Abstract
Background
Effect of the 2009 H1N1 influenza pandemic on viral epidemiology of upper and lower respiratory tract infections (URI and LRI) in healthy infants in the first year of life has not been well studied.
Methods
A total of 180 healthy infants were enrolled from birth and monitored for occurrences of URI and its LRI and acute otitis media (AOM) complications until the first AOM episode or between 6 and 12 months of age. Nasopharyngeal specimens collected during acute respiratory illnesses were tested for 18 viruses.
Results
Between October 2008 and April 2011, 373 URI episodes, including 20 with LRI, in 139 infants were documented. Viral studies were performed on 189 URI episodes; 87% were positive. Throughout the 31-month period (1386 patient-months), rhinovirus was the predominant virus causing URI (55%); RSV was the major cause of LRI (64%). While there was a significant increase in parent-initiated visit rate during the 15-month influenza pandemic as compared with pre- and post- pandemic periods, only 4 cases of influenza were detected (2 cases during and 2 cases pre- and post- pandemic).
Conclusion
The 2009 influenza A/H1N1 pandemic had no impact on the overall viral epidemiology of respiratory infections in healthy infants in the first year of life but resulted in increased parent-initiated visits due to respiratory symptoms. Maternal antibody and absence of co-morbidity may explain the low influenza burden while parental anxiety may explain the increased healthcare visit rate during the pandemic.
Keywords: influenza, influenza pandemic, upper respiratory tract infection, lower respiratory tract infection, infants
INTRODUCTION
Early life viral respiratory infection is an important cause of morbidity and mortality. Respiratory syncytial virus (RSV) is known to be the most common cause of severe lower respiratory infections (LRI) in infants in the first year of life. RSV infection is more severe than influenza infection in infants resulting in high rate of hospitalization1, 2. However, for more common but less severe upper respiratory tract infection (URI), there has been less information on the spectrum of causative viruses in young infants. Availability of molecular techniques has increased the sensitivity of viral diagnostic testing and allows studying of broad spectrum of viruses including relatively new viruses. Recent published studies on birth cohorts followed longitudinally for acute respiratory symptoms have come from Europe and Australia3,4,5 but not the United States. Further understanding of viral epidemiology of respiratory infections will be the basis of development of new interventions for treatment and prevention of infections in this vulnerable population.
In 2009, the United States experienced a pandemic caused by novel swine-origin influenza A/H1N1 strain. Two successive waves began in April and September of 2009, respectively. The Centers for Disease Control and Prevention declared the end of the pandemic in June of 20106. During this pandemic, there were increases in emergency department (ED) visits and hospital admissions, significant number of deaths, and social and economic problems globally7,8. Young infants were also infected with the pandemic virus although the incidence of H1N1 influenza in infants younger than 12 months was lower, compared with older children with high risk medical conditions 9, 10,11.
In our ongoing prospective study of infants in the first year of life, we observed increased parent-initiated visits for infants due to respiratory symptoms during the pandemic. The present report aims to define the spectrum of URI and LRI causative viruses in infants and to test the hypothesis that the 2009 pandemic caused increase in incidence of influenza infections in these infants, thus changing viral epidemiology of URI and LRI.
METHODS
Study design and subjects
The study was part of an ongoing, prospective study to evaluate the effect of host genetic and environment risks on URI and acute otitis media (AOM) development. Subjects were enrolled from near birth and followed to the first AOM episode, or between 6 and 12 months of age. Subjects completed the study at age 6 months if AOM developed prior to 6 months or followed up to 12 months for AOM occurrence. The study began enrolling infants in October 2008 at University of Texas Medical Branch (UTMB), Galveston and was approved by the Institutional Review Board. Written informed consent was obtained for all children. Study subjects were recruited from the newborn nursery or the primary care pediatric clinics at UTMB; they were healthy and resided in Galveston, TX. Preterm infants and those with major medical problems or anatomic/physiologic defects of the ear or nasopharynx were excluded. This report includes children enrolled and followed in the study from October 2008 to April 2011.
