Abstract
Context:
A diagnosis of Addison's disease means lifelong dependence on daily glucocorticoid and mineralocorticoid therapy and is associated with increased morbidity and mortality as well as a risk of unexpected adrenal crisis.
Objective:
The objective of the study was to determine whether immunomodulatory therapy at an early stage of autoimmune Addison's disease could lead to preservation or improvement in adrenal steroidogenesis.
Design and Intervention:
This was an open-label, pilot study of B lymphocyte depletion therapy in new-onset idiopathic primary adrenal failure. Doses of iv rituximab (1 g) were given on d 1 and 15, after pretreatment with 125 mg iv methylprednisolone.
Patients and Main Outcome Measures:
Six patients (aged 17–47 yr; four females) were treated within 4 wk of the first diagnosis of idiopathic primary adrenal failure. Dynamic testing of adrenal function was performed every 3 months for at least 12 months.
Results:
Serum cortisol levels declined rapidly and were less than 100 nmol/liter (3.6 μg/dl) in all patients by 3 months after B lymphocyte depletion. Serum cortisol and aldosterone concentrations remained low in five of the six patients throughout the follow-up period. However, a single patient had sustained improvement in both serum cortisol [peak 434 nmol/liter (15.7 μg/dl)] and aldosterone [peak 434 pmol/liter (15.7 ng/dl)] secretion. This patient was able to discontinue steroid medications 15 months after therapy and remains well, with improving serum cortisol levels 27 months after therapy.
Conclusion:
New-onset autoimmune Addison's disease should be considered as a potentially reversible condition in some patients. Future studies of immunomodulation in autoimmune Addison's disease may be warranted.
Autoimmune Addison's disease (AAD) is caused by immune-mediated destruction of the steroid-producing cells of the adrenal cortex, leading to reduced circulating cortisol and aldosterone concentrations (1–3). During the late 1940s, the availability of synthetic cortisone acetate transformed the prognosis of adrenal failure, changing it from a lethal condition to a chronic and manageable one (1). However, there have been no significant treatment advances in AAD for the last 50 yr, and patients with AAD have a lifelong dependency on daily treatment with replacement glucocorticoid and mineralocorticoid (1, 2). There is an ever-present risk of an unexpected adrenal crisis, and this curtails patients' activities (4), contributing to a reduction in the quality of life (5). In addition, there are specific complications associated with chronic glucocorticoid use, including osteoporosis, fracture, and impaired glucose tolerance (6, 7). Several longitudinal surveys have also documented a reduced life expectancy in individuals with Addison's disease (8, 9). Thus, although current endocrine replacement therapy makes patients with newly diagnosed AAD feel better almost immediately, in many cases, it does not fully restore their well-being, nor does it lead to ideal long-term health.
With the above provisos in mind, we wanted to explore novel therapeutic options for patients with newly diagnosed AAD. Type 1 diabetes has many pathophysiological features in common with AAD (2, 3), including immune-mediated destruction of hormone-secreting cells, circulating autoantibodies directed at tissue-specific antigens, and a complex genetic basis with several shared susceptibility alleles, notably those of the major histocompatibility complex (3, 10). And in recent years, several studies have targeted the autoimmune attack early on in the natural history of type 1 diabetes with some success, including with B lymphocyte-depleting anti-CD20 antibodies (11, 12). Importantly, adrenocortical plasticity is a regularly observed clinical phenomenon. In patients being weaned from chronic exogenous glucocorticoid therapy, there is functional adrenal failure, which usually recovers over a period of several months as secretion of the adrenocortical master regulatory hormone, ACTH, is reestablished. Thus, we hypothesized that if the immune attack in newly diagnosed AAD could be effectively quashed, then adrenocortical steroidogenesis might similarly recover. This manuscript reports on an exploratory, open-label study of B lymphocyte depletion therapy in six patients with newly diagnosed idiopathic Addison's disease.
