Abstract
Background and Objectives:
The diagnosis of central adrenal insufficiency (AI) continues to be challenging, especially when it is partial. We have recently demonstrated the value of measuring serum dehydroepiandrosterone sulfate (DHEA-S) in establishing the diagnosis of central AI. The current investigation examined the added value of measuring serum dehydroepiandrosterone (DHEA) levels during low-dose (1 μg) cosyntropin (LDC) stimulation in patients suspected to have central AI.
Methods:
Baseline and LDC-stimulated cortisol, DHEA, and DHEA-S were measured preoperatively in 155 consecutive patients with pituitary masses and 63 healthy subjects. Hypothalamic-pituitary adrenal (HPA) function was normal (NL-HPA) in 97 of the patients and was impaired (impaired HPA) in 58 patients. Patients with NL-HPA underwent surgical removal of the sellar masses and received no glucocorticoids before, during, or after surgery.
Results:
Baseline and LDC-stimulated serum cortisol, DHEA, and DHEA-S in patients with NL-HPA were similar to those of normal subjects. In contrast, patients with impaired HPA had lower baseline and LDC-stimulated serum cortisol, DHEA, and DHEA-S levels. There were 18 subjects in the latter group whose LDC-stimulated serum cortisol levels were greater than 18.0 μg/dl. In those 18 subjects, baseline and LDC-stimulated DHEA and DHEA-S levels were similar to the whole group of patients with impaired HPA function. The molar ratio of cortisol to DHEA did not change with LDC stimulation in normal subjects and those with NL-HPA. In contrast, patients with impaired HPA had a higher baseline cortisol to DHEA molar ratio that increased further with LDC stimulation.
Conclusions:
Patients with impaired HPA function have a more severe loss in DHEA secretion than that of glucocorticoids. Measurements of serum DHEA levels during LDC simulation provide additional valuable information that improves the diagnostic accuracy of LDC in patients suspected to have central AI. We recommend the inclusion of DHEA and DHEA-S measurements in the laboratory assessment of HPA function.
The integrity of the hypothalamic-pituitary-adrenal (HPA) axis is essential for maintaining adequate adrenal glucocorticoid secretion during various physiological functions. Central adrenal insufficiency (AI) is characterized by impaired cortisol production as a result of decreased CRH and/ or ACTH secretion. The deficiency of the latter two hormones is frequently partial, and thus, cortisol secretion is maintained albeit at a subphysiological level. In light of the latter phenomenon, establishing the diagnosis of central AI continues to be a challenge. This will be especially true when the diagnostic tests used include stimulation with CRH and/or cosyntropin (1–4).
Currently available methods to establish the biochemical diagnosis of central AI have many limitations. These tests include measurements of serum cortisol concentrations in the basal (i.e. unstimulated state) or after the introduction of stimuli such as hypoglycemia (insulin induced hypoglycemia) or an ACTH-like compound, namely, cosyntropin. Basal serum cortisol levels are helpful only when they are sufficiently elevated at greater than 12 μg/dl or greater than13 μg/dl (1, 2) or sufficiently low at less than 5 μg/dl (1, 2) to eliminate or confirm the diagnosis, respectively. Although the insulin-induced hypoglycemia test (IIH) remains the gold standard for diagnosis, it is often prohibitively unsafe in some patients such as children, the elderly, those with cardiac disease, and others with a history of seizures, to name a few (5, 6).
Dynamic testing with cosyntropin stimulation is the most commonly used method to evaluate HPA function. The standard-dose (250 μg, iv) cosyntropin test is severely limited by its poor sensitivity, which may be as low as 57% (3) and 79% (2). The low-dose (1 μg) cosyntropin test (LDC) has improved sensitivity, but it remains significantly flawed (1, 4, 7). The latter test lacks specificity and can give false-negative results, depending on the cutoff point used to define normality and the degree of ACTH deficiency. For example, when the cutoff point for defining a normal response to the LDC test is set at 18.1 μg/dl, approximately one third of patients with central AI (as defined by their inadequate response to IIH) will have levels higher than that and give a false-negative test result (4). The latter percentage reaches the 10% mark when the cutoff point defining normal is set at a higher level (20 μg/dl) and is virtually eliminated when the cutoff for defining normal is set at 22 μg/dl or greater (4). This is primarily because the partial nature of ACTH deficiency allows patients with central AI to achieve adequate responses to the LDC test (4, 8).
