Abstract
Context:
Combinations of testosterone (T) and nestorone (NES; a nonandrogenic progestin) transdermal gels may suppress spermatogenesis and prove appealing to men for contraception.
Objective:
The objective of the study was to determine the effectiveness of T gel alone or combined with NES gel in suppressing spermatogenesis.
Design and Setting:
This was a randomized, double-blind, comparator clinical trial conducted at two academic medical centers.
Participants:
Ninety-nine healthy male volunteers participated in the study.
Interventions:
Volunteers were randomized to one of three treatment groups applying daily transdermal gels (group 1: T gel 10 g + NES 0 mg/placebo gel; group 2: T gel 10 g + NES gel 8 mg; group 3: T gel 10 g + NES gel 12 mg).
Main Outcome Variable:
The main outcome variable of the study was the percentage of men whose sperm concentration was suppressed to 1 million/ml or less by 20–24 wk of treatment.
Results:
Efficacy data analyses were performed on 56 subjects who adhered to the protocol and completed at least 20 wk of treatment. The percentage of men whose sperm concentration was 1 million/ml or less was significantly higher for T + NES 8 mg (89%, P < 0.0001) and T + NES 12 mg (88%, P = 0.0002) compared with T + NES 0 mg group (23%). The median serum total and free T concentrations in all groups were maintained within the adult male range throughout the treatment period. Adverse effects were minimal in all groups.
Conclusion:
A combination of daily NES + T gels suppressed sperm concentration to 1 million/ml or less in 88.5% of men, with minimal adverse effects, and may be further studied as a male transdermal hormonal contraceptive.
Nearly 40% of pregnancies worldwide are unintended, which may result in undesirable outcomes including maternal mortality and morbidity, societal burden, and child abuse. Although many female contraceptive methods are available, discontinuation rates are high and hormonal methods may be contraindicated for many women (1). Male methods are limited to withdrawal, condom, and vasectomy. Condoms suffer from inconsistent use, whereas vasectomy requires surgery and is not reliably reversible. A majority of men surveyed internationally are interested and willing to use male contraceptive methods (2, 3) and female partners support male involvement (4). Unfortunately, no safe, effective, reversible method is available to men who are interested in sharing the responsibility of family planning.
Several studies have validated the efficacy, safety, and reversibility of male hormonal contraception (5–9). Development of an oral method is limited by the lack of an oral form of testosterone (T). A recent 2-yr study using monthly T undecanoate injections in more than 1000 healthy Chinese men showed efficacy rates similar to female hormonal contraceptives (5). However, monthly injections are inconvenient and contraceptive regimens using T alone are not as effective in non-Asian men (8, 10, 11). Addition of progestins to exogenous T enhances the rate and extent of suppression of spermatogenesis in men of all races with few short-term side effects (11–24). Male hormonal contraceptive strategies to date have relied on combining injections, patches, or pellets of T (or its esters) with injectable, oral, or implants of progestins in two delivery forms. A single method of provider-independent administration could streamline application, perhaps improving acceptability and compliance among users.
To provide steady-state delivery of both T and a progestin and avoid high peaks and low troughs observed with pills and injectables and to develop a user-friendly male contraceptive method, we evaluated the efficacy of transdermal delivery of both androgen and progestin in suppressing spermatogenesis. Progestins used in prior male contraceptive clinical trials, including 19-nortestosterone derivatives (levonorgestrel, etonogestrel, and norethisterone enanthate) or pregnane-derived medroxyprogesterone acetate, show significant androgenic activity in vitro and in vivo (25). Nestorone (NES; 16-methylen-17α-acetoxy-19-norpregn-4-ene-3, 20-dione) is the first 19-norprogesterone-derived progestin to be used for male contraception. NES has no androgenic, estrogenic, or glucocorticoid activity (25). The premise for using NES in male contraception is that it may inhibit gonadotropins by mechanisms other than via androgen receptors and/or act directly at the testis to inhibit local T production with less adverse effects than with androgenic progestins. A pilot study using T and NES transdermal gels in healthy men for 20 d showed effective gonadotropin suppression without any significant adverse events (26), prompting us to evaluate this gel-gel combination for potential use as a male hormonal contraceptive.
Subjects and Methods
Subjects
Healthy male volunteers between the ages of 18 and 50 yr were enrolled in two academic health centers, the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (LA BioMed), Los Angeles, CA, and the Center for Research in Reproduction and Contraception at the University of Washington, Seattle, WA. All subjects underwent screening including an evaluation of general and reproductive history; a detailed physical examination; and urine, blood, and semen analyses. Subjects were eligible if they had no chronic illnesses, a normal physical examination, normal baseline blood chemistries, no evidence of lower urinary tract symptoms by International Prostate Symptom Score (IPSS), and two consecutive semen analyses demonstrating sperm concentrations of 15 million/ml or more. Men who received study treatment but did not complete the study were not replaced.
Study medication
Testosterone gel (Testogel) was manufactured by Besins Healthcare S.A. (Brussels, Belgium) and supplied by GOOGLIFE Healthcare (Den Haag, The Netherlands). The hydroalcoholic gel containing 1% T (10 mg T per gram of gel) was packaged in 5-g sachets. Two sachets of testosterone gel (10 g gel) were applied to the skin over the upper arms delivering approximately 10 mg T daily.
NES and placebo gels were produced by Antares Pharma (Basel, Switzerland) based on a formulation developed by the Population Council (New York, NY). NES or placebo gel was supplied in a metered-dose pump calibrated to deliver 4 ml of gel containing 0, 8 (2 mg/ml gel), or 12 mg (3 mg/ml gel) of NES providing 0, 800, or 1200 μg/d of NES to the body because approximately 10% of the dose was absorbed. Subjects applied NES or placebo gel on the abdomen.