At enrollment, data were collected on family history and risk factors such as number of siblings, daycare attendance, cigarette smoke exposure and feeding type. During the follow-up period, parents were instructed to report to the study team as soon as the subject began to develop cold symptoms: rhinitis, cough, and sore throat, with or without constitutional symptoms such as fever, decreased appetite and restless sleep. The subjects were evaluated as soon as possible after URI onset and followed for AOM complication. At the initial URI visit, otoscopic examination and tympanometry were performed and nasopharyngeal specimens were collected for bacterial and viral studies; a follow-up visit was provided a few days later. Telephone calls were made weekly for follow-up of possible AOM symptoms within 4 weeks after URI onset. Parents were encouraged to bring the subject for examination by the study group anytime they suspected the infant might have an ear infection. AOM diagnosis was made based on presentations of acute symptoms (fever, irritability, otalgia), signs of tympanic membrane inflammation (intense redness, bulging, opaque tympanic membrane) and presence of middle ear fluid as documented by pneumatic otoscopy and/or tympanometry.
In addition to parent's self report of URI, the study personnel called the parents twice monthly to determine whether there were any current URI symptoms or occurrence of any URI, AOM or LRI episodes missed since prior contact. A comprehensive chart review was performed at the end of follow up to capture URI, AOM or LRI episodes that may have been seen by other providers. UTMB is the only primary healthcare and emergency care provider for infants and children on the Galveston Island and use the same electronic medical record system across all practice sites; thus most if not all of AOM and LRI diagnosis were captured in our medical records.
The 2009 influenza A/ H1N1 vaccine became available in our community in October 2009. Influenza vaccine (trivalent seasonal and 2009 A/H1N1) records in infants were reviewed from our electronic medical records. Infants who were 6 months old or younger at the time of discharge from the study were not eligible to receive influenza vaccine. Attempt was also made to review all available maternal influenza vaccine records during pregnancy or immediate postpartum as the majority of the subjects were born at our institution.
Virologic studies
Nasopharyngeal secretions were collected by vacuum suction; viral detection was performed by FilmArray® Respiratory Panel (FilmArray-RP), a multiplex PCR system (Idaho Technology, Inc. Salt Lake City, UT), according to the manufacturer's instructions. The pre-market version of the test was used and targeted 18 viruses: adenovirus, human bocavirus (hBoV), coronavirus (OC43, 229E, NL63, HKU1), influenza A, Influenza A/ H1N1, influenza A/ H1N1 2009, influenza A/ H3N2, influenza B, human metapneumovirus (hMPV), parainfluenza 1, 2, 3, 4, respiratory syncytial virus (RSV) and human rhinovirus12. The rhinovirus reagents may cross-react with enteroviruses and the system reported these results either rhinovirus or rhinovirus/enterovirus. Virologic studies from cases seen by other providers, which were documented in the medical records were also included; these cases were based on RSV and influenza rapid antigen detection tests and/or viral culture.