In an earlier study, we have demonstrated the utility of measuring the adrenal androgen dehydroepiandrosterone sulfate (DHEA-S) in the assessment of HPA function (4). Adrenal androgens are secreted by the zona reticularis of the adrenal cortex and are regulated primarily by ACTH. A major advantage of the latter physiological phenomenon is that adrenal androgen secretion can be viewed as another adrenal steroid product of ACTH action on the adrenal cortex. Similarly, measurements of adrenal androgen secretion can be viewed as an alternate method of evaluating the integrity of HPA function. In the latter study, we showed that patients with central AI as documented by IIH have drastically low serum DHEA-S levels, even those who achieved normal cortisol responses to LDC (4). In fact, the data indicated that an age- and gender-appropriate DHEA-S level effectively ruled out the diagnosis of central AI with a sensitivity approaching 100% (4). Observations reported by other investigators showed that serum DHEA-S levels were low in patients with central AI (9, 10). A major limitation of these observations is that they included data on patients previously or currently treated with glucocorticoids, which would by itself impair adrenal androgen secretion.
In the current investigation, we extend the earlier observations of basal serum DHEA-S measurements by determining serum dehydroepiandrosterone (DHEA) level changes during stimulation with cosyntropin in patients at risk for central AI. Specifically, we present a prospective study evaluating dynamic measurements of DHEA during LDC as an additional approach to evaluate HPA function. The results of the study indicate that measurements of serum DHEA and DHEA-S levels provide valuable additional information that are helpful in establishing the diagnosis of central AI. The data also indicate that age- and gender-adjusted levels of DHEA and those of DHEA-S are sensitive indicators of intact adrenal cortical function. The data also indicate that in patients with central AI, loss of adrenal androgen secretion precedes that of glucocorticoids.
Subjects and Methods
Subjects
Consecutive, adult patients with a new diagnosis of a large pituitary mass (>1.0 cm) were included in the study. All patients underwent a complete evaluation of pituitary function before surgery or any glucocorticoid administration, although only data pertaining to the HPA axis are presented here. Patients with hypoproteinemia, as well as others with ACTH-secreting tumors, glucocorticoid exposure, or those receiving medications that alter cortisol measurements, such as estrogen, were excluded. Tumor type was identified based on immunohistochemical staining of multiple sections of the resected adenoma, and in the case of unresected prolactinomas the diagnosis was made on the basis of serum prolactin levels greater than 200 μg/liter.
All patients and healthy subjects had the LDC stimulation test as described below. Although the serum cortisol data were used to define the patients' HPA function, the adrenal androgen data were obtained but not used in making clinical decision on the need for glucocorticoid replacement therapy.
Assessment of HPA function
HPA function was assessed in all patients as follows. A random serum cortisol and plasma ACTH levels were obtained at the patient's first visit. Patients with a normal plasma ACTH concentration and a random serum cortisol of 12 μg/dl or greater and who have no symptoms to suggest AI were considered to have a normal HPA function (NL-HPA). The latter group of patients had the LDC stimulation test as part of this investigation. All of these patients underwent surgical adenomectomy and were not given any glucocorticoids before, during, or after the procedure and were documented to have appropriate increases in their serum cortisol levels in the perioperative period.
Repeat testing in the morning was performed in patients who had a random serum cortisol of less than 5 μg/dl. A morning (0800–0900 h) serum cortisol of less than 5 μg/dl was considered an indication of impaired HPA function (IMPAIRED-HPA). The latter group of patients had the LDC-stimulation test as part of this investigation. Patients with a random serum cortisol level of 5–12 μg/dl had a LDC stimulation test done to define their HPA functional status. In such instances, an LDC-stimulated serum cortisol of 23 μg/dl or greater was considered an indication of NL-HPA, whereas a level less than 18 μg/dl was indicative of IMPAIRED-HPA. These cutoff values were chosen based on our extensive experience in more than 500 patients who had the test and were similar to conclusions reached by other investigators in a recent meta-analysis (2). When the LDC-stimulated serum cortisol level was 18–22 μg/dl, an IIH test was done and a normal response was defined as a peak serum cortisol of greater than 18.5 μg/dl, provided adequate hypoglycemia (glucose <40 mg/dl) was achieved.
The LDC was performed on all patients included in this study as well as a matching group of healthy volunteers. The LDC stimulation test was performed as described earlier (4) by a nursing staff with more than 20 yr of experience in performing this and other dynamic tests. Although serum cortisol, DHEA, and DHEA-S levels were measured at baseline, samples were drawn at 30 and 60 min to determine the LDC-stimulated cortisol and DHEA serum levels. The test was performed in a similar setting and at a similar time (0800–1000 h) in all subjects.
Thus, HPA function was defined on the basis of a random serum cortisol or in response to stimulation with LDC or hypoglycemia. Study subjects were considered to have NL-HPA when their baseline serum cortisol was 12 μg/dl or greater or when their LDC-stimulated serum cortisol levels were 23 μg/dl or greater or if their serum cortisol levels increased to greater than 18.5 μg/dl during the IIH. HPA was considered impaired (IMPAIRED-HPA) when the baseline morning serum cortisol level was less than 5 μg/dl or when the LDC-stimulated serum cortisol level was less than 18 μg/dl or when that level failed to reach18.5 μg/dl during the IIH. The clinical management of subjects with respect to glucocorticoid replacement was based on serum cortisol data defined above irrespective of the adrenal androgen data.