Patients and Methods
Patients
Six patients between the ages of 16 and 65 yr who had been diagnosed with idiopathic primary adrenal failure within the previous 28 d were recruited, either in Newcastle upon Tyne (n = 5) or Exeter, UK. Eligibility criteria included biochemical evidence of adrenocortical failure with subnormal serum cortisol response to 250 μg iv tetracosactide (synacthen; peak serum cortisol <350 nmol/liter) with biochemical evidence to confirm elevated ACTH or evidence of mineralocorticoid insufficiency. In addition, all patients underwent adrenal computed tomography scan, and normal or atrophic adrenal glands were confirmed before study medication was administered. Patients with active infective illness, other serious cardiorespiratory, renal, or hepatic comorbidity, or pregnancy or other condition that would preclude rituximab treatment were excluded (a full list of exclusion criteria is given in the Supplemental Information, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). The recruitment algorithm is shown as Supplemental Fig. 1. The study was registered as NCT00753597. The Northern and Yorkshire Research Ethics Committee gave approval (reference 08/H0903/32).
Design and treatment regimen
This was an open-label pilot study in which all participants received active treatment and were followed up for at least 12 months, with outcome assessments of adrenal steroidogenesis every 3 months. On d 1 and 15, patients were pretreated with 1 g oral paracetamol, 10 mg iv chlorpheniramine, and 125 mg iv methylprednisolone, followed by 1 g iv rituximab infused over 5.25 h (13). At diagnosis, all patients had been treated with oral hydrocortisone in doses ranging from 15 to 30 mg daily. After the first rituximab infusion, oral glucocorticoid and mineralocorticoid replacement was continued for at least 12 months (detailed in Supplemental Information).
Outcome measures and assessments
After baseline testing, outcome measurements were made at the end of months 3, 6, 9. and 12 (during wk 13, 26, 39, and 52). All steroid hormone measurements were made after 38–42 h of withdrawal from regular glucocorticoid and fludrocortisone replacement therapy. The morning before the day of assessment, all steroid replacement medication was omitted and patients were observed in the research facility until dynamic testing was complete on the following morning. Three of the 28 outcome visits were delayed by a week due to intercurrent illness (common cold, upper respiratory tract infection, urine infection), during which patients were judged as being potentially unsafe to stop medication. ACTH stimulation testing started between 0830 and 0900 h, with a basal serum sample for cortisol followed by a 250-μg iv bolus of tetracosactide and additional blood samples at 30 and 60 min.
Statistical analysis
All probability values shown are two sided from paired t tests, except where stated. Changes in serum 21-hydroxylase antibody concentration were analyzed by Wilcoxon ranked sign test (implemented in the R package) (14).
Results
All patients presented with features of acute adrenal insufficiency requiring hospitalization, associated with chronic weight loss and pigmentation. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics at diagnosis
| Patient ID | Sex, age (yr) | Duration of illness (months) | Serum Na+/K+ at diagnosis (mmol/liter)a | Peak serum cortisol (nmol/liter)b | Plasma ACTH (ng/liter)c | Adrenal cell Abd | 21-hydroxylase Ab (U/ml)e | HLA-DRB1 genotype | Personal or family autoimmunity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F 44 | 6 | 132/5.2 | 209 | >1250 | Neg | 0.68 | *0101/*1301 | Graves' disease |
| 2 | F 19 | 4 | 130/5.3 | 111 | >1250 | Pos | 345 | *0301/*0404 | Grandmother hypothyroid |
| 3 | F 38 | 12 | 132/4.4 | 80 | ND | Pos | 1669 | *0301/*0301 | Graves' disease; mother celiac |
| 4 | M 25 | 1.5 | 116/5.4 | 145 | 430 | Neg | 1.29 | *0101/*1501 | |
| 5 | F 17 | 6 | 118/4.9 | 235 | 629 | Pos | 19200 | *0301/*0801 | |
| 6 | M 47 | 4 | 130/5.0 | 70 | >1250 | Neg | 6.3 | *1301/*1501 |
F, Female; M, male; Neg, negative; Pos, positive; ND, not done.
Reference range for serum Na+ is 135–145 mmol/liter, K+ is 3.5–5.2 mmol/liter.
Reference range after tetracosactide is greater than 550 nmol. To convert from nanomoles per liter to micrograms per deciliter, divide by 27.6.
Reference range is 10–55 ng/liter.
Adrenal cell antibodies were measured by indirect immunofluorescence of patient sera on monkey adrenal slices (Prodiagnostics, Huntington, WV).
21-Hydroxylase antibodies were measured by immunoprecipitation.15 Reference range is less than 1.0 U/ml.