Study design
The study was a randomized, double-blind comparator trial. After the screening, 99 men were enrolled and randomized to three groups: 1) T 10 g + placebo gel (T+NES 0) (n = 32); 2) T 10 g + NES 8 mg (T+NES 8) (n = 33); or 3) T 10 g + NES 12 mg (T+NES 12) (n = 34). Gels were applied daily for 24 wk. After the treatment period, subjects were followed up for 12 wk or until two consecutive semen samples had sperm concentrations of 15 million/ml or more. Throughout the study, the subject and his partner were required to use a reliable method of contraception. Subjects were warned to shower or wear protective clothing to prevent skin-to-skin transfer of gel when in close contact with another person.
Serum concentrations of T, NES, LH, and FSH and semen samples were obtained at baseline and wk 4, 8, 16, 20, and 24 during treatment and at wk 26, 28, and 36 during recovery. Serum free T and SHBG were measured at baseline and wk 8, 16, 24, and 36. Additionally, serum T, LH, FSH, and NES were measured 24, 48, and 72 h (±1 h) after the end of treatment (wk 24), after stopping gel application to monitor remaining serum concentrations of NES and short-term recovery of serum LH and FSH. All serum samples were stored at −20 C until study completion. Safety laboratories included complete blood count; clinical chemistry (fasting glucose, urea nitrogen, creatinine, sodium, potassium, bicarbonate, chloride, and albumin); liver function tests (alanine aminotransferase, aspartate aminotransferase, γ-glutamine transferase, alkaline phosphatase, and bilirubin); a lipid panel [triglycerides, total and high-density lipoprotein (HDL), low-density lipoprotein (LDL) cholesterol]; and prostate-specific antigen. A history was taken, and a physical examination was performed, blood was drawn for safety laboratory tests, and the IPSS questionnaire was recorded at screening and wk 12, 24, and 36. Hemoglobin and hematocrit were assessed every 4 wk during the treatment phase. Adverse events and concomitant medications were reviewed at each visit. The Psychosexual Daily Questionnaire (27) was dispensed 7 d before the baseline visit and at wk 12, 24, and 36, and the responses were collected during those visits.
An external independent data safety monitoring board reviewed adverse events and safety considerations periodically. The institutional review boards of each institution approved the study protocol before study initiation, and all participants signed an informed consent form before any study procedure. This trial was registered (www.clinicaltrials.gov, National Clinical Trial no. 00891228 and 00229593).
Semen analyses, hormone assays, and safety laboratory tests
Semen volume, sperm concentration, motility, and morphology assessments were performed by trained technologists using standardized techniques according to the World Health Organization Laboratory Manual for the Examination of Human Semen (28). Sperm motility and morphology was assessed only in samples with more than 5000 spermatozoa/ml.
Safety laboratory tests were analyzed by clinical laboratories at each study site. NES concentrations were measured at the Population Council by a specific RIA method previously described with a lower limit of quantification (LLOQ) of 27 pmol/liter (10 pg/ml) (29). Intra- and interassay coefficients of variation (CV) varied from 3.4–13.9 and 9.3–13.5%, respectively. All other hormones were measured at the Endocrine and Metabolic Research Laboratory at LA BioMed using validated assays (see Supplemental Text File, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Serum T was measured by liquid chromatography tandem mass spectrometry as previously described (30). The within- and between-run CV were 5% or less. The LLOQ for T was 2 ng/dl (0.069 nmol/liter). The reference range for T in adult males is 265–972 ng/dl (9.2–33.7 nmol/liter). Free T was measured by equilibrium dialysis as described previously (31) with an adult male reference range of 4.7–18.0 ng/dl (0.16–0.62 nmol/liter). SHBG was measured by Delfia fluoroimmunometric assays (Wallace, Gaithersburg, MD). The intra- and interassay CV were 2.3 and 6.1%, respectively. The normal adult male range is 10.8–46.6 nmol/liter. Serum LH and FSH concentrations were measured by sensitive and highly specific fluoroimmunometric assays with reagents from Delfia (PerkinElmer Life and Analytical Sciences, Wallac Oy, Turku, Finland). The LLOQ for both assays is 0.2 IU/liter, the intra- and interassay CV were less than 6% and less than 10% for both LH and FSH (32).
Statistical analysis
Pharmacists used permuted block randomization schedules nested within each of the two study centers to assign treatments to subjects. The primary aim of the study is the estimation of the percentage of men in the efficacy eligible (per protocol) population in each treatment group who have sperm concentrations of 1 million/ml or less by 20–24 wk (33). The study size was determined by the precision of these estimates. We calculated the expected lower one-sided 95% confidence limits for percentages of 50, 80, and 90% and varying number of men in a group. From our prior study with T+NES gels (26), it is expected that at least 80% of men will reach sperm concentrations of 1 million/ml or less with T+NES gel, whereas in studies with transdermal T patches (34–36), no more than 25% of subjects reached this level of suppression. The gain in precision obtained from 30 compared with 20 men per group is about 5%. The study is thus designed to provide at least 30 men in each group in the efficacy eligible population. Secondary analyses include the percentage change from baseline to wk 24 for the following: sperm concentration; percentage motile sperm; and percentage spermatozoa with normal morphology, serum FSH, LH, T, free T, SHBG, and NES concentrations.
