Abstract
Background:
The intake of olive oil has been related to the prevention of osteoporosis in experimental and in in vitro models. Very few prospective studies have evaluated the effects of olive oil intake on circulating osteocalcin (OC) in humans.
Objective:
The objective of the study was to examine the longitudinal effects of a low-fat control diet (n = 34), a Mediterranean diet enriched with nuts (MedDiet+nuts, n = 51), or a Mediterranean diet enriched with virgin olive oil (MedDiet+VOO, n = 42) on circulating forms of OC and bone formation markers in elderly men at high cardiovascular risk.
Design:
Longitudinal associations between baseline and follow-up (2 yr) measurements of total OC, undercarboxylated osteocalcin, C-telopeptide of type I collagen, and procollagen I N-terminal propeptide (P1NP) concentrations were examined in 127 elderly men randomized to three healthy dietary interventions.
Results:
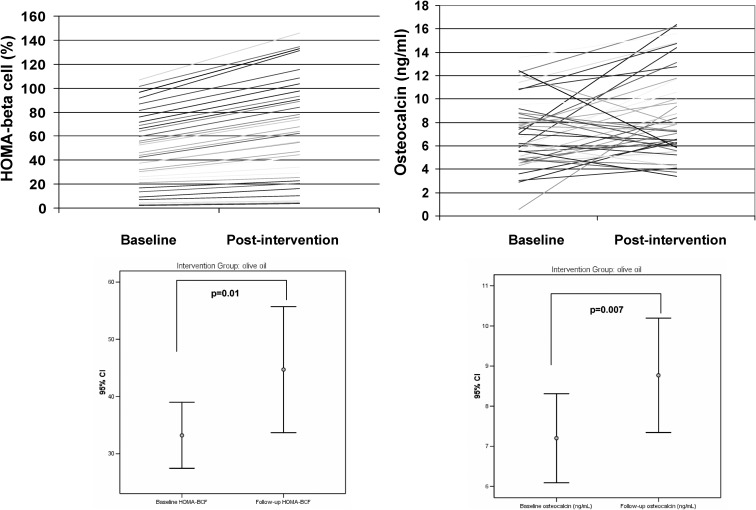
Baseline characteristics (age, body mass index, waist circumference, lipid profile, fasting insulin levels, and bone formation and resorption markers) were similar in all intervention groups. The total osteocalcin concentration increased robustly in the MedDiet+VOO group (P = 0.007) in parallel to increased P1NP levels (P = 0.01) and homeostasis model assessment-β-cell function (P = 0.01) but not in subjects on the MedDiet+nuts (P = 0.32) or after the control diet (P = 0.74). Interestingly, the consumption of olives was associated positively with both baseline total osteocalcin (r = 0.23, P = 0.02) and the 2-yr osteocalcin concentrations (r = 0.21, P = 0.04) in the total cohort.
Conclusions:
Consumption of a Mediterranean diet enriched with virgin olive oil for 2 years is associated with increased serum osteocalcin and P1NP concentrations, suggesting protective effects on bone.
Age-related bone mass loss and decreased bone strength is an almost invariable feature of human biology, affecting women and men alike as an important determinant of osteoporosis and fracture risk (1). Nutritional factors are known to be involved in age-related bone loss associated with osteoblast insufficiency during continuous bone remodeling, in interaction with a combination of genetic, metabolic, and hormonal factors (2).
Epidemiological studies have shown that the incidence of osteoporosis in Europe is lower in the Mediterranean basin (3, 4). The traditional Mediterranean diet, rich in fruit and vegetables, with a high intake of olives and olive products, mainly olive oil, could be one of the environmental factors underlying this difference.
Some reports have suggested that the consumption of olives (5), olive oil (6), and oleuropein, an olive oil polyphenol (7), can prevent the loss of bone mass in animal models of aging-related osteoporosis (8, 9). Recent in vitro studies have shown that oleuropein, the main phenolic compound in olive leaves and fruit and a constituent of virgin olive oil, reduced the expression of peroxisomal proliferator-activated receptor-γ, inhibiting adipocyte differentiation and enhancing differentiation of mesenchymal stem cells into osteoblasts. In addition, the gene expression of osteoblastogenesis markers, runt-related transcription factor II, osterix, collagen type I, alkaline phosphatase, and osteocalcin, was higher in osteoblast-induced oleuropein-treated cells (10).