Immune parameters
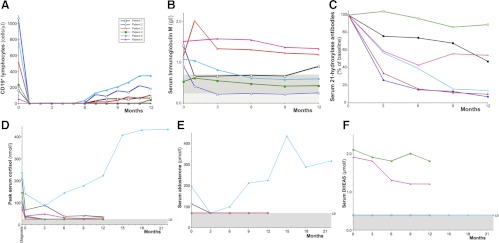
After the rituximab infusions, all patients had complete depletion of circulating B lymphocytes by 28 d, which lasted from 5 to 12 months (Fig. 1A). Serum IgM concentrations also declined [mean 1.09 g/liter at baseline to 0.78 g/liter at 12 months (P = 0.04)] (Fig. 1B). Five of six participants had concentrations of 21-hydroxylase greater than 1.0 U/ml (the threshold of normality) at baseline (Table 1), and all patients had reductions in concentration over follow-up (P = 0.031; Wilcoxon ranked sign test) (Fig. 1C).
Fig. 1.
Serial measurements of immune and endocrine parameters. A, Absolute peripheral B lymphocyte numbers over time after rituximab therapy. B lymphocytes were measured as CD19+ cells at flow cytometry (10,000 lymphocyte events counted twice for each measurement; complete depletion was judged as CD19+ < 0.1% of lymphocytes) (Becton Dickinson Instruments, Oxford, UK). The reference range for CD19+ is 90–660 cells/μl. B, The concentration of serum IgM, measured by immunometric nephelometry (BN2; Dade Behring, Surrey, UK), is shown over time after rituximab therapy. The lower limit of the adult female reference range is shown at the top of the gray shaded box (0.72 g/liter), and the lower limit of the adult male reference interval is shown at the top of the hatched box (0.24 g/liter). Three of the four female patients in the study (patients 1, 2, and 5) developed subnormal IgM levels during follow-up. C, The concentration of serum 21-hydroxylase autoantibodies over time is shown as a percentage of the baseline measurement. Measurements were made in duplicate by immunoprecipitation of radiolabeled recombinant in vitro translated 21-hydroxylase protein (RSR Ltd.) (15). For patient 5, sera were diluted 100-fold to measure the concentration. Absolute values of 21-hydroxylase antibody at baseline are shown for each subject in Table 1. D, Serial peak serum cortisol concentrations at diagnosis, at study baseline, and after rituximab treatment. All steroid hormone measurements were made after 38–42 h of withdrawal from regular glucocorticoid and fludrocortisone replacement therapy. For each time point, the highest serum cortisol is recorded, either the baseline sample or after the iv tetracosactide 250 μg (30 or 60 min). The normal response to this stimulus is a peak cortisol concentration greater than 550 nmol/liter (20 μg/dl). Measurements were made using a competitive chemoluminescent immunoassay on a Centaur platform (Siemens, Surrey, UK) with a limit of detection (LD) of 25 nmol/liter (0.91 μg/dl), shown as the shaded horizontal area. To convert serum cortisol from nanomoles per liter to micrograms per deciliter, divide by 27.6. E, Serial recumbent serum aldosterone concentrations at study baseline and after rituximab treatment. The reference range for recumbent serum aldosterone is 100–450 pmol/liter. Measurements were made by solid-phase RIA (DPC Coat-a-Count kit; Diagnostic Products Corp., Surrey, UK), with a LD of 70 pmol/liter, shown as the shaded horizontal area. Several patients' data are superimposed on this limit of detection line. To convert serum aldosterone from picomoles per liter to nanograms per deciliter, divide by 27.7. F, Serial serum DHEAS concentrations at study baseline and after rituximab treatment. Measurements were made by solid-phase competitive chemoluminescence on an Immulite platform (Siemens) with a LD of 0.4 μmol/liter (shown as the shaded horizontal area). The four female patients' data are superimposed on this limit of detection line. The female reference interval is 1.5–10.5 μmol/liter, and the male reference interval is 2.1–12.9 μmol/liter. To convert serum DHEAS from micromoles per liter to micrograms per deciliter, divide by 0.0271.
