Abstract
Self-folding broadly refers to self-assembly processes wherein thin films or interconnected planar templates curve, roll-up or fold into three dimensional (3D) structures such as cylindrical tubes, spirals, corrugated sheets or polyhedra. The process has been demonstrated with metallic, semiconducting and polymeric films and has been used to curve tubes with diameters as small as 2 nm and fold polyhedra as small as 100 nm, with a surface patterning resolution of 15 nm. Self-folding methods are important for drug delivery applications since they provide a means to realize 3D, biocompatible, all-polymeric containers with well-tailored composition, size, shape, wall thickness, porosity, surface patterns and chemistry. Self-folding is also a highly parallel process, and it is possible to encapsulate or self-load therapeutic cargo during assembly. A variety of therapeutic cargos such as small molecules, peptides, proteins, bacteria, fungi and mammalian cells have been encapsulated in self-folded polymeric containers. In this review, we focus on self-folding of all-polymeric containers. We discuss the mechanistic aspects of self-folding of polymeric containers driven by differential stresses or surface tension forces, the applications of self-folding polymers in drug delivery and we outline future challenges.
Keywords: lithography, three dimensional, spatio-temporal, controlled release, origami, hydrogels
1. Introduction
In drug delivery it is often required to package therapeutic cargo including small molecules, peptides, proteins, nucleic acids and living cells. Packaging provides a means to achieve enhanced solubility and accurate targeting, prevent premature degradation, permeate barriers, reduce dosage and limit side effects [1–3]. Several methods already exist to package therapeutic cargo within matrix, solid or reservoir based systems. These include micro or nanoparticles [4–6], liposomes [7–11], polymer capsules [12–16], and micromachined constructs [17–23]. However, in order to package drugs for delivery within the human body, which is a complex labyrinth of circulatory pathways and organs filled with small molecules, cross-linked biopolymers, cells and microorganisms, there is often a need to precisely structure drug encapsulation packages with a range of multi-functional attributes.
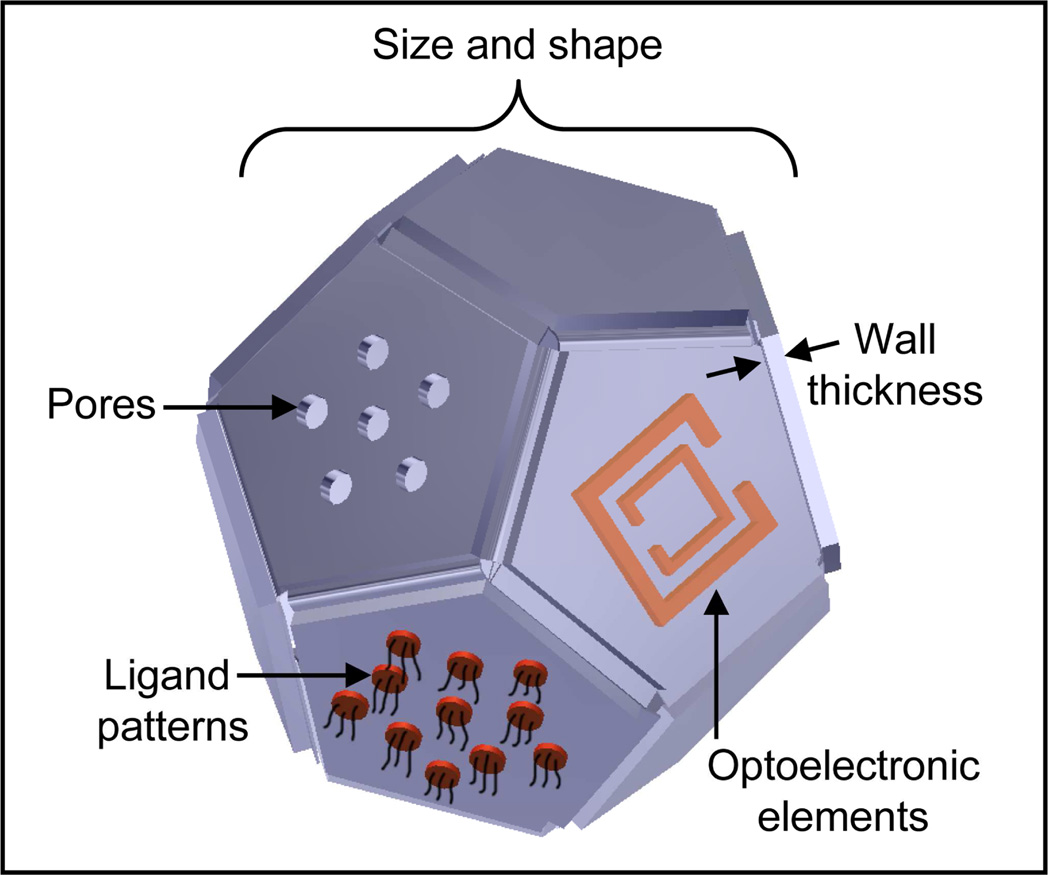
Important attributes of a drug delivery package, an illustration of which is shown in Fig. 1, include (a) material composition; (b) structural parameters such as monodispersity, size, shape, porosity, and reservoir wall thickness; (c) surface functionalization; (d) reconfigurability; and (e) manufacturability. The material composition of the package determines its toxicity, biodegradability and compatibility with different therapeutic cargo [24, 25]. The size and shape of the package strongly affect transport across different biological barriers and circulation times [26, 27]. The porosity is important for controlling semi-permeability for immunoisolation and spatial and temporal characteristics of drug release [28, 29]. The surface chemical functionalization determines immunocompatibility, cellular targeting and uptake [27, 30]. The incorporation of optoelectronic elements is important for imaging, remote communication and on-demand delivery [31, 32]. Reconfigurability enables stimuli-responsive and smart behaviors [33, 34], while manufacturability is important for practical considerations. Hence, synthesis or fabrication schemes that enable several of the aforementioned attributes to be achieved in a drug delivery package need to be seriously evaluated.
Figure 1. Important attributes of a drug delivery system.
Important attributes include precise size and shape, wall thickness, porosity, patterned targeting ligands and on-board optoelectronic elements (such as a split-ring resonator depicted in the illustration; illustration by Kate Laflin, Gracias Laboratory, JHU).
While existing drug delivery systems incorporate a few of the important attributes discussed above (Fig. 2), it is challenging to incorporate multiple attributes within a single fabrication or synthesis scheme. For example, many drug delivery constructs are fabricated using methods inspired by polymer and colloidal synthesis. Although these methods have advantages such as parallel cost-effective synthesis and encapsulation, ease of scale-up in manufacturing, and reasonable homogeneity, they are limited in that most of the particles have a predominantly spherical shape. As stated earlier, the importance of shape of drug delivery particles has been highlighted especially with respect to increased circulation times of particles with non-spherical shapes [35, 36]. This observation is perhaps not too surprising given that a large fraction of pathogens have non-spherical shapes. Additionally, spherical drug delivery particles allow only isotropic release of drugs and are not suitable for applications requiring directional release [21, 37]. Further, in cell encapsulation therapy, it has been challenging to form reproducible formations of gel capsules with adequate control of the thickness, uniformity and porosity of the semi-permeable membrane that form the walls of the capsule [38–41].
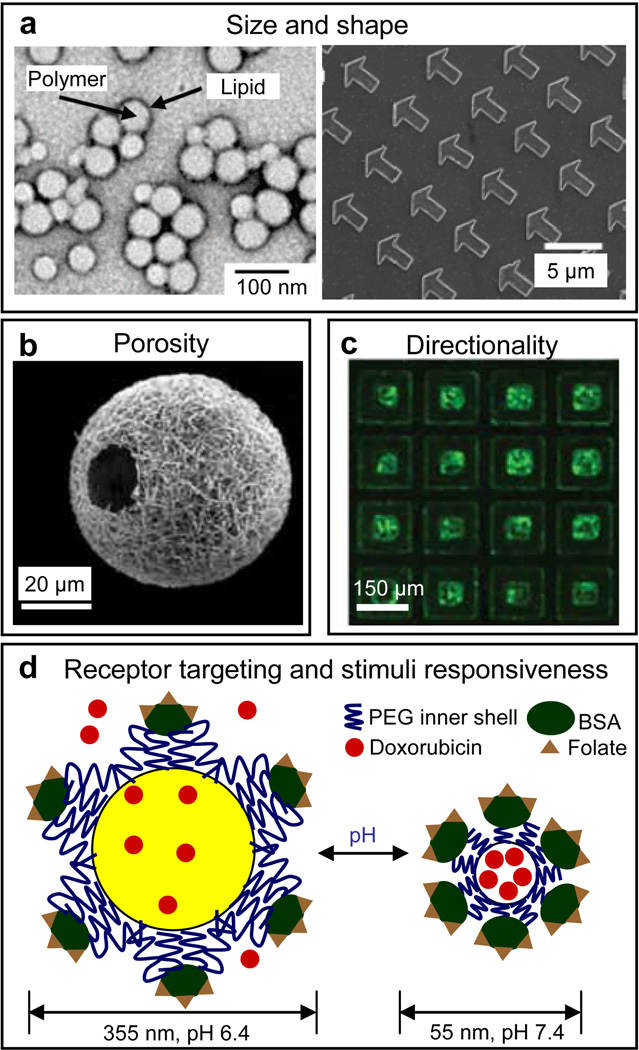
Figure 2. Features of current all polymeric drug delivery systems.
a) Size and shape: polymeric drug delivery systems have dimensions ranging from the nm to the mm scale with spherical and non-spherical geometries. Transmission electron microscopy (TEM) image of spherical lipid-polymer hybrid nanoparticles (left panel). Reprinted with permission from [140], © 2008, American Chemical Society). Scanning electron microscopy (SEM) image of 3 µm arrow-shaped polyethylene glycol based particles prepared using particle replication in non-wetting templates (PRINT, right panel). Reprinted with permission from [131], © 2005, American Chemical Society. b) Porosity: SEM image of a nanofibrous hollow microsphere prepared from star-shaped poly(L-lactic acid), showing the nanofibrous architecture and a hole of approximately 20 µm on the microsphere shell. Reprinted with permission from [141], © 2011, Nature Publishing Group. c) Directionality: Fluorescent micrograph of FITC-bovine serum albumin loaded SU-8-hydrogel bi-polymeric microparticles capable of directional release. [21] – Reproduced by permission of The Royal Society of Chemistry. d) Receptor targeting and stimuli responsiveness: schematic of a polymeric virus-mimetic nanogel vehicle that is surface functionalized with folate ligands. The nanogel vehicle delivers doxorubicin to targeted A2780/AD cells. Adapted and reprinted with permission, from [133] © Wiley-VCH Verlag GmbH & Co. KgaA.
