Abstract
Context:
The factors that govern skeletal responses to physical activity remain poorly understood.
Objective:
The aim of this study was to investigate whether gender or fat mass influences relationships between cortical bone and physical activity, after partitioning accelerometer outputs into low (0.5–2.1 g), medium (2.1–4.2 g), or high (>4.2 g) impacts, where g represents gravitational force.
Design/Setting:
We conducted a cross-sectional analysis in participants from the Avon Longitudinal Study of Parents and Children.
Participants:
We studied 675 adolescents (272 boys; mean age, 17.7 yr).
Outcome Measures:
We measured cortical bone parameters from peripheral quantitative computed tomography scans of the mid-tibia, adjusted for height, fat mass, and lean mass.
Results:
High-impact activity was positively associated with periosteal circumference (PC) in males but not females [coefficients (95% confidence intervals), 0.054 (0.007, 0.100) and 0.07 (−0.028, 0.041), respectively; showing sd change per doubling in activity]. There was also weak evidence that medium impacts were positively related to PC in males but not females (P = 0.03 for gender interaction). On stratifying by fat mass, the positive relationship between high-impact activity and PC was greatest in those with the highest fat mass [high impact vs. PC in males, 0.01 (−0.064, 0.085), 0.045 (−0.040, 0.131), 0.098 (0.012, 0.185), for lower, middle, and upper fat tertiles, respectively; high impact vs. PC in females, −0.041 (−0.101, 0.020), −0.028 (−0.077, 0.022), 0.082 (0.015, 0.148), P = 0.01 for fat mass interaction]. Similar findings were observed for strength parameters, cross-sectional moment of inertia, and strength-strain index.
Conclusions:
In late adolescence, associations between high-impact activity and PC are attenuated by female gender and low body fat, suggesting that the skeletal response to high-impact activity is particularly reduced in young women with low fat mass.
Sexual dimorphism in skeletal development is well recognized and likely to contribute to gender differences in fracture risk (1), such as the relatively high risk of hip fractures in elderly women (2). At the hip, these differences have been reported to emerge during the adolescent growth spurt and to be comprised largely of greater periosteal apposition in males, leading to greater femoral neck width and bending strength (3). More rapid periosteal bone formation in males, leading to greater cortical bone size, has also been reported at the mid-femur (prepubertal children and young adults combined) (4), mid-tibia (peripubertal children) (5), and distal tibia and femoral neck (18 yr olds) (6). Our previous cross-sectional study of hip structure in peripubertal children from the Avon Longitudinal Study of Parents and Children (ALSPAC) revealed that emergence of these differences coincided with the onset of puberty (7). Any suggestion that sex steroids contribute to sexual dimorphism of the skeleton is consistent with previous findings from rodent studies suggesting that estrogens and androgens respectively suppress and stimulate periosteal growth (8, 9).
Previous animal studies suggest that, as well as directly suppressing periosteal apposition, estrogen may reduce periosteal growth indirectly by inhibiting the osteogenic response to externally mechanical strain (10). The latter is an important determinant of skeletal growth and modeling, as exemplified by further animal studies which have demonstrated that bone strain (i.e. deformation relative to bone length) stimulates bone formation in proportion to its rate and magnitude (11, 12). Bone strain is directly related to the strength of applied force, which for the lower limbs comprises ground reaction forces generated by the musculature during locomotion, with body mass serving as resistance (13, 14). Recently, we examined these relationships in ALSPAC using an Actigraph accelerometer to provide an objective measure of habitual physical activity (PA), based on measurement of vertical accelerations, which provide an estimate of skeletal loading. Whereas relationships between vigorous PA and endocortical resorption were equivalent in both genders, there was some evidence that the association with periosteal growth was stronger in males (15). This raises the possibility that reduced periosteal apposition in response to PA contributes to sexual dimorphism of the skeleton.
