Abstract
Background
Although navigated THA provides improved precision in implant positioning and alignment, it is unclear whether these translate into long-term implant survival.
Questions/Purposes
We compared survivorship, dislocation rate, and incidence of radiographic failures such as loosening and bearing breakage after THA with and without navigation at a minimum 10-year followup.
Methods
We retrospectively reviewed 46 patients (60 hips) and 97 patients (120 hips) receiving THA with or without a CT-based navigation system, respectively, using cementless THA ceramic-on-ceramic bearing couples. There were no differences in age, sex, diagnosis, height, weight, BMI, or preoperative clinical score between groups. We evaluated survivorship, mode of acetabular and femoral component fixation, osteolysis, and implant wear or breakage at a minimum followup of 10 years (average, 11 years; range, 10–13 years).
Results
Survival at 13 years was 100% with navigation and 95.6% (95% CI, 88.4%–98.4%) without navigation. With navigation, all cups were placed within a zone of 40° (range, 30°–50°) of radiographic inclination and 15° (range, 5°–15°) of radiographic anteversion; without navigation, 31 cups (26%) were placed outside this zone. Hips treated without navigation had a higher rate of dislocation (8%) than the navigated cases (0%). Revision was performed in four nonnavigated cases, all of which showed evidence of neck impingement on the ceramic liner. Moreover, seven other cases without navigation showed posterior neck erosion on radiographs. These 11 impingement-related mechanical complications correlated with cup malorientation, and the incidence of impingement-related complications was higher in nonnavigated cases.
Conclusions
Navigation reduced the rates of dislocation and impingement-related mechanical complications leading to revision in cementless THA using ceramic-on-ceramic bearing couples over a minimum 10-year followup.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Accurate positioning of implants is of primary importance for the long-term survival of THA. Impingement of the femoral neck on the cup rim can cause dislocation and premature cup loosening [19, 23]. Edge loading due to high cup inclination or anteversion increases the risk of accelerated wear and breakage of the bearing couples even with modern wear-resistant bearing couples such as metal-on-metal bearings [11], ceramic-on-ceramic (COC) bearings [1], and highly crosslinked polyethylene [7]. However, standard mechanical instruments for cup orientation are not sufficient to reduce the cup orientation outliers [4].
Surgical navigation has been used to achieve a more precise placement of the total hip components [5, 21, 23]. The more precise placement of the acetabular component should lead to a lower dislocation rate and fewer bearing-related problems, resulting in a higher long-term survivorship. However, it is unclear whether the short-term achievement of more precise placement leads to long-term improvement in the survivorship, dislocation rate, and mechanical complication rate.
We therefore determined (1) survivorship (2) dislocation rate, and (3) incidence of radiographic failures such as loosening and bearing breakage after THA with and without navigation at a minimum 10-year followup.
Patients and Methods
We retrospectively reviewed all 143 patients (180 hips) treated with a cementless 28-mm Biolox® forte COC articulation (CeramTec, Plochingen, Germany) between April 1998 and April 2001. During that time, we treated 165 patients (231 hips) with other devices. In 60 of the 180 hips (navigation group), a CT-based navigation system was used. The remaining 120 hips (nonnavigation group) underwent THA without using the navigation system. There were 153 hips in 123 women and 27 hips in 20 men. The mean age at operation was 53 years (range, 27–74 years). The mean BMI was 23 kg/m2 (range, 16–36 kg/m2). The preoperative diagnosis was osteoarthritis in 153 hips, osteonecrosis of the femoral head in 21 hips, and rheumatoid arthritis in six hips. Osteoarthritis in 150 hips occurred secondary to hip dysplasia.
Selection of the patients for navigation was not randomized and navigation was used when the engineer’s support was available. However, there were no differences between the two groups in age, sex, diagnosis, or BMI (Table 1). Four patients (four hips) in the navigation group died within 10 years, but all these hips were functioning well until death. The remaining 42 patients (56 hips) were followed for a minimum of 10 years (mean, 11 years; range, 10–13 years). Nine patients (10 hips) in the nonnavigation group were lost to followup. Four patients (five hips) died within 10 years, but all these hips were functioning well until death. The remaining 84 patients (105 hips) were followed for a minimum of 10 years (mean, 11 years; range, 10–13 years). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Table 1.