A group of 63 healthy subjects with similar age and gender distributions as the study subjects also underwent LDC testing. Insulin-induced hypoglycemia and LDC tests were performed in an ambulatory setting according to the protocols described previously (4). The institutional review board approved the study, and informed, written consent was obtained from participants or their legal guardians.
Laboratory analysis
Baseline plasma ACTH concentration was measured using immunoradiometric assay kits (Quest Diagnostics, San Juan Capistrano, CA). Intra- and interassay coefficients of variation determined at different ranges in the assays were less than 4.5 and 5%, respectively. Serum cortisol measurements were made using the direct chemiluminescent assay method, using Centaur instrument (Siemens, Malvern, PA). The lower limit of detectability for the assay was 0.2 μg/dl (5.5 nmol/liter), whereas the inter- and intraassay coefficients of variation are 7.7 and 6.7%, respectively. Serum DHEA levels were measured with the HPLC-tandem mass spectroscopy method developed by Associated Regional University Pathologists Laboratory (ARUP). The lower limit of detectability for the latter assay is 0.01 ng/ml and the inter- and intraassay coefficients of variation were 8.5 and 7.4%, respectively. Serum DHEA-S levels were measured using a solid-phase competitive immunoassay using Immulite instrument (Siemens). The assay has an inter- and intraassay coefficient of variation of 12.4 and 8.0%, respectively. Each assay was performed at the same facility and using the same methodology for all study subjects.
Statistical analysis
Data are presented as mean ± sd, unless stated otherwise. The data from the two patient groups and controls were first analyzed using the Kruskal-Wallis test, as a nonparametric alternative to the ANOVA test. Comparisons between groups were done using the Wilcoxon rank sum test for nonparametric measurements. Categorical data were compared using χ2 and Fisher exact tests. Differences were considered significant when the two-sided P values were less than 0.05. Bonferroni's correction for multiple comparisons was used as appropriate. In light of the age dependence of DHEA and DHEA-S levels, we analyzed the data on these two steroids in patients and healthy subjects depending on their age group. To facilitate the latter comparison, we arbitrarily divided the age groups into three categories: younger than 40 yr, 40–60 yr old, and older than 60 yr of age. All data analysis was made using the SPSS program (SPSS Inc., Chicago, IL).
Results
A total of 155 patients with pituitary masses and 63 healthy subjects were included in the study. Of the subjects with pituitary masses, 97 were found to have NL-HPA function, whereas 58 had IMPAIRED-HPA function. Patients with NL-HPA had a similar age (53 ± 15.7 yr) to those with IMPAIRED-HPA (58.5 ± 13.7 yr) and healthy subjects (55.5 ± 15.5 yr). The three groups had similar gender distribution as well.
Serum cortisol levels
Baseline and LDC-stimulated serum cortisol levels in all subjects are shown in Table 1 and Figs. 1–3. Baseline and LDC-stimulated serum cortisol levels in patients with NL-HPA were identical with those of healthy subjects. In contrast, patients with IMPAIRED-HPA had baseline and LDC-stimulated serum cortisol levels that are lower than those with NL-HPA or healthy subjects (Table 1). All patients with a baseline serum cortisol level of 12 μg/dl or greater had an LDC-stimulated level of greater than 23 μg/dl, whereas those with a morning level of less than 5 μg/dl had an LDC-stimulated level of less than 18 μg/dl.
Table 1.
Baseline (cortisol, DHEA, and DHEA-S) and LDC-stimulated (cortisol and DHEA) serum levels in the three groups
| IMPAIRED-HPA | NL-HPA | Healthy subjects | P value: IMPAIRED-HPA vs. NL-HPA | P value: IMPAIRED-HPA vs. healthy subjects | |
|---|---|---|---|---|---|
| Cortisol (μg/dl) | |||||
| Baseline | 5.6 ± 2.3 | 11.0 ± 4.4 | 10.9 ± 5.6 | <0.001 | <0.001 |
| LDC stimulated | 17.1 ± 4.6 | 27.7 ± 6.7 | 28.1 ± 6.0 | <0.001 | <0.001 |
| DHEA (ng/ml) | |||||
| Baseline | 1.0 ± 0.7 | 3.3 ± 1.9 | 3.3 ± 2.2 | <0.001 | <0.001 |
| LDC stimulated | 2.1 ± 1.7 | 7.6 ± 4.9 | 8.4 ± 4.8 | <0.001 | <0.001 |
| DHEA-S (μg/dl) | |||||
| Baseline | 24.78 ± 19.6 | 73.7 ± 77.1 | 96.5 ± 81.3 | <0.001 | <0.001 |
Baseline and LDC-stimulated serum cortisol, DHEA, and DHEA-S levels in three groups of subjects: those with IMPAIRED-HPA (impaired HPA function); those with normal HPA function (NL-HPA); and healthy subjects. Baseline serum cortisol, DHEA, and DHEA-S serum levels in patients with NL-HPA were similar to those of healthy subjects. The LDC-stimulated serum cortisol and DHEA levels in patients with NL-HPA were similar to those of healthy subjects.