A protective effect of olive oil and oleuropein has also been observed in experimental models. Femoral failure load and diaphyseal bone mineral density were increased after consumption of oleuropein and olive oil in ovariectomized mice (6). In addition to its role as bone marker, osteocalcin has also been related to glucose homeostasis. Mice lacking osteocalcin displayed decreased β-cell proliferation, glucose intolerance, and insulin resistance when compared with wild-type mice (12). We are unaware of studies evaluating the effects of olive oil on circulating osteocalcin and its possible relationship with insulin secretion/resistance in humans. The objective of this study was to explore circulating bone formation and resorption markers in association with the intake of olive oil. For comparison, we also studied the effects of consuming nuts and the effects of a low-fat diet.
Subjects and Methods
Study subjects
The participants in this study were 127 community-dwelling men aged 55–80 yr randomly selected from one of the Prevención con Dieta Mediterránea (PREDIMED) study centers (PREDIMED-Reus) who had at least 2 yr of follow-up. The PREDIMED study is a large, parallel-group, randomized, controlled trial aimed to assess the effect of the Mediterranean diet on the primary prevention of cardiovascular diseases. Full details of the PREDIMED protocol have been published elsewhere (13). Subjects were elderly without prior cardiovascular disease but having a diagnosis of type 2 diabetes or harboring at least three cardiovascular risk factors, namely hypertension; dyslipidemia [low density lipoprotein (LDL) cholesterol level of ≥ 4.14 mmol/liter [≥ 160 mg/dl] (or treatment with hypolipidemic drugs) or high density lipoprotein (HDL) cholesterol level ≤1.04 mmol/liter (≤40 mg/dl)]; overweight [body mass index (BMI) ≥ 25 kg/m2]; or a family history of premature cardiovascular disease. Exclusion criteria were any severe chronic illness; alcohol or drug abuse; a BMI greater than 40 kg/m2; and loss of more than 5% body weight in the last year. For the present analysis, we further excluded current smokers, subjects treated with insulin, those with secondary hypertension, subjects using medications known to affect bone or calcium metabolism, such as corticosteroids, bisphosphonates, thiazides, and vitamin or mineral supplements; and those receiving large amounts of aspirin or anticoagulant medication that would interfere with vitamin K absorption. Renal function was systematically assessed in all subjects, and only subjects with normal renal function (as estimated using serum creatinine concentration or estimated glomerular filtration rate) were included in this study.
Design of the study
Participants were randomly assigned to three intervention groups: advice on the MedDiet plus supplementation with virgin olive oil (MedDiet+VOO); advice on the MedDiet plus supplementation with mixed nuts (MedDiet+nuts); and advice on a low-fat diet (control group). Dietitians gave personalized dietary advice to participants randomized to the different diets, with instructions directed to upscale the score, including, among others, the following: 1) abundant use of olive oil for cooking and dressing; 2) increased consumption of fruit, vegetables, legumes, and fish; 3) reduction in total meat consumption, recommending white meat instead of red or processed meat; 4) preparation of homemade sauce with tomato, garlic, onion, and spices with olive oil to dress vegetables, pasta, rice, and other dishes; 5) avoidance of butter, cream, fast food, sweets, pastries, and sugar-sweetened beverages; and 6) in alcohol drinkers, moderate consumption of red wine. Participants allocated the low-fat diet received recommendations to reduce all types of fat from both animal and vegetable sources. In the MedDiet+VOO intervention group, those individuals taking refined olive oil were advised to change this type of oil by an extra virgin olive oil that retains all the phytochemical and antioxidants compounds. Those taking other sources of vegetable oil also were advised to change the oil to extra virgin olive oil. Refined oil does not have such an amount of phytochemicals. The advice on virgin olive oil was to consume at least 50 ml/d, whereas mixed nuts (walnuts, almonds, and hazelnuts) were provided in daily allotments of 30 g (13). The dietary changes that occurred in the PREDIMED study have been previously described (14). Energy restriction was not advised nor physical activity promoted. Changes in physical activity were not observed during the follow-up (13). The local institutional review board approved the study protocol and all participants provided written informed consent.