Efficacy analyses included efficacy eligible subjects who applied at least 80% of study medication (determined by counting T gel sachets returned and change in weight of NES gel containers), provided semen and blood samples within wk 20–24, and had detectable NES levels if assigned to the NES 8- or 12-mg groups. Safety and side effect analyses included all randomized subjects with available data at each time point. No imputation for incomplete data was performed. Due to nonnormal distributions, we used nonparametric Kruskal-Wallis tests to compare continuous outcomes and Fisher's exact tests to compare percentages (except that the Cochran-Mantel-Haenszel test was used for race and ethnicity), with medians (and their 95% confidence intervals for outcome effects and 25th to75th percentiles for baseline characteristics and for graphic presentation) or percentages used for summarization. Power transformation (37) was performed and optimized as fourth root for sperm concentration and fifth root total spermatozoa/ejaculate (see Fig. 3) using the Shapiro-Wilks statistics.
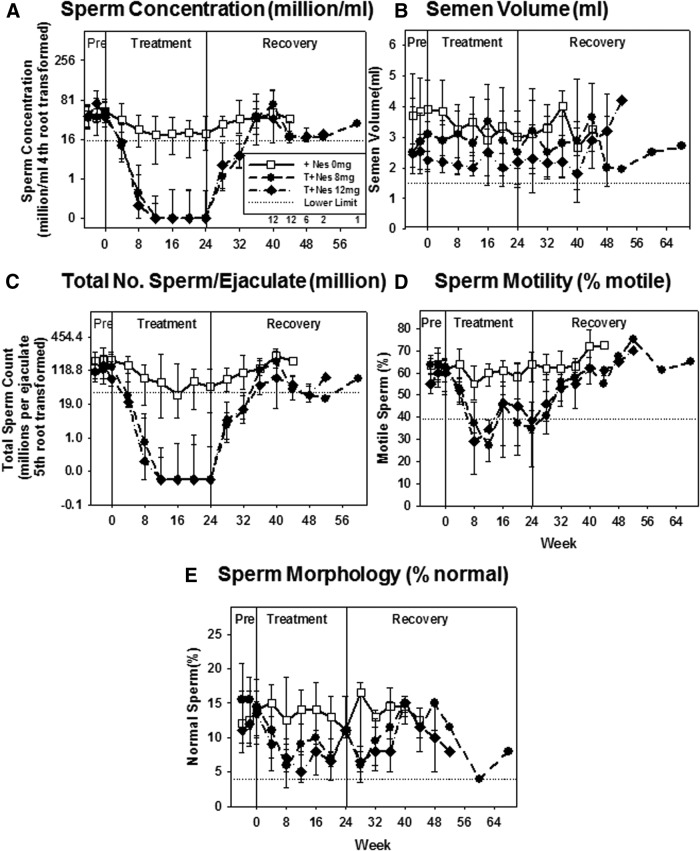
Fig. 3.
Sperm concentration (A), semen volume (B), total sperm count per ejaculate (C), percentage motility (D), and percentage normal morphology (E) (median, 25th, and 75th percentile) in each treatment group during each assessment week in the pretreatment (PRE), treatment, and recovery periods. Note that a fourth root transformation was used for the sperm concentration (A) and the fifth root for total sperm count per ejaculate (C). Sperm motility and morphology were assessed only in samples with more than 5000 spermatozoa/ml. Dotted lines represent the lower reference range in adult men.
Results
Study participants
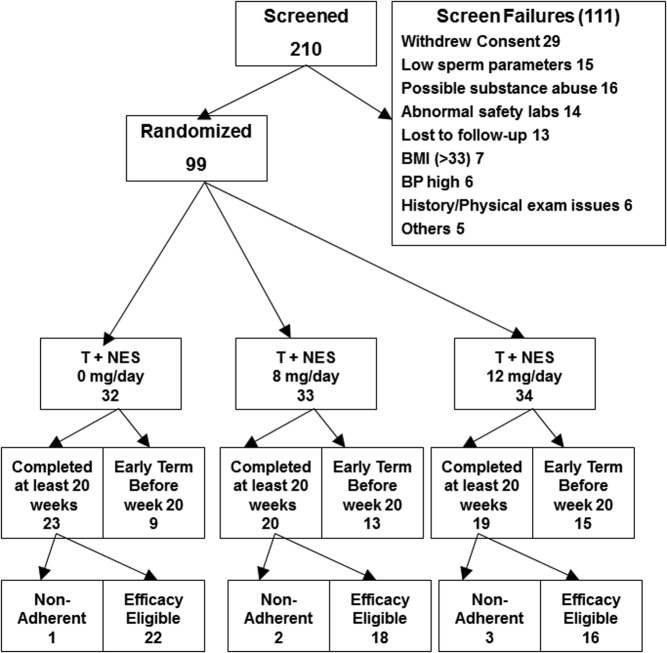
Two-hundred ten men were screened and 99 were enrolled and randomized (Fig. 1). Subject characteristics were not different among the groups at baseline except for a significantly higher sperm concentration in the T+NES 0 group (Table 1). Sixty-two subjects continued the study until wk 20 or longer: 23 men in the T+NES 0, 20 men in the T+NES 8, and 19 men in the T+NES 12 groups, respectively. Of these subjects, 22 in the T+NES 0, 18 in the T+NES 8, and 16 in the T+NES 12 groups were included in the efficacy analysis as defined above (Fig. 1). One volunteer in the T+NES 8 group met the efficacy eligible criteria but was subsequently excluded for nonadherence. This subject had no measurable serum NES concentration from wk 8 to 20 but had detectable NES concentration at wk 24 indicating he did not take NES for at least 16 of 24 wk of treatment.
Fig. 1.
Subject enrollment, randomization, and disposition. BMI, Body mass index; BP, blood pressure.
Table 1.