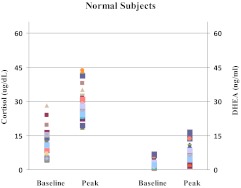
Fig. 1.
Baseline and LDC-stimulated cortisol (left two panels) and DHEA (right two panels) serum concentrations in healthy subjects. Each character on the graph represents a data point on a subject.
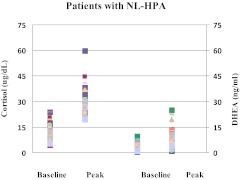
Fig. 2.
Baseline and LDC-stimulated cortisol (left two panels) and DHEA (right two panels) serum concentrations in patients with normal HPA function. Each character on the graph represents a data point on a subject. Both baseline and LDC-stimulated cortisol and DHEA levels were similar to the respective values in healthy subjects.
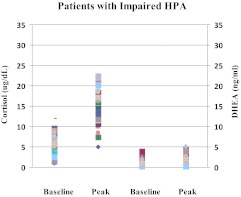
Fig. 3.
Baseline and LDC-stimulated cortisol (left two panels) and DHEA (right two panels) serum concentrations in patients with IMPAIRED-HPA function. Each character on the graph represents a data point on a subject. Both the baseline and LDC-stimulated concentrations of cortisol and DHEA are significantly lower than the respective values in healthy subjects and also those with normal HPA.
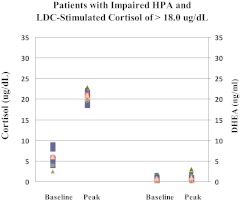
Importantly, 18 of the 58 patients with IMPAIRED-HPA had an LDC-stimulated serum cortisol level that was greater than 18.0 μg/dl and would have been considered a normal response by many (Fig. 4). The mean LDC-stimulated serum cortisol level in these 18 patients was 20.9 ± 1.4 μg/dl and all 18 patients had poor responses to IIH.
Fig. 4.
Baseline and LDC-stimulated cortisol (left two panels) and DHEA (right two panels) serum levels in 18 patients with central AI (IMPAIRED-HPA) who achieved an LDC- stimulated cortisol concentration of greater than 18.0 μg/dl are shown here. Although the cortisol responses may suggest an adequate cortisol response to LDC, DHEA levels are very low at baseline with a much blunted response to LDC. All 18 subjects are confirmed to have significantly reduced DHEA levels when compared with age- and gender-appropriate levels.
Serum DHEA-S levels
Baseline serum DHEA-S levels in the group of patients with NL-HPA were similar to those of healthy subjects (Table 1). Baseline serum DHEA-S levels were lower than age- and gender-adjusted normal values in five of the 97 patients with NL-HPA. In contrast, all patients with IMPAIRED-HPA had very low baseline serum DHEA-S, even those who had an LDC-stimulated serum cortisol of greater than 18.0 μg/dl (Table 1). All 58 patients with IMPAIRED-HPA function had lower than normal age- and gender-adjusted serum DHEA-S levels. This is in keeping with the findings in our previous work that demonstrated consistently decreased DHEA-S levels in subjects with central AI (4).
Serum DHEA levels
Mean baseline and LDC-stimulated serum DHEA levels in the group of patients with NL-HPA were similar to those of normal subjects (Table 1 and Figs. 1 and 2). However, both baseline and LDC-stimulated serum DHEA levels were markedly decreased (P < 0.001) in subjects with IMPAIRED-HPA (Table 1 and Fig. 3). Large sd in DHEA levels were noted because of the wide range of age groups in study subjects and healthy volunteers. It is important to point out that the marked suppression of DHEA and DHEA-S levels in patients with IMPAIRED-HPA remained statistically evident when data of different age groups of subjects were compared (ages: 20–39, 40–60, and 61 yr of age or older; data not shown).
An important finding in this regard is the additive value of measuring serum DHEA levels. This was especially important in subjects in the IMPAIRED-HPA group whose LDC-stimulated serum cortisol levels were greater than 18.0 μg/dl (Fig. 4). In those 18 subjects, mean baseline and LDC-stimulated serum DHEA levels were distinctly low (0.78 ± 0.47 and 1.1 ± 0.8 ng/ml, respectively) and were similar to those seen in other subjects with IMPAIRED-HPA and clearly lower than those observed in patients with NL-HPA of comparable age and gender.