Since the precision and versatility with which the aforementioned attributes can be varied in conventional polymer drug delivery synthesis methods is somewhat limited, researchers have begun to explore the use of highly precise micro and nanofabrication methods to structure polymeric drug delivery systems. Many of these methods were initially developed in the microelectronics and microelectromechanical systems (MEMS) industries for use with metals, semiconductors and inorganic dielectrics and are now being adapted for use with polymers and gels. The approaches include spin coating or solution casting of polymers or gels into prefabricated molds or on substrates for optical patterning using photomasks [18, 21, 22]. Uniform non-spherical and intricately patterned 3D polymeric and hydrogel structures can be formed using microfluidic [42], electrospinning [43], 3D printing, and multi-photon methods [44]. However, many of these methods can be limited especially in the micro and nanoscale patterning capabilities that can be achieved in 3D. Self-folding methods leverage the precision and versatility of existing planar micro and nanofabrication methods and additionally translate their capabilities into 3D, in a highly parallel manner. Hence, self-folding is a promising approach to create encapsulants which simultaneously incorporate many of the attributes mentioned earlier.
2. What is self-folding?
Everyone can relate to the action of folding a sheet of paper to package a gift on the macroscale, so it should come as no surprise that a folding approach might be similarly explored to encapsulate cargo at other length scales. However, at sub-mm length scales, even miniaturized probes or automated machines are unable to perform such complex 3D folding tasks. Hence, “hands-free” mechanisms are required to fold the package around the object. Self-folding is a word used to represent these methods, and broadly refers to these self-assembly mechanisms wherein thin films or patterned templates spontaneously curve, roll-up or fold into three dimensional (3D) structures such as cylindrical tubes, spirals, corrugated sheets or polyhedra from their two dimensional (2D) precursors. Self-folding can occur spontaneously when 2D planar structures are released from a substrate, typically on dissolution of a sacrificial layer, or in response to stimuli such as electrical signals, pH, temperature, magnetic fields or chemicals (see Leong et al. [45] for a comprehensive review).
Folded structures are intellectually compelling since they are widely observed in nature [46, 47] and in many tissues such as vasculature, ducts, gyri/sulci and villi in the human body [48]. From a technological perspective, several hollow structures such as precisely patterned polyhedra and nanotubes that are challenging to fabricate using existing 3D fabrication methods have been constructed using self-folding. For example, patterned polyhedra have been self-folded with sizes as small as 100 nm [49]. As compared to patchy particles [50, 51], which have been synthesized with very limited surface patterns, self-folded nanopolyhedra have been fabricated with a variety of patterns including letters of the alphabet with a line resolution as small as 15 nm [49, 52]. Similarly rolled-up tubes have been fabricated with radii as small as 2 nm [53] and with curved patterns with a resolution line width as small as 20 nm [54]. Self-folding is also compatible with a range of materials including metals, semiconductors, ceramics and polymers [55–58]. Self-folding structures with bi-directional curvature and thousands of folds have also been created [59]. In the subsequent sections, we limit our discussion to the self-folding of all polymeric containers for encapsulation of therapeutic cargo.
3. Self-folding of polymeric containers
Self-folding is not new to polymer science as polymers themselves can be self-folding molecular chains. In nature, biopolymers such as proteins and nucleic acids spontaneously fold into complex 3D structures. Spontaneous curving of thin molecular films such as lipid bilayers also often involves a flat sheet to spherical transition, as is observed when dry phospholipid films swell in excess water to form multilamellar vesicles [60], or in the formation of multi-compartment drug delivery vehicles or vesosomes from interdigitated lipid-ethanol sheets [61]. There has been a large effort directed at synthesizing shape memory polymers which have been used to create novel stimuli-responsive structures for drug delivery and biomedical engineering [62]. Stimuli responsive behavior in these materials is due to a specific molecular network architecture consisting of hard and switching segments [63]. While many of these shape memory polymers have several attractive properties for drug delivery applications such as biocompatibility and biodegradability [64, 65], the structures formed have been primarily macroscopic such as mm to cm scale sutures [66], stents [67], cubes [68] and electrodes [69]. It is conceivable that future advances in micro and nanostructuring of these materials and the development of strategies to program behavior of smaller structures could result in the creation of smaller, sub-mm scale shape memory polymeric containers for other routes of drug delivery e.g. intravenous or inhalation. There is also a vibrant research effort directed at self-folding oligomeric and polymeric containers at much smaller size scales. For example, scientists have created synthetic self-folding DNA polyhedra [70–73] and foldamers [74, 75]; such molecular folding methods are beyond the scope of this review.
Self-folding of polymeric thin films has been achieved at mm to 100 nm length scales and driven by physical forces such as differential stress or surface tension. It is noteworthy that despite the differences in size and self-folding driving forces as compared to molecular folding, there is some evidence that simple geometric design principles may apply across self-folding length scales [76, 77]. Research in the broader area of self-folding of polymeric thin films has many foci such as the fabrication of polymeric actuators [78–81], the fabrication of complex meso scale structures inspired by protein folding [82], the area of robotics [83] and the synthesis of biomimetic materials and scaffolds [48, 84–86].
To create polymeric containers by self-folding, it is necessary to deposit one or more layers of a polymer or gel on a flat substrate or a mold so that they can be patterned on the micro or nanoscale. Most polymers and gels can be spin or dip coated from solution as thin films with precise, controlled thickness. The thickness of these thin films can be readily controlled by varying the concentration of the solution from which the polymer is cast, the spin speed, substrate surface treatments and bake times. The thickness of the deposited films determines the thickness of walls of the polymeric container. By varying the thickness it is possible to control the mechanical stability of the container and the chemical diffusion characteristics. It may be necessary to deposit a sacrificial layer in between the self-folding films and the substrate so as to release the polymeric films and trigger self-folding. Typically, this sacrificial layer is dissolved by dry etching with plasmas or wet etching with organic solvents, acids or bases. A variety of sacrificial layers including polysilicon or silicon dioxide; metals such as copper, chromium or aluminum; water soluble polymers such as polyvinyl alcohol (PVA) or polyacrylic acid (PAA) [87]; acetone soluble polymers such as polymethylmethacrylate (PMMA) and fluoropolymers such as CYTOP can be utilized. Alternatively electrical [81] or thermal [88] stimuli responsive behavior of one or more components of the polymer layers can de-adhere or peel the films off the substrate during self-folding. Apart from precise control over the thickness of the films, they can be patterned with existing micro and nanofabrication methodologies. Patterning can be used to define pores with specific sizes, shapes and densities or define patches for surface modification with ligands or integration of electrical or optical modules such as an antennas or split-ring resonators (Fig. 1) that form the basis of many sensors and actuators for advanced multi-functional behavior. While depositing or patterning the thin films, self-assembly forces must be programmed into them so that they can spontaneously curve or fold either on release from the substrate or in response to specific stimuli.
3. 1. Self-folding mechanisms
A variety of mechanisms have been utilized to self-fold polymeric structures that could be used to grip or encapsulate objects; a detailed list is reviewed in the literature [45]. Many of these methods, however, require the use of a wire or tether through which electricity, air or fluids flow controls folding or unfolding. For example, controlled folding using electroactive polymers such as polypyrrole/dodecylbenzenesulfonate, has been used to open and close lids on reservoirs for cell encapsulation, but requires a wire to make an electrical connection that is required for folding and unfolding [89]. Similarly, pneumatically actuated Parylene balloons interconnecting rigid silicon phalanges [90] and polydimethylsiloxane (PDMS)-based pneumatic networks [83] have been demonstrated to control folding and curving of patterned polymeric gripping devices. These approaches are widely applicable for array based technologies and robotics but the need for wires or tethers limits their applicability in the design of mass-deployable, substrate-free sub-mm scale drug delivery containers.
With regards to self-folding without wires and tethers, there are mechanisms that require the use of heterogeneous compositions of metals and polymers. For example, Boncheva et al. described a macroscale demonstration of the self-folding of flat planar sheets to form spherical PDMS shells using magnetic forces [91]. This approach required the creation of elastomeric sheets patterned with magnetic dipoles; self-folding was driven by the interplay between the elastic bending energy and the magnetic energy. Similarly, Randhawa et al., have described the concept of microchemomechanical systems (MCMS) which incorporate polymeric triggers on pre-stressed metallic thin films to achieve chemical stimuli-responsive gripping devices [80]. These devices include wireless surgical grippers those that fold (close) and un-fold (open) on exposure to enzymes such as trypsin and cellulase [33, 34]. Recently, controlled folding of gold (Au)-polyelectrolyte brush bilayers [92] and thermally responsive PDMS/Au bilayers [93] has also been demonstrated.
Strategies that can be used to self-fold all-polymeric containers in the absence of any wires or tethers include those based predominantly on differentially stressed polymeric films and surface tension based effects. The mechanism based on differentially stressed films has been used primarily to create curved structures such as polymeric micro and nanotubes; surface tension self-folding methods have been utilized to form polyhedra. These structures and the associated mechanisms are described in detail below.
3. 2. Differentially stressed polymers
Since the seminal work of Stoney in the early 1900s [94] it is known that well-adhered thin film multilayers with differential stress will equilibrate by curving. Although initially discovered in electrodeposited metallic films, this process also occurs with polymer thin films and the spontaneous curling of peeling paint demonstrates this phenomenon. Hence, a rather straightforward way to create a spontaneously curling polymeric structure is to deposit two polymers with differential stimuli-responsive properties atop each other or to generate stress within a single polymer film by differential crosslinking (Fig. 3). Then on differential swelling or drying, the bilayer will spontaneously curl up. For example, Luchnikov et al. reported the creation of a bilayer composed of polystyrene (PS) and poly (4-vinylpyridine) (P4VP) which swells differentially due to differences in hydrophobicity of the two polymers [88]. Around the same time, Guan et al. created self-curling microstrips of chitosan/poly(ethylene glycol methacrylate)-co-poly(ethylene glycol dimethacrylate) (PEGMA-co-PEGDMA) and poly(methacrylic acid)/poly(ethylene glycol dimethacrylate) (PMAA/PEGDMA) based on differential bilayer swelling [95]. More recently Zakharchenko et al. has extended this concept to the creation of biodegradable polysuccinimide/polycaprolactone (PS/PCL) tubes [96] and Shim et al. have demonstrated the creation of microcarriers by pH triggered folding of snowman and flower-shaped bilayer films composed of poly(2-hydroxyethyl methacrylate-co-acrylic acid), p(HEMA-co-AA), and PHEMA) [97]. The radii of curvature of the self-curling structures depend on the thickness of the component films and their relative swelling ratios. Polymeric tubes with diameters as small as 100 nm have been reported [98]. Additionally, the wall thickness of the tubes can be controlled by varying the thickness of the polymer thin films, the number of turns can be altered by varying the lateral dimensions, and the tubes can also be patterned. A number of mathematical models [99, 100] can be utilized to approximate the radii of curvature of the multi-layer films based on the thickness, mechanical properties and strain in the films; however, accurate estimations that take into account lateral geometry of the patterned films require finite element modeling. While much of the research has focused on utilizing polymer bilayers with two different materials to drive differential stress on exposure to a stimulus, Jamal et al. have recently shown how differential photocrosslinking within a single polymer film and solvent conditioning can cause spontaneous curving and folding on polymeric SU-8 structures on immersion in water [85]. Since many biodegradable gels can be photocrosslinked, it is conceivable that this methodology could be utilized to create containers composed of these materials.