Baseline characteristics of the randomized subjects (median, 25th, and 75th percentile in parentheses)
| Treatment group | T+NES 0 mg/d | T+NES 8 mg/d | T+NES 12 mg/d | P value |
|---|---|---|---|---|
| Subjects, n | 32 | 33 | 34 | |
| Age (yr) | 27 (22, 37) | 29 (22, 34) | 23 (21, 39) | 0.61 |
| Ethnicity | 0.92 | |||
| Hispanic | 25% | 27% | 29% | |
| Non-Hispanic | 75% | 73% | 71% | |
| Race | 0.08 | |||
| White | 50% | 73% | 74% | |
| African-American | 16% | 9% | 21% | |
| Asian | 22% | 3% | 3% | |
| Others | 13% | 15% | 3% | |
| Weight (kg) | 78.2 (76.4, 83.6) | 75.4 (70.0, 82.7) | 80.0 (74.6, 88.6) | |
| BMI (kg/m2) | 25.0 (23.5, 26.8 | 24.8 (22.3, 26.7) | 25.4 (24.1, 26.7) | 0.68 |
| Height (cm) | 154 (147, 158) | 152 (147, 156) | 156 (150, 161) | 0.13 |
| Serum hormone levels | ||||
| Total T (nmol/liter) | 21.1 (17.1, 25.7) | 20.2 (15.1, 23.7) | 20.1 (15.8, 24.2) | 0.66 |
| LH (IU/liter) | 3.4 (2.8, 5.0) | 3.2 (2.5, 4.7) | 3.5 (2.2, 4.3) | 0.41 |
| FSH (IU/liter) | 3.2 (2.1, 4.7) | 3.1 (2.1, 4.5) | 3.5 (2.2, 4.3) | 0.93 |
| Sperm concentration (106/ml) | 53.4 (34.8, 74.1) | 50.4 (33.3, 74.0) | 48.2 (29.3, 64.4) | 0.57 |
| Total sperm per ejaculate (×106) | 172 (126, 263) | 134 (83, 186) | 129 (62, 193) | 0.05 |
| Testicular size (both sides in milliliter) | 50 (44, 53) | 50 (42, 52) | 50 (46, 50) | 0.82 |
BMI, Body mass index.
Spermatogenesis suppression
Primary efficacy analyses showed men whose sperm concentration suppressed to 1 million/ml or less at wk 20–24 was significantly higher in T+NES 8 (89%, P < 0.0001) and T+NES 12 (88%, P = 0.0002) groups compared with the T+NES 0 group (23%) (Fig. 2 and Supplemental Table 1). Significantly more men became azoospermic in the T+NES 8 (78%, P = 0.001) and T+NES 12 (69%, P = 0.008) groups compared with T+NES 0 (23%) (Supplemental Table 1). Suppression of spermatogenesis was rapid in the groups applying NES 8 or 12 mg gel. By wk 8, the numbers of subjects whose sperm concentration were suppressed to 1 million/ml or less in the efficacy evaluable group were four of 22 (18%), nine of 18 (61%), and nine of 16 (62%) subjects in the T+NES 0, T+NES 8, and T+NES 12 groups, respectively (Fig. 2). In four subjects in the T+NES 12 group, the suppressed sperm concentration transiently rebounded to greater than 1 million/ml (1.4, 1.6, 1.7, and 7 million/ml). The median sperm concentration in the T+NES 8 (0 million/ml, P < 0.0001) and T+NES 12 (0 million/ml, P = 0.002) at wk 24 were significantly lower than the T+NES 0 group (20.7 million/ml) (Fig. 3 and Supplemental Table 2). The decrease in sperm motility and sperm normal morphology followed the decreases in sperm concentration. All subjects recovered to a normal sperm concentration of 15 million/ml or greater, and there were no significant changes from baseline to the final evaluation of sperm concentration at recovery (Supplemental Table 2A). The median time to recovery in the T+NES 0 group was 1 d compared with 115 d in the T+NES 8 and 116 d in the T+NES 12 groups (Supplemental Table 2B).
Fig. 2.
Percent of subjects with sperm concentration suppressed to 0, 1 million or less, 3 million or less, and more than 3 million/ml in the three treatment groups: T + NES 0 mg (A), T + NES 8 mg (B), and T + NES 12 mg group (C) during the treatment and recovery periods.
Serum hormone levels
After T gel application, serum T levels showed large variations both within and between subjects. Median serum total T concentration increased at wk 24 by 20.4, 3.2, and 24.8% and free T concentration increased by 16.8, 25.6, and 33.5% in the T+NES 0, T+NES 8, and T+NES 12 groups, respectively, without significant differences between groups. Both median total and free T concentrations remained within the physiological range, irrespective of treatment group (Fig. 4, A and B, and Supplemental Table 2). In 15 subjects, T concentrations were above 62 nmol/liter (1800 ng/dl) but on more than two occasions in only three subjects.
Fig. 4.
Serum T (A), free T (B), NES (C), SHBG (D), LH (E), and FSH (F) concentrations (median, 25th, and 75th percentiles) in each treatment group during each assessment week in the pretreatment (PRE), treatment, and recovery period. Dotted lines represent the reference range in adult men.
Median serum NES concentrations correlated with the dose applied (372 pmol/liter in the T+NES 8 group and 632 pmol/liter in the T+NES 12 group at wk 24) (Fig. 4C); however, NES concentrations varied greatly both within individuals and between treatment groups. After the last application of NES gel at wk 24, the serum NES concentration remaining at 24, 48, and 72 h was 91.3, 42.8, and 34.8% and 94.0, 38.5, and 27.4% for the T+NES 8 and T+NES 12 groups, respectively. One week after stopping NES application, serum NES concentrations approached the LLOQ. Median SHBG concentrations remained within the normal range but decreased by 11.2, 22.7, and 6% in the T+NES 0, T+NES 8, and T+NES 12 groups, respectively, without significant differences between groups (Fig. 4D). At wk 24, median serum LH and FSH levels were significantly more suppressed in the T+NES 8 and T+NES 12 groups compared with the T+NES 0 group (Fig. 4, E and F, and Supplemental Table 2).