Steroidogenic function
Before any study intervention, peak serum cortisol concentrations decreased, from a mean of 141 ± 28 (sem) nmol/liter (5.1 ± 1.0 μg/dl) at the time of first clinical diagnosis to 57 ± 18 nmol/liter (2.1 ± 0.65 μg/dl) (P = 0.004) at baseline retesting 14–22 d later (Fig. 1D). After the trial therapy, five of six patients had serum cortisol concentrations that remained below 100 nmol/liter (3.2 μg/dl) between 3 and 12 months' follow-up. Similarly, five of the six patients had serum aldosterone concentrations below the threshold for detection [<70 pmol/liter (< 2.53 ng/dl)] from the 3-month assessment onward (Fig. 1E). In contrast, patient 5 had a steady increase in both peak serum cortisol and serum aldosterone after the 3-month visit the current time. At 15 months after B cell depletion therapy, her peak serum cortisol was 407 nmol/liter (14.7 μg/dl) and serum aldosterone 434 pmol/liter (15.7ng/dl), so steroid medications were progressively reduced and stopped. She has remained well for 12 months without medication, with current peak serum cortisol 434 nmol/liter (15.7 μg/dl), serum aldosterone 316 pmol/liter (11.4 ng/dl), with a corresponding plasma ACTH of 569 ng/liter (10–55) and renin activity 14.5 pmol/ml·h (1.0–5.5). Her weight has progressively increased from 50.6 kg at diagnosis to a most recent weight of 64.6 kg. Serum dehydroepiandrosterone sulfate (DHEAS) concentrations remained below the limit of detection in all female patients and low in the male participants (Fig. 1F).
Safety and tolerability
The rituximab infusions were well tolerated with only two minor infusion reactions during the first infusion in different participants (headache, nausea; both grade 1). There was one serious adverse event that may have been causally related to the interventions (coliform urine infection).
Discussion
This study is the first, to our knowledge, that has attempted to modify the natural history of autoimmune Addison's disease using an immunomodulatory treatment. Although five of the six patients had an early and persistent decline in adrenocortical steroidogenesis after the diagnosis of Addison's disease, one patient had a steady improvement in serum cortisol and aldosterone secretion during the observation period. Fifteen months after therapy, we were able to discontinue glucocorticoid and mineralocorticoid replacement, and this patient has now felt well without medication for 12 months. Even though this patient does not have entirely normal adrenal function, as judged by a persistent elevation in plasma ACTH and renin activity, such an improvement of adrenal steroidogenic function is still remarkable. This has changed our perception of autoimmune Addison's disease from one of a chronic but largely manageable autoimmune condition to one in which disease-modifying therapy directed against the immune-response could lead to a cure, at least in the short term, in certain patients. A similar therapeutic model has been investigated in type 1 diabetes for several years, with some notable successes (11, 12).
Regeneration of adrenal steroidogenesis after the immunomodulation of autoimmune Addison's disease might be predicted from several observations. First, plasticity of adrenocortical function is regularly observed in clinical practice in several situations. In Cushing's disease and congenital adrenal hyperplasia, excess ACTH stimulation leads to adrenal growth and, in the former, excess glucocorticoid secretion. Conversely, after treatment with exogenous glucocorticoids, there is adrenal atrophy and functional adrenal failure, which reverses as the exogenous steroid therapy is withdrawn. Second, islands of hyperplastic adrenocortical cells were observed at postmortem examination of Addisonian patients more than 80 yr ago (16) and suggest that the plasticity of adrenal cortex remains until late on in the natural history of the untreated disease. Lastly, spontaneous recovery of Addison's disease has been reported in two cases, one with clear evidence of autoimmune etiology (17, 18). In a third case, recovery of early adrenal failure was observed after high-dose oral steroid therapy for inflammatory thyroid orbitopathy (19). Thus, the intrinsic plasticity of adrenal function makes AAD an attractive theoretical target for disease-modifying therapies. This study shows an important proof of this concept, that such a therapeutic approach can be successful in practice.
Although one patient has had a successful short-term outcome from B cell depletion therapy in our study, five patients had no benefit from the intervention. The identification of patients who are the most likely to benefit from such a treatment is therefore a key issue for future studies. Importantly, patient 5 had the highest concentration of both cortisol and aldosterone at baseline testing of all the patients, so her presentation with AAD may be regarded as earlier on in the natural history of declining steroidogenesis and hence more tractable to recovery. Patient 5 also had the highest level of circulating autoantibodies to 21-hydroxlase. These antibodies may be considered as a biomarker for the humoral immune response in AAD, and this may be a contributing factor to the efficacy of B lymphocyte depletion in her case. For future clinical trials of disease-modifying therapy in AAD, we would recommend an early assay of 21-hydroxylase antibodies as well as commencing any disease-modifying treatment as soon as possible.