Cortisol to DHEA molar ratio
We evaluated the baseline and the LDC-stimulated serum DHEA and cortisol levels in each of the three groups and calculated the molar ratio of cortisol to DHEA in the groups before and after LDC stimulation. In healthy subjects, the cortisol to DHEA molar ratio did not change after LDC stimulation, suggesting that equimolar amounts of the two steroids are secreted in response to cosyntropin administration (Table 2). The same features were noted in patients with NL-HPA (Table 2). In contrast, those with IMPAIRED-HPA had a higher baseline cortisol to DHEA molar ratio that increased even further with LDC stimulation (Table 2). These findings suggest that in patients with IMPAIRED-HPA, cortisol is being secreted preferentially over DHEA and that becomes more apparent after LDC stimulation. The latter feature was also noted in the unique subject group with IMPAIRED-HPA but whose LDC-stimulated cortisol responses were greater than 18.0 μg/dl (Table 2).
Table 2.
Molar ratio of cortisol/DHEA at baseline and after LDC stimulation in healthy subjects, patients with NL-HPA, and others with IMPAIRED-HPA
| Cortisol/DHEA molar ratio |
P value baseline vs. LDC stimulated | ||
|---|---|---|---|
| Baseline | LDC stimulated | ||
| Healthy subjects | 35.3 ± 27.7 | 35.2 ± 21.6 | 0.93 |
| NL- HPA | 43.7 ± 28.9 | 47.9 ± 34.5 | 0.38 |
| IMPAIRED-HPA | 84.4 ± 124a | 145 ± 238a | 0.03 |
| IMPAIRED-HPA who had an LDC-stimulated cortisol of >18.0 μg/dl | 115 ± 26a | 245 ± 115a | 0.02 |
The molar ratio of cortisol to DHEA in patients with NL-HPA and healthy subjects were similar at baseline and did not change appreciably after LDC stimulation in either of these groups. However, in patients with impaired HPA function, the ratio was higher at baseline and increased further after LDC stimulation. The same finding was noted in the select group of patients with IMPAIRED-HPA but whose LDC-stimulated serum cortisol levels were greater than 18.0 μg/dl.
P = 0.03 as compared with respective values in healthy subjects and those with NL-HPA.
Discussion
The data demonstrate that patients with central adrenal insufficiency consistently have low mean serum DHEA levels at baseline and after LDC stimulation. Serum DHEA-S levels are similarly decreased in all patients with central AI, validating our earlier observations (4). With very few exceptions, subjects with normal HPA function have age- and gender-appropriate DHEA and DHEA-S levels. As we have previously shown, the presence of normal DHEA and DHEA-S levels are strong indicators of normal ACTH secretion and adequate adrenal cortical function.
The need for additional methods to evaluate HPA function stems from the known limitations of the LDC test. Although the LDC provides serum ACTH levels that mimic physiological stress and more closely approximate responses to insulin tolerance test than the standard-dose cosyntropin (11), its sensitivity is variable, depending on the cortisol level cutoff used and is primarily influenced by the partial nature of ACTH deficiency in central AI. In an earlier study (4), we have shown that nearly one third of subjects with central AI, as defined by the response to IIH, were capable of mounting adequate stimulated cortisol levels of greater than 18.1 μg/dl (499 nmol/liter) with LDC stimulation. Similar findings are noted in the current study in which 18 of 58 patients with IMPAIRED-HPA had a greater than 18.0 μg/dl peak response to LDC administration. The diagnosis of central AI would have been missed in this subset of patients on the basis of the LDC stimulated cortisol levels alone. It is in this specific clinical setting that the use of the LDC can be misleading, and further evaluation of the HPA is needed. It is worth mentioning that although technical limitations to the LDC have been described (7), the LDC protocol used at our institution has been effective for more than 2 decades and is carried out by long-term nurses, thus limiting variability from one test to the next.
The rationale for using the adrenal androgen DHEA and its sulfated conjugate, DHEA-S, to assess HPA function is based on several of their properties. Most importantly, they are secreted by the zona reticularis almost exclusively, under the trophic effect of ACTH. Normally, DHEA and DHEA-S are secreted synchronously with cortisol from the adrenal cortex (12). In healthy subjects, the administration of incremental doses of cosyntropin results in a rise in both cortisol and DHEA serum levels in similar time frames and in incrementally higher levels as the cortrosyn dose is increased (13). Furthermore, there is minimal contribution from the testes (14) toward the production of DHEA and DHEA-S, hence rendering their levels almost unaltered by hypogonadal states. We have previously demonstrated the lack of effect of hypogonadism on DHEA-S levels as well as the absence of change in these levels with menopause (4). Another property of DHEA-S that lends it helpful in the evaluation of HPA function is the markedly lesser degree of diurnal variation compared with that of cortisol. DHEA-S has a long half-life of around 20 h that provides constant levels throughout the day (15, 16), whereas DHEA has a shorter half-life of 1–3 h and has been shown to have less diurnal variation than cortisol (14). Finally, adrenal androgen levels decline with age (17), requiring the interpretation of individual results using age-appropriate references. In the current investigation, adrenal androgen levels with LDC were analyzed after stratifying patients into three age groups, which confirmed the uniformly low levels of DHEA and DHEA-S in central AI compared with age-appropriate normal values. One limitation of this study is that the sample size did not allow for further stratification of the subjects by gender as well. It is important to note, however, that adrenal androgen levels are subject to much more variation by age group than by gender.