Procedures
At baseline, 1 yr, and 2 yr of dietary intervention, a short-questionnaire about lifestyle variables, medical conditions, and medication use was obtained. Once a year, usual dietary intakes were assessed during the study using a previously validated, semiquantitative, 137-item food frequency questionnaire. Energy and nutrient intakes were calculated from Spanish food composition tables, as described (15). Physical activity was evaluated using the validated Spanish version of the Minnesota Leisure Time Physical Activity Questionnaire (16).
Biochemical analyses
Biochemical measurements were performed at baseline and after 2 yr of follow-up on fasting blood samples. Serum levels of glucose, total cholesterol, HDL-cholesterol, and triglycerides were measured by standard enzymatic methods using a biochemistry autoanalyzer. The LDL cholesterol concentration was calculated by the Friedewald's formula [total cholesterol − ([HDL-cholesterol] + ([triglycerides]/5) (in milligrams per deciliter)]. Fasting insulin was measured by a chemiluminiscent immunoassay (Linco Research, St. Charles, MO; intra and inter-assay coefficients of variation 6 and 10%, respectively). Insulin resistance was estimated by the homeostasis model assessment (HOMA) method as HOMA-IR. Altered β-cell function was studied using HOMA-BCF, as previously described (17). Serum total and uncarboxylated osteocalcin levels were measured by electrochemiluminiscence immunoassay (N-mid osteocalcin, electrochemiluminescence immunoassay; Roche, Indianapolis, IN; the intra- and interassay coefficients of variation of 3.6 and 6.6%, respectively). Human cross-linked C-telopeptide of type I collagen (CTX) and procollagen I N-terminal propeptide (P1NP) concentrations in serum were measured by commercial ELISA kits (E90665Hu and E90639Hu, respectively; Uscn, Life Science Inc., Wuhan, China) according to the manufacturer's protocol. The intra- and interassay coefficients of variation were less than 10% and less than 12%, respectively.
Statistical analyses
Means (se) or percentages were used to describe the participants' baseline characteristics. χ2 tests and one-way ANOVA were used to compare the qualitative traits and means of quantitative variables, respectively. Fasting glucose and insulin levels and markers of insulin resistance were logarithmically transformed to reduce skewness and the geometric mean and 95% confidence intervals were used. Mean differences in glucose and insulin metabolic markers during follow-up were normally distributed. Interaction tests for age (product terms, age osteocalcin, age undercarboxylated osteocalcin) showed that there were no statistically significant differences by age regarding the associations between osteocalcin and glucose and insulin concentrations. Analyses were performed using the SPSS software version 17.0 (SPSS Inc., Chicago, IL).
Results
The clinical and biochemical characteristics of the study subjects are shown in Table 1. Baseline characteristics (age, BMI, waist circumference, lipid profile, fasting insulin, and osteocalcin) were similar in all intervention groups. Serum glucose, however, was significantly lower in the group allocated to MedDiet+nuts. After intervention for 2 yr, total cholesterol, HDL-cholesterol, and fasting triglycerides decreased significantly in subjects allocated to the MedDiet+nuts group.
Table 1.
