Abstract
We develop a life history model with two sexes, and study the optimal energy allocation strategy of males and females. We join Darwin and others in suggesting that the origin of sexual dimorphism and sexual selection is the difference between male and female reproduction costs. Due to this assumed cost difference, the resulting Bellman equations of gene dynamics in our two-sex life history model imply a large “energy surplus” on the part of males. This allows the male form to devote energy to the development of some costly male traits that help the males to compete for access to females. These costly male traits are sexually dimorphic. Using this life history model, we are able to explain important features of sexual dimorphism, as well as why males often transfer less to their offspring than do females, and why only females have menopause.
Keywords: Sexual dimorphism, sexual selection, intergenerational transfers, menopause, optimal life history, two-sex life history, male competition, viability, handicap principle
1 Introduction
Over the past two decades a number of researchers (e.g. Bergstrom [1995], Rogers [1994], Robson and Kaplan [2003], Chu and Lee [2006], Chu and Lee [2008a,b]) have applied economic approaches to the evolutionary processes leading to particular characteristics in various species. A recent survey can be found in Cox (2007). A topic mentioned but not seriously studied in the economics literature is sexual dimorphism, the phenomenon that male and female adults possess significantly different biological traits, whereas juveniles are more similar (see the classic paper by Trivers, 1972). A related topic is sexual selection, in which either direct male-male competition for access to females or females’ preferences for certain male traits shape the evolution of those traits. It is well known from evolutionary research that sexual dimorphism across species is highly correlated with the intensity of competition for mating partners. In practice, this competition is usually between males, although it is increasingly recognized that female competition is pervasive (Clutton-Brock, 2007). In this paper we propose a unified economic theory that can explain the core features of sexual dimorphism and sexual selection, although not all the nuances such as female competition for mates.
1.1 Previous Literature
A brief introduction to the abundant biological literature should be helpful to general readers. According to Maynard Smith (1991), there are two broad types of sexual interactions: intrasexual competition and intersexual selection. The latter involves both male traits and female preferences; and the former does not consider the preference side of females, but emphasizes the male-male competition.
For intrasexual competition, the classic article is a contribution by Trivers (1972), who argued that relative parental investment is the key to understanding sexual competition. By parental investment, Trivers meant “any investment by the parent in an individual offspring that increases the off-spring’s chance of surviving (and hence reproductive success) at the cost of the parent’s ability to invest in other offspring” (p.139, italics added). From the italicized words and the discussion in his paper, we know that the resources Trivers refers to are mostly parental transfers after the offspring is born. From the perspective of life history theory, however, parental transfers are part of the evolved life history strategy that is to be explained. We believe that sexual dimorphism can be explained based on more fundamental characteristics of a species such as the difference between the sexes in the size of investment in gametes.
Other than Trivers (1972), Emlen and Oring (1977) emphasized the importance of operational sex ratio (OSR) on sexual selection, where the OSR is defined as the ratio of active males to fertilizable females. In practice, the OSR is evidently related to ecological, geographical, and spatial factors that may influence the environment of male-female interactions. Kokko and Johnstone (2002) considered the joint influence of parental investment, mortality, mate-encounter rate and OSR, and concluded that the importance of parental investment outweighs that of OSR. In this paper, we leave most environmental factors to the background, and concentrate on the role of parental investment decisions.
In the analysis of sexual interaction, the literature sometimes addresses the correlation of dimorphic traits with other characteristics of a species, and the interpretation or any correlation. For instance, in studies of primates, Leutenegger and Choverud (1982) found and analyzed a correlation between sexual dimorphism and body size; however, Gaulin and Sailer (1984) suggested a very different interpretation of the same correlation. Partly because of such difficulties of interpretation, we set up a life history model to study the problem of sexual dimorphism. In the framework of an optimal life history, species’ traits such as body size and tooth size are control variables chosen by species, and the interpretation follows naturally from the model. Basically, the only fundamentals in a life history model are the energetic costs of various control variables, and we shall interpret most observations by sex-specific differences of these fundamentals, thereby avoiding paradoxical interpretations of correlations.
In our analysis, we go beyond Trivers (1972) and differentiate afterbirth parental transfers from fertility costs, which were bundled together as “parental investments” by Trivers. We will take sex-specific fertility costs as given for each species, whereas parental transfers are something each species “chooses” in their life history. This distinction is important to the understanding of evolutionary dynamics. For example, our theory suggests that viviparous species are likely to have sexual dimorphism, for viviparous males and females have drastically different reproduction costs.
For intersexual selection, Zahavi (1975) argued that males may have some advantageous traits which are unobservable to females. In this case, males may employ a seemingly useless trait (a handicap), such as the plumage of some birds, to signal their superior quality to females. However, Fisher (1958) and Lande and Arnold (1985) all pointed out that the evolution of such trait-preference may be selectively neutral. Using a simple 2-trait 2-allele model, Kirkpatrick (1982) showed that even the initial selective advantages of evolution are not necessary. This pretty much makes the evolutionary outcome of intersexual selection indeterminate. Later, Iwasa et al. (1991) showed that this neutrality result breaks down if there is a small cost of female searching. They also demonstrated that the handicap hypothesis is valid only if there exists a connection between the male trait and viability parameters. Using our model we are able to provide a neat economic interpretation for this connection.
1.2 Contribution of this Paper
The existing literature explains various features of sexual dimorphism and sexual selection using different models, including quantitative genetics (Iwasa et al. [1991]), Malthusian competition (Hausken and Hirshleifer [2008]), and a very insightful verbal analysis (Trivers [1972]). In this paper, we propose a unified framework of two-sex life history, along the lines of Stearns (1972), that can explain many of the core features of sexual selection and its consequences.
The origin of sexual dimorphism and sexual selection as proposed by (Darwin 1871) is the difference between male and female reproduction costs: the cost of producing sperm is trivial for males and that of producing eggs by females is significantly larger, not to mention the cost of pregnancy and delivery for many viviparous species. This leads to a higher potential rate of reproduction (PRR) for males than females, so that the female costs and PRR constrain the male possibilities, leaving them with surplus energy that can be devoted to costly traits that may increase their access to female mates. Our formal analysis of the optimal energy allocation strategy of males and females over the course of a life history incorporates these features.