RESULTS
Subject characteristics
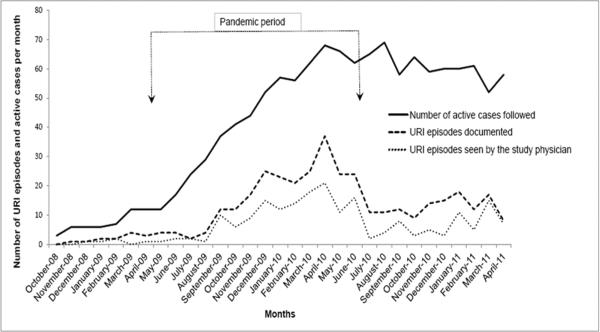
During the 31-month study period, 180 subjects were enrolled and followed. Due to the effect of hurricane Ike, which hit Galveston in September 2008 and caused displacement of Galveston population, the total number of cases enrolled was low in October 2008 (n=3). The number of active patients enrolled and followed gradually increased to 40 in the month of October 2009, and stabilized at 52–68 (average 61) per month by March 2010 (Figure 1). Demographic characteristics of the study subjects and duration of follow-up are shown in Table 1. Total duration of follow-up of all subjects in the study was 1409 patient-months. A total of 139 (77%) had at least one documented URI episode during the follow-up period (up to age 12 months); of these, 26% completed the study at 6 months; 20% completed between 6 and before 12 months corresponding to the times of their first AOM diagnosis; 44% completed at 12 months with no AOM diagnosis; 10% were lost to follow up and dropped from the study before 6 months of age. Of the 41 subjects who had no documented URI, mean duration of follow-up in the study was 3.9 months. Overall, a total of 373 URI episodes were documented. Viral studies of nasopharyngeal specimens were performed in 189 episodes; FilmArray-RP was performed in 175 URI episodes (in 86 subjects) seen by the study physician. In 14 URI episodes with LRI complications, which were seen by other healthcare providers, viral studies were performed by RSV and influenza antigen detection and/or viral culture. The number of URI episodes seen by the study physician by month paralleled the overall number of URI episodes documented (Figure 1).
Figure 1. Documented URI episodes and episodes seen by the study group.
The arrows represent the beginning (April 2009) and the end (June 2010) of the influenza A/H1N1 pandemic.
URI frequencies in relation to pandemic and non-pandemic periods
During the influenza pandemic period (April 2009 to June 2010), there was increase in number of documented URI episodes (Figure 1); 236 URI episodes were documented during the 15-month pandemic period, compared with 137 episodes during 16 months of pre- and post-pandemic periods (October 2008 to March 2009 and July 2010 to April 2011). Because the number of subjects followed in the study was lower at the beginning of the study, we calculated the ratio of the documented URI episodes per number of the subjects followed by month. During the pandemic, URI episode/patient ratio by month ranged from 0.08 to 0.54 (mean=0.33, median=0.36); during pre- and post- pandemic, the ratio ranged 0.14 to 0.33 (mean=0.23, median=0.21); the difference was significant (p = 0.01 by Mann Whitney Rank Sum test). During the pandemic, proportion of URI episodes seen by the study group and other health care providers was 61% and 33%, respectively, compared to 42% and 55% in the pre- and post- pandemic periods. The remaining URI episodes were documented by the study team's interval phone calls; these cases did not receive medical attention.
Virologic data and influenza cases
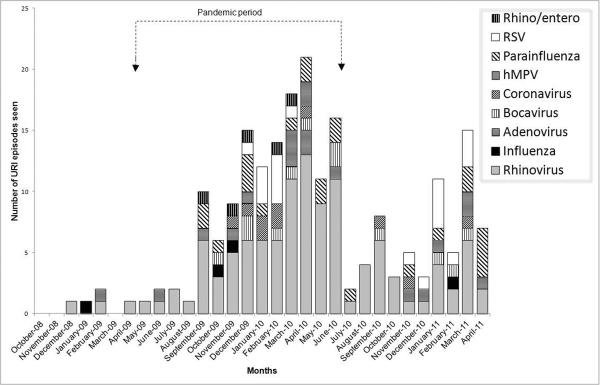
Of 189 URI episodes for which virologic assays were performed, 87% were virus positive. There were 208 viruses detected from 164 samples; 124 samples with one virus, 36 with two viruses, and 4 with 3 viruses. Types of respiratory viruses detected are shown in Table 2; distribution of virus types by month is shown in Figure 2. Rhinovirus accounted for 55% of all viruses detected and 69% of specimens for which a single virus was detected. Although there was increased URI visits documented during the pandemic, rhinovirus continued to be the predominant virus. Table 2 also shows type of viruses detected during the pandemic and pre- and post-pandemic periods.
Figure 2. Distribution of respiratory viruses detected between October 2008 and April 2011.
The arrows represent the beginning (April 2009) and the end (June 2010) of influenza A/H1N1 pandemic.