Anthropometric and biochemical characteristics of study subjects at baseline and after 2 yr of follow-up
| Control diet |
MedDiet+nuts |
MedDiet+VOO |
Pa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | Before | After | P | ||
| n | 34 | 51 | 42 | |||||||
| Age (yr) | 68.4 ± 6 | 67.6 ± 6 | 67.9 ± 6.9 | 0.85 | ||||||
| BMI (kg/m2) | 28.8 ± 3.08 | 29.1 ± 3.1 | 0.13 | 28.6 ± 3.3 | 28.7 ± 3.009 | 0.13 | 28.6 ± 2.8 | 28.6 ± 2.9 | 0.75 | 0.83 |
| Waist circumference (cm) | 102.6 ± 8.7 | 103.1 ± 8.3 | 0.42 | 101.7 ± 8.1 | 102.7 ± 7.8 | 0.06 | 102.5 ± 7.03 | 103.02 ± 7.8 | 0.47 | 0.74 |
| SBP (mm Hg) | 152 ± 18 | 146 ± 15 | 0.08 | 149 ± 14.9 | 147.3 ± 16.3 | 0.4 | 155.6 ± 20 | 149.9 ± 16 | 0.02 | 0.23 |
| DBP (mm Hg) | 82.2 ± 10 | 79.7 ± 9.6 | 0.11 | 83.5 ± 9.6 | 80 ± 8.8 | 0.01 | 82.2 ± 10.7 | 79.4 ± 10.9 | 0.09 | 0.51 |
| Cholesterol (mg/dl) | 197.7 ± 39.1 | 198.1 ± 40.1 | 0.93 | 205.4 ± 36.6 | 199.9 ± 38.7 | 0.03 | 204.8 ± 43.7 | 194.3 ± 36.2 | 0.04 | 0.66 |
| LDL-cholesterol (mg/dl) | 118.8 ± 37.4 | 121.4 ± 34.8 | 0.65 | 124.2 ± 33.4 | 125.9 ± 33.1 | 0.43 | 128.4 ± 37.4 | 118.9 ± 27 | 0.04 | 0.55 |
| HDL-cholesterol (mg/dl) | 48.1 ± 9.4 | 46.4 ± 9.8 | 0.17 | 54.7 ± 13.7 | 51.3 ± 11.4 | 0.01 | 54.3 ± 15.2 | 51.4 ± 14.7 | 0.07 | 0.06 |
| Triglycerides (mg/dl) | 151.5 ± 91.5 | 157.2 ± 107.5 | 0.62 | 129.2 ± 72 | 112.6 ± 51.7 | 0.01 | 115.6 ± 68.01 | 123.8 ± 62.3 | 0.22 | 0.12 |
| Fasting glucose (mg/dl) | 126.6 ± 30.8 | 127.8 ± 36.8 | 0.74 | 109.6 ± 26.2 | 109.9 ± 26.6 | 0.81 | 121.6 ± 33.4 | 128.8 ± 44.05 | 0.24 | 0.01 |
| Fasting insulin (mU/liter) | 6.1 ± 3.9 | 5.2 ± 2.8 | 0.13 | 6.1 ± 4.6 | 5.7 ± 4.01 | 0.52 | 4.9 ± 2.5 | 5.7 ± 4.4 | 0.11 | 0.34 |
| HOMA-IR | 1.92 ± 1.3 | 1.6 ± 0.95 | 0.14 | 1.64 ± 1.23 | 1.45 ± 1.1 | 0.13 | 1.5 ± 0.8 | 1.79 ± 1.5 | 0.11 | 0.35 |
| HOMA-BCF | 39.6 ± 28.8 | 45.4 ± 43.7 | 0.34 | 50.3 ± 44.06 | 52.7 ± 42.08 | 0.62 | 33.2 ± 16.5 | 44.7 ± 31.5 | 0.01 | 0.22 |
| Serum calcium (mg/dl) | 9.71 ± 0.42 | 9.42 ± 0.40 | 0.001 | 9.67 ± 0.47 | 9.51 ± 0.43 | 0.02 | 9.59 ± 0.44 | 9.51 ± 0.41 | 0.30 | 0.45 |
| Serum phosphate (mg/dl) | 3.20 ± 0.50 | 3.38 ± 0.69 | 0.14 | 3.23 ± 0.47 | 3.26 ± 0.40 | 0.62 | 3.36 ± 0.59 | 3.36 ± 0.63 | 0.95 | 0.57 |
| CTX (ng/ml) | 1.13 ± 0.83 | 0.37 ± 0.25 | 0.0001 | 0.99 ± 0.84 | 0.44 ± 0.33 | 0.0001 | 1.02 ± 0.52 | 0.54 ± 0.36 | 0.0001 | 0.49 |
| P1NP (ng/ml) | 243.2 ± 167.3 | 240.1 ± 105.4 | 0.92 | 253.2 ± 177.3 | 260.4 ± 131.2 | 0.82 | 205.4 ± 143.2 | 277 ± 108.3 | 0.01 | 0.39 |
| UC osteocalcin (ng/ml) | 1.9 ± 1.7 | 1.9 ± 1.8 | .93 | 1.6 ± 0.7 | 1.9 ± 1.01 | 0.06 | 1.7 ± 1.3 | 1.8 ± 1.6 | 0.36 | 0.84 |
| Osteocalcin (ng/ml) | 9 ± 9.1 | 9.3 ± 6.9 | 0.72 | 8.3 ± 4.9 | 9.12 ± 5.4 | 0.32 | 6.9 ± 3 | 8.4 ± 3.8 | 0.007 | 0.27 |
Data are expressed as mean ± sd. DBP, diastolic blood pressure; SBP, systolic blood pressure; UC, undercarboxylated.