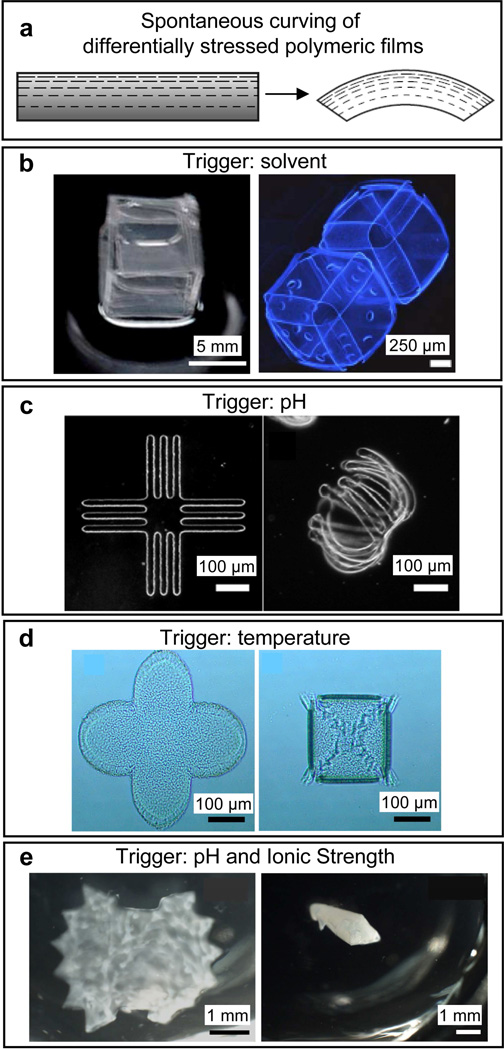
Figure 3. Spontaneous curving of differentially stressed polymeric films in response to solvents, temperature, pH and ionic strength.
a) Schematic depicting self-folding of differentially stressed polymeric thin films. Reprinted with permission from [85], © 2011, Nature Publishing Group. b) Solvent triggered folding: self-folding of a polydimethylsiloxane (PDMS) and a UV-curable hydrophilic polyurethane (PU)/2-hydroxyethyl methacrylate (HEMA) bilayer into a cube upon submerging into hexane (left panel). [79] – Reproduced by permission of The Royal Society of Chemistry. Self-folding of patterned and unpatterned SU-8 cubes using flat, highly crosslinked faces and hinges with crosslinking gradients (right panel). Reprinted with permission from [85], © 2011, Nature Publishing Group. c) pH triggered folding: self-folding of a poly(methacrylic acid) (PMAA)/poly-(ethylene glycol dimethacrylate) PEGDMA bilayer upon release in water. PMAA is pH-sensitive. Reprinted with permission from [95], © 2005, American Chemical Society. d) Temperature triggered folding: self-folding of star-like patterned polycaprolactone (PCL)/poly(N-isopropylacrylamide) (PNIPAM) bilayers in response to lowering of temperature. PNIPAM swells at lower temperatures. [142] – Reproduced by permission of The Royal Society of Chemistry. e) pH and ionic strength triggered folding: reversible self-folding of poly N-isopropyl-acrylamide-acrylic acid (NIPAm-AA) and polyethylene glycol diacrylate (PEGDA) bilayers in the shape of a Venus flytrap in response to reduced pH and increased ionic strength. Reprinted with permission from [78], © 2010, Elsevier.
3. 3. Surface tension driven self-folding
Anyone who has seen a solid cube of ice melt into a curved droplet of water is familiar with the spontaneous transformation in its shape. Liquid water is deformable and spontaneously adopts a shape that minimizes its surface energy. That this phenomenon could be used in micro and nanofabrication is less obvious. However, it was discovered that if solid materials are patterned in between rigid microstructures and if they are subsequently liquefied, these liquids can pull the microstructures into alignment [101–103] or even rotate them out of plane [104, 105]. This approach has been widely used to self-align integrated circuit chips and to rotate micromirrors. It was later shown that closed-form structures such as polyhedral particles with metallic or semiconducting faces would spontaneously fold around a deforming molten solder drop [106]. Although several metallic and semiconducting polyhedral shapes were demonstrated, the polyhedra were filled with solder after assembly, thereby limiting their applicability as containers. In order to create hollow polyhedra capable of encapsulating cargo, it was necessary to refine the approach by selectively patterning solder only within hinges and at the edges to create both patterned micropolyhedra [107, 108] and nanopolyhedra [49]. The key innovation was the use of both folding and locking hinges (Fig. 4a). Folding hinges were patterned between panels to rotate them approximately into place (Fig 4a, center panel). Once approximately in place, locking hinges that were patterned with the same hinge material but at the peripheral edges of the panels self-aligned in a cooperative manner (Fig. 4a, right panel) so that even complex polyhedral hollow containers such as dodecahedra with 12 faces and truncated octahedra with 14 faces could be formed [77, 109]. These experiments suggest that complex polyhedral geometries that are not easily synthesized by conventional drug delivery approaches can be generated by self-folding. It is noteworthy that many viruses which so efficiently target specific cells have polyhedral shapes [110] suggesting that these shapes may be important for targeted delivery to cells. Understanding folding pathways of nanoscale polyhedra may also be important in understanding viral self-assembly [111]. Additionally, many viruses also have patterns on their protein coat, and the self-folding approach allows any desired pattern that can be defined by planar lithography to be incorporated on the faces of the polyhedra.
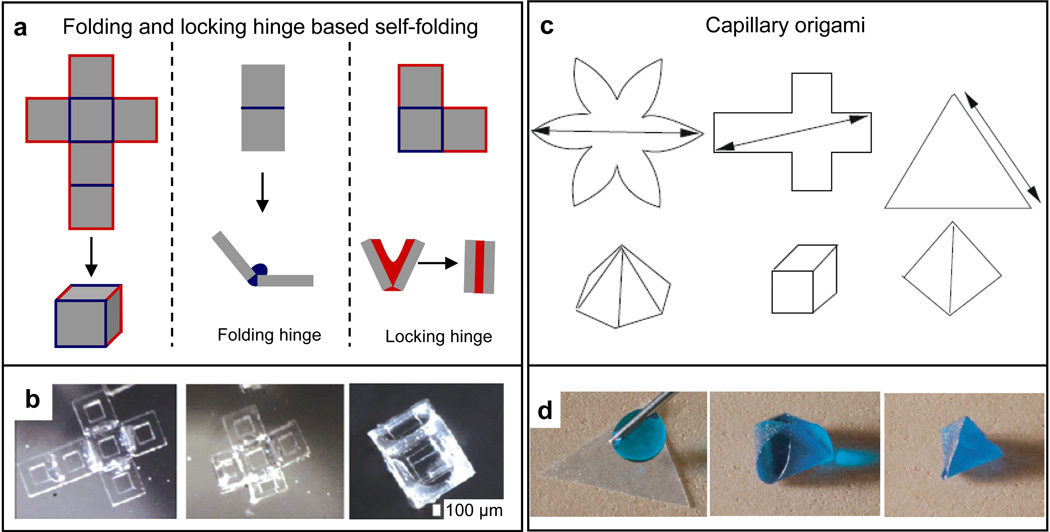
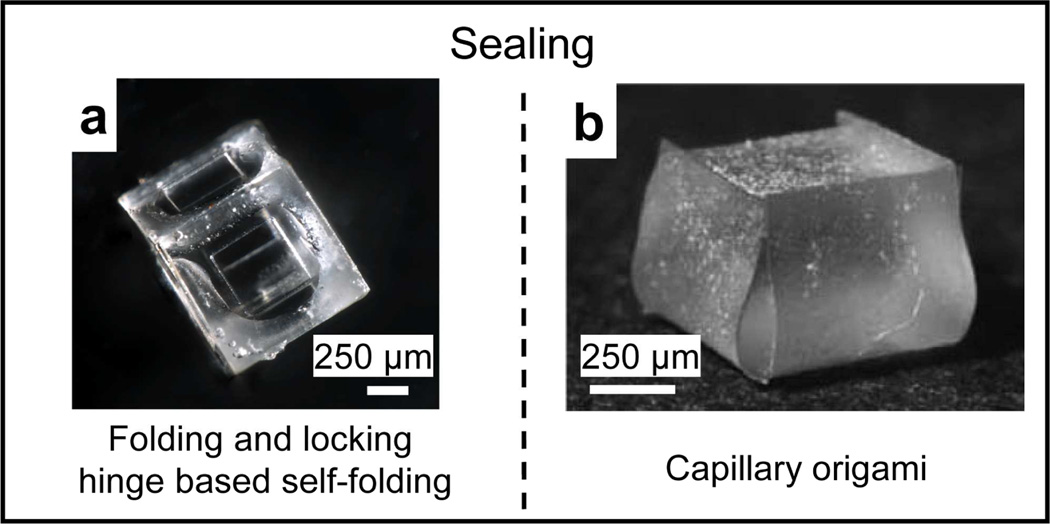
Figure 4. Surface tension based polymeric self-folding.
a) Self-folding based on folding and locking hinges. Schematic showing 2D to 3D self-folding of a cube using surface tension based self-folding (left panel). The 2D template or net is patterned with hinges between panels that assist in folding (folding hinges; blue; center panel) and also at the edges that assist in sealing of the polymeric structure (locking hinges; red; right panel). b) Video capture sequence (over 15 s) showing a 1 mm sized, six-windowed PCL/SU-8 container self-folding at 60°C. Reprinted with permission from [112], © Springer. c) Self-folding based on capillary origami. Schematic showing 2D to 3D self-folding of a elastomeric sheets cut into a variety of shapes (star, cube, triangle) via capillary origami. Reprinted with permission from [143], © 2010, Elsevier. d) Time sequence images of a triangular, millimeter-scale, elastomeric sheet folding into a pyramid by capillary origami. Reprinted with permission from [144], © EDP Sciences, Springer-Verlag 2009.