Changes in psychosexual diary scores
Prospective assessment of psychosexual function showed no significant difference in sexual desire, sexual activity, sexual enjoyment, satisfaction with erections, and fullness of erections scores among groups at baseline and during and at the end of treatment in any treatment group. The T+NES 8 group showed small but significant increased positive and decreased negative mood scores when compared with the T+NES 12 group but not different from the T+NES 0 group (Fig. 5 and Supplemental Table 3).
Fig. 5.
Psychosexual diary scores (median, 25th, and 75th percentiles) for each treatment group at wk 0 (baseline), 12 and 24 wk (treatment), and 36 wk (recovery). A, Sexual activity; B, sexual desire; C, sexual enjoyment with partner; D, sexual enjoyment without partner; E, positive mood; and F, negative mood scores.
Changes in clinical and safety laboratory parameters
The median levels and change from baseline of safety parameters in all randomized subjects are shown in Supplemental Tables 4 and 5. Subjects in all three groups gained weight. The increases were significantly higher in the T+NES 8 group (5.2 kg, P = 0.004) but not in the T+NES 12 group (2.5 kg, P = 0.057) compared with the T+NES 0 group (1.1 kg). Weight decreased during the recovery period but did not reach baseline levels. There were no significant changes in systolic or diastolic blood pressure or other vital signs. Serum hemoglobin increased by 0.3 g/dl and hematocrit by 1.0% without significant differences between groups. There was no significant change in the lipid panel in the T+NES 0 mg group. The lipid panel in the T+NES 8 group at wk 24 was not different from baseline, whereas in the T+NES 12 group, the decrease (−4 mg/dl) in serum HDL cholesterol at wk 24 was significantly different from baseline. In the T +NES 8 and T+ NES 12 groups, changes in serum HDL cholesterol level and the HDL to LDL cholesterol ratio were significantly different compared with the T+NES 0 group. Serum fasting glucose concentrations were not changed during treatment in the T+NES 0 and T+NES 8 groups. In the T+NES 12 group, there was a small but significant increase in serum glucose (+6 mg/dl) from baseline to wk 24. There were no significant changes in other blood cell counts, liver enzymes, or renal function tests. Serum prostate-specific antigen and IPSS did not change with treatment, nor did any subject experience a clinically significant event related to the prostate.
Discontinuation and adverse events
During this study, no serious adverse events occurred. Common adverse events possibly related to the study medications are listed in Table 2. The main reasons for discontinuation were inconvenience of study visits or failure to come for follow-up visits. Five men discontinued because of adverse events: one subject each reported irritability and nightmares, decreased libido, increased appetite, mood swings, and asthma exacerbation. The most common adverse event was acne (21% of subjects, mild in 16%, moderate in 5%) without differences among treatment groups. Changes in sexual function were reported by four subjects (4%). Subjective depressed mood was reported in one, depression in two, altered mood in one, and mood swings in three subjects. Insomnia was reported in 6% of subjects. Headaches, reported in 17% of subjects, were higher in the T+NES 12 vs. the T+NES 0 group (P = 0.021).
Table 2.
Number of subject (percentage in parentheses) with adverse events that may be at least possibly related to the medications (using MedDRA terms)
| System | T+NES 0 (n = 32) | T+NES 8 (n = 33) | T+NES 12 (n = 34) | All subjects (n = 99) |
|---|---|---|---|---|
| Skin | ||||
| Acne | 9 (28.1) | 7 (21.2) | 5 (14.7) | 21 (21.2) |
| Dry skin | 1 (3.1) | 2 (6.1) | 1 (2.9) | 4 (4.0) |
| Exfoliation | 1 (3.1) | 0 | 0 | 1 (1.0) |
| Weight increase | 1 (3.1) | 4 (12.1) | 2 (5.9) | 7 (7.1) |
| Psychosexual/mood | ||||
| Aggression | 0 | 0 | 1 (2.9) | 1 (1.0) |
| Anorgasmia | 0 | 1 (3.0) | 0 | 1 (1.0) |
| Depressed mood | 0 | 1 (3.0) | 0 | 1 (1.0) |
| Depression | 1 (3.1) | 0 | 1 (2.9) | 2 (2.0) |
| Insomnia | 2 (6.3) | 2 (6.1) | 2 (5.9) | 6 (6.1) |
| Libido decreased | 1 (3.1) | 2 (6.1) | 0 | 3 (3.0) |
| Libido increased | 0 | 0 | 1 (2.9) | 1 (1.0) |
| Mood altered | 0 | 1 (3.0) | 0 | 1 (1.0) |
| Mood swings | 0 | 2 (6.1) | 1 (2.9) | 3 (3.0) |
| Energy increased | 0 | 0 | 1 (2.9) | 1 (1.0) |
| Fatigue | 1 (3.1) | 1 (3.0) | 0 | 2 (2.0) |
| Irritability | 1 (3.1) | 1 (3.0) | 0 | 2 (2.0) |
Discussion
This is the first study of a transdermal male hormonal contraceptive regimen showing effective suppression of spermatogenesis using T gel combined with the nonandrogenic progestin NES gel. Transdermal delivery offers the advantage of steady-state serum levels of both steroids released from the stratum corneum reservoir after absorption through the skin. With 10 g T gel administered in this study, serum T and free T concentrations showed variations within and between subjects and were increased from baseline but remained within the physiological range in the majority of time in all men. Acne was a common complaint, and lower doses of T may result in fewer androgen-related effects. Nestorone also was absorbed well transdermally and was associated with dose proportional median serum levels during treatment, but the NES serum concentrations were variable both between and within subjects. As shown previously (26), serum LH and FSH were markedly more suppressed at wk 2 of treatment in both groups applying T + NES gels compared with T + placebo gel.