P values for comparisons of baseline parameters.
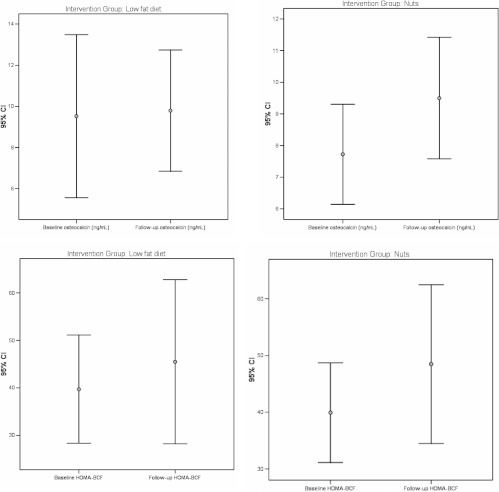
Fasting insulin concentration and HOMA values tended to decrease in the control group and in participants in the MedDiet+nuts group, whereas a significant increase in HOMA β-cell was observed in the MedDiet+VOO group (P = 0.01). In subjects not taking oral antidiabetic drugs, baseline osteocalcin concentrations were positively associated with higher fasting insulin concentrations and HOMA-BCF at follow-up, even after adjustment for BMI, physical activity, intervention group, presence of type 2 diabetes mellitus, and baseline values of each dependent variable. Undercarboxylated osteocalcin tended to increase in subjects in the MedDiet+nuts group (P = 0.06) in parallel with the tendency toward decreased HOMA-IR. However, these changes became nonsignificant when subjects taking oral antidiabetic drugs (n = 17 in the control group, n = 12 in the in the MedDiet+nuts group, and n = 16 in the in the MedDiet+VOO group) were excluded from the analysis.
Total osteocalcin increased in the MedDiet+VOO group (P = 0.007) but not in the MedDiet+nuts or control groups (Table 1 and Figures 1 and 2). Baseline total osteocalcin varied widely (Fig. 1). Interestingly, although the bone resorption marker CTX decreased significantly in all study groups (P = 0.0001), the bone formation marker P1NP increased significantly only in the MedDiet+VOO group (P = 0.01, Table 1). These findings were in parallel to nonsignificant changes in serum calcium in this group, whereas serum calcium decreased significantly in the other two groups.
Fig. 1.
Effects of the MedDiet+VOO on individual HOMA-BCF and circulating osteocalcin concentrations (upper panels). Error bars show median and 95% confidence intervals.
Fig. 2.
Effects of the MedDiet+nuts (left panels) and control diet (right panels) on HOMA-BCF and circulating osteocalcin concentrations. Error bars show median and 95% confidence intervals.
The increase in total osteocalcin after the MedDiet+VOO was still significant when subjects taking oral antidiabetic drugs were excluded from the analysis (8.8 ± 4.2 vs. 6.8 ± 3.5 ng/ml, P = 0.013). Olive consumption was positively associated with both baseline total osteocalcin concentrations (r = 0.23, P = 0.02) and follow-up osteocalcin (r = 0.21, P = 0.04) in the total cohort (n = 127).
