% of the patients could be determined. Second, our study was a medium-term study. However, we judged it important to determine whether, after a minimum 5-year followup, cementless fixation of a dual mobility socket using hydroxyapatite would lower the risk of acetabular loosening. In addition, this study emphasized a specific complication of dual mobility sockets, and we believe this information was important to surgeons’ practices.
We observed no dislocations in our patients between the polyethylene insert and the metal. This was consistent with other reports (Table 1) and further validated the ability of dual mobility sockets to provide THA stability [2, 5, 9, 12–14, 16–19, 21, 26–29]. However, we observed four (2%) dislocations at the inner bearing between the femoral head and the mobile polyethylene component inside the metal shell. This so-called intraprosthetic dislocation was a specific complication of dual mobility socket described by Lecuire et al. [20] with the original Bousquet design. Several authors have reported the incidence of this complication to be from 0% to 5% (Table 1). The mechanism of intraprosthetic dislocation usually included femoral neck to mobile polyethylene insert (third-articulation) impingement, leading to rim fatigue and wear of the insert at the capturing area [1]. Patients complained of a slight and progressive discomfort in the groin and were encouraged to have radiographic analysis performed when such symptoms happened to allow for early revision and avoid metallosis. The diagnosis required careful examination of the AP view of the hip that showed the femoral neck was off-center when compared to the center of the metal shell. The reported risk factors for intraprosthetic dislocation include a large-diameter femoral neck with an aggressive surface, skirted femoral heads, and a small head-to-neck ratio [20]. None of these established risk factors was present in the current series. The use of a long-neck option that left the base of the Morse taper uncovered in 53 hips was responsible for four dislocations (7.5%). There were no intraprosthetic dislocations when the Morse taper was completely covered. This phenomenon was related to the rough and aggressive surface of the Morse taper. The fact that a long-neck option left the base of the Morse taper uncovered might be specific to its design. We redesigned the Morse taper so that it was fully covered by a 22.2- and 28-mm femoral head with a long-neck option. Periprosthetic ossifications and major intraarticular fibrosis could have also contributed to intraprosthetic dislocation [20]. Additionally, design of the polyethylene insert at the capturing area, shape of the metal shell, and eccentric centers of rotation of the insert and the shell could have played a role in intraprosthetic dislocation. However, to date, no design has shown any advantage over another.
Table 1.
Main published results of dual mobility sockets in primary THA
| Study | Number of hips | Mean followup (years) | Dislocation (%) | Intraprosthetic dislocation (%) | Radiographic loosening (%) | Survival (%)* |
|---|---|---|---|---|---|---|
| Original Bousquet design | ||||||
| Boyer et al. [5] | 240 | 22 | 0 | 4.1 | 8.3 | 73.9 |
| Farizon et al. [9] | 135 | 12 | 0 | 2 | 3 | 95.4 |
| Lautridou et al. [17] | 437 | 16.5 | 1.1 | 0.7 | 12 | 88.4 |
| Philippot et al. [26] | 106 | 10 | 0 | 1.9 | 1.9 | 94.6 |
| Philippot et al. [28] | 384 | 15.3 | 0 | 3.6 | 3.3 | 94.6 |
| Philippot et al. [29] | 438 | 17 | 0 | 5.2 | 3 | 89.2 |
| Current dual mobility designs | ||||||
| Guyen et al. [13] | 167 | 3 | 0 | 0 | 0 | |
| Leclercq et al. [19] | 200 | 6 | 0 | 0 | 0 | 100 |
| Vielpeau et al. [31] | 231 | 5.2 | 0 | 0 | 9 | |
| Current study | 168 | 6 | 0 | 2.4 | 0 | 94.2 |
* Survival provided at mean followup of the series.
Of the 119 hips, 100 had no pain in the operated hip, and the range of flexion was greater than 90° in 104 hips, with a mean of 106°. These findings were in accordance with other studies using a dual mobility socket [2, 5, 9, 12–14, 16–19, 21, 26–29] and in vitro data [11].
Radiographic analysis revealed no radiolucent lines on the acetabular and femoral sides. Additionally, no component loosened and no femoral component subsided. These findings were comparable with other studies that evaluated cementless fixation of dual mobility sockets using hydroxyapatite at similar followup [13, 18, 31].