Patient demographic data
| Variable | Navigation group | Nonnavigation group | p value |
|---|---|---|---|
| Age (years)* | 53 (37–74) | 53 (27–72) | 0.89† |
| Sex (number of hips) | 0.51‡ | ||
| Male | 7 | 20 | |
| Female | 53 | 100 | |
| Diagnosis (number of hips) | |||
| Osteoarthritis | 55 | 98 | |
| Osteonecrosis | 4 | 17 | 0.18§ |
| Rheumatoid arthritis | 1 | 5 | |
| BMI (kg/m2)* | 23 (19–23) | 23 (16–36) | 0.70 (−1.128 to 0.894)|| |
* Values are expressed as mean, with range in parentheses; †Mann-Whitney test; ‡Fisher’s exact test; §chi-square test; ||unpaired t-test, with 95% CI in parentheses.
In the navigation group, transverse CT images from the level of the superior anterior iliac spine to the level of the isthmus of the femoral canal were preoperatively obtained using a helical CT scanner. The slice thickness was 3 mm and the slice pitch was 3 mm, with the exception of a region between 2 cm below the lesser trochanter and the isthmus of the femoral canal, where the slice pitch was 10 mm. Several reference images of the femoral condyles were also taken to measure anteversion, with a slice pitch of 3 mm. Radiation dose of this protocol was calculated to be less than 3 mSV. The navigation system consisted of an optical 3D localizer (Optotrak® 3020; Northern Digital, Waterloo, Canada), a custom-made dynamic reference frame with active light-emitting diodes, custom-made surgical tools designed to work with the dynamic reference frame, an Optotrak® pen probe, and a UNIX®-based Sun UltraSPARC® workstation (Sun Microsystems, Santa Clara, CA, USA). The navigation software was developed in our Medical Image Analysis Division, using an open source software system (Visualization Toolkit; Kitware, Inc, Clifton Park, NY, USA). We reconstructed three-dimensional acetabular and femoral bone surface models from the CT data of each patient. To measure cup orientation (inclination and anteversion), we used the functional pelvic zero position where the pelvis in supine on the CT scan table was axially rotated until the bilateral anterior iliac spines touched the same horizontal plane and then the interteardrop line was used as the mediolateral axis [20]. We defined the ideal cup orientation as 40° of radiographic inclination and 20° of anatomic anteversion (13.2° of radiographic anteversion or 17.0° of operative anteversion), by considering the ROM without impingement during various daily activities and avoiding a high inclination angle to prevent the acetabular ceramic liner from developing a chipping fracture when the mean average femoral anteversion was 30° [14]. This was close to the center of the safe zone described by Lewinnek et al. [12].
All operations were performed by one of four surgeons (NS, TN, TS, KO) via a posterolateral approach under general anesthesia. In the operating room, shape-based surface registration of the patient’s bones to the previously constructed bone models of the pelvis and femur was performed using 30 surface points [21]. The accuracy of registration was verified by touching bony landmarks. The navigation system was used to guide the femoral neck osteotomy level, position of the cup center, cup orientation, femoral anteversion, and limb length discrepancy by comparing these parameters to the CT-based preoperative planning. Acetabular osteophyte was removed after cup placement when it overhung the cup rim. A modular Biolox® forte alumina ceramic liner was inserted in a hemispherical titanium porous-bead-coated shell (AnCA-Fit™ cup; Cremascoli Ortho, Milan, Italy). The modular ceramic head was fixed to a 12/14 taper cone, resulting in 128° of component ROM (oscillation angle) before impingement between the taper and the rim of the liner. The stem position was measured with navigation by using a neck adaptor with light-emitting diode markers. A modular changeable neck was used to change the anteversion to approach 30°. When the stem anteversion ranged from 25° to 35°, a straight modular neck was chosen. When the stem anteversion ranged from 35° to 40° or from 20° to 25°, an 8° retroverted or anteverted neck was used. When the femoral anteversion ranged from 40° to 50° or from 10° to 15°, a 15° retroverted or anteverted neck was used [15]. The prosthetic head position was measured with navigation and the mean measured femoral neck anteversion was 30° (range, 16°–43°).