In the MedDiet+VOO group, total osteocalcin increased significantly (8.7 ± 4.5 vs. 6.9 ± 2.7 ng/ml, P = 0.02) in subjects not taking statins (n = 24), subjects in whom a statin was added during follow-up (10.5 ± 4.7 vs. 8.2 ± 3.5 ng/ml, P = 0.018, n = 8), or subjects who maintained the same treatment, whether taking statins or not (8 ± 3.5 vs. 6.5 ± 2.9 ng/ml, P = 0.02, n = 32). Interestingly, serum P1NP levels also increased in these subjects (280.1 ± 100 vs. 198.1 ± 142 ng/ml, P = 0.012). In contrast, no significant changes were observed in total osteocalcin or serum P1NP in the MedDiet+nuts group or in the control group in subjects taking or not taking statins. On the other hand, the changes in serum osteocalcin were similar in subjects taking or not antihypertensives.
Discussion
To the best of our knowledge, the present randomized clinical trial is the first assessing the effects of the MedDiet on total osteocalcin concentrations in humans. The main finding is that consumption of a MedDiet enriched with olive oil, but not a MedDiet enriched with nuts or a control diet, was associated with a significant increase of total osteocalcin concentrations that paralleled an increase of HOMA-BCF. These findings were strengthened by the simultaneous observation of increased serum P1NP in subjects taking olive oil, whereas circulating CTX levels decreased. The concentration of P1NP in serum is a sensitive indicator of the synthesis of type I collagen and is mostly affected by changes in bone metabolism. Also in line with preserved bone metabolism, we observed no significant changes in serum calcium in subjects taking olive oil, whereas serum calcium decreased significantly in the other two groups.
Recent findings disclosed that mice lacking osteocalcin displayed decreased β-cell proliferation, glucose intolerance, and insulin resistance when compared with wild-type mice (12). There is previous evidence in the literature of increased bone density in type 2 diabetes, characteristically associated with hyperinsulinemia and insulin resistance (18). We have described that circulating osteocalcin was positively associated with insulin secretion in humans (19), and these findings have been confirmed by other authors (20).
There is limited information in the literature concerning the effects of olive oil on circulating osteocalcin concentration in humans. In a recent study, participants were randomly assigned into two groups, one receiving vitamin K2 supplementation and the other placebo capsules containing olive oil. After 12 months, there were no between-group differences in bone loss rates at the total hip or any other measurement site. Serum levels of undercarboxylated osteocalcin decreased in the treatment vs. the placebo group (P < 0.001) (21), suggesting that in fact placebo (olive oil) led to a significant increase of undercarboxylated osteocalcin when compared with vitamin K2. We did not find significant changes in undercarboxylated osteocalcin after consumption of natural olive oil, in agreement with another study designed to test the effects of diets enriched with corn oil or an olive oil/sunflower oil mixture on vitamin K metabolism and vitamin K-dependent proteins in young men (22).
Interestingly, we also found that a higher intake of olives was associated with higher serum osteocalcin concentrations. Taken together, our findings concur with experimental reports that associate the consumption of olives (5), olive oil (6), and oleuropein (7) with the prevention of bone mass loss in animal models of osteoporosis (8, 9). In vitro studies have also shown that oleuropein intake leads to increased osteocalcin concentrations (10).
We have found decreased circulating CTX with all interventions. On the contary, several studies have reported elevations in serum and urinary markers of bone resorption after modest (5–15%) weight reduction over 6–12 months in both pre- and postmenopausal women and adult men and women induced via moderate energy restriction (reviewed in Ref. 23).
Serum calcium concentration was found to decrease in subjects under a low-fat diet and MedDiet+nuts. Individuals vary in their ability to absorb the calcium they consume. In one study, fractional calcium absorption was positively associated with dietary fat intake (r = 0.29, P = 0.001) (24). Subjects in the lowest tertile of the ratio of dietary fat to fiber had 19% lower fractional calcium absorption values (24). These differences could be behind decreased serum calcium concentration in subjects under a low-fat diet and MedDiet+nuts but not in those subjects consuming MedDiet+VOO.
Statins have been described to increase circulating osteocalcin (11). For this reason, we controlled for statin intake. We found that statin intake did not influence significantly the results regarding osteocalcin or P1NP changing levels in subjects of the MedDiet+VOO group.