The fact that males have an energy surplus implies that their overall reproductive output is constrained by females. This constraint greatly simplifies the analysis, and helps us derive clear-cut sex-dimorphism results. Furthermore, this constraint also implies that the analyses of female fitness and male traits can be done recursively. As a result, we find that the analytics of inter-sexual interactions is not very different from that of male-male intra-sexual competition.
Consider the scenario that many males compete to become an alpha male, who alone can mate. Since there is only a small chance for a male to become an alpha male, ex ante each male child can be seen as a risky lottery with a small probability to mate many females and a large probability to mate none. Female children almost surely can mate with the alpha male, and hence the corresponding lottery is degenerate. From the parents’ point of view, their children are assets for the proliferation of their genes. Just as an investor’s portfolio of assets may include some risky ones which may yield a high rate of return, and some safe ones that will likely yield a low rate of return, so we can view the parents’ portfolio of children. The parents have a sex-specific portfolio, with male children who are high-risk high-return, and females who are low-risk and low-return. We shall explain why this particular phenomenon arises from a life history model.
Male competition favors the evolution of some particular male traits, and eventually to the phenomenon of sexual dimorphism. Using this two-sex life history model we are also able to explain, for instance, why for most species males provide smaller intergenerational transfers than females, and why menopause occurs occasionally in females but never in males. Thus, we elaborate the discussion of Trivers (1972), and extend it to some other sex-specific differences that have not been analyzed in the literature. In his review article, Clutton-Brock (2007) concludes that “…the theory of sexual selection still provides a robust framework that explains much of the variation in the development of secondary sexual characters in males….” He discusses the increasing evidence of the prevalence and importance of sexual selection in females, but finds that the recognition of these complexities “does not undermine its basic structure”. It is this basic structure of the classic theory that we model, analyze and interpret in the following.
1.3 Paper Structure
The rest of this paper is arranged as follows. Section 2 introduces the model of life history and energy constraints, and derives the two-sex Bellman equation for maximizing fitness. Section 3 discusses intrasexual competition, and relates various phenomena of sexual dimorphism to the difference of distinct sex-specific reproduction costs. Section 4 discusses intersexual selection. The fifth and sixth sections are about differences in male-female transfers and menopause. The final section summarizes and concludes.
2 A Two-sex Model of Selfish Genes
In all but one section of this paper we adopt a 1-age or 2-age setting. The two ages are denoted as (0, 1). The principle of our modelling is to choose the simplest setting to the extent that the key message can be delivered.
There are two sexes, male and female. Let Ma,t be the value of a genome carried by a male at age-a and time-t, and Fa,t be the value of a genome carried by a female at age-a and time-t. The value of a genome is the maximum reproductive fitness that could be achieved starting at some particular age and time given all the prior life history strategic allocations (e.g. allocations of energy to survival, fertility, or somatic growth at earlier ages). Let m(f ) be the reproductive effort by males (females), and pa,m (pa,f ) be the survival probability for males (females) aged-a. In practical context, m refers to the male effort of producing sperm and successful insemination (not including the costs of gaining access to a female through competition based on costly traits and not including post-birth parental investments), and f refers to the female effort of producing ova and conducting a successful pregnancy and delivery, but not including costs of mate selection. At each age, energy is assumed to be allocated among four uses: maintenance, reproduction, somatic growth and parental downward transfers.
2.1 Event Order and Preliminaries
Life begins with a juvenile stage of growth and survival but no reproduction (age-0), followed by an adult stage of reproduction and survival but no growth (age-1), that is, a “determinate” life history.3 The juvenile at age-0 allocates energy between maintenance and growth. However, we must specify he order of events for an age-1 adult. We assume that at the beginning of age-1, each adult first hunts or forages, generating the energy to be used throughout this life cycle stage. Then, each adult decides how to allocate this energy between reproduction and maintenance. This event order is somewhat arbitrary, but some order must be specified in this discrete-time model. None of our results will be affected qualitatively if this order is changed.
In some parts of our analysis, we consider reproductive access as a lottery. For instance, monogamy and polygamy are two different marriage-fertility lotteries for males: the former has a high probability of mating one partner and a low probability of mating none, and the latter has a low probability of mating many partners and a high probability of mating none. When the random outcome of the lottery is realized, each adult allocates the available energy to maintenance, transfers and reproduction. Nature then determines whether an adult survives, and only survivors can reproduce.
In this 2-sex model, individuals of opposite sexes combine their reproductive efforts to successfully produce an offspring. However, only one sex actually bears the child, and we call this sex “female”. There are two unique features associated with the female sex. First, as Trivers (1972 p. 138) pointed out, for almost all two-sex species (and especially for viviparous ones), the metabolic energy of reproduction is particularly large for females, but is very small for males. Second, the number of male impregnations by males can never exceed the number of female pregnancies, whereas it is the female’s pregnancies that determine the final births. The first property specifies a difference in energy coefficient associated with reproduction, and the second property suggests an aggregation constraint on male/female reproduction.
2.2 The Two-sex Bellman Equations
We shall first write down Bellman’s principle of optimality for the maximal replication of genes (Bellman 1957), and then explain its meaning. Following Chu and Lee (2006), we see that the Bellman equations for selfish genes in this 2-sex model look like
| (B) |
In the above expression, the fitness maximization is over age-specific controls including (pa,mi, pa,fj, mi, fj ), a = 0, 1 and some others to be described shortly, where mi refers to the individual’s ith fertility choice, and p(a, mi) refers to the individual’s survival probability at age-a, when the ith fertility option is chosen.
2.3 Interpreting the Bellman Equations
Individuals at age-0 only grow and survive but do not give birth, so the value of the genes they carry derives from successful survival to age 1 one period later. This explains the first two equations. For genes of females at age 1, the F1,t equation is straightforward. If a female lioness has fj pregnancies, we assume, as in Trivers (1972 p.140), that the probability is 1/2 of having a newborn of either sex.4 The values in the next generation of genes carried by males and females at age-0 are denoted respectively M0,t−1 and F0,t−1. Because each birth only carries 1/2 of the original genes, and because there is a 1/2 probability that a given birth will be male or female, we divide the M0,t−1 and F0,t−1 by 4 on the right hand side of the third equation. This explains the F1,t−1 equation.