Patients were followed up at 3 months, 6 months, 1 year, and annually after surgery. At each visit, we performed clinical assessment using the Merle d’Aubigné-Postel hip score [13], with a maximum score of 6 points for pain, mobility, and gait function, respectively. An AP radiograph of bilateral hips in supine and a lateral radiograph of the operated side of the proximal femur were taken 1 and 2 weeks after surgery and at each routine followup visit. We radiographically evaluated the mode of acetabular and femoral component fixation, osteolysis, and implant wear or breakage. One of us (MT) who was not a treating surgeon evaluated all radiographs. The interobserver variability was tested using a set of 20 radiographs by two of us (MT, HM) and the Pearson R was 0.929 for inclination and 0.854 for anteversion. There were no missing radiographs. To measure the cup orientation, an ellipse was fitted to the rim of the acetabular component on the early postoperative AP radiographs using computer software (CAM of THA; Kyocera Corp, Kyoto, Japan). To eliminate measurement error due to pelvic axial rotation on AP radiographs, measurements were performed on selected postoperative radiographs taken within 1 month after surgery on which the pubic symphysis and the spinous processes of the sacrum were located along a vertical line. The mode of femoral component fixation was radiographically classified as bone-ingrown stable, stable fibrous, or unstable, according to the criteria of Engh et al. [6]. This was performed 2 years postoperatively and annually thereafter. We assessed migration of femoral components using the following measurements: the vertical distance from the shoulder of the stem to the midpoint of the lesser trochanter and the varus angle of the stem formed by the stem axis and the proximal femoral axis. Subsidence in a vertical direction of greater than 4 mm [3] or a change in the varus angle of greater than 2° was considered to indicate stem migration and loosening. Loosening of acetabular components was defined as migration of greater than 2 mm or a change in the abduction angle of the acetabular component of greater than 5° [3]. Osteolysis was also evaluated by comparing the latest followup radiographs and the postoperative radiographs taken within 3 months of surgery. Osteolysis was defined as a sharply demarcated radiolucent space with a rounded or scalloped appearance that extends away from the surface of the implant [24].
We performed Kaplan-Meier survival analysis with revision as the end point. The 95% CI was also calculated. The log-rank test was used to see whether there was a difference in the survival between the navigation and nonnavigation groups. We determined differences in the Merle d’Aubigné-Postel hip score, age, cup inclination, and cup anteversion between the groups using the Mann-Whitney U test. Differences in variance of cup inclination and anteversion between the groups were analyzed using the F-test. We determined differences in dislocation rate between the groups using Fisher’s exact probability test.
Results
In the navigation group, no revision was needed during the followup period. The survival rate at 13 years was 100%. In the nonnavigation group, cup revision was performed due to aseptic loosening in one hip at 3 years. Three hips graded as bone-ingrown stable at 2 years postoperatively developed an acetabular ceramic liner chipping fracture at 4 and 8 years, respectively. Both of these patients noticed a clicking noise that started without any trauma 2 years after surgery. The noise increased and the radiographic examination showed several fragments in the hip with the femoral head subluxation later. These two hips and one hip with recurrent dislocation 7 years after surgery underwent cup revision. All four revised implants showed a wear scar on the neck. The survival rate at 13 years was 95.6% (95% CI, 88.4%–98.4%). However, there was no difference (p = 0.14) in the survival rate between the navigation and nonnavigation groups.
During the followup period, dislocation occurred more frequently (p = 0.030) in the nonnavigation group (nine hips) than in the navigation group (no hips) (Fig. 1). There were one anterior dislocation and eight posterior dislocations. Eight dislocations were seen within 3 months after surgery and three hips showed recurrence only once. One hip posterior dislocation was seen at 6 years after surgery and dislocation became iterative. Cup revision was performed at 7 years after surgery in this case. More hips (p < 0.001) were outside the Lewinnek safe zone in the nonnavigation group (32 hips) than in the navigation group (no hips) (Fig. 1). However, there was no difference (p = 0.24) in the dislocation rates between hips inside and outside the safe zone. There was no difference (p = 0.35) in mean inclination between the two groups, but the variance was greater (p < 0.001) in the nonnavigation group than in the navigation group (Table 2). Mean cup anteversion was lower (p < 0.001) in the navigation group than in the nonnavigation group, with a difference in variance (p < 0.001) between the groups as well (Table 2).