Although it had been theoretically predicted that this approach would work with a range of hinge materials [108], including polymers, it took several years to create all-polymeric micropolyhedra by this self-folding approach [112], mainly due to appropriate selection of polymeric materials that could be micropatterned and also liquefied at relatively low temperatures. The first self-folded polyhedra were formed with SU-8 panels and biodegradable polycaprolactone (PCL) hinges (Fig. 4b). Self-folding was driven on heating above 58°C to cause PCL melting; upon cooling, the polyhedra retained their shape and were mechanically rigid. Through the use of locking hinges, the polyhedra were well sealed at the edge, which is an important attribute ensuring that therapeutic chemicals are released only through lithographically defined pores on the faces of the polyhedra. Of importance to drug delivery is the fact that any desired pattern of pores or patches of receptors can be incorporated on some or all of the faces of these polyhedra. The thickness of the walls of the polyhedra can also be precisely controlled, and the SU-8 faces can be replaced by alternate photopatternable polymers such as PEGDA.
It has also been observed that appropriately shaped thin polymeric films would deform when a water droplet was placed on them and allowed to evaporate; this approach was termed capillary origami [113] (Fig. 4c). In contrast to earlier work with rigid metallic or semiconducting panels [106], the researchers used thin deformable polymeric (PDMS) membranes and folding occurred during evaporation of water from these hydrophobic membrane surfaces (Fig. 4d). Capillary origami is an attractive process since it is relatively straightforward, requiring only one layer of patterning, and it has been theoretically argued that self-folding with water droplets could work even at the nm length scale [114]. One limitation of this approach is that although polyhedral structures have been formed, edges are only weakly held together and would need to be sealed prior to use in drug delivery applications. In contrast, locking hinge based self-folding results in well-bonded and sealed polyhedra (Fig. 5). It is noteworthy that Shim et al. have also reported robust sealing of their p(HEMA) bilayer gel based microcapsules; sealing presumably occurs when edges of the swollen gel meet [97].
Figure 5. Hinged-based self-folding yields sealed containers.
a) Photograph of a sealed, porous SU-8/PCL cube formed via folding and locking hinge based self-folding (Image by Anum Azam, Gracias Laboratory, JHU). b) Image of an unsealed folded cube formed via capillary origami. Figure reprinted with permission from [113], © 2007 by the American Physical Society.
4. Applications
Although the field of self-folding is still in its infancy, the relevance of self-folding polymeric containers in drug delivery applications is evident on account of its capability of incorporating many advantageous attributes for drug delivery applications within a single fabrication approach. Self-folding has been shown to work at many length scales ranging from centimeters to nanometers (Figs. 6 a and b) [115–116]. Limitations in experimentally realized sizes in the surface tension driven self-folding of all-polymeric polyhedral devices are a consequence of limitations in 2D nanopatterning of gels and polymers. Theoretical models suggest that if 2D templates of polymer or gels layers could be appropriately lithographically patterned at 10–100 nm size scales, they would self-fold at that size scale [108]. This favorable scaling follows from the fact that surface forces become increasingly important as compared to gravitational forces at small size scales; hence, even materials with low surface tension could be utilized as hinges. Theoretical models are further corroborated by experiments showing that precisely patterned metals and ceramics can be self-folded at these small length scales [49, 52].
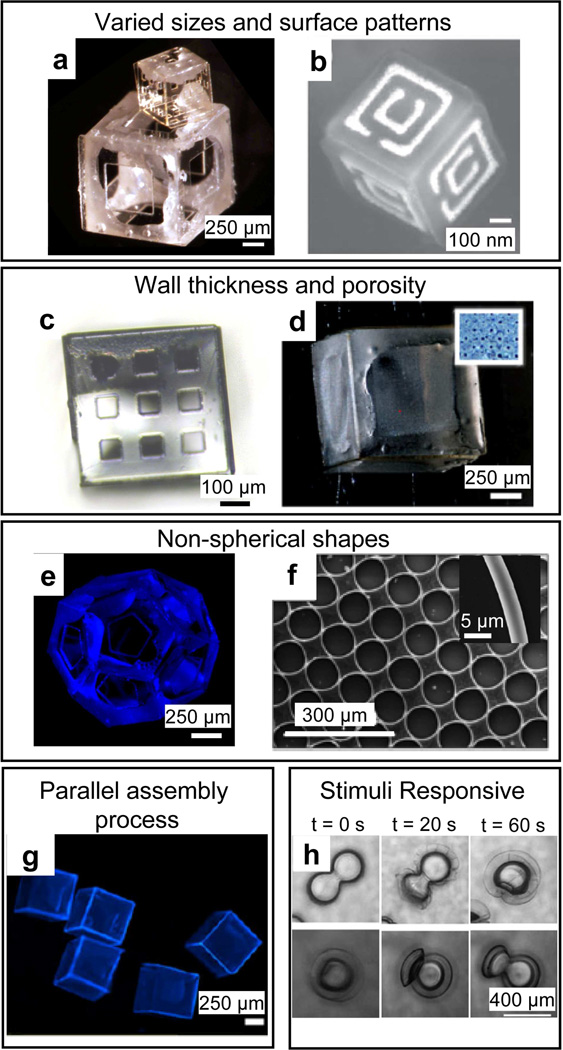
Figure 6. Controllable features of self-folding, polymeric, drug delivery devices.
Polymeric containers can be fabricated from the sub-mm scale (a) to the nanoscale (b). a) Image of folded, sub-mm scale SU-8/PCL containers. Reprinted with permission from [145], © 2010, American Chemical Society. b) SEM of a metallic nanoscale container formed via tin reflow (nanoorigami). Reprinted with permission from [52], © Wiley-VCH Verlag GmbH & Co. KgaA. Polymeric containers can be fabricated with controllable wall thickness and porosity (c–d). c) Optical image of 500 µm sized polymeric cubes with isotropic porosity. The pores are square shaped with dimensions of 73×73 µm and are precisely arranged in a 3×3 array on each face (Image by Anum Azam, Gracias Laboratory, JHU). d) Bright-field image of a 1 mm sized SU-8/PCL polymeric container with 8 µm diameter circular-shaped pores in a 20×20 array. e) Fluorescence image of a self-folding SU-8/PCL dodecahedron with 500 µm sized faces and a single 250 µm sized pentagonal pore on each face. f) SEM of rolled-up toroidal microtubes comprised of a bilayer of polystyrene and poly (4-vinylpyridine). Reprinted with permission from [117], © 2008 Institute of Physics. g) Fluorescence image of a group of non-porous, 1 mm sized SU-8/PCL containers. d, e and g are reprinted with permission from [112], © Springer. Polymeric containers can be fabricated to be stimuli-responsive (h). h) Optical time-lapse images showing reversible folding of poly(2-hydroxyethyl methacrylate-co-acrylic acid)/ poly(2-hydroxyethyl methacrylate) microcapsule bilayers upon pH increase from 4 to 9 (folding; upper row) and pH decrease from 9 to 4 (unfolding; lower row). Reprinted with permission from [97], © Wiley-VCH Verlag GmbH & Co. KgaA.
Lithographic patterning allows accurate control over the wall thickness (Fig. 6c), porosity (Fig. 6d) and shape (Figs. 6 e and f) [112, 117] of the polymeric containers which are all important for drug delivery applications as discussed earlier. Accurate 3D surface patterning also can enable more advanced functionalities, such as the creation of patterns of ligands for targeting cell surface receptors or the incorporation of electromagnetic modules such as antenna or split ring resonators [52] for sensing, and remote communication (depicted schematically in Fig. 1). Self-folded devices can be made in a variety of geometries: as long as a 3D structure can be mapped onto a planar substrate and lithographically patterned, it can be self-folded. Additionally, self-folding is a highly parallel process (Fig. 6g) which is good for manufacturability. Further, a recent study suggests that self-folding polymeric microcarriers can be made stimuli responsive (Fig. 6h) [97]. Some noteworthy examples of self-folding polymeric containers in drug delivery applications are described below.
4.1. Directional release
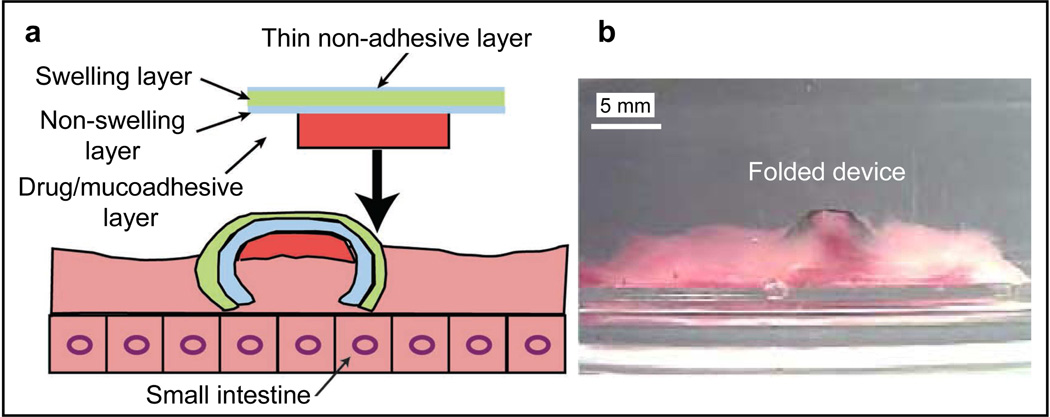
The lithographic patterning of differentially swelling polymeric bilayers can be used to create self-folding devices enabling directional release of encapsulated therapeutics. For example, He et al. fabricated a tri-layered, polymeric, mucoadhesive drug delivery system (Fig. 7) that consisted of a swelling layer; a non-swelling layer and a mucoadhesive (drug loaded) layer [37]. The swelling layer was a crosslinked, pH sensitive PMAA-based hydrogel. The non-swelling layer was a poly(hydroxylethyl methacrylate) (PHEMA)-based hydrogel and acted as a diffusion barrier. A PVA/carbopol based mucoadhesive layer containing the drug was attached to the bilayer (Fig. 7a). The device successfully gripped onto the walls of a porcine small intestine filled with a pH 6.5 buffer on account of PMAA swelling (Fig. 7b) and provided a longer residence time (as compared with controls patches comprised either of PCL or PHEMA) by maximizing its contact with the porcine small-intestinal walls and minimizing its contact with the fluid flow through the intestines. The PHEMA layer in the device acted as a diffusion barrier; hence, the fractional drug leakage from the device was observed to be lower than controls. The fractional drug release from the device across the mucosal epithelium (i.e. directional drug release) was verified to be significantly higher than controls. Hence, such directional release devices could reduce systemic dosage and side effects.