Earlier reports showed that serum DHEA-S were low in patients with central AI (9, 10), and similar findings were recently published (18). The report by Fischli et al. (18) showed similar data in patients with pituitary tumors, especially those who are young in whom serum DHEA-S levels are expected to be higher. In contrast to our data, however, there was some overlap between subjects' serum DHEA-S levels. The discordance between our study and that of Fischli et al. is likely due to the fact that their patients received glucocorticoid supplement for several weeks before testing, whereas ours were tested de novo and before exposure to exogenous glucocorticoids. As stated earlier, glucocorticoid supplementation causes suppression of DHEA and DHEA-S secretion that persists for some time after glucocorticoid secretion is normal.
In this investigation, we delineated the concept of cortisol/adrenal androgen dissociation in the setting of central AI. This observation has been previously made primarily in patients with chronic illness (19) and others with critical illness (20, 21). The mechanism for this dissociation is not clear, but it appears to be physiologically advantageous to divert the synthetic process toward the production of glucocorticoids rather than adrenal androgens, given the limited adrenal reserve with ACTH deficiency. In this investigation, subjects with impaired HPA function did not demonstrate the equimolar secretion of cortisol and DHEA seen in the group with normal HPA function and healthy subjects. In this group, the ratio of cortisol to DHEA was elevated at baseline and rose further with LDC stimulation. This portrays the relatively larger loss of DHEA compared with cortisol in central AI in the basal state and a more magnified loss of DHEA upon stimulation. Measurements of the cortisol to DHEA ratio with LDC in patients at risk for central AI can therefore prove to be a useful adjunct to the methods available in the evaluation of HPA function.
Thus, it appears that in patients with central AI, impairment of adrenal androgen secretion precedes that of the glucocorticoid class. Interestingly, similar findings were noted in patients at risk of, or in the early phases of, primary adrenal insufficiency (22, 23). The exact mechanism for the loss of adrenal androgens before glucocorticoids in patients with adrenal insufficiency is not known. However, a recent intriguing study by Topor et al. (24) shed some light on a potential mechanism. In the latter study, Topor et al. demonstrated that at physiological intraadrenal concentrations, cortisol stimulated DHEA secretion in a dose-dependent manner in human adrenocortical cells in vitro (24). The study also demonstrated that the intraadrenal stimulation of DHEA by cortisol was through its inhibition of the enzyme, 3β-hydroxysteroid dehydrogenase type II (24). Thus, a decrease in the intraadrenal cortisol concentration results in lower DHEA secretion as a result of increased 3β-hydroxysteroid dehydrogenase type II activity. Additional studies are needed to fully elucidate the latter mechanism.
Our data demonstrate that dynamic measurements of DHEA with LDC testing can improve the ability to identify subjects with central AI. This is particularly important in the subset of patients who achieved adequate stimulated cortisol levels despite their documented diagnosis of central AI. Those subjects had uniformly low DHEA levels at baseline and did not respond adequately to LDC stimulation. From a practical sense, it would seem reasonable to suggest that the DHEA-S determination would be helpful when the LDC-stimulated serum cortisol levels are not clearly normal (≥23 μg/dl) or abnormal (<18 μg/dl) but are in the gray zone. In such instances, measurements of LDC-stimulated serum DHEA levels on blood samples obtained during the test would improve the diagnostic accuracy of the test. An alternative and simpler approach that we use in our practice is to measure the serum DHEA-S along with serum cortisol levels at the outset. Based on our experience, we believe that a normal serum cortisol and DHEA-S (age and gender adjusted) level makes the diagnosis of AI untenable. The LDC-stimulation test can be performed when the data are not concordant.
It is important to emphasize that low DHEA and DHEA-S levels are not always synonymous with AI because their levels may remain low for weeks or months after exposure to exogenous glucocorticoids. Conversely, almost all subjects with normal HPA function had age-appropriate DHEA levels and DHEA-S. This is in keeping with the conclusions of our previous work and therefore strengthens the argument that normal levels of adrenal androgen effectively exclude the diagnosis of adrenal insufficiency.