The strengths of this study include the randomization of study subjects in a representative sample of men followed up during 2 yr. We did not measure bone density or β-cell function by using indexes more reliable and sensitive than HOMA-β-cell, and these are study limitations. Potentially important confounding factors, such as thyroid function or serum testosterone concentration were not systematically measured in these subjects. Furthermore, we performed only single measurements at baseline and at 2 yr of follow-up. However, the findings of P1NP were in parallel to those of osteocalcin.
In summary, the consumption of a MedDiet enriched with virgin olive oil for 2 yr is associated with increased serum osteocalcin concentrations that parallel an increase in β-cell function in elderly men at high cardiovascular risk, suggesting a protective effect on bone. To discern whether increased osteocalcin is the cause or the consequence of increased β-cell function awaits further studies, ideally in healthy volunteers.
Acknowledgments
Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición is an initiative of the Instituto de Salud Carlos III (Madrid, Spain). J.M.F.-R. participated in the experimental design and wrote and edited the manuscript; M.B. recollected the samples and performed the biochemical analysis; J.M.M.-N. performed the biochemical analysis; W.R., E.R., and R.E. revised the manuscript; and J.S.-S. designed the experiments and revised the manuscript.
This work was partially supported by research grants from the Ministerio de Educación y Ciencia (Grant SAF2008-02073) and by CIBERobn (CIBER Patofisiología Obesidad y Nutrición).
Disclosure Summary: J.S.-S. is a nonpaid member of the Scientific Advisory Board of the International Nut Council. E.R. is a nonpaid member of the Scientific Advisory Board of the California Walnut Commission. The other authors have no conflict of interest affecting the conduct or reporting of the work submitted.
Footnotes
- BMI
- Body mass index
- CTX
- C-telopeptide of type I collagen
- HDL
- high-density lipoprotein
- HOMA
- homeostasis model assessment
- HOMA-BCF
- HOMA-β-cell function
- HOMA-IR
- HOMA insulin resistance index
- LDL
- low-density lipoprotein
- P1NP
- procollagen I N-terminal propeptide
- PREDIMED
- Prevención con Dieta Mediterránea.
References
- 1. Seeman E, Delmas PD. 2006. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261 [DOI] [PubMed] [Google Scholar]
- 2. Raisz LG. 2005. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnell O, Gullberg B, Allander E, Kanis JA. 1992. The apparent incidence of hip fracture in Europe: a study of national register sources. MEDOS Study Group. Osteoporos Int 2:298–302 [DOI] [PubMed] [Google Scholar]
- 4. Kanis JA. 1993. The incidence of hip fracture in Europe. Osteoporos Int 3(Suppl 1):10–15 [DOI] [PubMed] [Google Scholar]
- 5. Puel C, Mardon J, Kati-Coulibaly S, Davicco MJ, Lebecque P, Obled C, Rock E, Horcajada MN, Agalias A, Skaltsounis LA, Coxam V. 2007. Black Lucques olives prevented bone loss caused by ovariectomy and talc granulomatosis in rats. Br J Nutr 97:1012–1020 [DOI] [PubMed] [Google Scholar]
- 6. Puel C, Quintin A, Agalias A, Mathey J, Obled C, Mazur A, Davicco MJ, Lebecque P, Skaltsounis AL, Coxam V. 2004. Olive oil and its main phenolic micronutrient (oleuropein) prevent inflammation-induced bone loss in the ovariectomised rat. Br J Nutr 92:119–127 [DOI] [PubMed] [Google Scholar]
- 7. Puel C, Mathey J, Agalias A, Kati-Coulibaly S, Mardon J, Obled C, Davicco MJ, Lebecque P, Horcajada MN, Skaltsounis AL, Coxam V. 2006. Dose-response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clin Nutr 25:859–868 [DOI] [PubMed] [Google Scholar]