Figure 7. A tri-layered, polymeric, mucoadhesive drug delivery system.
The device consists of a swelling polymethacrylic acid hydrogel, a non-swelling poly(hydroxylethyl methacrylate)-based hydrogel and a drug containing polyvinyl alcohol/carbopol based mucoadhesive layer. A side-view schematic (a) and optical image (b) of the 3-layer device when folded on the porcine small intestinal surface. Folding is triggered by the differential swelling of the constituent polymeric layers. Adapted and reprinted with permission from [37], © 2006, Elsevier.
4.2. Spatio-temporal release
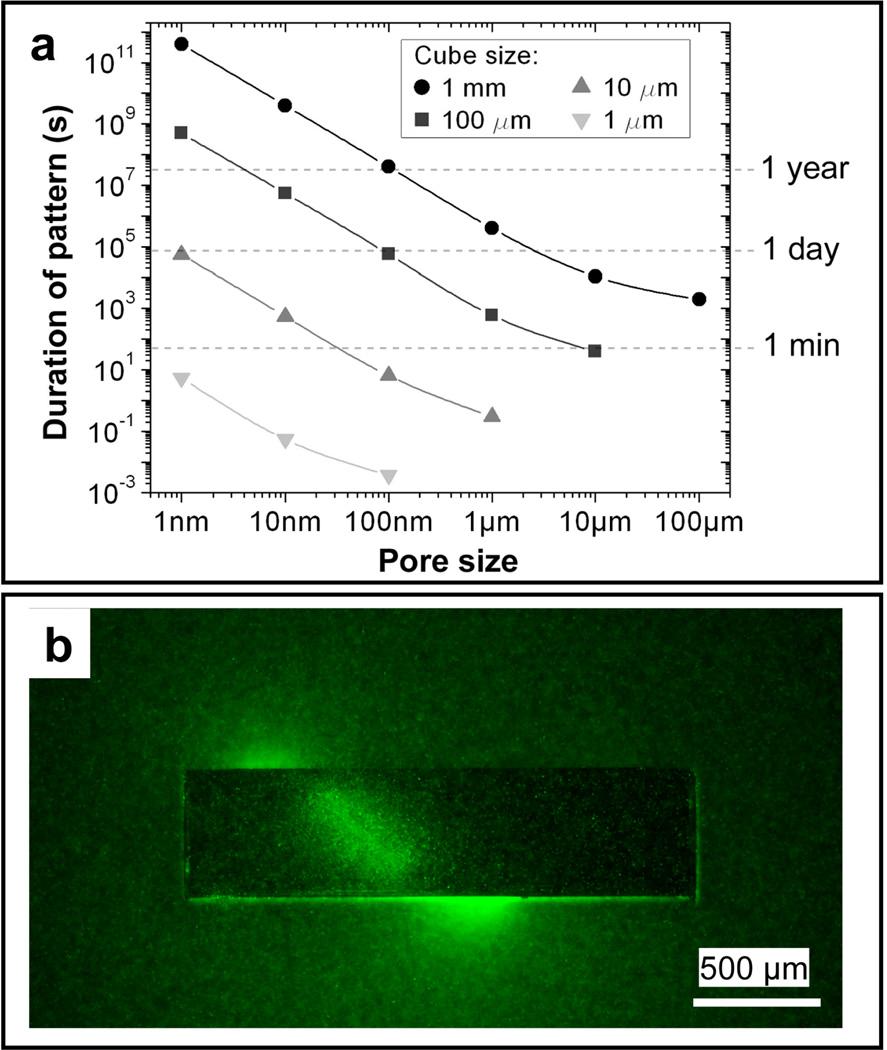
The lithographic patterning of porosity allows the user to vary the pore size, uniformity, placement and density. Self-folding extends this control to 3D, thereby offering unprecedented precision of spatio-temporal controlled release which is important for replicating 3D cellular microenvironments [118]. Further, precise pore patterning can enable semi-permeability which is important for cell encapsulation therapy applications [119]. For example, Kalinin et al. have simulated the release of a chemical from a porous, self-folded cube over time-scales ranging from a fraction of a second to a human lifetime by simply varying the size of the cubic container and the pore size (Fig. 8a) [118]. They showed that one can also release chemicals with precise spatial control, such as in helical shapes that are not readily achieved using conventional drug delivery devices. Additionally, by controlling the pore diameter and wall thickness of the device, temporal control over drug release is achieved. Kalinin et al. demonstrated 3D (helical) self-organization of chemotactic Escherichia coli in response to chemoattractant released in a spatio-temporally controlled manner from the container (Fig. 8b). Spatio-temporal factors play an important role in chemotaxis, cell signaling, angiogenesis, homeostasis and immune surveillance [120–124].
Figure 8. Spatio-temporal controlled release from a polymeric drug delivery system.
A drug delivery system can be designed to have pore sizes that enable drug release from a container in a few seconds to over the life span of a human (a) and in a spatio-temporally controlled manner in 3D (b). a) Plot showing relation between cube size, pore size and duration of release from a container. b) Fluorescent image showing 3D self-organization (helical) of chemotactic Escherichia coli in response to chemoattractant released in a spatio-temporally controlled manner from the container. Reprinted with permission from [118], © Wiley-VCH Verlag GmbH & Co. KgaA.
4.3. Cell encapsulation applications
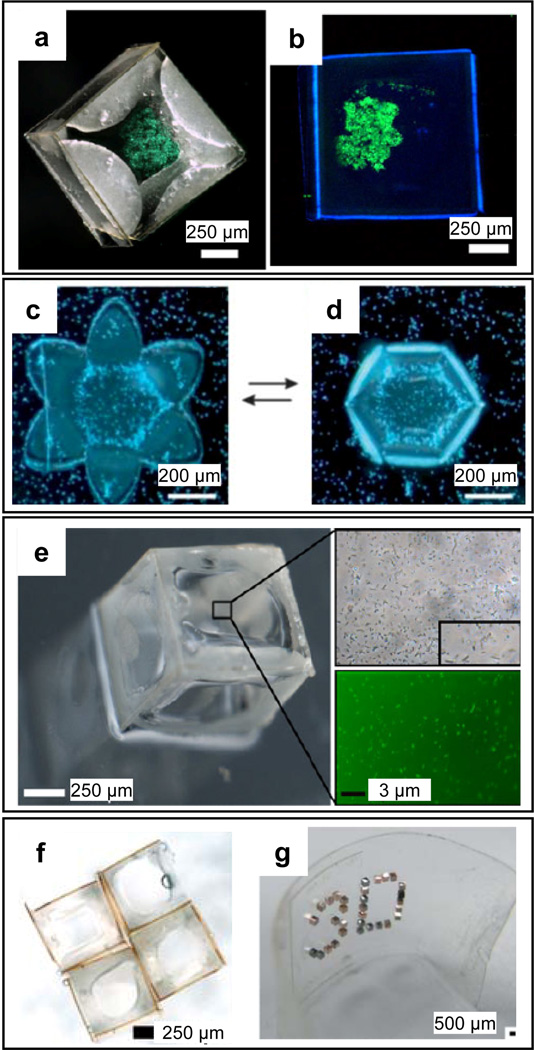
Self-folding polymeric containers have been successfully used to encapsulate a variety cell types. Azam et al. demonstrated the encapsulation of viable fibroblasts (Fig. 9a) and pancreatic beta cells (Fig. 9b) within self-folding SU-8/PCL containers. The encapsulated cells were verified to be viable for over a week post-encapsulation [112]. Stoychev et al. demonstrated the temperature-dependent, reversible capture of yeast cells within self-folding PCL/poly(N-isopropylacrylamide (PNIPAM) capsules (Figs. 9 c and d). Azam et al. also demonstrated the encapsulation of viable bacteria within self-folding SU-8/PCL containers (Fig. 9e). For cell encapsulation, the ability to precisely pattern porosity on the container in all three dimensions enhances diffusion and consequently cell viability. Porous self-folding containers can also be used as building blocks for bottom-up assembly [125, 126] of clusters (Fig 9f) or arrays on rigid or flexible (Fig. 9g) substrates for cell and tissue engineering.
Figure 9. Self-folding polymers for cell encapsulation therapy.
a) Bright-field z-plane stack image of stained fibroblast cells encapsulated within a non-porous SU-8/PCL container. b) Fluorescent image of pancreatic beta cells 180 h following encapsulation inside an SU-8/PCL container. The green fluorescence in panels a and b indicates that the cells are alive. c–d) Dark field optical microscopy images of the temperature-dependent, reversible encapsulation of yeast cells inside thermoresponsive self-folding PCL/PNIPAM capsules. [142] – Reproduced by permission of The Royal Society of Chemistry. e) Bright-field and fluorescence images of Syto 9 stained E. coli encapsulated within an SU-8/PCL container, 24 h after encapsulation. a, b and e reprinted with permission from [112], © Springer. f–g) Cell-laden polymeric containers can, in principle, be used as building blocks to construct rigid (f) or flexible arrays (g). f) Array of four SU-8/PCL containers (Image by Anum Azam and Jatinder Randhawa, Gracias Laboratory, JHU). g) Optical image of an ordered 3D microwell array on a flexible surface; 3D microwells enhance encapsulated cell viability. [146] – Reproduced by permission of The Royal Society of Chemistry.