Two cases of influenza were documented during the pandemic and two cases during the pre- and post-pandemic period. In October 2009, a two-month old child presented to the ED with fever and decreased intake, cultures of blood and urine were obtained and IM ceftriaxone was given. URI symptoms were also noted on the following day when point-of-care rapid antigen detection was negative for influenza A and B. Nasopharyngeal secretion was positive for 2009 influenza A/ H1N1 by FilmArray-RP tested on the fourth day of illness by the study team. The second case was a two-month old child who presented to the ED in November 2009 with fever and nasal congestion. Point-of-care testing of nasal wash was positive for influenza A by rapid antigen detection, no subtyping was performed. No specific treatment was given.
In January 2009 prior to the pandemic, a one-month old child presented to the study physician with URI symptoms and poor feeding. Nasopharyngeal secretion was positive for influenza B by FilmArray-RP. A post-pandemic case was a five-week old child presented to ED in February 2011 with fever, URI symptoms, and irritability. The infant was admitted to the hospital for neonatal fever. Bacterial cultures were negative; nasal wash was positive for influenza A by rapid antigen detection; no subtyping was performed.
AOM and LRI Complications
There were 90 AOM episodes documented; all occurred with URI symptoms (median day of diagnosis = day 3 of URI onset). A total of 20 LRI diagnoses were documented in the medical records (bronchiolitis, 17; bronchitis, 1; and croup, 2); all began with URI symptoms, and 14 were diagnosed during the pandemic. The median age at LRI was 5.1 months. Of these, 14 (70%) were diagnosed at the ED and 8 were hospitalized; 6 episodes were diagnosed by the study physician and viral studies were performed by FilmArray- RP. Of the 14 LRI cases for whom viral studies were performed, RSV was detected in 9 (64%), rhinovirus together with parainfluenza virus in 2 (14%), metapneumovirus in 1 (7%), and 2 (14%) were virus negative; no influenza was detected among cases with LRI.
Influenza vaccine records
Of 180 infants enrolled in the study, 85 (47%) were 6 months of age or younger at the time of discharge or dropped from the study and therefore, were not eligible to receive influenza vaccine. Of the 95 infants eligible for influenza vaccine, 33 (35%) received 2 doses of seasonal influenza vaccines and 23 (24%) received one dose. There was no documentation on seasonal influenza vaccine in 39 (41%) infants.
A total of 22 infants were older than 6 months and followed in the study during the pandemic; 7 (32%) of these received 2 doses of the vaccine and 4 (18%) received only one dose. There were no documented vaccine records for the remaining.
For maternal immunization records, 157 (87%) mothers delivered their babies at UTMB; maternal influenza immunization records during pregnancy and immediate postpartum were reviewed. Of 75 mothers whose infants were not influenza vaccine-eligible; records of the mothers receiving influenza vaccines at UTMB were documented in 19 of 75 (25%) for seasonal and 6 of 32 (19%) for H1N1 vaccine (during the pandemic), respectively.
DISCUSSION
In this cohort of 180 healthy infants followed from birth up to 12 months of age, we found rhinovirus to be the most common cause of URI (55%) and RSV as the most common cause of LRI (64%). The most interesting and unexpected finding was that increase in respiratory disease activities during the 2009 influenza pandemic, as documented by increased parent-initiated healthcare visits, was not due to the pandemic virus. The spectrum of viruses affecting our infants did not change throughout the 31-month pre-, during and post- pandemic periods. To our knowledge, this is the first study on the impact of the influenza pandemic on changes in viral epidemiology of both URI and LRI among healthy infants in the first year of life in the United States. The data do not support our original hypothesis on the effect of the pandemic on viral epidemiology of respiratory infections in this age group. Increased parent-initiated visits likely reflect parental awareness and an anxiety about the pandemic. Our data also emphasize the significance of rhinovirus infections in infants in the first year of life.