Sperm suppression was rapid; by wk 8, greater than 60% of subjects receiving NES had sperm concentrations of 1 million/ml or less. A sperm concentration compatible with contraceptive efficacy (≤1 million/ml) was achieved in 89% of subjects from wk 20 to 24 when T gel was combined with NES 8 mg/d and was not further improved with a higher NES dose of 12 mg/d (88%). Azoospermia was achieved in 78% of the T+NES 8- and 69% of the T+NES 12-treated subjects. Thus, NES 8 mg/d appears to be the optimal dose to be used with 10 g T gel for male hormonal contraception. After suppression, four men receiving T+NES 12 showed a rebounded sperm concentration transiently, three remained less than 2 million/ml, but one reached a concentration of 7 million/ml. Such rebound during suppression has been reported in clinical trials using T transdermal preparations (35) and T undecanoate injections (5). This gel-gel combination offers a novel regimen that may be more appealing to men for long-term use than the injection-containing contraceptive regimens (3).
As with female contraceptive trials with self-administration methods, nonadherence with daily gel application in some subjects may contribute to the lower spermatogenesis suppression rate as well as sperm rebound after suppression observed in this study. Complete suppression of spermatogenesis is not universal with male hormonal contraceptive regimens tested to date (5, 7–9). In the present study, 11 and 12% of men in the T+NES 8 and T+NES 12 groups did not suppress to sperm concentrations less than 1 million/ml. Six of the 62 subjects who completed the treatment period were nonadherent and were excluded from the efficacy analyses. Nonadherence in the T+NES 0 group may be higher than presented as the only method to assess T gel adherence was counting empty T gel sachets. In contrast to real life, when men may use this as a method of contraception, men were asked to use an additional method of contraception during this clinical trial, which may contribute to higher nonadherence rates. The subjects did not expect any benefit from the study. This is a proof-of-concept study in which the two gels are applied in two areas. Formulation of the T and NES into a single gel with less total volume may also improve adherence. No biomarker has yet been identified that predicts who will fail to achieve this threshold. Therefore, seminal fluid testing should be used to confirm severe spermatogenesis suppression before further efficacy testing of this, or other, male hormonal contraceptive regimens.
More than one third of the subjects discontinued during the study, mostly due to personal reasons rather than adverse effects. The high discontinuation rate in this study is likely due to the burden of the study to subjects with 18 clinic visits in 6 months and consecutive daily clinic visits at the beginning and end of the treatment period. In addition, there are two substudies of self-measurement of sperm concentration using a dipstick and daily text messages to remind subjects to apply the gels. In prior, less burdensome contraceptive clinical trials conducted at our two centers in which suppression of spermatogenesis is the end point, the discontinuation rate ranged from 10 to 25% (19, 23, 36, 38). In contraceptive clinical trials in women, subjects are motivated to participate due to a small reimbursement and a desire to avoid pregnancy. Despite this, the discontinuation rates for women participating in self-administration contraceptive methods such as spermicide averaged 44%, vaginal rings 32%; and female condom 49% at 6 months (personal communication with D. L. Blithe, Ph.D., NIH NICHD). Currently available methods of contraception, the discontinuation rate over 6 months ranges from 31% for the pill up to 69% for male condoms. Discontinuation rates are higher at 12 months, even for very effective methods (1).
No significant safety concerns arose during this study. No clinically significant changes occurred in sexual function or decreased mood according to a validated psychosexual questionnaire administered during the study (27). Acne was reported in one fifth of subjects, but none discontinued because of acne. A few subjects reported mood swings (4%), changes in sexual function (4%), or depressed mood/depression (3%). Weight gain occurred in all treatment groups but was not related to the dose of NES because more weight gain was observed in men receiving the lower dose of the NES gel. Serum HDL cholesterol and HDL to LDL ratios decreased during treatment in both T+NES groups, more so in the T+NES 12 group, suggesting that changes in lipoproteins may be related to the dose of NES rather than T gel. No such changes were observed in women using lower doses of NES alone for contraception (39). It is known that progestins may affect insulin sensitivity (40). There was a small but significant increase in fasting serum glucose in the group using the higher NES dose (12 mg/d), but fasting glucose levels remained within the normal range in all men. There were small and nonsignificant increases in hemoglobin and hematocrit as may be expected with transdermal T administration (41).
In summary, the addition of transdermal NES to T gel significantly enhances the effectiveness of suppression of gonadotropins and spermatogenesis. NES 8 mg daily in combination with 10 g T gel appears to be the optimal dosing regimen, with no improvement in suppression of spermatogenesis observed using a higher dose of NES. It should be noted that this dose of gel was associated with acne in 21% of the subjects and thus may be supraphysiological. This 10-g dose of T gel used in this study could have masked any dose effects of NES gel that may be apparent if a lower dose of T gel had been administered. The next study should test a lower concentration of T gel to confirm that NES 8 mg is indeed the optimal dose for sperm suppression in men. Combining T and NES into a single gel preparation and keeping the gel volume as low as possible should simplify application and may help with acceptability, compliance, and effectiveness in the suppression of spermatogenesis to exceed the rate demonstrated in this study. Future studies should assess the pharmacokinetics of a combined gel regimen to ensure that reformulation does not affect the absorption and the distribution of both T and NES.