When applying these observations to individual patients, several considerations need to be kept in mind. DHEA and DHEA-S levels should be interpreted in the context of each patient's age- and gender-appropriate normal values. In elderly subjects, this may be challenging because normal values are quite low and may not necessarily reflect ACTH deficiency. We have previously demonstrated that DHEA-S levels unadjusted for age carry nearly 100% sensitivity at values above 53.5 μg/dl (185.6 nmol/liter) and 100% specificity below 14.0 μg/dl (386 nmol/liter) (4). Normal DHEA-S levels for elderly patients fall between these values and are therefore difficult to interpret. Another limitation to the application of our observations is the recent administration of a long-acting glucocorticoid as we have already described. In such instances, suppression of adrenal androgen secretion persists for some time, even after endogenous glucocorticoid secretion becomes normal. Hyperprolactinemic states are also associated with elevated DHEA-S levels that may not be reflective of the integrity of HPA function. The mechanism is not entirely clear but may be related to decreased clearance of DHEA-S (25). Notably, the effect of hyperprolactinemia on DHEA-S levels does not seem to apply in subjects with ACTH deficiency, as was shown by Yamaji et al. (9) in evaluating DHEA-S levels in eight subjects with prolactinoma and documented central AI by gold standard tests. The number of patients with prolactinomas and others with silent corticotroph adenomas is too small in our patient population to make any impact on our findings or to make any firm conclusions. Finally, the use of DHEA supplements has become more popular and can definitely cause falsely elevated DHEA and DHEA-S measurements.
Our study did not investigate the need for glucocorticoid therapy in subjects with central AI who achieved an LDC-stimulated serum cortisol level of 18–22 μg/dl. It is evident that the AI in these patients is partial and would not likely require routine daily supplementation with glucocorticoids. It is, however, likely that such patients may decompensate during major stressful events and critical illnesses and would require glucocorticoid therapy during that time. Clinical judgment should be exercised in deciding on need for glucocorticoid therapy in such patients. Additional studies are needed to address this issue before more firm recommendations can be offered.
In conclusion, our data clearly show that DHEA and DHEA-S measurements are valuable markers of the integrity of the HPA axis. Assessment of HPA function should rely on determination of baseline and/or LDC-stimulated serum cortisol concentrations. Dynamic measurements of DHEA with LDC can improve the accuracy of establishing the diagnosis of central AI. A more practical and less costly approach would be to determine the serum level of the sulfated conjugate of DHEA at baseline (DHEA-S). We believe that measurements of baseline serum DHEA-S level are very valuable in the assessment of HPA function and a normal age- and gender-adjusted level makes the diagnosis of AI extremely unlikely. We therefore recommend the inclusion of DHEA and DHEA-S measurements in the laboratory evaluation of HPA function, particularly in subjects who achieve borderline cortisol responses with LDC stimulation.
Acknowledgments
This work was supported, in part, by the Case Western Reserve University School of Medicine Clinical and Translational Science Award from The Division of Research Resources, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AI
- Adrenal insufficiency
- DHEA
- dehydroepiandrosterone
- DHEA-S
- dehydroepiandrosterone sulfate
- HPA
- hypothalamic-pituitary-adrenal
- IIH
- insulin-induced hypoglycemia
- IMPAIRED-HPA
- impaired HPA function
- LDC
- low-dose cosyntropin
- NL-HPA
- normal HPA function.
References
- 1. Al-Aridi R, Abdelmannan D, Arafah BM. 2011. Biochemical diagnosis of adrenal insufficiency: the added value of dehydroepiandrosterone sulfate (DHEA-S) measurements. Endocr Pract 17:261–270 [DOI] [PubMed] [Google Scholar]
- 2. Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, Choi CH, Clayton RN, Courtney CH, Gonc EN, Maghnie M, Rose SR, Soule SG, Tordjman K; Consortium for Evaluation of Corticotropin Test in Hypothalamic-Pituitary Adrenal Insufficiency 2008. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab 93:4245–4253 [DOI] [PubMed] [Google Scholar]
- 3. Dorin RI, Qualls CR, Crapo LM. 2003. Diagnosis of adrenal insufficiency. Ann Intern Med 139:194–204 [DOI] [PubMed] [Google Scholar]
- 4. Nasrallah MP, Arafah BM. 2003. The value of dehydroepiandrosterone sulfate measurements in the assessment of adrenal function. J Clin Endocrinol Metab 88:5293–5298 [DOI] [PubMed] [Google Scholar]