Actigraph accelerometers may have a limited role in evaluating PA in this context because these are calibrated according to maximal oxygen uptake (VO2 max), rather than the magnitude of vertical acceleration. To provide more detailed information about the relationship between PA exposure and the skeleton, a Newtest accelerometer (Newtest Oy, Oulu, Finland) was developed to measure vertical impacts within discrete ranges of intensity as reflected by g (where g represents gravitational force) (16). In our recent study where a similar device was worn by young adults (mean age, 17.9) from ALSPAC and the output was categorized as low (0.5–2.1 g), medium (2.1–4.2 g) or high impact (>4.2 g) counts, high impact showed the strongest relationship with hip bone mineral density (BMD) (18). In the present study, we explored whether this approach provides more accurate assessment of possible gender differences in the way cortical bone responds to PA, by examining relationships between low, medium, and high impacts obtained in ALSPAC, as described above, and peripheral quantitative computed tomography (pQCT) measurements from the mid-tibia.
Subjects and Methods
Study participants
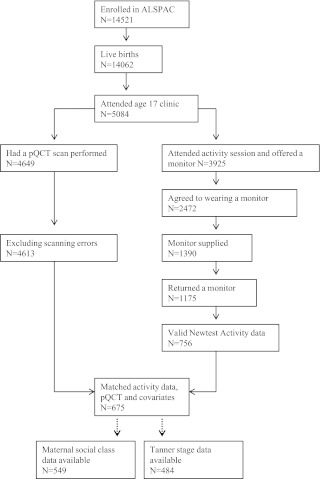
ALSPAC is a geographically based birth cohort study investigating factors influencing the health, growth, and development of children. All pregnant women resident within a defined part of the former county of Avon in southwest United Kingdom with an expected date of delivery between April 1991 and December 1992 were eligible for recruitment, and 14,541 of them were enrolled (17) (http://www.alspac.bristol.ac.uk). Ethical approval was obtained from the ALSPAC Law and Ethics Committee and relevant local ethics committees, written informed consent was provided by all parents, and young people provided written assent. Data in ALSPAC are collected by self-completion postal questionnaires sent to parents, by linkage to computerized records, by abstraction from medical records, and from examination of the children at research clinics. The present study was based on the research clinic held at approximately 17 yr of age, between December 2008 and June 2011, to whom all ALSPAC participants were invited. A total of 5084 adolescents attended this clinic, of whom 3925 attended the activity session where they were asked whether they would like to wear an accelerometer (see flowchart of participants in Fig. 1). A total of 2472 participants agreed to wear a device, which was available for 1390 participants; 1175 subjects returned the monitor, which was damaged or nonfunctional in 22 cases and unworn in 189 cases. A further 232 participants were excluded because they returned the monitors without completed diaries or indicated that the monitors had been worn for less than 8 h/d for 2 d. This left 756 participants with valid recordings, of whom 675 (272 males) had information about pQCT data and other covariates, who formed the basis of the present analysis.
Fig. 1.
Flow diagram showing the number of participants at each stage of the data preparation.
PA measurements
Those who agreed to participate in the accelerometer substudy, subject to availability, were fitted with a version of the Newtest monitor produced for this study. This uniaxial device records accelerations within 33 separate bands across the range 0.3–9.9 g above gravitational force (1 g), as previously described for other research applications (16). Participants were asked to wear the monitor for 7 consecutive days during waking hours, recharge it overnight, and only take it off at other times for contact sports or when it might get wet. Participants were also asked to record a diary when the monitor was worn, a valid recording being defined as a minimum of 8 h recording per day for 2 d. Raw data were read into Stata 11.2 (StataCorp, College Station, TX) using custom-designed code. Number of counts per subject was calculated and expressed as number of counts per day for each of three different acceleration bands as previously described (18), namely 0.5–2.1 g (equates to low impacts), 2.1–4.2 g (medium impacts), and above 4.2 g (high impacts). These relate to walking, jogging/slow running (<10 km/h), and faster running (>10 km/h)/jumping, respectively, as determined by our previous calibration study based on a separate group of 22 school children (mean, 17.1 yr; 15 males) who were asked to wear Newtest monitors while performing a series of supervised activities (19).
pQCT measurement
Cortical bone mineral content (BMCC), cortical BMD (BMDC), and cortical bone area (BAC) were measured using the Stratec XCT2000L (StraTec Medizintechnik, Pforzheim, Germany) in 4649 participants, of which 36 participants were excluded due to scanning errors. pQCT images were analyzed with XCT custom software version 6.00B. Periosteal circumference (PC) and cortical thickness (CT) were derived from a circular ring model. A threshold routine was used for defining cortical bone, which specified a voxel with a density of more than 650 mg · cm−3 as cortical bone. Strength strain index (SSI) and cross-sectional moment of inertia (CSMI) were derived based on standard methods (20).