Because the expected number of impregnations, which is p1,mimi, times the number of age-1 males, must be equal to the expected number of pregnancies, which is p1,fjfj times the number of age-1 females, an aggregation constraint must be taken into account as we solve the Bellman equation. If the numbers of surviving male and female adults are roughly equal, and if the unconstrained p1,mimi is expected to be larger than the unconstrained p1,fjfj, then the male’s choices will be suppressed. We shall come back to this point later.
Some choice of the control variables, designated by an asterisk, will maximize the Euler-Lotka growth rate λ that is implied by the Bellman equations above. Replacing the controls by these optimal values, we can iterate the Bellman equations (B) to obtain the dynamic equations for (M0,t, F0,t), as in Chu and Lee (2006). The dimension of the state variables can be further reduced, once we observe that we can define xt = (M0,t + F0,t)/2, and re-write everything in terms of xt. The Bellman equations (B) can now be expressed compactly as
| (1) |
From demographic theory it is well known that as long as the controls (mi*, fj*, pa,s) are time invariant, the dynamics of (1) will guarantee the convergence of xt to an exponential growth path, growing at a rate called the Euler-Lotka parameter. Suppose λ is this steady state growth rate of xt. Then the above discussion suggests a simple characterization of the Euler-Lotka parameter λ for the two-sex model:
| (2) |
On the right hand side of (2), terms in the square brackets are the expected number of replications of each gene over the lifetime of its carrier, similar to the lifetime birth criterion or Net Reproduction Ratio (R0) adopted in many of the one-sex (female) models (see e.g. Sozou and Seymour [2003]). Note that in a one-age setting, we can simply assume p0,m = p0,f = 1, drop the age subscript of pm and pf, and re-write the Euler-Lotka parameter as
2.4 Energy Constraints and Assumptions
Consider the energy constraint in adulthood (at age 1). Let the unit cost of m and f be respectively cm and cf. Similarly, the unit cost of p1,s is assumed to be b1,s. These are the energetic costs per unit of survival and fertility. These fertility costs exclude post birth transfers which we denote separately as Tm and Tf. The fertility costs also exclude pre-impregnation costs of competition or searching for mates. Then, if a male and a female choose i and j respectively, we have the following adult energy constraints:
where zf (zm) is the initial somatic state of female (male) adults, most simply size, for example. We assume that the energy generated is increasing in z, given by g(zs). In Trivers (1972), cf fj and Tf are combined under the heading “female investment”, and similarly for males. Here we separate them and shall provide respective interpretations later. In a one-age setting, we simply assume that Ts is zero. Later in a two-age setting we shall study how Tm and Tf are determined.
3 How Does Sexual Dimorphism Arise?
Since juvenile growth is not important for the discussion of sexual dimorphism, we shall consider a one-age scenario in this section, set p0,s = 1 and Ts = 0 (s = m, f ), and drop the age subscripts of (pm, pf ). We will return to the two age group model later. When mi is chosen by a male, using the energy constraint (C), pmi can be written as pmi = (g(zm) − cmmi)/bm. Similarly, pfj can be written as pfj = (g(zf ) − cf fj )/bf. Substituting these into (2′), we can rewrite the fitness-maximizing problem as solving
| (3) |
There is actually one more constraint for the male’s maximization, which we shall soon add.
We first look at the female problem, because the male’s choice does not affect the female reproduction solution (whatever the male impregnation effort, the female’s gene is passed on in each of her pregnancies). Let fj* be the value of f that maximizes the expected number of pregancies u (f ) given by the quadratic formula fj (g(zf )− cf fj )/bf. The optimal policy for femalesis certainly to choose . Considering the simplifying approximation that f is a continuous variable, then the optimal f from maximizing the quadratic objective function can be written as
3.1 A Male-Female Interacting Constraint
Now we look at the male’s choice. Let u (m) = pmimi be the expected number of impregnations by a male. Since this must be equal to the expected number of pregnancies, u (f ) = pfjfj, the following constraint should hold
| (4) |
If the male’s outcome is constrained by the female’s optimum, then the male’s choice of reproduction is redundant. Thus, in view of (3), we see that the constraint in (4) makes the Euler-Lotka parameter in (2′) simply λ = F*/2.
But intuitively, why is the male the constrained sex? For the time being we ignore the constraint in (4), and seek the male’s “ideal” solution, similar to the concept of the potential rate of reproduction or PRR. If males are not constrained by (4), their optimal choice of m (when it can be chosen continuously) could be solved from the maximization mi[g(zm) − cmmi]/bm. Since mipmi is quadratic in mi, it is easy to see that under a continuous approximation,
Suppose that the energy available to the adult male and female does not differ much, so that the difference between g(zm) and g(zf ) is not significant. Then, comparing pm*m* = [g(zm)]2/(4cmbm) with pf*f* = [g(zf )]2/(4cf bf ), we see that pm*m* would be significantly larger than pf*f*, because cm is significantly smaller than cf. Thus, the male is likely to be the constrained sex.
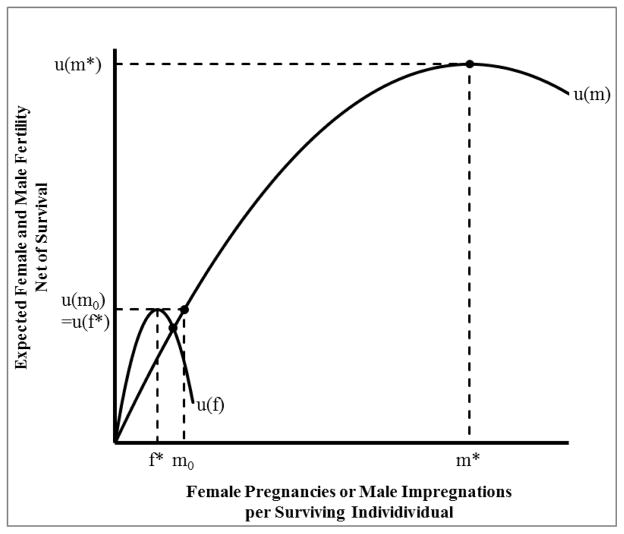
When males’ choice is constrained by (4), the ideal m* is out of the question. From (4), for instance, the male can choose a certain m0 such that u (m0) = m0pm0 = m0[g(zm) − cmm0]/bm = pf*f*. This m0 is the male reproduction sufficient to match the female’s f*. The fact that pf*f* and pm*m* differ a lot indicates that males have much lower fertility at their compromised choice of m0 than their potential maximum.5 Letting u(m) = [g(zm) − cmm]m/bm, we see from Figure 1 that monogamous males are very inefficient in using their energy, producing an expected number of impregnations, u(m0) = u (f*), that is much lower than u(m*).