The natural history of steroidogenesis in newly diagnosed AAD has not previously been studied in detail. We were surprised by the rapid decline in circulating cortisol levels that, on average, more than halved within 3 wk of diagnosis. We presume this is a combination of the continuing aggressive immune-mediated destruction of the adrenal cortex, coupled with the loss of tropic stimulation owing to reductions in plasma ACTH, consequent to steroid replacement medication resulting in less steroid biosynthesis. The previous report of recovery of early Addison's disease in a patient treated with high-dose glucocorticoids for another indication was consistent with a possible immunomodulatory effect of glucocorticoid therapy in AAD, (19) as was a previous study in adrenal cell antibody positive patients without overt adrenal dysfunction (20). However, our data concerning adrenocortical function after commencement of replacement doses of glucocorticoid and mineralocorticoid suggest that at the symptomatic stage of AAD, this likely contributes to a rapid decline in cortisol by removing the tropic influence of ACTH. Having observed this phenomenon in patients 1–4, we changed the study protocol to give a much lower dose of glucocorticoid in the early part of the study in the remaining two patients. This may have contributed to the improved outcome in patient 5. In future studies of new-onset Addison's disease, we would suggest that replacement steroid medications are carefully titrated to avoid overtreatment and to maintain some endogenous tropic drive to adrenal cortisol and aldosterone production. AAD is generally assumed to be a monophasic autoimmune disorder with an inevitable decline to complete adrenocortical failure, and this is supported by a study reexamining adrenal function in a cohort of 27 patients (21). Nevertheless, if the immune attack on the adrenal cortex was fluctuant, then a relapsing-remitting disease course could occur, which might also explain certain of our findings.
In summary, we treated six patients with new-onset Addison's disease with B cell depletion therapy. One patient had a recovery of adrenal function. We believe that autoimmune Addison's disease should be considered as a reversible condition in some patients and that future studies using immunomodulatory approaches in new-onset disease are now warranted.
Supplementary Material
Acknowledgments
Infrastructure support to Newcastle upon Tyne Hospitals Foundation National Health Service Trust was provided by the U.K. Comprehensive Local Research Network. We thank the trial oversight committee: Professor G. Ford, Sr. Kim Johnson, Dr. P. Perros, and Mrs. K. White. We are grateful to the Clinical Research Facility nursing and administration staff, in particular Jan Gebbie, John Wilson, Jill Moran, and Tracy Hill, and to RVI, and the National Blood Transfusion, laboratory services including Mr. Steve Turner, Mr. Roy Ward, Ms. Dawn Barge, and Mr. Vaughan Carter. We thank Dr. M. Santibanez-Koref for statistical advice. We are also grateful to the participants in this study and to the U.K. Addison's disease self-help group (www.addisons.org.uk) for comments on the protocol and for help in recruiting. Trial registration is NCT00753597 (www.clinicaltrials.gov).
This work was supported by the Medical Research Council, Experimental Medicine-2 panel (Grant G07017632), United Kingdom. B.R.S. and S.Che. are employees of RSR Ltd.
Disclosure Summary: S.H.S.P. and J.D.I. conceived the study; S.H.S.P., A.L.M., J.D.I., and B.V. wrote the protocol; S.H.S.P., A.L.M., S.B., P.K., S.Cha., S.N., and B.V. managed the patients day to day in the study; S.Che. and B.R.S. performed the 21-hydroxylase assays; S.H.S.P. wrote the first draft of the manuscript. All authors commented and made amendments to the final version. B.R.S. and S.Che. are employees of RSR Ltd. RSR Ltd. is a developer of medical diagnostics including kits for measuring 21-hydroxylase autoantibodies. No other author declares any potential conflict of interest.
Footnotes
- AAD
- Autoimmune Addison's disease
- DHEAS
- dehydroepiandrosterone sulfate.