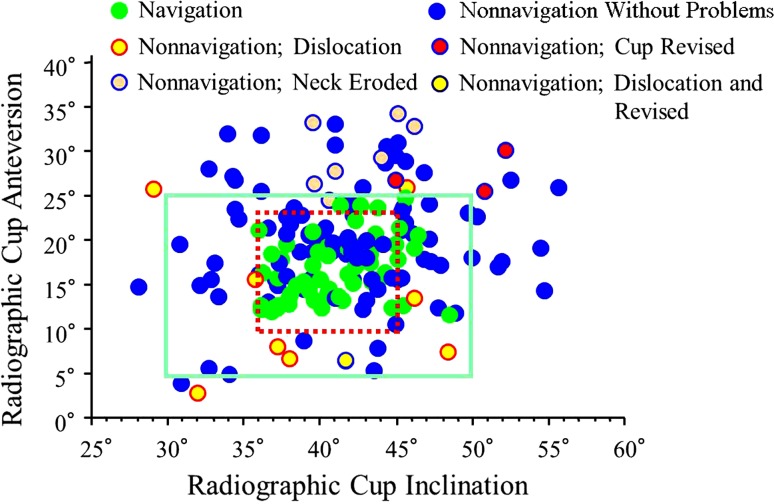
Fig. 1.
A scattergram of cup orientation plots cup anteversion against cup inclination. All cups inserted with navigation are within the Lewinnek safe zone (outer rectangle). Dislocation occurred more frequently in the nonnavigation group (eight hips) than in the navigation group (no hips), but there was no difference in the dislocation rate between hips inside and outside the safe zone. The impingement-related mechanical complications comprised four revisions and seven neck erosions, all of which were seen in the nonnavigation group. The true safe zone without dislocation or mechanical complications seems to be 36° to 45° of inclination and 10° to 24° of anteversion (inner dotted rectangle).
Table 2.
Cup orientation
| Parameter | Navigation group | Nonnavigation group |
|---|---|---|
| Inclination (°)* | 40.9 (35.9–44.8) | 41.7 (28.0–55.7) |
| Anteversion (°)† | 16.8 (11.7–24.8) | 20.1 (2.9–34.4) |
Values are expressed as mean, with range in parentheses; * p < 0.001 (F-test), p = 0.35 (Mann-Whitney U test); †p < 0.001 (F-test), p < 0.001 (Mann-Whitney U test).
Radiographically, all hips in the navigation group were classified as bone-ingrown stable. No osteolysis or expanding radiolucent lines at the bone-implant interface were observed on the latest followup radiographs. The unrevised 101 hips in the nonnavigation group were graded as bone-ingrown stable, and no osteolysis or expanding radiolucent lines at the bone-implant interface were observed on the latest followup radiographs. Seven hips showed posterior neck erosion on the lateral views of the femur without osteolysis or pain. The location of neck erosion was near the head-neck junction where the neck could impinge on the liner rim in hip extension and/or external rotation. The erosion became visible on radiographs at 3 to 5 years after surgery. The depth was 1 to 2 mm at the latest followup. These seven patients noticed a clicking noise at the followup visit of 6 to 12 months when the hip was extended. The average age of these seven patients was 53.6 years, which was not different (p = 0.54) from that of the remaining patients. The average cup anteversion was 29.5°, which was higher (p < 0.001) than that of the remaining hips (Fig. 1). There had been no noticeable change in noise during the followup period. The impingement-related mechanical complications comprised one cup loosening, two liner fractures, one recurrent dislocation, and seven neck erosions (Fig. 1). The incidence of impingement-related mechanical complications was higher (p = 0.016) in the nonnavigation group than in the navigation group.
The mean Merle d’Aubigné-Postel hip score improved from 9.5 preoperatively to 17.5 at the latest followup in both groups. However, there was no difference (p = 0.50) in the mean hip scores between the two groups. None of the 180 subjects developed early or late infections or pulmonary embolism. There were no navigation-related complications such as pin site fracture or infection.
Discussion
Surgical navigation has been used to achieve a more precise placement of the total hip components than conventional methods [5, 17, 20]. The more precise placement of the acetabular component should lead to a lower dislocation rate and fewer bearing-related problems, resulting in a higher long-term survivorship. However, it is unclear whether more precise placement of the cups really leads to a higher survivorship of implants and lower mechanical complication rates. We therefore asked whether the survival, dislocation rate, and incidence of radiographic failures such as loosening and bearing breakage after THA with navigation were better than those without navigation at a minimum 10-year followup.