Figure 1.
There is a large difference between the fitness-maximizing fertility of females (f*) and males (m*) abstracting from their interdependence. u(m) and u(f) are the expected number of female and male births taking into account the relation of fertility to survival through the energy constraint. Taking into account the interdependence of the sexes, the male level of expected births, u(m), is constrained to equal the female level, u(f). The hypothetical separate male optimum u(m*) violates this constraint and cannot be attained. Instead at the optimum male expected fertility is u(m0).
3.2 The Male’s Motivation for Competition
Because male energy is used “inefficiently” due to the female constraint, there is room for an invading mutation to exploit the unused potential energy in order to improve a male’s competitive access to females. Specifically, a mutation may shift some of the male’s energy to somatic investment I which may improve the spread of his gene. The somatic investment may be enlarging canine teeth, improving muscle strength, growing bigger antlers, or larger body size, and so on. These somatic investments increase the probability of his successful impregnation of a female either by being more attractive to females, or by fighting and driving away other competitors. But of course, all investments are at the cost of reducing survival probability or gamete production (see Section 2.4).6
For exposition purposes, we assume that females always live in a pride of size n, and that the male faces a lottery for mating opportunity which is specified as having probability q of mating all n females in this pride, and probability 1 − q of mating none of them. In the latter case, we can think of it as a scenario in which the male in question loses the competition and is driven away. Formally, for a male indexed k, let the probability of winning these n females be written as q(Ik, I~k), where Ik is k’s somatic investment and I~k is the vector of investment for all other competitors. We shall consider a mutation of the male-k gene that increases Ik, and see if this will help k win over other gene lines.
Given our specification, once k becomes an alpha lion, he can impregnate all n females. The constraint in (4) now becomes
| (5) |
First, we check whether a mutation of male investment could invade the original gene line in which there was no sexual competition, or all males were equal. To simplify our discussion we shall consider the symmetric case. Suppose the original investment for all relevant males is the same, and is equal to , which may well be zero. In such a symmetric equilibrium, each male is equally efficient in mate competition, and we know that q(Ik, Ik) = 1/n must hold at any symmetric equilibrium. If a mutation now leads male k to increases his investment slightly, while all other males make the original investment, the impact of this change on q can be written as7
| (6) |
We assume φ(Ik) > 0 because more unilateral investment by k usually implies higher likelihood of winning the competition.
Noting that q(Ik, Ik) = 1/n in any symmetric equilibrium, by differentiating the left hand side of (5) we see that the mutation will invade successfully as long as (6) holds (note that in (6), bm and cm are parameters).
3.3 Relating Sexual Dimorphism to Reproduction Costs
How would this arms race in Ik and I~k end? Recall from (5) that in any symmetric equilibrium the following constraint must hold:
or equivalently
| (7) |
where we have used the formulae f* = g(z)/(2cf ), and pf* = [g(z)− cf f*]/bf. This is the equation that determines the equilibrium Ik. Since g(zm) − Ik must be positive by the energy constraint, we see that ∂h/∂Ik < 0 in the equilibrium. Furthermore, ∂h/∂cm (∂h/∂cf ) is obviously negative (positive). Thus, we know that Ik and cm/cf move in opposite directions along the equilibrium. In fact, we have shown from (7) that when cm (cf ) is smaller (larger), the equilibrium Ik* is larger. Therefore, we directly relate the degree of equilibrium male competition (Ik*) in sexual competition to the relative size of male/female reproduction costs.
This is intuitively appealing, for male species with smaller cm can produce a relatively large m costlessly, and hence they feel particularly depressed by the constraint in (4). Their excess energy, therefore, is more likely to be directed to investment on I*.
3.4 Other Factors
In the above analysis, we put most of our emphasis on the difference in reproduction costs. But what about the ecological and territorial costs emphasized by Brown (1964) and Emlen and Oring (1977) as key factors explaining sexual dimorphism? In our setup, these environmental factors are implicitly embodied in the q(Ik, I~k) function. For instance, in an environment where potential mates are not defendable, it is even difficult for a strong male to maintain his control over females. In our model, this implies that f is almost zero, and hence a mutation that raises investment in Ik does not pay off. Thus, sexual dimorphism in this dimension cannot arise. For life history specification of genes, environmental factors are taken as given, but our model is consistent with the resource-environment argument of Brown, and Emlen and Oring.
Our theory is also consistent with models of intersexual selection with female preferences. Conceptually, the q function in (5) may be determined, not by nature, but by female preferences, and hence only investments that appeal to females can possibly have a rewarding φ. We shall come back to this discussion later in section 4.
3.5 The Sex-specific “Portfolio”
When a competition equilibrium is reached, each male devotes I*; but since all individuals in the new symmetric equilibrium have the same I*, the resulting probability of winning the mating privilege must still be 1/n. Thus, what we observe is that the female faces a degenerate lottery of f = f*, whereas the male faces a risky lottery: [m = m* with probability 1/n and m = 0 with probability 1 − (1/n)]. Because realizing a small f* is certain, the female strategy is low-risk and low-return. The male strategy on the other hand is high-risk and high-return, for m* is large and its probability of realization is small (1/n). Treating the male and female children as assets, we observe that a parent has a reproductive portfolio of some lotteries with high-risk high-return and some lotteries with low-risk low-return. It is well-known from the finance literature that it is efficient to combine low-risk low-return assets with high-risk high-return ones. But can we claim any efficiency here in the case of the portfolio of children?