5. Outlook
Since self-folding allows one to transform patterned planar templates into 3D structures, it is an attractive methodology to synthesize precisely structured polymeric containers for drug delivery applications. Important attributes of self-folding polymeric devices for drug delivery are highlighted in Table 1. While the outlook looks promising, a few challenges need to be overcome prior to widespread applicability. One challenge lies in further miniaturization of self-folded polymeric containers to the sub-micron scale. The challenge is rooted in the extension of planar lithographic methods developed for metals and semiconductors to polymers so that they can be deposited and patterned with nanoscale dimensions. Promising methods to deposit ultrathin polymer films such as atom transfer radical polymerization (ATRP) [127, 128] and layer-by-layer (LbL) deposition [129, 130] may be required. Similarly, methods such as particle replication in non-wetting templates (PRINT) [131] or imprint lithography methods [132] will need to be further developed to enable parallel patterning of 2D polymeric templates at the nanoscale. It is likely that a combination of the above techniques will be needed to fabricate nanoscale self-folding polymeric containers. Another challenge involves the sealing and mechanical strength of self-folding polymeric containers. This challenge can be overcome by using self-aligning and locking peripheral hinges or sealants as discussed above (Figs. 4a and 5a). With regards to large scale manufacturability, although many lithographic patterning methods are highly parallel, they are still likely to be more expensive than traditional colloidal synthesis methods such as emulsion polymerization. Consequently, these structures may be more appropriate for high value drug delivery applications. However, it is noteworthy that the mechanism of self-folding itself is a highly parallel process.
Table 1.
Summary of self-folding polymeric systems
| Self-Folding Mechanism |
Polymers | Trigger | Biodegradability | Reversibility | Primary Application |
Geometry | References |
|---|---|---|---|---|---|---|---|
| Differential stress (polymer bilayer) |
Chitosan, PEGMA, PEGDMA, PMAA, PSI, PCL, PS, P4VP, PPy, PNIPAM, PEGDA, PAA, PHEMA |
Water, pH, ionic strength, electric field, temperature |
Yes (some) | Yes | Chemical release, cell encapsulation, tissue engineering |
Tubes, capsules, actuators, gripping devices |
[19], [37], [78], [81], [88], [95], [96], [97] |
| Differential stress (single polymer layer) |
SU-8 | Water, acetone | No | Yes | 3D microfluidic cell culture scaffolds |
Polyhedra, undulatory sheets, tubes, volutes |
[85] |
| Surface tension (folding by evaporation of water or liquefying polymers) |
PDMS, SU-8/PCL | Water evaporation, temperature |
Biodegradable hinges |
No | Chemical release, cell encapsulation |
Polyhedra | [112], [113], [144], [145] |
An attractive but relatively unexplored area is the fabrication of reconfigurable or stimuli responsive polymeric structures that can fold or un-fold at specific locations or in response to specific stimuli (such as pH changes or local light absorption) to enable smart drug delivery [33, 34, 97, 133–134]. The integration of optoelectronic nanoscale elements such as antennas, split ring resonators or plasmonic modules can enable frequency selective imaging, remote communication for on-demand drug delivery release [135–137] or heating for hyperthermia applications [138, 139]. In principle, as with vesosomes, it should also be possible to engineer hierarchically self-folded structures to create containers with multiple compartments. In summary, self-folding methods look very promising for drug delivery applications although further research is needed to transform the current proof-of-concept demonstrations to the clinic.
Acknowledgments
This work was supported by the NSF CBET-1066898 grant and the NIH Director's New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant number 1-DP2-OD004346-01 and DP2-OD004346-01S1. Information about the NIH Roadmap can be found at http://nihroadmap.nih.gov. The authors acknowledge helpful suggestions from Yevgeniy V. Kalinin and thank Kate Laflin for suggestions and help with the illustrations.
Abbreviations
- 2D
two dimensional
- 3D
three dimensional
- MEMS
microelectromechanical Systems
- MCMS
microchemomechanical systems
- LbL
layer by layer
- ATRP
atom transfer radical polymerization
particle replication in non-wetting templates
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- PAA
poly acrylic acid
- PHEMA
poly(hydroxyethyl methacrylate)
- PMAA
poly(methacrylic acid)
- PCL
polycaprolactone
- PDMS
poly(dimethylsiloxane)
- PEGDMA
poly(ethylene glycol dimethacrylate)
- PEGMA
poly(ethylene glycol methacrylate)
- PEGDA
poly(ethylene glycol diacrylate)
- PMMA
poly(methylmethacrylate)
- PNIPAM
poly(N-isopropylacrylamide)
- PSI
polysuccinimide
- PS
polystyrene
- P4VP
poly(4-vinylpyridine)
- PVA
polyvinyl alcohol
- PPy
polypyrrole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 2.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 3.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 4.Slowing, Trewyn BG, Giri S, Lin VSY. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Func. Mater. 2007;17:1225–1236. [Google Scholar]
- 5.Liu Z, Robinson JT, Tabakman SM, Yang K, Dai HJ. Carbon materials for drug delivery & cancer therapy. Mater. Today. 2011;14:316–323. [Google Scholar]
- 6.Lai SK, Suk JS, Pace A, Wang YY, Yang M, Mert O, Chen J, Kim J, Hanes J. Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials. 2011;32:6285–6290. doi: 10.1016/j.biomaterials.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langer R. New methods of drug delivery. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 8.Gregoriadis G. Liposome Technology, Volume II: Entrapment of Drugs and Other Materials. Second ed. Boca Raton: CRC-Press; 1992. [Google Scholar]
- 9.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 10.Boyer C, Zasadzinski JA. Multiple lipid compartments slow vesicle contents release in lipases and serum. ACS Nano. 2007;1:176–182. doi: 10.1021/nn7002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Jamal WT, Kostarelos K. Liposomes: From a Clinically Established Drug Delivery System to a Nanoparticle Platform for Theranostic Nanomedicine. Acc. Chem. Res. 2011;44:1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 12.Stadler B, Price AD, Chandrawati R, Hosta-Rigau L, Zelikin AN, Caruso F. Polymer hydrogel capsules: en route toward synthetic cellular systems. Nanoscale. 2009;1:68–73. doi: 10.1039/b9nr00143c. [DOI] [PubMed] [Google Scholar]
- 13.Schlaad H, You L, Sigel R, Smarsly B, Heydenreich M, Mantion A, Masic A. Glycopolymer vesicles with an asymmetric membrane. Chem. Commun. 2009:1478–1480. doi: 10.1039/b820887e. [DOI] [PubMed] [Google Scholar]
- 14.van Dongen SFM, de Hoog HPM, Peters R, Nallani M, Nolte RJM, van Hest JCM. Biohybrid Polymer Capsules. Chem. Rev. 2009;109:6212–6274. doi: 10.1021/cr900072y. [DOI] [PubMed] [Google Scholar]
- 15.Esser-Kahn AP, Odom SA, Sottos NR, White SR, Moore JS. Triggered Release from Polymer Capsules. Macromolecules. 2011;44:5539–5553. [Google Scholar]
- 16.Stadler B, Price AD, Zelikin AN. A Critical Look at Multilayered Polymer Capsules in Biomedicine: Drug Carriers, Artificial Organelles, and Cell Mimics. Adv. Func. Mater. 2011;21:14–28. [Google Scholar]
- 17.Santini JT, Richards AC, Scheidt R, Cima MJ, Langer R. Microchips as controlled drug-delivery devices. Angew. Chem. Int. Ed. 2000;39:2397–2407. [PubMed] [Google Scholar]
- 18.Ziaie B, Baldi A, Lei M, Gu Y, Siegel RA. Hard and soft micromachining for BioMEMS: review of techniques and examples of applications in microfluidics and drug delivery. Adv. Drug Deliv. Rev. 2004;56:145–172. doi: 10.1016/j.addr.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Guan J, Ferrell N, James Lee L, Hansford DJ. Fabrication of polymeric microparticles for drug delivery by soft lithography. Biomaterials. 2006;27:4034–4041. doi: 10.1016/j.biomaterials.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Randall CL, Leong TG, Bassik N, Gracias DH. 3D lithographically fabricated nanoliter containers for drug delivery. Adv. Drug Deliv. Rev. 2007;59:1547–1561. doi: 10.1016/j.addr.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Ainslie KM, Desai TA. Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab Chip. 2008;8:1864–1878. doi: 10.1039/b806446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, Murthy N, Compans RW, Skountzou I, Prausnitz MR. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 25.Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MN. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release. 2007;119:77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 27.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat. Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klose D, Siepmann F, Elkharraz K, Krenzlin S, Siepmann J. How porosity and size affect the drug release mechanisms from PLGA-based microparticles. Int. J. Pharm. 2006;314:198–206. doi: 10.1016/j.ijpharm.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Luciani A, Coccoli V, Orsi S, Ambrosio L, Netti PA. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials. 2008;29:4800–4807. doi: 10.1016/j.biomaterials.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D, Li C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30:1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamad-Schifferli K, Schwartz JJ, Santos AT, Zhang S, Jacobson JM. Remote electronic control of DNA hybridization through inductive coupling to an attached metal nanocrystal antenna. Nature. 2002;415:152–155. doi: 10.1038/415152a. [DOI] [PubMed] [Google Scholar]