There are several limitations to this study. First, this was not a randomized controlled study. However, there were no differences between the groups in age, sex, diagnosis, BMI, or preoperative clinical score. Accordingly, we believe this study offers valuable information about the clinical efficacy of navigation on the long-term results of COC THA. Second, we measured only cup orientation; however, variability in femoral neck anteversion can affect ROM and stability of the hip. In this series, we used modular necks to try to achieve 30° of femoral neck anteversion to reduce the effect of variability in femoral neck anteversion on the clinical results. Finally, the safe zone defined by Lewinnek et al. [12] was used to measure the extent of variance in cup orientation, but there is little evidence to prove this is a true safe zone. Therefore, we used this safe zone as a target zone in which complications such as dislocation and impingement-related mechanical problems could presumably be avoided to an acceptable degree.
Our study confirmed navigation revealed a higher precision to achieve the cup orientation within a target zone than conventional methods as previously reported (Table 3). However, the previous literature did not correlate the precise cup placement to the clinical benefits partly due to a short period of followup. We found 100% of survival with revision as the end point with navigation at 13 years while it was 95.6% without navigation. Although the difference in survival was not statistically significant, we suspect it is just a matter of time until the difference becomes significant because the other seven hips without navigation showed femoral neck erosion on the lateral radiographs while there was no neck erosion in the navigation group. We may have to consider early revision of these hips to avoid catastrophic failures such as neck and ceramic fractures. When we combined revision and femoral neck erosion as impingement-related mechanical complications, the incidence was higher in the nonnavigation group than in the navigation group.
Table 3.
Comparison of our results to those in recent literature
| Study | Followup (years) | Navigation | Nonnavigation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of hips | % of cups in safe zones | Dislocation rate (%) | Survival rate (%) | Type of navigation | Number of hips | % of cups in safe zones | Dislocation rate (%) | Survival rate (%) | ||
| Haaker et al. [8] | 1 | 98 | 93 | NA | NA | CT-based | 69 | 82 | NA | NA |
| Kalteis et al. [9] | 0.1 | 30 | 83 | NA | NA | CT-based | 30 | 50 | NA | NA |
| Murphy et al. [15] | 0.5 | 185 | NA–98* | 0.54 | NA | CT-based | 189 | NA–95* | 0.53 | NA |
| Najarian et al. [16] | NA | 96 | 86–96* | NA | NA | Imageless | 53 | 79–87* | NA | NA |
| Parratte and Argenson [18] | NA | 30 | 80 | NA | NA | Imageless | 30 | 43 | NA | NA |
| Wixson et al. [22] | 0.1 | 82 | 30 | NA | NA | Imageless | 50 | 6 | NA | NA |
| Current study | 11 | 60 | 100 | 0 | 100 | CT-based | 120 | 73.3 | 6.7 | 95.6 |
* Anteversion-inclination; NA = not available.
We found no differences in the Merle d’Aubigné-Postel hip score between the groups after surgery. However, we found the rate of dislocation was higher for hips without navigation than for hips with navigation. The cause of dislocation is multifactorial, and it has been reported dislocation is related not only to cup orientation but also to cup rim design, ball size, femoral neck angle, femoral neck dimension, soft tissue tension, and surgical approach [2, 10]. In this study, a ball diameter of 28 mm with a femoral neck diameter of 14 mm, a ceramic acetabular liner without an elevated rim, and the posterolateral approach without repair of the posterior soft tissue were the factors that may have influenced the likelihood of dislocation. Although cup malorientation defined by the Lewinnek safe zone did not correlate with dislocation, the true safe zone with this implant design and approach may be smaller (inclination: 36°–45°; anteversion: 10°–24°) than the Lewinnek safe zone (Fig. 1).
In conclusion, CT-based navigation was associated with lower rates of dislocation and impingement-related mechanical complications in cementless THA using COC bearing couples over a minimum 10-year followup.
Acknowledgment
The authors thank Dr Kenji Ohzono for his contribution to this study as one of the operating surgeons.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Osaka University Graduate School of Medicine, Suita, Osaka, Japan.