While RSV has long been accepted as a major cause of severe respiratory disease and hospitalization in the first year of life, recent data have shown similar rate of hospitalization13 and severity of illness14 caused by rhinovirus. We only had 2 cases of rhinovirus LRI in this cohort and both were co-infected with parainfluenza virus. Lack of specific detection for rhinovirus by molecular testing in 14 of 20 LRI cases may explain this relative low rate. However, FilmArray-RP, which included rhinovirus target was performed in 175 of 189 (93%) of URI episodes studied for viruses. The results show high rate of rhinovirus infection in this cohort. This observation is consistent with data from other recent studies showing the overall burden of rhinovirus in infants in the first year of life3, 4, 15,4, 16. It is likely that availability of sensitive molecular methods to detect rhinovirus has helped recent discovery of the larger role of rhinovirus in more serious respiratory diseases. Using molecular diagnostics, however, investigators have also shown that rhinovirus nucleic acids may persist in the respiratory tract for 4–5 weeks17; therefore, rhinovirus detection may not always represent the cause of acute respiratory illness. The sensitive molecular diagnostic methods, therefore, contribute to detection of multiple viruses, including the pathogen as well as persistent viruses. Further studies on pathogenicity of rhinovirus, in relation to other respiratory viruses are needed. To date, a few studies have already begun to use quantitative viral diagnostics (viral load) to help indicate the cause-and-effect relationship in rhinovirus infection14, 18.
Influenza in young infants often presents as an undifferentiated febrile illness, with or without respiratory symptoms1, 19, 20; this often leads to work up for serious bacterial diseases and hospitalization. All 4 of our influenza cases were younger than 3 months, 3 of 4 presented to the ED, 2 of whom were worked up for serious bacterial infections, including one who was hospitalized. The overall number of influenza cases diagnosed in this cohort over the span of 31 months (n=4), including 15 months of the pandemic, was much lower than the number of cases diagnosed with RSV infection (n=19). This finding is consistent with the reports on higher impact of RSV than influenza on young infants during the influenza pandemic or otherwise. In a study from Austria between October 2004 and May 2009, the frequencies of hospitalization of infants younger than 12 months due to RSV were 10 times higher than from influenza2. Another study from Mexico during the pandemic21 also reported influenza to be the most common reason for hospitalization in adults, while RSV was still the most common reason for hospitalization of young children.
The influenza pandemic caused significant increase in acute respiratory diseases, ED visits, hospital admissions, and deaths all over the globe. Severe illnesses were associated with co-morbidities such as asthma, diabetes, other chronic medical conditions and immunosuppression22–25. While some studies reported increased hospitalization or mortality rate in infants due to the pandemic virus compared to seasonal influenza22, 23, others found equivalent or lower rate of hospitalization and death in infants < than 2 years from the pandemic virus9, 26. The relatively low incidence of the pandemic influenza in the very young (0–2 years) compared school age children9, 26, 27 may help explain the low overall impact of the pandemic on young infants.
There may be several reasons to explain the low impact of the influenza pandemic on this study. First, only healthy, term-born infants, without any co-morbidity were enrolled in the study. Second, during the pandemic, very young infants may be protected from exposure to symptomatic index cases. Third, the use of influenza vaccines, both pandemic and seasonal in our population may help protect the infants although only about half of our eligible infants were vaccinated. Lastly, maternal immunity and immunization may have also played a role in protection of these infants. Our findings on low impact of the pandemic on our infants are supported by results from a recent study from the United Kingdom; in a cohort of 150 prematurely born infants followed longitudinally, hospitalization rate for pandemic influenza was low and similar to that of term-born infants in the same geographic area2. Influenza activities in Galveston area always mirror those in Houston and the state of Texas. Our initial peak of the influenza pandemic was in May and June and a second peak was between September and November, 2009. Our increased research visits coincided with the community peaks. During the 2010–2011 influenza season post pandemic, influenza A/H1N1, A/H3N2, and B were in circulation in our community.