Confounders
Data on lean mass and fat mass were obtained from total body dual-energy x-ray absorptiometry (DXA) scans performed on a Lunar Prodigy at the same clinic visit. Height was measured using a Harpenden stadiometer (Holtain Ltd., Crymych, UK). Maternal social class was derived from self-report questionnaire administered at 32 wk gestation.
Statistical methods
Participants were divided into quartiles for low-, medium-, and high-impact activity, based on number of counts per day within each g-band and a trend in pQCT parameters across quartiles, and then analyzed by linear regression. Regression analysis was subsequently used to examine relationships between number of counts per person per day and pQCT parameters, after log transformation of activity data, based on numbers of counts recorded for each individual within the three different g-bands. β-Coefficients were standardized, so as to represent an sd change in pQCT parameter per doubling in number of impacts. Our minimally adjusted model included age, gender, and height, whereas our fully adjusted model included fat and lean mass in addition. Gender differences were explored by comparing β coefficients between separate analyses in males and females and by testing for gender interactions in analyses performed in males and females combined. A similar approach was used to examine interactions with fat mass (height-adjusted) tertile. All analyses were performed by K.D. in Stata 11.2 (StataCorp).
Results
Description of participants
Those included in this study had a mean age of 17.7 yr. Fat mass was substantially higher in females compared with males, whereas the converse held for lean mass, whereas body mass index (BMI) was similar in both sexes (Table 1). BMDC was greater in females, whereas BMCC, BAC, PC, and CT were higher in males. Compared with other ALSPAC participants measured at the first research clinic held at age 9, those included in the present study were broadly similar in terms of height, BMI, fat mass, and lean mass (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). However, a lower proportion of boys were included in the present study compared with the proportion who attended the age 9 clinic (41.7 vs. 50.0%, respectively). Moreover, mothers of participants had a higher socioeconomic status at initial enrollment of the cohort in pregnancy compared with the remainder of the cohort, as exemplified by a greater proportion in class I and lower proportion in class V (Supplemental Table 1). Accelerometers were worn for a mean of 5.8 d.
Table 1.
Descriptive statistics
| Outcome | Sex | Mean | sd | Median | p25 | p75 | p(diff.) |
|---|---|---|---|---|---|---|---|
| Age (yr) | Males | 17.7 | 0.3 | 17.7 | 17.6 | 17.8 | |
| Females | 17.7 | 0.3 | 17.7 | 17.6 | 17.8 | 0.244 | |
| All | 17.7 | 0.3 | 17.7 | 17.6 | 17.8 | ||
| Height (cm) | Males | 178.4 | 6.9 | 177.6 | 174.0 | 182.9 | |
| Females | 164.9 | 5.8 | 164.2 | 161.1 | 168.9 | <0.001 | |
| All | 170.3 | 9.1 | 169.5 | 163.2 | 176.3 | ||