We first should note that the male and female lotteries appear for different reasons. The female chooses a degenerated birth strategy because her pf ·f = [g(zf )− cf f]f/bf is a concave function of f, and therefore it does not pay to randomize over f. For males, it is not that they “choose” a randomized strategy; rather, it is that they compete with one another, and each has a small probability of winning a pack of females. In fact, their competition has nothing to do with the equilibrium λ, which is solely determined by females.
As one can see, when (6) is positive, a mutation leading to more trait investment will successfully invade. However, all competitive outcomes will end up with the same success fitness index, given the constraint in (4). Whatever lottery a male gene mutates into, the fate of having fitness λ = pf*f*/2 is simply preordained. Therefore, in a symmetric evolutionary equilibrium, the male competition is like an arms race, in which a unilateral mutation of increasing I may be a dominant strategy when (6) is positive, although the eventual equilibrium result actually provides exactly the same evolutionary equilibrium. This “fight-for-nothing” property actually implies some kind of inefficiency from intrasexual competition. However, those gene lines that do not invest in Ik were out-competed and went extinct.
4 Inter-Sexual Selection
The typical description of inter-sexual selection is that the male has a gene controlling the size of some trait (such as the plumage of a peacock), and the female has a gene controlling her preference for mating males that excel tin his trait. The question is: if such traits appear to be useless or even harmful (e.g. the excessively large plumage of male peacocks which reduce their agility), why and how would this trait-preference interaction arise from evolution?
There are two strands of theories that try to explain this evolution. The first was proposed by Fisher (1930) who suggested that the advantage of preferences lies in the choice of mates who will father attractive sons. If females on average prefer a particular male trait, then males having that trait will have a mating advantage. If this trait is heritable, then females would also prefer to mate with males with this trait. Fisher argued that this evolution may lead to a runaway process, leading to an inefficient trait size, such as the excessively large plumage of peacocks. However, as pointed out by Pomiankowski et al. (1991), this runaway argument is valid only when there is no cost associated with female preferences. If there is some cost (say of searching), no matter how small, Fisher’s argument cannot go through.
The second hypothesis suggests that the benefit of female preferences hinges upon the improved survival of offspring. Zahari (1975) argued in his well-known “handicap” theory that the male trait provides the female with information about heritable male quality, very much like the idea of signaling in economics. Iwasa et al. (1991) showed that a correlation between male trait and viability is necessary to create an equilibrium with female preferences. With respect to this handicap theory, most existing literature uses quantitative genetic theories to present their arguments. In this section, we shall use our life history model to demonstrate this theory in a very neat way. The key, as we shall see, is the dominance of the female reproduction constraint in (4).
4.1 The Correlation Between Viability and Female Search
Again, consider a species that has polygamous marriages of one-male mating n females, and each surviving female bears f* children. However, there is some probability 1 − q that a male cannot find a female willing to mate with him, and hence has zero impregnations. The chance that a male can find a mate is determined by competition with other males to attract females, based on their trait value Y. We assume that females like to mate with males with larger Y, and we shall justify this assumption later.
For females, they have to spend some search energy S in order to find a male with larger Y. Both Y and S are controlled by sex-specific genes. Other than S and Y, there is a viability gene v that affects both males and females. We assume that v affects bm and bf, the efficiency coefficients of maintenance, with and . The gene affects adult maintenance efficiency but of course it is carried by offspring (see Iwasa et al. (1991)).
From our discussion in section 3, we know that the intrinsic growth rate of the species is dominated by females. Assuming that there is no investment or transfer by females, her available energy net of her search cost is g(zf )− S. Thus, maximizing F = pf · f subject to bf (v)pf + cf f ≤ g(zf ) − S yields
Note that F* is the equilibrium intrinsic growth rate irrespective of the trait competition among males.
Since ∂F*/∂S < 0, meaning that searching costs some female energy, in order for us to observe an evolutionary equilibrium that involves female search, it must be the case that there is some connection between S and v. Specifically, since
and since ∂F*/∂v > 0,8 we must have a positive correlation between S and v (dv/dS > 0) in order to have dF*/dS > 0. Thus, we have used a very simple argument to establish the result in Iwasa (1991). The key reason we have this neat derivation is that female dominance is always true, even when the species in question has inter-sexual interactions. Note that this analysis is based on maximization of F* across gene lines that have different amounts of energy devoted to search.
4.2 The Correlation Between Viability and Male Trait
What about the male competition based on trait size Y ? The fact of female dominance in reproduction shows that Y does not affect the equilibrium λ= F*/2 at all. Suppose the probability q̃ for a male k to be selected by females is a function of his trait Y k relative to others’ Y~k. Then we have q̃ = q̃(Yk, Y~k). For any given amount of energy spent on trait Y, the male chooses m to maximize his q̃ ·(nF*). We also note that in a symmetric equilibrium, q(Y k, Y k) = 1/n must hold. Thus, as in (7), the evolutionary result must be constrained by the following equation in a symmetric equilibrium:
| (8) |
Evidently, since , from (8) we see that Y k and v move in the same direction for any constrained pf*f*, meaning that only viable males are capable of growing larger Y: cov(Y, v) > 0. This is the essence of the handicap theory. And this is also what is needed to justify the search of larger Y on the female side. By searching, the female finds males with larger Y. These males happen to be more viable ones because cov(Y, v) > 0, and their gene controlling larger Y helps the energy efficiency of their offspring. This justifies the female expenditure of energy-on search.
Note that our analysis of Y is not different from our analysis of male investment (I) on canine teeth in section 3. The model in this section is one with inter-sexual selection, whereas the one in section 3 is of intra-sexual competition. However, the fact of female dominance makes the superficial sexual interaction between females and males degenerate. It turns out that we can still solve the female problem first, and then work on the male problem afterwards. Intra-sexual male competition or male-female interaction turn out to be technically the same.