References
- 1. Chakera AJ, Vaidya B. 2010. Addison disease in adults: diagnosis and management. Am J Med 123:409–413 [DOI] [PubMed] [Google Scholar]
- 2. Michels AW, Eisenbarth GS. 2010. Immunologic endocrine disorders. J Allergy Clin Immunol 125:S226–S237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitchell AL, Pearce SH. 2012. Pathogenesis and genetic complexity of autoimmune Addison's disease. Nat Rev Endocrinol 8:306–316 [DOI] [PubMed] [Google Scholar]
- 4. White K, Arlt W. 2010. Adrenal crisis in treated Addison's disease: a predictable but under-managed event. Eur J Endocrinol 162:115–120 [DOI] [PubMed] [Google Scholar]
- 5. Øksnes M, Bensing S, Hulting AL, Kämpe O, Hackemann A, Meyer G, Badenhoop K, Betterle C, Parolo A, Giordano R, Falorni A, Papierska L, Jeske W, Kasperlik-Zaluska AA, Chatterjee VK, Husebye ES, Løvås K. 2012. Quality of life in European patients with Addison's disease: validity of the disease-specific questionnaire AddiQoL. J Clin Endocrinol Metab 97:568–576 [DOI] [PubMed] [Google Scholar]
- 6. Björnsdottir S, Sääf M, Bensing S, Kämpe O, Michaëlsson K, Ludvigsson JF. 2011. Risk of hip fracture in Addison's disease: a population-based cohort study. J Intern Med 270:187–195 [DOI] [PubMed] [Google Scholar]
- 7. Soule S. 1999. Addison's disease in Africa—teaching hospital experience. Clin Endocrinol (Oxf) 50:115–120 [DOI] [PubMed] [Google Scholar]
- 8. Bensing S, Brandt L, Tabaroj F, Sjöberg O, Nilsson B, Ekbom A, Blomqvist P, Kämpe O. 2008. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf) 69:697–704 [DOI] [PubMed] [Google Scholar]
- 9. Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, Johannsson G. 2006. Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab 91:4849–4853 [DOI] [PubMed] [Google Scholar]
- 10. Baker PR, Baschal EE, Fain PR, Triolo TM, Nanduri P, Siebert JC, Armstrong TK, Babu SR, Rewers MJ, Gottlieb PA, Barker JM, Eisenbarth GS. 2010. Haplotype analysis discriminates genetic risk for DR3-associated endocrine autoimmunity and helps define extreme risk for Addison's disease. J Clin Endocrinol Metab 95:E263–E270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waldron-Lynch F, Herold KC. 2011. Immunomodulatory therapy to preserve pancreatic β-cell function in type 1 diabetes. Nat Rev Drug Discov 10:439–452 [DOI] [PubMed] [Google Scholar]
- 12. Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS. 2009. Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N Engl J Med 361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. 2004. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350:2572–2581 [DOI] [PubMed] [Google Scholar]
- 14. R Development Core Team 2011. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 15. Tanaka H, Perez MS, Powell M, Sanders JF, Sawicka J, Chen S, Prentice L, Asawa T, Betterle C, Volpato M, Smith BR, Furmaniak J. 1997. Steroid 21-hydroxylase autoantibodies: measurements with a new immunoprecipitation assay. J Clin Endocrinol Metab 82:1440–1446 [DOI] [PubMed] [Google Scholar]
- 16. Guttman PH. 1930. Addison's disease: a statistical analysis of five hundred and sixty-six cases and a study of the pathology. Arch Pathol 10:742–785 [Google Scholar]
- 17. Smans LC, Zelissen PM. 2008. Partial recovery of adrenal function in a patient with autoimmune Addison's disease. J Endocrinol Invest 31:672–674 [DOI] [PubMed] [Google Scholar]
- 18. Chakera AJ, Vaidya B. 10 September 2011. Spontaneously resolving Addison's disease. Q J Med 10.1093/qjmed/hcr162 [DOI] [PubMed] [Google Scholar]
- 19. De Bellis A, Falorni A, Laureti S, Perrino S, Coronella C, Forini F, Bizzarro E, Bizzarro A, Abbate G, Bellastella A. 2001. Time course of 21-hydroxylase antibodies and long-term remission of subclinical autoimmune adrenalitis after corticosteroid therapy: case report. J Clin Endocrinol Metab 86:675–678 [DOI] [PubMed] [Google Scholar]
- 20. De Bellis A, Bizzarro A, Rossi R, Paglionico VA, Criscuolo T, Lombardi G, Bellastella A. 1993. Remission of subclinical adrenocortical failure in subjects with adrenal autoantibodies. J Clin Endocrinol Metab 76:1002–1007 [DOI] [PubMed] [Google Scholar]
- 21. Smans LC, Zelissen PM. 2011. Does recovery of adrenal function occur in patients with autoimmune Addison's disease? Clin Endocrinol (Oxf) 74:434–437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.