A current cementless dual mobility socket prevented dislocation at the large bearing after primary THA and provided a pain-free and mobile hip, along with durable radiographic fixation of the acetabular component, at a minimum of 5 years. However, fatigue damage and wear of the mobile insert at the capturing area could have led to intraprosthetic dislocation requiring revision. Surgeons should be aware of this specific complication, and aggressive contact at the femoral neck to mobile insert articulation should be avoided. Two of the authors (HA, BB) continue to systematically use dual mobility sockets in primary THA, using the redesigned Morse taper that is fully covered by the femoral head. Longer-term studies are warranted to assess whether cementless dual mobility sockets could represent a reliable treatment option in primary THA in the absence of risk factors for instability.
Footnotes
One or more of the authors (MH, HA, BB) have received or may receive payments or benefits, in any 1 year, in excess on $10,000, from a commercial entity (Aston® Medical, Saint Étienne, France) related to this work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution has approved or waived approval for the human protocol of this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Clinical Orthopaedic Research Center (Paris, France), Centre Hospitalier Fleyriat (Bourg en Bresse, France), and Institut Calot (Berck sur Mer, France).
References
- 1.Adam P, Farizon F, Fessy MH. Dual articulation retentive acetabular liners and wear: surface analysis of 40 retrieved polyethylene implants] [in French. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:627–636. doi: 10.1016/S0035-1040(05)84466-6. [DOI] [PubMed] [Google Scholar]
- 2.Aubriot J, Lesimple P, Leclercq S. Study of Bousquet’s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component): average 5-year follow-up] [in French] Acta Orthop Belg. 1993;59(suppl 1):267–271. [PubMed] [Google Scholar]
- 3.Berry DJ. Unstable total hip arthroplasty: detailed overview. Instr Course Lect. 2001;50:265–274. [PubMed] [Google Scholar]
- 4.Bousquet G, Gazielly DF, Girardin P, Debiesse JL, Relaye M, Israeli A. The ceramic coated cementless total hip arthroplasty: basic concepts and surgical technique. J Orthop Surg Tech. 1985;1:15–28. [Google Scholar]
- 5.Boyer B, Philippot R, Geringer J, Farizon F. Primary total hip arthroplasty with dual mobility socket to prevent dislocation: a 22-year follow-up of 240 hips. Int Orthop. 2012;36:511–518. doi: 10.1007/s00264-011-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandler RW, Dorr LD, Perry J. The functional cost of dislocation following total hip arthroplasty. Clin Orthop Relat Res. 1982;168:168–172. [PubMed] [Google Scholar]
- 7.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 8.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement: the factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 9.Farizon F, Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility: a twelve-year follow-up study. Int Orthop. 1998;22:219–224. doi: 10.1007/s002640050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 11.Guyen O, Chen QS, Bejui-Hugues J, Berry DJ, An KN. Unconstrained tripolar hip implants: effect on hip stability. Clin Orthop Relat Res. 2007;455:202–208. doi: 10.1097/01.blo.0000238796.59596.1f. [DOI] [PubMed] [Google Scholar]
- 12.Guyen O, Pibarot V, Vaz G, Chevillotte C, Bejui-Hugues J. Use of a dual mobility socket to manage total hip arthroplasty instability. Clin Orthop Relat Res. 2009;467:465–472. doi: 10.1007/s11999-008-0476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyen O, Pibarot V, Vaz G, Chevillotte C, Caret JP, Bejui-Hugues J. Unconstrained tripolar implants for primary total hip arthroplasty in patients at risk for dislocation. J Arthroplasty. 2007;22:849–858. doi: 10.1016/j.arth.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Hamadouche M, Biau DJ, Huten D, Musset T, Gaucher F. The use of a cemented dual mobility socket to treat recurrent dislocation. Clin Orthop Relat Res. 2010;468:3248–3254. doi: 10.1007/s11999-010-1404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.[Health Authority. Evaluation of total hip prostheses: generic descriptions revising the list of reimbursable products and services of hip joint implants] [in French]. September 2007. Available at: www.has-sante.fr/portail/upload/docs/application/pdf/rapport_evaluation_des_prothese_de_hanche.pdf. Accessed January 18, 2012.