Supplementary Material
Acknowledgments
The authors acknowledge the gift of testosterone gel through Paul Piette, M.D., Besins Health Care International; the advice and support on the development of the statistical analyses plan and data analyses from Peter D. Christenson, Ph.D.; the help and assistance provided by Sharon Midler and her team for monitoring the study at Healthy Decisions; Elizabeth Ruiz, Xiao-Dan Han, Kathy Winter, Robert Bale Jr., Iris Nielsen, Connie Pete, and Dorothy McGuinness for their help in coordinating the study; Christin Snyder, M.D., for helping recruit and evaluate patients at the University of Washington site; Andrew Leung, High Technician Certificate, Sima Baravarian, Ph.D., and the technologists of the Hormone and Metabolic Research Laboratory at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center for all the hormone analyses; and Laura Hull, M.A., with the preparation of the tables and graphs for the manuscript.
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Contracts HHSN275200403369I and HHSN2751008060044U and the Population Council. The Los Angeles Center was supported by the Endocrinology, Metabolism, and Nutrition Training Grant NIH T32 DK007571, and the General Clinical Research Center Grant MO1 NIH RR00425 and the University of California, Los Angeles, Clinical and Translational Science Institute Grant NIH 1UL1-RR033176 at Harbor-University of California, Los Angeles, Medical Center and Los Angeles Biomedical Research Institute. The Seattle center was supported by Contract HHSN27520040337 from the NICHD, the Center for Research in Reproduction and Contraception Grant NIH U54 HD 04245 (NICHD), and NICHD Grant 5K12HD053984.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CV
- Coefficient of variation
- HDL
- high-density lipoprotein
- IPSS
- International Prostate Symptom Score
- LDL
- low-density lipoprotein
- LLOQ
- lower limit of quantification
- NES
- nestorone
- T
- testosterone.
References
- 1. Vaughan B, Trussell J, Kost K, Singh S, Jones R. 2008. Discontinuation and resumption of contraceptive use: results from the 2002 National Survey of Family Growth. Contraception 78:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin CW, Anderson RA, Cheng L, Ho PC, van der Spuy Z, Smith KB, Glasier AF, Everington D, Baird DT. 2000. Potential impact of hormonal male contraception: cross-cultural implications for development of novel preparations. Hum Reprod 15:637–645 [DOI] [PubMed] [Google Scholar]
- 3. Heinemann K, Saad F, Wiesemes M, White S, Heinemann L. 2005. Attitudes toward male fertility control: results of a multinational survey on four continents. Hum Reprod 20:549–556 [DOI] [PubMed] [Google Scholar]
- 4. Glasier AF, Anakwe R, Everington D, Martin CW, van der Spuy Z, Cheng L, Ho PC, Anderson RA. 2000. Would women trust their partners to use a male pill? Hum Reprod 15:646–649 [DOI] [PubMed] [Google Scholar]
- 5. Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, Bo L, Xiong C, Wang X, Liu X, Peng L, Yao K. 2009. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab 94:1910–1915 [DOI] [PubMed] [Google Scholar]
- 6. Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK, Huang ZJ, Zhang GY. 2003. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab 88:562–568 [DOI] [PubMed] [Google Scholar]
- 7. Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, Handelsman DJ. 2003. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab 88:4659–4667 [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization Task Force on Methods for the Regulation of Male Fertility 1990. Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet 336:955–959 [PubMed] [Google Scholar]
- 9. World Health Organization Task Force on Methods for the Regulation of Male Fertility 1996. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril 65:821–829 [PubMed] [Google Scholar]
- 10. Ilani N, Liu PY, Swerdloff RS, Wang C. 2011. Does ethnicity matter in male hormonal contraceptive efficacy? Asian J Androl 13:579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, Gu YQ, Kersemaekers WM, McLachlan RI, Meriggiola MC, Nieschlag E, Sitruk-Ware R, Vogelsong K, Wang XH, Wu FC, Zitzmann M, Handelsman DJ, Wang C. 2008. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab 93:1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu YQ, Tong JS, Ma DZ, Wang XH, Yuan D, Tang WH, Bremner WJ. 2004. Male hormonal contraception: effects of injections of testosterone undecanoate and depot medroxyprogesterone acetate at eight-week intervals in Chinese men. J Clin Endocrinol Metab 89:2254–2262 [DOI] [PubMed] [Google Scholar]
- 13. Handelsman DJ, Conway AJ, Howe CJ, Turner L, Mackey MA. 1996. Establishing the minimum effective dose and additive effects of depot progestin in suppression of human spermatogenesis by a testosterone depot. J Clin Endocrinol Metab 81:4113–4121 [DOI] [PubMed] [Google Scholar]
- 14. Kamischke A, Ploger D, Venherm S, von Eckardstein S, von Eckardstein A, Nieschlag E. 2000. Intramuscular testosterone undecanoate with or without oral levonorgestrel: a randomized placebo-controlled feasibility study for male contraception. Clin Endocrinol (Oxf) 53:43–52 [DOI] [PubMed] [Google Scholar]
- 15. Kamischke A, Venherm S, Plöger D, von Eckardstein S, Nieschlag E. 2001. Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab 86:303–309 [DOI] [PubMed] [Google Scholar]
- 16. Meriggiola MC, Costantino A, Saad F, D'Emidio L, Morselli Labate AM, Bertaccini A, Bremner WJ, Rudolph I, Ernst M, Kirsch B, Martorana G, Pelusi G. 2005. Norethisterone enanthate plus testosterone undecanoate for male contraception: effects of various injection intervals on spermatogenesis, reproductive hormones, testis, and prostate. J Clin Endocrinol Metab 90:2005–2014 [DOI] [PubMed] [Google Scholar]