Our study has some limitations. Due to the study design, not all infants were followed for the whole 12 months. Therefore, we could not accurately calculate the incidence of URI and LRI in the entire first year of life. Furthermore, while we relied on parent reports of the respiratory symptoms, bi-weekly telephone interview, monthly home visits and comprehensive chart review; milder cold symptoms may have been missed. This may explain why during the pandemic, with more awareness and concern of parents, there was a surge in parent-initiated visits due to respiratory symptoms, even if mild. Another limitation was that only about half of documented URI episodes, with or without LRI were studied for viruses. We have shown, however, that the URI episodes with study samples paralleled in time with the episodes without study samples. This is unlikely to have caused bias in our result; missing influenza cases should have occurred more during the pre- and post- pandemic periods than during the pandemic.
In summary, in our cohort of healthy infants in the first year of life, rhinovirus was the most frequent cause of URI, while RSV was the most frequent cause of LRI. The H1N1 influenza pandemic did not change viral epidemiology of URI and LRI in this population.
ACKNOWLEDGEMENTS
We thank our patients and families and the UTMB General Academic Pediatric physicians for allowing us to recruit and follow patients from their practice. We also thank Alejandro Diego, Stella Kalu, Johanna Nokso-Koivisto, Esther Valdivia, and Lilia Rodriquez for their assistance with research subjects, Ying Xiong for laboratory assistance, Victor Welch for data management, and Kristofer Jennings for statistical assistance.
Source of funding This work was supported by the National Institutes of Health grant R01DC005841 and R01DC005841-08S1 (to TC) and a grant from Idaho technology, Inc. (to MJL). The study was conducted at the General Clinical Research Center at the University of Texas Medical Branch at Galveston, funded by grant UL1RR029876 from the National Center for Research Resources, NIH. MJL received grant support and honorarium from Idaho Technology, Inc.
Footnotes
conflicts of interest: The authors have no other conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Resch B, Eibisberger M, Morris N, Müller W. Respiratory syncytial virus- and influenza virus-associated hospitalizations in infants less than 12 months of age. Pediatr Infect Dis J. 2011;30(9):797–799. doi: 10.1097/INF.0b013e318215cf3e. [DOI] [PubMed] [Google Scholar]
- 2.Drysdale SB, Alcazar M, Wilson T, Smith M, Zuckerman M, Wedderburn CJ, et al. Pandemic influenza A (H1N1) virus 2009 in a prospectively followed cohort of prematurely born infants. Pediatr Infect Dis J. 2012;31(1):91–92. doi: 10.1097/INF.0b013e3182324a1f. [DOI] [PubMed] [Google Scholar]
- 3.van der Zalm MM, Uiterwaal CS, Wilbrink B, de Jong BM, Verheij TJ, Kimpen JL, et al. Respiratory pathogens in respiratory tract illnesses during the first year of life: a birth cohort study. Pediatr Infect Dis J. 2009;28(6):472–476. doi: 10.1097/inf.0b013e318195e26e. [DOI] [PubMed] [Google Scholar]
- 4.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25(8):680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 5.Regamey N, Kaiser L, Roiha HL, Deffernez C, Kuehni CE, Latzin P, et al. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J. 2008;27(2):100–105. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services [Accessed 9 December, 2011];WHO Declares End to 2009 H1N1 Influenza Pandemic. http://www.hhs.gov/news/press/2010pres/08/20100810b.html. Published 2010. Updated January 03, 2011.