| Weight (cm) | Males | 70.0 | 10.7 | 69.1 | 63.1 | 76.5 | |
| Females | 61.5 | 9.9 | 60.7 | 54.1 | 66.6 | <0.001 | |
| All | 64.9 | 11.0 | 63.9 | 57.2 | 71.4 | ||
| Fat mass (kg) | Males | 12.5 | 7.8 | 10.0 | 6.9 | 16.1 | |
| Females | 20.6 | 7.7 | 19.4 | 15.0 | 24.8 | <0.001 | |
| All | 17.3 | 8.7 | 16.2 | 10.6 | 22.5 | ||
| Lean mass (kg) | Males | 54.2 | 5.7 | 54.3 | 50.4 | 58.0 | |
| Females | 37.9 | 3.8 | 37.6 | 35.2 | 40.3 | <0.001 | |
| All | 44.5 | 9.3 | 41.5 | 36.9 | 52.7 | ||
| BMI (kg/m2) | Males | 22.0 | 3.1 | 21.4 | 19.8 | 23.7 | |
| Females | 22.6 | 3.4 | 22.2 | 20.2 | 24.3 | 0.012 | |
| All | 22.4 | 3.3 | 21.9 | 20.0 | 24.1 | ||
| BMDC (mg/cm3) | Males | 1105.8 | 30.1 | 1111.4 | 1094.9 | 1124.1 | |
| Females | 1134.0 | 28.4 | 1137.1 | 1122.5 | 1148.9 | <0.001 | |
| All | 1122.7 | 32.2 | 1126.0 | 1109.0 | 1141.3 | ||
| BMCC (mg) | Males | 371.1 | 50.8 | 371.7 | 336.6 | 404.2 | |
| Females | 308.5 | 40.8 | 307.9 | 285.2 | 334.2 | <0.001 | |
| All | 333.7 | 54.5 | 328.7 | 296.7 | 368.1 | ||
| BAC (mm2) | Males | 335.6 | 44.8 | 334.9 | 305.9 | 365.4 | |
| Females | 272.0 | 35.3 | 271.1 | 250.6 | 295.2 | <0.001 | |
| All | 297.6 | 50.3 | 290.7 | 262.6 | 329.7 | ||
| PC (mm) | Males | 76.3 | 4.8 | 76.7 | 72.9 | 79.7 | |
| Females | 68.4 | 4.5 | 68.4 | 65.5 | 71.5 | <0.001 | |
| All | 71.6 | 6.0 | 71.1 | 67.2 | 75.9 | ||
| CT (mm) | Males | 5.8 | 0.6 | 5.8 | 5.4 | 6.1 | |
| Females | 5.2 | 0.6 | 5.2 | 4.8 | 5.6 | <0.001 | |
| All | 5.4 | 0.6 | 5.5 | 5.0 | 5.9 | ||
| Buckling ratio | Males | 2.1 | 0.2 | 2.1 | 2.0 | 2.3 | |
| Females | 2.1 | 0.2 | 2.1 | 1.9 | 2.2 | 0.094 | |
| All | 2.1 | 0.2 | 2.1 | 2.0 | 2.3 | ||
| CSMI (cm4) | Males | 1.6 | 0.4 | 1.6 | 1.3 | 1.9 | |
| Females | 1.0 | 0.3 | 1.0 | 0.9 | 1.2 | <0.001 | |
| All | 1.3 | 0.4 | 1.2 | 1.0 | 1.5 | ||
| SSI | Males | 1.2 | 0.2 | 1.2 | 1.0 | 1.4 | |
| Females | 0.9 | 0.2 | 0.9 | 0.8 | 1.0 | <0.001 | |
| All | 1.0 | 0.3 | 1.0 | 0.8 | 1.2 |
Table showing characteristics of participants, including pQCT-derived parameters, as mean, sd, median, 25th (p25) and 75th (p75) centiles (n = 675; males = 272; females = 403). p(diff.), Gender difference calculated with t test.
Activity quartiles
Participants were divided into quartiles of low activity, medium activity, and high activity, based on their number of counts per day within the three corresponding g-bands. The median number of counts per day for each quartile of low-, medium-, and high-impact activity is shown in Table 2. There was a profound fall in number of counts on moving from lower to higher impact activity. The number of counts was greater in males compared with females across all three activity bands. In analyses based on boys and girls combined, there was evidence of a positive trend in BAC, PC, and CT across quartiles of medium-impact and particularly high-impact activity. A similar relationship was observed for BMCC (P = 0.013 and P < 0.001 for associations with medium- and high-impact activity, respectively).
Table 2.