5 Why Do Males Transfer Less than Females?
Now we take into account the role of parental transfers, a variable we distinguished from the cost of fertility in section 1. This discussion is also related to the endogeneity of zs, s = m, f, which was assumed to be exogenous (that is, a given in the analysis) in a previous analysis but will be derived here as part of the life history strategy. In Trivers (1972), parental transfers and reproduction costs were bundled together as a single variable. When male (female) adults provide Tm (Tf ) transfers to their children, from the energy budget constraint we see that survival at age-1 should be revised to p1,m = [g(zm) − cmm − Tm]/b1,m for males and p1,f = [g(zf ) − cf f − Tf ]/b1,f for females. From the previous discussion, we know that the female should choose f* = [g(zf ) − Tf ]/(2cf ).
In a setting with 2-ages, parental transfers are an energy endowment for juveniles age 0. In general, such energy is used by the young for maintenance and somatic growth. To simplify the analysis, we assume that juvenile survival p0,s is mostly determined by parental guarding and watching, and therefore that parental transfers are all used for somatic growth zs.
5.1 Male Transfers in Monogamy and Polygyny
Given any planned transfer Tf we have shown that the female’s expected fertility is
| (9) |
Now consider the case of polygyny where each alpha male mates n females. In a more general interpretation, the case of monogamy is approximated when n → 1. In this way, n characterizes the mating scenario. The mate-competition that has occurred before the actual mating is not important here for the discussion of transfers, because transfers happen only after a male achieved the privilege to impregnate a female.
Consider a pride of lions with one male and n female lionesses. Suppose for simplicity that male and female juveniles share parental transfers equally.9 Then, given that there are p0,s age-1 adults of sex s, the average energy shared by each of the f* newborns in an n-female cooperative breeding group would be
| (10) |
For any given Tm, the female should choose a Tf to maximize F* in (9) subject to the constraint in (10).
The male lion, after becoming the king, may use his energy in different ways. Because his total number of impregnations is constrained by the females, there is not much choice in that direction. The lion king, of course, has an incentive to make a transfer Tm which increases the starting z of his offspring and raises λ. But there are also other uses for his energy, such as roaring, patrolling, killing hyaenas, etc. Suppose the opportunity cost of other uses of energy is a constant C. We shall investigate the marginal benefit he derives from devoting energy to transfers.
Note that zf = z is a function of Tm by (10). Differentiating nF* with respect to Tm, we have
The formula of z in (10) tells us that
Since f* is determined solely by females, we see that the marginal benefit of Tm is independent of n. However, the larger the female pride size (n), the more costly it is for the alpha male to lose control of the females in his pride, hence the higher the opportunity cost of diverting energy away from patroling-roaring to transfers to his offspring. In other words, the marginal cost of Tm for larger n is likely to be higher. This means that other things being equal, fathers of polygamous species have less incentive to transfer to offspring. This is mainly because a single father’s contribution is diluted by the group of mothers in a cooperative breeding species. We can look at the this finding from another angle: Consider a mutation that shifts male energy from maintenance to investment in trait-competition to increase the chances of becoming a polygynous alpha male. If this mutation is selected, then a mutation that reduces total transfers by the alpha male to the young would also be selected.
Concerning the relationship between transfers and investment in a mate-competition trait, equations (7) and (8) can be combined and re-written as
| (11) |
It is evident from (11) that Ik and Tm must move in opposite directions. Thus, the more energy that males invest in Ik, other things being equal, the less energy that males transfer to their offspring. We therefore expect to have a negative correlation between male investment in mate-competition traits and male transfers. This seems to be consistent with what we observe in nature.
5.2 Transfers and the Risk of Cuckoldry
Now consider the case when the pride includes other males. This will happen when male competition generates an alpha male but he cannot drive away other males. Well-known examples include baboons, wolves, and sea lions. Then, the alpha male has the privilege of priority in mating, but he cannot completely prevent access to females by the other males and consequently he is never sure whether a pregnant female actually carries his genes. In other words, we have paternal uncertainty, or the possibility of cuckoldry for the alpha male. Facing such a cuckoldry risk, how would the alpha male change his transfers?
Suppose each female still has a certain number of births, but that now on average f* of them are with the alpha male and f′ of them are with other males. The female does not care because in either case the baby carries her genes. Since a female seeks to maximize f · [g(zf ) − cf f − Tf ], her optimal fertility is f* + f′ = [g(zf ) − Tf ]/(2cf ). However, because only f* births are from the alpha male, his transfers are even more diluted in this cooperative breeding group. This result is further strengthened if the non-alpha males also make some transfers to the young. It is easy to see that his marginal benefit from providing a transfer is further reduced by the risk of cuckoldry, not to mention the dilution effect of male transfers as in the case of subsection 5.1.
In short, Cuckoldry dilutes the probability that a particular child is the descendant of the alpha male, and therefore reduces his incentive to transfer.
6 The Sex Asymmetry of Menopause
Very few species in the world have menopause. According to Carey and Gruenfelder (1997), only human beings, killer whales, and dolphins have adaptive menopause. In terms of life history, an adaptive menopause means that the species has an optimal life history path that corresponds to a corner solution of zero fertility for an old female. In the traditional evolutionary framework of maximizing gene proliferation, the life history of a species should not include survival to ages in which fertility is zero. However, for species that make intergenerational transfers, even post-reproductive old age can still contribute to gene proliferation so long as these old individuals provide net transfers to the young (Lee, 2003). Most researchers, for instance Hawkes et al. (1997), Carey and Gruenfelder (1997),b and Chu and Lee (2008b), explain menopause as a special kind of division of labor between a grandmother and mothers, where the grandmother specializes in non-reproductive tasks such as food gathering and care of young, while the mothers do all these tasks plus bearing children. However, all these papers implicitly consider a model of females, and therefore don’t address the logical question of why males never have menopause. In this section we shall provide an answer to this question.
To consider menopause in our framework, we must extend our model to two mature ages, so that a choice of zero fertility in old age is meaningful. However, with two mature ages, we have the possibility of a female age 1 or 2 mating with a male age 2 or 1, and also the possibility of pairings lasting 1 period or two periods. To avoid all these technical complications, we make the following assumptions. We consider a polygamous mating system where the females living together have a size of n1 age-1 adults and n2 age-2 adults. Only males at age-1 can compete for the alpha status, and the probability of victory is q1. If a male wins, he will impregnate all the females in his pride. He will be the alpha male for two consecutive periods if he survives to old age. When he dies, either at age 1 or age 2, another alpha male arises from a new round of competition. None of these assumptions is crucial to our results, but they do make it easier to derive insights. As in our 2-age model of the previous sections, adult females determine their optimal fertility independent of the male decisions. We denote females’ optimal decisions at age-1 and age-2 by f1 and f2 respectively.