- 7.Vaillant L, La Ruche G, Tarantola A, Barboza P, InVS eita Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14(33) doi: 10.2807/ese.14.33.19309-en. [DOI] [PubMed] [Google Scholar]
- 8.Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362(1):45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 9.Cox CM, Blanton L, Dhara R, Brammer L, Finelli L. 2009 Pandemic influenza A (H1N1) deaths among children--United States, 2009–2010. Clin Infect Dis. 2011;52(Suppl 1):S69–74. doi: 10.1093/cid/ciq011. [DOI] [PubMed] [Google Scholar]
- 10.Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122(4):805–811. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 11.Nieto-Guevara J, Sosa N, García M, Martinez A, Castillo M. 2009 Influenza A (H1N1) in Panama: a disease affecting children with a benign course. J Infect Dev Ctries. 2011;5(9):664–668. doi: 10.3855/jidc.1495. [DOI] [PubMed] [Google Scholar]
- 12.Loeffelholz MJ, Pong DL, Pyles RB, Xiong Y, Miller AL, Bufton KK, et al. Comparison of the FilmArray Respiratory Panel and Prodesse Real-Time PCR Assays for Detection of Respiratory Pathogens. J Clin Microbiol. 2011;49(12):4083–4088. doi: 10.1128/JCM.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piotrowska Z, Vázquez M, Shapiro ED, Weibel C, Ferguson D, Landry ML, et al. Rhinoviruses are a major cause of wheezing and hospitalization in children less than 2 years of age. Pediatr Infect Dis J. 2009;28(1):25–29. doi: 10.1097/INF.0b013e3181861da0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Leeuwen JC, Goossens LK, Hendrix RM, Van Der Palen J, Lusthusz A, Thio BJ. Equal virulence of rhinovirus and respiratory syncytial virus in infants hospitalized for lower respiratory tract infection. Pediatr Infect Dis J. 2012;31(1):84–86. doi: 10.1097/INF.0b013e31823345bf. [DOI] [PubMed] [Google Scholar]
- 15.van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154(3):396–400. 400.e391. doi: 10.1016/j.jpeds.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legg JP, Warner JA, Johnston SL, Warner JO. Frequency of detection of picornaviruses and seven other respiratory pathogens in infants. Pediatr Infect Dis J. 2005;24(7):611–616. doi: 10.1097/01.inf.0000168747.94999.aa. [DOI] [PubMed] [Google Scholar]
- 17.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72(4):695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 18.Peltola V, Jartti T, Putto-Laurila A, Mertsola J, Vainionpää R, Waris M, et al. Rhinovirus infections in children: a retrospective and prospective hospital-based study. J Med Virol. 2009;81(10):1831–1838. doi: 10.1002/jmv.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342(4):225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 20.Bender JM, Ampofo K, Gesteland P, Sheng X, Korgenski K, Raines B, et al. Influenza virus infection in infants less than three months of age. Pediatr Infect Dis J. 2010;29(1):6–9. doi: 10.1097/INF.0b013e3181b4b950. [DOI] [PubMed] [Google Scholar]
- 21.Lovato-Salas F, Matienzo-Serment L, Monjarás-Ávila C, Godoy-Lozano EE, Comas-García A, Aguilera-Barragán M, et al. Pandemic influenza A(H1N1) 2009 and respiratory syncytial virus associated hospitalizations. J Infect. 2010;61(5):382–390. doi: 10.1016/j.jinf.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Jhung MA, Swerdlow D, Olsen SJ, Jernigan D, Biggerstaff M, Kamimoto L, et al. Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis. 2011;52(Suppl 1):S13–26. doi: 10.1093/cid/ciq008. [DOI] [PubMed] [Google Scholar]
- 23.Pebody RG, McLean E, Zhao H, Cleary P, Bracebridge S, Foster K, et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill. 2010;15(20) [PubMed] [Google Scholar]
- 24.Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7):e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skarbinski J, Jain S, Bramley A, Lee EJ, Huang J, Kirschke D, et al. Hospitalized patients with 2009 pandemic influenza A (H1N1) virus infection in the United States--September-October 2009. Clin Infect Dis. 2011;52(Suppl 1):S50–59. doi: 10.1093/cid/ciq021. [DOI] [PubMed] [Google Scholar]
- 26.Aguirre E, Papenburg J, Ouakki M, Fontela PS, Guimont C, De Serres G, et al. Comparison of pandemic and seasonal influenza in the pediatric emergency department. Pediatr Infect Dis J. 2011;30(8):633–639. doi: 10.1097/INF.0b013e3182103d54. [DOI] [PubMed] [Google Scholar]
- 27.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]