- 32.Anglin EJ, Cheng L, Freeman WR, Sailor MJ. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 2008;60:1266–1277. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassik N, Brafman A, Zarafshar AM, Jamal M, Luvsanjav D, Selaru FM, Gracias DH. Enzymatically triggered actuation of miniaturized tools. J. Am. Chem. Soc. 2010;132:16314–16317. doi: 10.1021/ja106218s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leong TG, Randall CL, Benson BR, Bassik N, Stern GM, Gracias DH. Tetherless thermobiochemically actuated microgrippers. Proc. Natl. Acad. Sci. USA. 2009;106:703–708. doi: 10.1073/pnas.0807698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He HY, Guan JJ, Lee JL. An oral delivery device based on self-folding hydrogels. J. Control. Release. 2006;110:339–346. doi: 10.1016/j.jconrel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TM, De Vos P, Hortelano G, Hunkeler D, Lacik I, Shapiro AM, Pedraz JL. Cell encapsulation: promise and progress. Nat. Med. 2003;9:104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- 39.Orive G, Hernandez RM, Rodriguez Gascon A, Calafiore R, Chang TM, de Vos P, Hortelano G, Hunkeler D, Lacik I, Pedraz JL. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 2004;22:87–92. doi: 10.1016/j.tibtech.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez RM, Orive G, Murua A, Pedraz JL. Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 2010;62:711–730. doi: 10.1016/j.addr.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Choi DH, Park CH, Kim IH, Chun HJ, Park K, Han DK. Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. J. Control. Release. 2010;147:193–201. doi: 10.1016/j.jconrel.2010.07.103. [DOI] [PubMed] [Google Scholar]
- 42.Teh SY, Lin R, Hung LH, Lee AP. Droplet microfluidics. Lab Chip. 2008;8:198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- 43.George MC, Braun PV. Multicompartmental Materials by Electrohydrodynamic Cojetting. Angew. Chem. Int. Ed. 2009;48:8606–8609. doi: 10.1002/anie.200904089. [DOI] [PubMed] [Google Scholar]
- 44.Lu Y, Chen SC. Micro and nano-fabrication of biodegradable polymers for drug delivery. Adv. Drug Deliv. Rev. 2004;56:1621–1633. doi: 10.1016/j.addr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Leong TG, Zarafshar AM, Gracias DH. Three-dimensional fabrication at small size scales. Small. 2010;6:792–806. doi: 10.1002/smll.200901704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahadevan L, Rica S. Self-organized origami. Science. 2005;307:1740. doi: 10.1126/science.1105169. [DOI] [PubMed] [Google Scholar]
- 47.Yin J, Cao Z, Li C, Sheinman I, Chen X. Stress-driven buckling patterns in spheroidal core/shell structures. Proc. Natl. Acad. Sci. USA. 2008;105:19132–19135. doi: 10.1073/pnas.0810443105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jamal M, Bassik N, Cho JH, Randall CL, Gracias DH. Directed growth of fibroblasts into three dimensional micropatterned geometries via self-assembling scaffolds. Biomaterials. 2010;31:1683–1690. doi: 10.1016/j.biomaterials.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho JH, Gracias DH. Self-assembly of lithographically patterned nanoparticles. Nano Lett. 2009;9:4049–4052. doi: 10.1021/nl9022176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pawar AB, Kretzschmar I. Fabrication, Assembly, and Application of Patchy Particles. Macromol. Rapid Commun. 2010;31:150–168. doi: 10.1002/marc.200900614. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi E, Blaak R, Likos CN. Patchy colloids: state of the art and perspectives. Phys. Chem. Chem. Phys. 2011;13:6397–6410. doi: 10.1039/c0cp02296a. [DOI] [PubMed] [Google Scholar]
- 52.Cho JH, Keung MD, Verellen N, Lagae L, Moshchalkov VV, Van Dorpe P, Gracias DH. Nanoscale Origami for 3D Optics. Small. 2011;7:1943–1948. doi: 10.1002/smll.201100568. [DOI] [PubMed] [Google Scholar]
- 53.Prinz VY, Seleznev VA, Gutakovsky AK, Chehovskiy AV, Preobrazhenskii VV, Putyato MA, Gavrilova TA. Free-standing and overgrown InGaAs/GaAs nanotubes, nanohelices and their arrays. Physica E. 2000;6:828–831. [Google Scholar]
- 54.Cho JH, James T, Gracias DH. Curving nanostructures using extrinsic stress. Adv. Mater. 2010;22:2320–2324. doi: 10.1002/adma.200904410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt OG, Eberl K. Nanotechnology. Thin solid films roll up into nanotubes. Nature. 2001;410:168. doi: 10.1038/35065525. [DOI] [PubMed] [Google Scholar]
- 56.Golod SV, Prinz VY, Wagli P, Zhang L, Kirfel O, Deckhardt E, Glaus F, David C, Grutzmacher D. Freestanding SiGe/Si/Cr and SiGe/Si/SixNy/Cr microtubes. Appl. Phys. Lett. 2004;84:3391–3393. [Google Scholar]
- 57.Songmuang R, Rastelli A, Mendach S, Schmidt OG. SiOx/Si radial superlattices and microtube optical ring resonators. Appl. Phys. Lett. 2007;90:091905. [Google Scholar]
- 58.Guo X, Li H, Ahn BY, Duoss EB, Hsia KJ, Lewis JA, Nuzzo RG. Two- and three-dimensional folding of thin film single-crystalline silicon for photovoltaic power applications. Proc. Natl. Acad. Sci. USA. 2009;106:20149–20154. doi: 10.1073/pnas.0907390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bassik N, Stern GM, Gracias DH. Microassembly based on hands free origami with bidirectional curvature. Appl. Phys. Lett. 2009;95:091901. doi: 10.1063/1.3212896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lasic DD. The mechanism of vesicle formation. Biochem. J. 1988;256:1–11. doi: 10.1042/bj2560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kisak ET, Coldren B, Evans CA, Boyer C, Zasadzinski JA. The vesosome-- a multicompartment drug delivery vehicle. Curr. Med. Chem. 2004;11:199–219. doi: 10.2174/0929867043456197. [DOI] [PubMed] [Google Scholar]
- 62.Lendlein A, Kelch S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002;41:2034–2057. [PubMed] [Google Scholar]
- 63.Ratna D, Karger-Kocsis J. Recent advances in shape memory polymers and composites: a review. J. Mater. Sci. 2008;43:254–269. [Google Scholar]
- 64.Serrano MC, Carbajal L, Ameer GA. Novel biodegradable shape-memory elastomers with drug-releasing capabilities. Adv. Mater. 2011;23:2211–2215. doi: 10.1002/adma.201004566. [DOI] [PubMed] [Google Scholar]
- 65.Wischke C, Neffe AT, Steuer S, Lendlein A. Evaluation of a degradable shape-memory polymer network as matrix for controlled drug release. J. Control. Release. 2009;138:243–250. doi: 10.1016/j.jconrel.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 66.Lendlein A, Langer R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science. 2002;296:1673–1676. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- 67.Baer GM, Small W, IV, Wilson TS, Benett WJ, Matthews DL, Hartman J, Maitland DJ. Fabrication and in vitro deployment of a laser-activated shape memory polymer vascular stent. Biomed. Eng. Online. 2007;6:43. doi: 10.1186/1475-925X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Behl M, Razzaq MY, Lendlein A. Multifunctional Shape-Memory Polymers. Adv. Mater. 2010;22:3388–3410. doi: 10.1002/adma.200904447. [DOI] [PubMed] [Google Scholar]
- 69.Sharp AA, Panchawagh HV, Ortega A, Artale R, Richardson-Burns S, Finch DS, Gall K, Mahajan RL, Restrepo D. Toward a self-deploying shape memory polymer neuronal electrode. J. Neural Eng. 2006;3:L23–L30. doi: 10.1088/1741-2560/3/4/L02. [DOI] [PubMed] [Google Scholar]
- 70.Dietz H, Douglas SM, Shih WM. Folding DNA into twisted and curved nanoscale shapes. Science. 2009;325:725–730. doi: 10.1126/science.1174251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Y, Ye T, Su M, Zhang C, Ribbe AE, Jiang W, Mao C. Hierarchical self-assembly of DNA into symmetric supramolecular polyhedra. Nature. 2008;452:198–201. doi: 10.1038/nature06597. [DOI] [PubMed] [Google Scholar]
- 72.Rothemund PW. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440:297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 73.Seeman NC. DNA nanotechnology: novel DNA constructions. Annu. Rev. Biophys. Biomol. Struct. 1998;27:225–248. doi: 10.1146/annurev.biophys.27.1.225. [DOI] [PubMed] [Google Scholar]
- 74.Gellman SH. Foldamers: A manifesto. Acc. Chem. Res. 1998;31:173–180. [Google Scholar]
- 75.Guichard G, Huc I. Synthetic foldamers. Chem. Commun. 2011;47:5933–5941. doi: 10.1039/c1cc11137j. [DOI] [PubMed] [Google Scholar]
- 76.Azam A, Leong TG, Zarafshar AM, Gracias DH. Compactness determines the success of cube and octahedron self-assembly. PLoS One. 2009;4:e4451. doi: 10.1371/journal.pone.0004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pandey S, Ewing M, Kunas A, Nguyen N, Gracias DH, Menon G. Algorithmic design of self-folding polyhedra. Proc. Natl. Acad. Sci. USA. 2011;108:19885–19890. doi: 10.1073/pnas.1110857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bassik N, Abebe BT, Laflin KE, Gracias DH. Photolithographically patterned smart hydrogel based bilayer actuators. Polymer. 2010;51:6093–6098. [Google Scholar]
- 79.Jeong KU, Jang JH, Kim DY, Nah C, Lee JH, Lee MH, Sun HJ, Wang CL, Cheng SZD, Thomas EL. Three-dimensional actuators transformed from the programmed two-dimensional structures via bending, twisting and folding mechanisms. J. Mater. Chem. 2011;21:6824–6830. [Google Scholar]
- 80.Randhawa JS, Laflin KE, Seelam N, Gracias DH. Microchemomechanical Systems. Adv. Func. Mater. 2011;21:2395–2410. [Google Scholar]
- 81.Smela E, Inganas O, Lundstrom I. Controlled folding of micrometer-size structures. Science. 1995;268:1735–1738. doi: 10.1126/science.268.5218.1735. [DOI] [PubMed] [Google Scholar]
- 82.Clark TD, Boncheva M, German JM, Weck M, Whitesides GM. Design of three-dimensional, millimeter-scale models for molecular folding. J. Am. Chem. Soc. 2002;124:18–19. doi: 10.1021/ja0120633. [DOI] [PubMed] [Google Scholar]
- 83.Ilievski F, Mazzeo AD, Shepherd RF, Chen X, Whitesides GM. Soft robotics for chemists. Angew. Chem. Int. Ed. 2011;50:1890–1895. doi: 10.1002/anie.201006464. [DOI] [PubMed] [Google Scholar]
- 84.Singamaneni S, McConney ME, Tsukruk VV. Spontaneous Self-Folding in Confined Ultrathin Polymer Gels. Adv. Mater. 2010;22:1263–1268. doi: 10.1002/adma.200903052. [DOI] [PubMed] [Google Scholar]