Given these assumptions, we can write down the expanded Bellman equation for our 3-age setup. Details are given in the Appendix, and the interpretation is the same as in section 2, and is therefore omitted.
Chu and Lee (2008b) showed that a key reason for menopause to appear is the division of labor between grandmothers and mothers. To specialize in care of the young, grandmothers have to give up their foraging time, which in turn reduces their available energy. As the grandmothers move closer and closer to complete specialization care of the young, their energy constraint pushes them toward a corner solution for fertility, to conserve energy, ultimately leading to menopause. From our model we can provide three reasons for males not to have menopause:
Female menopause corresponds to a life history with f2 = 0, a particular corner solution. This would be a reasonable choice only if the opportunity cost of f2 (which is cf ) is high, so that avoiding fertility at age-2 saves substantial energy on the part of the potential mothers. But for males, since cm is trivially small, it does not pay to avoid m2. Even if males do have m2 = 0, they cannot save much energy thereby in any case. Thus, an efficient division of labor is unlikely to involve a corner solution of m2 = 0 for old males.
We see from Chu and Lee (2008b) that the division of labor is the key feature of menopausal species. However, we have shown in the previous sections that the alpha male has little incentive to transfer to the young, due to the dilution effect and paternity uncertainty in a cooperative breeding group. Even if the male does save some energy by choosing m2 = 0 he is likely to move it to investment in competitive traits or somatic maintenance. Thus, because the male does not tend to transfer energy anyway, it is difficult to form a division of labor involving the male. However, this reason is diminished when the male can make public good type transfers that are not subject to dilution, such as defense of all offspring from a single infanticidal competitor.
In a polygynous pride, if an old male were to stop producing sperm, then all females in the pride would be unable to reproduce. There would be a high fitness cost if the alpha male were to have m2 = 0, while still maintaining his alpha status.
7 Conclusion
In this paper, we provide a unified approach to explain sexual dimorphism, intrasexual competition, and intersexual selection in biology. In terms of analytical structure, we adopt a model of two-sex life history. We suggest that the fundamental driving force is the male-female difference in direct costs of reproduction (as opposed to mating costs and post-birth investments). Using our model we are able to explain why males are motivated to grow canine teeth, why fathers provide smaller transfers to children, why there is a positive correlation between polygyny and sex-specific size dimorphism, how environmental factors influence sex competition, why males can sometimes mate only through a risky lottery for access to females, and why only females are likely to have menopause. Finally, we explain the negative correlation between male traits and viability using our framework of optimal life history.
Not surprisingly, the richness of nature does not always conform to our predictions. For instance, hyenas have a matriarch instead of an alpha male, and among wolves and African hunting dogs there are both alpha males and alpha females, with only the alpha female permitted to breed. Indeed, we believe the hyena and wolf cases indicate scenarios not covered by our model. For instance, wolves and African wild dogs are pack hunters who need agile individuals to join the hunt, including non-pregnant females. Efficiency is gained by exploiting economies of scale in breeding and lactation. In terms of our model, the energy-generating function g would have to be specified differently. Hyenas have many predators such as lions, and they cannot provide effective protection for too many juveniles. This implies that few or even one pregnant female might be enough. In either case, these examples fall into the broad-sense category of Emlen and Oring (1977) that some resources are “undefendable”, and hence certain sexual interaction phenomena do not arise. In fact, this is also why there is not much sexual dimorphism in species such as hyenas and wolves.
Appendix: The 3-age Model for Analyzing Menopause
The Bellman equation for the 3-age model described in the text can be written as follows.
where there is also a reproduction constraint on the alpha male. Males compete to become the alpha male, and the probability of success depends on their respective investments in a competitive trait. Let the age of the alpha male be α. The energy budget constraint for this cooperative breeding pride is
In the above expression, the left hand side adds up the energy output by all members in the pride. The first and second terms on the right hand side are the female’s energy expense on her age-1 and age-2, and the third term is the energy expense of the alpha male. Other than this constraint, the somatic investment for each youth is determined by
| (*) |
The stationary state of the pride can be solved from the above expressions, with the understanding that the constancy of n1 and n2 holds only in a density-dependent equilibrium. In a stationary state equilibrium, the population age structure will appear, and the size of females aged a will be determined by the environmental density. We do not want to go into details here, for that is not the purpose of our research.
Footnotes
We are indebted to Meng-yu Liang, and Eirc Maskin for their comments and suggestions on earlier drafts.
Lee’s research for this paper was funded by a grant from NIA, P01 AG022500.
It is well known from the literature that determinate growth is the optimal strategy in a life history model with a linear energy constraint. See Chu and Lee (20060 for details.
Since our purpose here is to find the optimal life history (mi, fj, pa,s) and to discuss sexual dimorphism, we will not digress to sex ratios at birth different from unity.
This is what Gaulin and Sailer (1984) called “sex differences in reproduction potentials.”
In a life history with deterministic growth, somatic growth occurs only prior to initiation of reproduction. Here we are allowing further growth of a sort during the reproductive period. But this investment does not enhance foraging success as does greater z. This investment is for the purpose of intraspecific competition or intraspecific competition for access to females. We assume it takes place in adulthood after foraging success is revealed and is finished before decisions are made about allocations to reproduction and survival.
There is a bit of notation abuse here: I~k is a vector and Ik is a scalar. As we write , we actually mean that every element of I~k is equal to .
Because ∂F*/∂bf < 0, and ∂bf/∂v < 0 by assumption.
This will be the scenario in the lion case, for instance, when the hunted food is left and shared by juvenile lions of both sexes freely, so that no juvenile of a particular sex has a systematic advantage of taking more food.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
C. Y. Cyrus Chu, Email: cyruschu@gate.sinica.edu.tw, Institute of Economics, Academia Sinica, 128 Academia Road Sec. 2, Nankang, Taipei, TAIWAN.