- 17. Meriggiola MC, Farley TM, Mbizvo MT. 2003. A review of androgen-progestin regimens for male contraception. J Androl 24:466–483 [DOI] [PubMed] [Google Scholar]
- 18. Nieschlag E, Zitzmann M, Kamischke A. 2003. Use of progestins in male contraception. Steroids 68:965–972 [DOI] [PubMed] [Google Scholar]
- 19. Page ST, Amory JK, Anawalt BD, Irwig MS, Brockenbrough AT, Matsumoto AM, Bremner WJ. 2006. Testosterone gel combined with depomedroxyprogesterone acetate is an effective male hormonal contraceptive regimen and is not enhanced by the addition of a GnRH antagonist. J Clin Endocrinol Metab 91:4374–4380 [DOI] [PubMed] [Google Scholar]
- 20. Wu FC, Aitken RJ. 1989. Suppression of sperm function by depot medroxyprogesterone acetate and testosterone enanthate in steroid male contraception. Fertil Steril 51:691–698 [DOI] [PubMed] [Google Scholar]
- 21. Bebb RA, Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, Matsumoto AM. 1996. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab 81:757–762 [DOI] [PubMed] [Google Scholar]
- 22. Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, Behre HM, Beynon J, Bouloux PM, Costantino A, Gerbershagen HP, Grønlund L, Heger-Mahn D, Huhtaniemi I, Koldewijn EL, Lange C, Lindenberg S, Meriggiola MC, Meuleman E, Mulders PF, Nieschlag E, Perheentupa A, Solomon A, Väisälä L, Wu FC, Zitzmann M. 2008. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab 93:2572–2580 [DOI] [PubMed] [Google Scholar]
- 23. Wang C, Wang XH, Nelson AL, Lee KK, Cui YG, Tong JS, Berman N, Lumbreras L, Leung A, Hull L, Desai S, Swerdloff RS. 2006. Levonorgestrel implants enhanced the suppression of spermatogenesis by testosterone implants: comparison between Chinese and non-Chinese men. J Clin Endocrinol Metab 91:460–470 [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Swerdloff RS. 2004. Male hormonal contraception. Am J Obstet Gynecol 190:S60–S68 [DOI] [PubMed] [Google Scholar]
- 25. Sitruk-Ware R, Nath A. 2010. The use of newer progestins for contraception. Contraception 82:410–417 [DOI] [PubMed] [Google Scholar]
- 26. Mahabadi V, Amory JK, Swerdloff RS, Bremner WJ, Page ST, Sitruk-Ware R, Christensen PD, Kumar N, Tsong YY, Blithe D, Wang C. 2009. Combined transdermal testosterone gel and the progestin nestorone suppresses serum gonadotropins in men. J Clin Endocrinol Metab 94:2313–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee KK, Berman N, Alexander GM, Hull L, Swerdloff RS, Wang C. 2003. A simple self-report diary for assessing psychosexual function in hypogonadal men. J Androl 24:688–698 [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization 2010. World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. 5th ed Geneva: World Health Organization [Google Scholar]
- 29. Lähteenmäki P, Weiner E, Lähteenmäki P, Johansson E, Luukkainen T. 1981. Contraception with subcutaneous capsules containing ST-1435. Pituitary and ovarian function and plasma levels of ST-1435. Contraception 23:63–75 [DOI] [PubMed] [Google Scholar]
- 30. Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. 2008. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clin Chem 54:1855–1863 [DOI] [PubMed] [Google Scholar]
- 31. Qoubaitary A, Meriggiola C, Ng CM, Lumbreras L, Cerpolini S, Pelusi G, Christensen PD, Hull L, Swerdloff RS, Wang C. 2006. Pharmacokinetics of testosterone undecanoate injected alone or in combination with norethisterone enanthate in healthy men. J Androl 27:853–867 [DOI] [PubMed] [Google Scholar]
- 32. Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N. 2000. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab 85:4500–4510 [DOI] [PubMed] [Google Scholar]
- 33. Nieschlag E. 2007. 10th Summit Meeting consensus: recommendations for regulatory approval for hormonal male contraception. October 22–23, 2006. Contraception 75:166–167 [DOI] [PubMed] [Google Scholar]
- 34. Büchter D, von Eckardstein S, von Eckardstein A, Kamischke A, Simoni M, Behre HM, Nieschlag E. 1999. Clinical trial of transdermal testosterone and oral levonorgestrel for male contraception. J Clin Endocrinol Metab 84:1244–1249 [DOI] [PubMed] [Google Scholar]
- 35. Hair WM, Kitteridge K, O'Connor DB, Wu FC. 2001. A novel male contraceptive pill-patch combination: oral desogestrel and transdermal testosterone in the suppression of spermatogenesis in normal men. J Clin Endocrinol Metab 86:5201–5209 [DOI] [PubMed] [Google Scholar]
- 36. Gonzalo IT, Swerdloff RS, Nelson AL, Clevenger B, Garcia R, Berman N, Wang C. 2002. Levonorgestrel Implants (Norplant II) for male contraception clinical trials: combination with transdermal and injectable testosterone. J Clin Endocrinol Metab 87:3562–3572 [DOI] [PubMed] [Google Scholar]
- 37. Handelsman DJ. 2002. Optimal power transformations for analysis of sperm concentration and other semen variables. J Androl 23:629–634 [PubMed] [Google Scholar]
- 38. Anawalt BD, Bebb RA, Bremner WJ, Matsumoto AM. 1999. A lower dosage levonorgestrel and testosterone combination effectively suppresses spermatogenesis and circulating gonadotropin levels with fewer metabolic effects than higher dosage combinations. J Androl 20:407–414 [PubMed] [Google Scholar]
- 39. Laurikka-Routti M. 1992. Serum lipids, blood pressure, body weight, and serum chemistry in women using subcutaneous contraceptive implants releasing the progestin ST 1435. Obstet Gynecol 80:855–859 [PubMed] [Google Scholar]
- 40. Lopez LM, Grimes DA, Schulz KF. 2007. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev CD006133. [DOI] [PubMed] [Google Scholar]
- 41. Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. 2004. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 89:2085–2098 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.