References
- 1.Barrack RL, Burak C, Skinner HB. Concerns about ceramics in THA. Clin Orthop Relat Res. 2004;429:73–79. doi: 10.1097/01.blo.0000150132.11142.d2. [DOI] [PubMed] [Google Scholar]
- 2.Berry DJ, Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2456–2463. doi: 10.2106/JBJS.D.02860. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan JJ, Dysart SH, Savory CG. The uncemented porous-coated anatomic total hip prosthesis: two-year results of a prospective consecutive series. J Bone Joint Surg Am. 1988;70:337–346. [PubMed] [Google Scholar]
- 4.Callanan MC, Jarrett B, Bragdon CR, Zurakowski D, Rubash HE, Freiberg AA, Malchau H. The John Charnley Award. Risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res. 2011;469:319–329. doi: 10.1007/s11999-010-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiGioia AM, Jaramaz B, Blackwell M, Simon DA, Morgan F, Moody JE, Nikou C, Colgan BD, Aston CA, Labarca RS, Kischell E, Kanade T. The Otto Aufranc Award. Image-guided navigation system to measure intraoperatively acetabular implant alignment. Clin Orthop Relat Res. 1998;355:8–22. doi: 10.1097/00003086-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Engh CA, Glassman AH, Suthers KE. The case for porous-coated hip implants: the femoral side. Clin Orthop Relat Res. 1990;261:63–81. [PubMed] [Google Scholar]
- 7.Furmanski J, Anderson M, Bal S, Greenwald AS, Halley D, Penenberg B, Ries M, Pruitt L. Clinical fracture of cross-linked UHMWPE acetabular liners. Biomaterials. 2009;30:5572–5582. doi: 10.1016/j.biomaterials.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22:151–159. doi: 10.1016/j.arth.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Kalteis T, Handel M, Bathis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT-based navigation? J Bone Joint Surg Br. 2006;88:163–167. doi: 10.1302/0301-620X.88B2.17163. [DOI] [PubMed] [Google Scholar]
- 10.Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop Relat Res. 2006;447:34–38. doi: 10.1097/01.blo.0000218746.84494.df. [DOI] [PubMed] [Google Scholar]
- 11.Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90:1143–1151. doi: 10.1302/0301-620X.90B9.20785. [DOI] [PubMed] [Google Scholar]
- 12.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 13.Merle d’Aubigné R, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed] [Google Scholar]
- 14.Miki H, Yamanashi W, Nishii T, Sato Y, Yoshikawa H, Sugano N. Anatomic hip range of motion after implantation during total hip arthroplasty as measured by a navigation system. J Arthroplasty. 2007;22:946–952. doi: 10.1016/j.arth.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Murphy SB, Ecker TM, Tannast M. THA performed using conventional and navigated tissue-preserving techniques. Clin Orthop Relat Res. 2006;453:160–167. doi: 10.1097/01.blo.0000246539.57198.29. [DOI] [PubMed] [Google Scholar]
- 16.Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24:15–21. doi: 10.1016/j.arth.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Nogler M, Kessler O, Prassl A, Donnelly B, Streicher R, Sledge JB, Krismer M. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop Relat Res. 2004;426:159–163. doi: 10.1097/01.blo.0000141902.30946.6d. [DOI] [PubMed] [Google Scholar]
- 18.Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty: a prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89:494–499. doi: 10.2106/JBJS.F.00529. [DOI] [PubMed] [Google Scholar]
- 19.Shon WY, Baldini T, Peterson MG, Wright TM, Salvati EA. Impingement in total hip arthroplasty a study of retrieved acetabular components. J Arthroplasty. 2005;20:427–435. doi: 10.1016/j.arth.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 20.Sugano N, Nishii T, Miki H, Yoshikawa H, Sato Y, Tamura S. Mid-term results of cementless total hip replacement using a ceramic-on-ceramic bearing with and without computer navigation. J Bone Joint Surg Br. 2007;89:455–460. doi: 10.1302/0301-620X.89B4.18458. [DOI] [PubMed] [Google Scholar]
- 21.Sugano N, Sasama T, Sato Y, Nakajima Y, Nishii T, Yonenobu K, Tamura S, Ochi T. Accuracy evaluation of surface-based registration methods in a computer navigation system for hip surgery performed through a posterolateral approach. Comput Aided Surg. 2001;6:195–203. doi: 10.3109/10929080109146083. [DOI] [PubMed] [Google Scholar]
- 22.Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(suppl 3):51–56. doi: 10.1016/j.arth.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Wroblewski BM, Siney PD, Fleming PA. Effect of reduced diameter neck stem on incidence of radiographic cup loosening and revisions in Charnley low-frictional torque arthroplasty. J Arthroplasty. 2009;24:10–14. doi: 10.1016/j.arth.2008.01.312. [DOI] [PubMed] [Google Scholar]
- 24.Zicat B, Engh CA, Gokcen E. Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg Am. 1995;77:432–439. doi: 10.2106/00004623-199503000-00013. [DOI] [PubMed] [Google Scholar]