- 5. Arafah BM, Kailani SH, Nekl KE, Gold RS, Selman WR. 1994. Immediate recovery of pituitary function following transsphenoidal resection of pituitary macroadenoma. J Clin Endocrinol Metab 79:348–354 [DOI] [PubMed] [Google Scholar]
- 6. Oelkers W. 1996. Adrenal insufficiency. N Engl J Med 335:1206–1212 [DOI] [PubMed] [Google Scholar]
- 7. Wade M, Baid S, Calis K, Raff H, Sinaii N, Nieman L. 2010. Technical details influence the diagnostic accuracy of the 1 μg ACTH stimulation test. Eur J Endocrinol 162:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Streeten DH, Anderson GH, Jr, Bonaventura MM. 1996. The potential for serious consequences from misinterpreting normal responses to the rapid adrenocorticotropin test. J Clin Endocrinol Metab 81:285–290 [DOI] [PubMed] [Google Scholar]
- 9. Yamaji T, Ishibashi M, Takaku F, Itabashi A, Katayama S, Ishii J. 1987. Serum dehydroepiandrosterone sulfate concentrations in secondary adrenal insufficiency. J Clin Endocrinol Metab 65:448–451 [DOI] [PubMed] [Google Scholar]
- 10. Aimaretti G, Baffoni C, Di Vito L, Grottoli S, Gaia D, Gasco V, Giordano R, Zadik Z, Camanni F, Ghigo E, Arvat E. 2003. Hypopituitaric patients with corticotropin insufficiency show marked impairment of the cortisol response to ACTH (1–24) independently of the duration of disease. J Endocrinol Invest 26:49–55 [DOI] [PubMed] [Google Scholar]
- 11. Abdu TA, Elhadd TA, Neary R, Clayton RN. 1999. Comparison of the low dose short synacthen test (1 μg), the conventional dose short synacthen test (250 μg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab 84:838–843 [DOI] [PubMed] [Google Scholar]
- 12. Rosenfeld RS, Hellman L, Gallagher TF. 1972. Metabolism and interconversion of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J Clin Endocrinol Metab 35:187–193 [DOI] [PubMed] [Google Scholar]
- 13. Arvat E, Di Vito L, Lanfranco F, Maccario M, Baffoni C, Rossetto R, Aimaretti G, Camanni F, Ghigo E. 2000. Stimulatory effect of adrenocorticotropin on cortisol, aldosterone, and dehydroepiandrosterone secretion in normal humans: dose-response study. J Clin Endocrinol Metab 85:3141–3146 [DOI] [PubMed] [Google Scholar]
- 14. Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. 1999. DHEA and DHEA-S: a review. J Clin Pharmacol 39:327–348 [DOI] [PubMed] [Google Scholar]
- 15. Migeon C, Keller A, Lawrence B, Shepard T. 1957. Dehydroepiandrosterone and androsterone levels in human plasma. Effect of age and sex; day-to-day and diurnal variations. J Clin Endocrinol Metab 50:286–296 [DOI] [PubMed] [Google Scholar]
- 16. Rosenfeld RS, Rosenberg BJ, Fukushima DK, Hellman L. 1975. 24-Hour secretory pattern of dehydroisoandrosterone and dehydroepiandesterone sulfate. J Clin Endocrinol Metab 40:850–855 [DOI] [PubMed] [Google Scholar]
- 17. Orentreich N, Brind JL, Rizer RL, Vogelman JH. 1984. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59:551–555 [DOI] [PubMed] [Google Scholar]
- 18. Fischli S, Jenni S, Allemann S, Zwahlen M, Diem P, Christ ER, Stettler C. 2008. Dehydroepiandrosterone sulfate in the assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab 93:539–542 [DOI] [PubMed] [Google Scholar]
- 19. Okonko DO, Crosato M, Kalra PR, Cicoira M, John M, Doehner W, Coats AJ, Poole-Wilson PA, Anker SD. 2005. Association of deranged adrenal steroid metabolism with anemia in chronic heart failure. Am J Cardiol 96:101–103 [DOI] [PubMed] [Google Scholar]
- 20. Lephart ED, Baxter CR, Parker CR., Jr 1987. Effect of burn trauma on adrenal and testicular steroid hormone production. J Clin Endocrinol Metab 64:842–848 [DOI] [PubMed] [Google Scholar]
- 21. Parker CR, Baxter CR., Jr 1985. Divergence in adrenal secretory pattern after thermal injury in adult patients. J Trauma 25:508–510 [DOI] [PubMed] [Google Scholar]
- 22. Laureti S, Arvat E, Candeloro P, Di Vito L, Ghigo E, Santeusanio F, Falorni A. 2000. Low dose (1 μg) ACTH test in the evaluation of adrenal dysfunction in pre-clinical Addison's disease. Clin Endocrinol 53:107–115 [DOI] [PubMed] [Google Scholar]
- 23. Laureti S, Candeloro P, Aglietti MC, Giordano R, Arvat E, Ghigo E, Santeusanio F, Falorni A. 2002. Dehydroepiandrosterone, 174α hydroxyprogesterone and aldosterone responses to the Low dose (1 μg) ACTH test in subjects with preclinical Adrenal autoimmunity. Clin Endocrinol 57:677–683 [DOI] [PubMed] [Google Scholar]
- 24. Topor LS, Asai M, Dunn J, Majzoub JA. 2011. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3β-HSD2. J Clin Endocrinol Metab 96:E31–E39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schiebinger RJ, Chrousos GP, Cutler GB, Jr, Loriaux DL. 1986. The effect of serum prolactin on plasma adrenal androgens and the production and metabolic clearance rate of dehydroepiandrosterone sulfate in normal and hyperprolactinemic subjects. J Clin Endocrinol Metab 62:202–209 [DOI] [PubMed] [Google Scholar]