Ronald D. Lee, Email: rlee@demog.berkeley.edu, Departments of Demography and Economics, University of California, 2232 Piedmont Avenue, Berkeley
References
- 1.Abrams PA, Ludwig D. Optimality Theory, Gompertz’ Law, and the Disposable Soma Theory of Senescence. Evolution. 1995;49:1055–1066. doi: 10.1111/j.1558-5646.1995.tb04433.x. [DOI] [PubMed] [Google Scholar]
- 2.Bellman R. Dynamic Programming. Princeton Univ. Press; Englewood Cliffs, NJ: 1957. [Google Scholar]
- 3.Bergstrom Theodore C. On the Evolution of Altruistic Ethical Rules for Siblings. American Economic Review. 1995;85:58–81. [Google Scholar]
- 4.Brown Jerram L. The Evolution of Diversity in Avian Territorial Systems. The Wison Bulletin. 1964;76(2):160–169. [Google Scholar]
- 5.Clutton-Brock Tim. Sexual Selection in Males and Females. Science. 2007 Dec 21;318:5858, 1882–1885. doi: 10.1126/science.1133311. [DOI] [PubMed] [Google Scholar]
- 6.Chu CY Cyrus, Lee Ronald D. The Coevolution of Intergenerational Transfers and Longevity: An Optimal Life History Approach. Theoretical Population Biology. 2006;69:193–201. doi: 10.1016/j.tpb.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu CY Cyrus, Chien Hung-ken, Lee Ronald. Explaining the Optimality of U-shaped Age-specific Mortality. Theoretical Population Biology. 2008a;73:171–180. doi: 10.1016/j.tpb.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu CY Cyrus, Lee Ronald D. “Menopause and the Division of Labor: An Economic Analysis,” mimeo 2008b [Google Scholar]
- 9.Cox Donald. Biological Basics and the Economics of the Family. Journal of Economic Perspectives. 2007 Spring;21(2):91–108. [Google Scholar]
- 10.Crowley Philip H. Sexual Dimorphism with Female Demographic Dominance: Age, Size, and Sex Ratio at Maturation. Ecology. 2000;81:2592–2605. [Google Scholar]
- 11.Darwin Charles. The Descent of Man, and the Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 12.Emlen Stephen T, Oring Lewis W. Ecology, Sexual Selection, and the Evolution of Mating Systems. Science. 1977;197(4300):215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RA. The Genetical Theory of Natural Selection. Clarendon Press; Oxford, UK: 1930. [Google Scholar]
- 14.Gaulin Steven JC, Sailer Lee Douglas. Sexual Dimorphism in Weights Among the Primates: The Relative Impact of Allometry and Sexual Selection. International Journal of Primatology. 1984;5(6):515–535. [Google Scholar]
- 15.Hausken Kjell, Hirshleifer Jack. Truthful Signalling, the Heritability Paradox, and the Malthusian Equi-marginal Principle. Theoretical Population Biology. 2008;73:11–28. doi: 10.1016/j.tpb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Iwasa Yoh, Pomiankowski Andrew, Nee Sean. The Evolution of Costly Mate Preferences II. The ‘Handicap’ Principle. Evolution. 1991;45:1431–1442. doi: 10.1111/j.1558-5646.1991.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick Mark. Sexual Selection and the Evolution of Female Choice. Evolution. 1982;36:1–12. doi: 10.1111/j.1558-5646.1982.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 18.Kokko Hanna, Johnstone Rufus A. Why is Mutual Mate Choice Not the Norm? Operational Sex Roles and the Evolution of Sexually Dimorphic and Monomorphic Signalling. Philosophical Transactions: Biological Sciences. 2002;357:319–330. doi: 10.1098/rstb.2001.0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lande Russell, Arnold Stevan J. Evolution of Mating Preference and Sexual Dimorphism. Journal of Theoretical Biology. 1985;117:651–664. doi: 10.1016/s0022-5193(85)80245-9. [DOI] [PubMed] [Google Scholar]
- 20.Lee RD. Rethinking the evolutionary theory of aging: transfers, not birth, shape senescence in social species. Proceedings of the National Academy of Sciences. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luentenegger W, Cheverud J. Correlates of Sexual Dimorphism in Primates: Ecological and Size Variables. International Journal of Primatology. 1982;3:387–402. [Google Scholar]
- 22.Maynard Smith John. Theories of Sexual Selection. Trends in Ecology and Evolution. 1991;6:146–151. doi: 10.1016/0169-5347(91)90055-3. [DOI] [PubMed] [Google Scholar]
- 23.Pomiankowski Andrew, Iwasa Yoh, Nee Sean. The Evolution of Costly Mate Preferences, I. Fisher and Biased Mutation. Evolution. 1991;45:1422–1430. doi: 10.1111/j.1558-5646.1991.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 24.Robson Arthur J, Kaplan Hillard S. The Evolution of Human Longevity and Intelligence in Hunter-Gatherer Economies. American Economic Review. 2003;93:150–169. doi: 10.1257/000282803321455205. [DOI] [PubMed] [Google Scholar]
- 25.Rogers Alan R. Evolution of Time Preferences by Natural Selection. American Economic Review. 1994;84:460–481. [Google Scholar]
- 26.Servedio Maria R, Lande Russell. Population Genetic Models of Male and Mutual Mate Choice. Evolution. 2006;60:674–685. [PubMed] [Google Scholar]
- 27.Sozou Peter D, Seymour Robert. Augmented Discounting: Interaction between Ageing and Time-preference Behavior. Proceedings of the Royal Society, B. 2003;270:1047–1053. doi: 10.1098/rspb.2003.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stearns SC. The Evolution of Life Histories. New York: Oxford University Press; 1992. [Google Scholar]
- 29.Trivers Robert L. Parental Investment and Sexual Selection. In: Campbell BG, editor. Sexual Selection and the Descent of Men: 1871–1971. Chicago: Aldine Publishing Co; 1972. [Google Scholar]
- 30.Zahavi Amotz. Mate Selection – A Selection for a Handicap. Journal of Theoretical Biology. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]