- 16.Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop Relat Res. 2008;466:389–395. doi: 10.1007/s11999-007-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lautridou C, Lebel B, Burdin G, Vielpeau C. Survival of the cementless Bousquet dual mobility cup: minimum 15-year follow-up of 437 total hip arthroplasties] [in French. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:731–739. doi: 10.1016/j.rco.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Leclercq S, Benoit JY, Rosa JP, Euvrard P, Leteurtre C, Girardin P. Results of the Evora dual mobility socket: five years follow-up] [in French. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:37–42. doi: 10.1016/j.rco.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Leclercq S, Blidi S, Aubriot JH. Bousquet’s device in the treatment of recurrent dislocation of a total hip prosthesis: apropos of 13 cases] [in French. Rev Chir Orthop Reparatrice Appar Mot. 1995;81:389–394. [PubMed] [Google Scholar]
- 20.Lecuire F, Benareau J, Rubini J, Basso M. Intra-prosthetic dislocation of the Bousquet dual mobility socket] [in French. Rev Chir Orthop Reparatrice Appar Mot. 2004;90:249–255. doi: 10.1016/S0035-1040(04)70101-4. [DOI] [PubMed] [Google Scholar]
- 21.Leiber-Wackenheim F, Brunschweiler B, Ehlinger M, Gabrion A, Mertl P. Treatment of recurrent THR dislocation using a cementless dual mobility cup: a 59 cases series with a mean 8 years follow-up. Orthop Traumatol Surg Res. 2011;97:8–13. doi: 10.1016/j.otsr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration: an experimental study. J Arthroplasty. 1989;4:245–251. doi: 10.1016/S0883-5403(89)80020-8. [DOI] [PubMed] [Google Scholar]
- 23.Merle d’Aubigné R. [Numerical evaluation of hip function] [in French] Rev Chir Orthop Reparatrice Appar Mot. 1970;56:481–486. [PubMed] [Google Scholar]
- 24.Murray DW, Carr AJ, Bulstrode C. Survival analysis of joint replacement. J Bone Joint Surg Br. 1993;75:697–704. doi: 10.1302/0301-620X.75B5.8376423. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrini VD, Hughes SS, Evarts CM. A collarless cobalt-chrome femoral component in uncemented total hip arthroplasty: five- to eight-year follow-up. J Bone Joint Surg Br. 1992;74:814–821. doi: 10.1302/0301-620X.74B6.1447240. [DOI] [PubMed] [Google Scholar]
- 26.Philippot R, Adam P, Farizon F, Fessy MH, Bousquet G. Survival of cementless dual mobility sockets: ten-year follow-up] [in French. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:326–331. doi: 10.1016/S0035-1040(06)75762-2. [DOI] [PubMed] [Google Scholar]
- 27.Philippot R, Adam P, Reckhaus M, Delangle F, Verdot FX, Curvale G, Farizon F. [Prevention of dislocation in total hip revision surgery using a dual mobility design] [in French. Rev Chir Orthop Reparatrice Appar Mot. 2009;95:407–413. doi: 10.1016/j.otsr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Philippot R, Camilleri JP, Boyer B, Adam P, Farizon F. The use of a dual-articulation acetabular cup system to prevent dislocation after primary total hip arthroplasty: analysis of 384 cases at a mean follow-up of 15 years. Int Orthop. 2009;33:927–932. doi: 10.1007/s00264-008-0589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippot R, Farizon F, Camilleri JP, Boyer B, Derhi G, Bonnan J, Fessy MH, Lecuire F. Survival of dual mobility socket with a mean 17 years follow-up] [in French. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:43–48. doi: 10.1016/j.rco.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Sotelo J, Haidukewych GJ, Boberg C. Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88:290–294. doi: 10.2106/JBJS.D.02799. [DOI] [PubMed] [Google Scholar]
- 31.Vielpeau C, Lebel B, Ardouin L, Burdin G, Lautridou C. The dual mobility socket concept: experience with 668 cases. Int Orthop. 2011;35:225–230. doi: 10.1007/s00264-010-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]