- 8. Visioli F, Galli C. 2002. Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nutr 42:209–221 [DOI] [PubMed] [Google Scholar]
- 9. Habauzit V, Horcajada MN. 2008. Phenolic phytochemicals and bone. Phytochem Rev 7:21 [Google Scholar]
- 10. Santiago-Mora R, Casado-Díaz A, De Castro MD, Quesada-Gómez JM. 2011. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: the effect on differentiation in stem cells derived from bone marrow. Osteoporos Int 22:675–684 [DOI] [PubMed] [Google Scholar]
- 11. Kanazawa I, Yamaguchi T, Yamauchi M, Sugimoto T. 2009. Rosuvastatin increased serum osteocalcin levels independent of its serum cholesterol-lowering effect in patients with type 2 diabetes and hypercholesterolemia. Intern Med 48:1869–1873 [DOI] [PubMed] [Google Scholar]
- 12. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martínez-González MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol M, Wärnberg J, Arós F, Ruíz-Gutiérrez V, Lamuela-Raventós RM, Lapetra J, Muñoz MÁ, Martínez JA, Sáez G, Serra-Majem L, Pintó X, Mitjavila MT, Tur JA, Portillo Mdel P, Estruch R; for the PREDIMED Study Investigators 2012. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 41:377–385 [DOI] [PubMed] [Google Scholar]
- 14. Zazpe I, Sanchez-Tainta A, Estruch R, Lamuela-Raventos RM, Schröder H, Salas-Salvado J, Corella D, Fiol M, Gomez-Gracia E, Aros F, Ros E, Ruíz-Gutierrez V, Iglesias P, Conde-Herrera M, Martinez-Gonzalez MA. 2008. A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean-type diets: the PREDIMED study. J Am Diet Assoc 108:1134–1144; discussion 1145 [DOI] [PubMed] [Google Scholar]
- 15. Mataix J. 2009. Tabla de composición de alimentos. 5th ed Granada, Spain: Universidad de Granada [Google Scholar]
- 16. Elosua R, Garcia M, Aguilar A, Molina L, Covas MI, Marrugat J. 2000. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish women. Investigators of the MARATHON Group. Med Sci Sports Exerc 32:1431–1437 [DOI] [PubMed] [Google Scholar]
- 17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 18. Thrailkill KM, Lumpkin CK, Jr, Bunn RC, Kemp SF, Fowlkes JL. 2005. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab 289:E735–E745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernández-Real JM, Izquierdo M, Ortega F, Gorostiaga E, Gómez-Ambrosi J, Moreno-Navarrete JM, Frühbeck G, Martínez C, Idoate F, Salvador J, Forga L, Ricart W, Ibañez J. 2009. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab 94:237–245 [DOI] [PubMed] [Google Scholar]
- 20. Winhofer Y, Handisurya A, Tura A, Bittighofer C, Klein K, Schneider B, Bieglmayer C, Wagner OF, Pacini G, Luger A, Kautzky-Willer A. 2010. Osteocalcin is related to enhanced insulin secretion in gestational diabetes mellitus. Diabetes Care 33:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Emaus N, Gjesdal CG, Almås B, Christensen M, Grimsgaard AS, Berntsen GK, Salomonsen L, Fønnebø V. 2010. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int 21:1731–1740 [DOI] [PubMed] [Google Scholar]
- 22. Schurgers LJ, Shearer MJ, Soute BA, Elmadfa I, Harvey J, Wagner KH, Tomasch R, Vermeer C. 2002. Novel effects of diets enriched with corn oil or with an olive oil/sunflower oil mixture on vitamin K metabolism and vitamin K-dependent proteins in young men. J Lipid Res 43:878–884 [PubMed] [Google Scholar]
- 23. Hinton PS, LeCheminant JD, Smith BK, Rector RS, Donnelly JE. 2009. Weight loss-induced alterations in serum markers of bone turnover persist during weight maintenance in obese men and women. J Am Coll Nutr 28:565–573 [DOI] [PubMed] [Google Scholar]
- 24. Wolf RL, Cauley JA, Baker CE, Ferrell RE, Charron M, Caggiula AW, Salamone LM, Heaney RP, Kuller LH. 2000. Factors associated with calcium absorption efficiency in pre- and perimenopausal women. Am J Clin Nutr 72:466–471 [DOI] [PubMed] [Google Scholar]