- 85.Jamal M, Zarafshar AM, Gracias DH. Differentially photo-crosslinked polymers enable self-assembling microfluidics. Nat. Commun. 2011;2:527. doi: 10.1038/ncomms1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan B, Jin Y, Sun Y, Wang D, Sun J, Wang Z, Zhang W, Jiang X. A Strategy for Depositing Different Types of Cells in Three Dimensions to Mimic Tubular Structures in Tissues. Adv. Mater. 2012;24:890–896. doi: 10.1002/adma.201104589. [DOI] [PubMed] [Google Scholar]
- 87.Linder V, Gates BD, Ryan D, Parviz BA, Whitesides GM. Water-soluble sacrificial layers for surface micromachining. Small. 2005;1:730–736. doi: 10.1002/smll.200400159. [DOI] [PubMed] [Google Scholar]
- 88.Luchnikov V, Sydorenko O, Stamm M. Self-rolled polymer and composite polymer/metal micro- and nanotubes with patterned inner walls. Adv. Mater. 2005;17:1177–1182. [Google Scholar]
- 89.Carpi F, Smela E. Biomedical Applications of Electroactive Polymer Actuators. Hoboken: Wiley; 2009. [Google Scholar]
- 90.Lu YW, Kim CJ. Microhand for biological applications. Appl. Phys. Lett. 2006;89:164101. [Google Scholar]
- 91.Boncheva M, Andreev SA, Mahadevan L, Winkleman A, Reichman DR, Prentiss MG, Whitesides S, Whitesides GM. Magnetic self-assembly of three-dimensional surfaces from planar sheets. Proc. Natl. Acad. Sci. USA. 2005;102:3924–3929. doi: 10.1073/pnas.0500807102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelby TS, Wang M, Huck WTS. Controlled Folding of 2D Au-Polymer Brush Composites into 3D Microstructures. Adv. Func. Mater. 2011;21:652–657. [Google Scholar]
- 93.Simpson B, Nunnery G, Tannenbaum R, Kalaitzidou K. Capture/release ability of thermo-responsive polymer particles. J. Mater. Chem. 2010;20:3496–3501. [Google Scholar]
- 94.Stoney GG. The tension of metallic films deposited by electrolysis. Proc. Roy. Soc. Lond. Ser. A. 1909;82:172–175. [Google Scholar]
- 95.Guan JJ, He HY, Hansford DJ, Lee LJ. Self-folding of three-dimensional hydrogel microstructures. J. Phys. Chem. B. 2005;109:23134–23137. doi: 10.1021/jp054341g. [DOI] [PubMed] [Google Scholar]
- 96.Zakharchenko S, Sperling E, Ionov L. Fully biodegradable self-rolled polymer tubes: a candidate for tissue engineering scaffolds. Biomacromol. 2011;12:2211–2215. doi: 10.1021/bm2002945. [DOI] [PubMed] [Google Scholar]
- 97.Shim TS, Kim SH, Heo CJ, Jeon HC, Yang SM. Controlled origami folding of hydrogel bilayers with sustained reversibility for robust microcarriers. Angew. Chem. Int. Ed. 2012;51:1420–1423. doi: 10.1002/anie.201106723. [DOI] [PubMed] [Google Scholar]
- 98.Kumar K, Luchnikov V, Nandan B, Zakharchenko S, Ionov L. Polymer tubes by rolling of polymer bilayers. Mater. Res. Soc. Symp. Proc. Vol. 1272 1272-OO01-09. [Google Scholar]
- 99.Nikishkov GP. Curvature estimation for multilayer hinged structures with initial strains. J. Appl. Phys. 2003;94:5333–5336. [Google Scholar]
- 100.Timoshenko S. Analysis of bi-metal thermostats. J. Opt. Soc. Am. Rev. Sci. Instr. 1925;11:233–255. [Google Scholar]
- 101.Miller LF. Controlled collapse reflow chip joining. IBM J. Res. Dev. 1969;13:239–250. [Google Scholar]
- 102.Clark TD, Tien J, Duffy DC, Paul KE, Whitesides GM. Self-assembly of 10-micromsized objects into ordered three-dimensional arrays. J. Am. Chem. Soc. 2001;123:7677–7682. doi: 10.1021/ja010634l. [DOI] [PubMed] [Google Scholar]
- 103.Wale MJ, Edge C, Randle FA, Pedder DJ. Proc. 15th Eur. Conf. Optical Comm; 1989. pp. 368–371. [Google Scholar]
- 104.Harsh KF, Bright VM, Lee YC. Solder self-assembly for three-dimensional microelectromechanical systems. Sens. Actuat. A Phys. 1999;77:237–244. [Google Scholar]
- 105.Syms RRA, Yeatman EM. Self-assembly of 3-dimensional microstructures using rotation by surface-tension forces. Electron. Lett. 1993;29:662–664. [Google Scholar]
- 106.Gracias DH, Kavthekar V, Love JC, Paul KE, Whitesides GM. Fabrication of micrometer-scale, patterned polyhedra by self-assembly. Adv. Mater. 2002;14:235–238. [Google Scholar]
- 107.Gimi B, Leong T, Gu Z, Yang M, Artemov D, Bhujwalla ZM, Gracias DH. Self-assembled three dimensional radio frequency (RF) shielded containers for cell encapsulation. Biomed. Microdev. 2005;7:341–345. doi: 10.1007/s10544-005-6076-9. [DOI] [PubMed] [Google Scholar]
- 108.Leong TG, Lester PA, Koh TL, Call EK, Gracias DH. Surface tension-driven self-folding polyhedra. Langmuir. 2007;23:8747–8751. doi: 10.1021/la700913m. [DOI] [PubMed] [Google Scholar]
- 109.Filipiak DJ, Azam A, Leong TG, Gracias DH. Hierarchical self-assembly of complex polyhedral microcontainers. J. Micromech. Microeng. 2009;19:075012. doi: 10.1088/0960-1317/19/7/075012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andersson S. The structure of virus capsids. Z. Anorg. Allg. Chem. 2008;634:2161–2170. [Google Scholar]
- 111.Caspar DLD, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 112.Azam A, Laflin KE, Jamal M, Fernandes R, Gracias DH. Self-folding micropatterned polymeric containers. Biomed. Microdev. 2011;13:51–58. doi: 10.1007/s10544-010-9470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Py C, Reverdy P, Doppler L, Bico J, Roman B, Baroud CN. Capillary Origami: Spontaneous wrapping of a droplet with an elastic sheet. Phys. Rev. Lett. 2007;98:156103. doi: 10.1103/PhysRevLett.98.156103. [DOI] [PubMed] [Google Scholar]
- 114.Patra N, Wang B, Kral P. Nanodroplet activated and guided folding of graphene nanostructures. Nano Lett. 2009;9:3766–3771. doi: 10.1021/nl9019616. [DOI] [PubMed] [Google Scholar]
- 115.Ionov L. Soft microorigami: self-folding polymer films. Soft Matter. 2011;7:6786–6791. [Google Scholar]
- 116.Randall CL, Gultepe E, Gracias DH. Self-folding devices and materials for biomedical applications. Trends Biotechnol. 2012;30:138–146. doi: 10.1016/j.tibtech.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luchnikov V, Kumar K, Stamm M. Toroidal hollow-core microcavities produced by self-rolling of strained polymer bilayer films. J. Micromech. Microeng. 2008;18:035041. [Google Scholar]
- 118.Kalinin YV, Randhawa JS, Gracias DH. Three-Dimensional Chemical Patterns for Cellular Self-Organization. Angew. Chem. Int. Ed. 2011;50:2549–2553. doi: 10.1002/anie.201007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Randall CL, Kalinin YV, Jamal M, Shah A, Gracias DH. Self-folding immunoprotective cell encapsulation devices. Nanomed. 2011;7:686–689. doi: 10.1016/j.nano.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 120.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 122.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Engineering tumors with 3D scaffolds. Nat. Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 125.Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 126.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kato M, Kamigaito M, Sawamoto M, Higashimura T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine)ruthenium(II)/methylaluminum bis(2,6,-di-tert-butylphenoxide) initiating system : possibility of living radical polmerization. Macromolecules. 1995;28:1721–1723. [Google Scholar]
- 128.Wang JS, Matyjaszewski K. Controlled/"living" radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995;117:5614–5615. [Google Scholar]
- 129.Decher G, Hong JD, Schmitt J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces Thin Solid Films. 1992;210:831–835. [Google Scholar]
- 130.Poon Z, Lee JB, Morton SW, Hammond PT. Controlling in Vivo Stability and Biodistribution in Electrostatically Assembled Nanoparticles for Systemic Delivery. Nano Lett. 2011;11:2096–2103. doi: 10.1021/nl200636r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 132.Chou SY, Krauss PR, Renstrom PJ. Imprint of sub-25 nm vias and trenches in polymers Appl. Phys. Lett. 1995;67:3114–3116. [Google Scholar]
- 133.Lee ES, Kim D, Youn YS, Oh KT, Bae YH. A virus-mimetic nanogel vehicle. Angew. Chem. Int. Ed. 2008;47:2418–2421. doi: 10.1002/anie.200704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y, Boyles JK, Genzer J, Dickey MD. Self-folding of polymer sheets using local light absorption. Soft Matter. 2012;8:1764–1769. [Google Scholar]
- 135.Prescott JH, Lipka S, Baldwin S, Sheppard NF, Jr, Maloney JM, Coppeta J, Yomtov B, Staples MA, Santini JT., Jr Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat. Biotechnol. 2006;24:437–438. doi: 10.1038/nbt1199. [DOI] [PubMed] [Google Scholar]
- 136.Ye H, Randall CL, Leong TG, Slanac DA, Call EK, Gracias DH. Remote radiofrequency controlled nanoliter chemistry and chemical delivery on substrates. Angew. Chem. Int. Ed. 2007;46:4991–4994. doi: 10.1002/anie.200604414. [DOI] [PubMed] [Google Scholar]
- 137.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li W, Cai X, Kim C, Sun G, Zhang Y, Deng R, Yang M, Chen J, Achilefu S, Wang LV, Xia Y. Gold nanocages covered with thermally-responsive polymers for controlled release by high-intensity focused ultrasound. Nanoscale. 2011;3:1724–1730. doi: 10.1039/c0nr00932f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 140.Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R, Farokhzad OC. Self-assembled lipid--polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2:1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu X, Jin X, Ma PX. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater. 2011;10:398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stoychev G, Puretskiy N, Ionov L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter. 2011;7:3277–3279. [Google Scholar]
- 143.de Langre E, Baroud CN, Reverdy P. Energy criteria for elasto-capillary wrapping. J. Fluid. Struct. 2010;26:205–217. [Google Scholar]
- 144.Py C, Reverdy P, Doppler L, Bico J, Roman B, Baroud CN. Capillarity induced folding of elastic sheets. Eur. Phys. J. Sp. Top. 2009;166:67–71. [Google Scholar]
- 145.Cho JH, Azam A, Gracias DH. Three dimensional nanofabrication using surface forces. Langmuir. 2010;26:16534–16539. doi: 10.1021/la1013889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Randall CL, Kalinin YV, Jamal M, Manohar T, Gracias DH. Three-dimensional microwell arrays for cell culture. Lab Chip. 2010;11:127–131. doi: 10.1039/c0lc00368a. [DOI] [PubMed] [Google Scholar]