Median counts-per-day by quartile of g-band
| g-band quartiles | n | Median per day | Range | BAC (mm2) | P | CT (mm) | P | PC (mm) | P |
|---|---|---|---|---|---|---|---|---|---|
| Boys | |||||||||
| Low | |||||||||
| 1 | 68 | 1,687 | 652–2,264 | 340.7 | 5.8 | 76.6 | |||
| 2 | 68 | 3,359 | 2,476–4,443 | 340.0 | 5.8 | 76.8 | |||
| 3 | 68 | 5,603 | 4,446–6,987 | 331.5 | 5.7 | 75.7 | |||
| 4 | 68 | 9,388 | 7,063–29,591 | 330.2 | 0.101 | 5.7 | 0.071 | 76.2 | 0.344 |
| Medium | |||||||||
| 1 | 69 | 48 | 7–67 | 329.2 | 5.7 | 75.3 | |||
| 2 | 67 | 105 | 70–149 | 337.5 | 5.7 | 77.2 | |||
| 3 | 68 | 198 | 149–261 | 338.5 | 5.8 | 76.6 | |||
| 4 | 68 | 499 | 261–2,588 | 337.3 | 0.295 | 5.8 | 0.388 | 76.4 | 0.312 |
| High | |||||||||
| 1 | 68 | 8 | 1–12 | 330.7 | 5.8 | 75.5 | |||
| 2 | 69 | 18 | 12–24 | 337.5 | 5.8 | 76.5 | |||
| 3 | 67 | 35 | 24–54 | 329.2 | 5.6 | 76.3 | |||
| 4 | 68 | 95 | 57–727 | 344.7 | 0.167 | 5.9 | 0.543 | 77.1 | 0.063 |
| Girls | |||||||||
| Low | |||||||||
| 1 | 101 | 1,649 | 711–2,375 | 273.8 | 5.3 | 68.6 | |||
| 2 | 101 | 3,199 | 2,377–3,904 | 273.1 | 5.2 | 68.8 | |||
| 3 | 101 | 4,764 | 3,909–6,079 | 271.9 | 5.2 | 68.3 | |||
| 4 | 100 | 8,115 | 6,086–22,224 | 269.2 | 0.347 | 5.2 | 0.661 | 68.1 | 0.319 |
| Medium | |||||||||
| 1 | 101 | 33 | 5–52 | 272.3 | 5.2 | 68.8 | |||
| 2 | 101 | 82 | 53–113 | 270.3 | 5.2 | 68.4 | |||
| 3 | 101 | 154 | 113–229 | 272.3 | 5.3 | 68.3 | |||
| 4 | 100 | 366 | 229–1,768 | 273.0 | 0.800 | 5.3 | 0.194 | 68.3 | 0.444 |
| High | |||||||||
| 1 | 102 | 5 | 1–7 | 268.8 | 5.2 | 68.3 | |||
| 2 | 101 | 12 | 8–17 | 271.1 | 5.2 | 68.3 | |||
| 3 | 100 | 25 | 17–35 | 271.3 | 5.3 | 68.0 | |||
| 4 | 100 | 69 | 35–1,605 | 276.9 | 0.120 | 5.3 | 0.147 | 69.2 | 0.244 |
| All | |||||||||
| Low | |||||||||
| 1 | 169 | 1,656 | 652–2,377 | 300.0 | 5.5 | 71.8 | |||
| 2 | 169 | 3,278 | 2,387–4,024 | 294.6 | 5.4 | 71.4 | |||
| 3 | 169 | 4,954 | 4,084–6,301 | 297.5 | 5.5 | 71.5 | |||
| 4 | 168 | 9,037 | 6,371–22,224 | 298.4 | 0.914 | 5.4 | 0.691 | 71.8 | 0.855 |
| Medium | |||||||||
| 1 | 169 | 37 | 5–60 | 290.6 | 5.4 | 70.9 | |||
| 2 | 169 | 91 | 60–123 | 296.1 | 5.4 | 71.7 | |||
| 3 | 169 | 176 | 124–236 | 300.0 | 5.5 | 71.8 | |||
| 4 | 168 | 417 | 239–1,761 | 303.8 | 0.012 | 5.5 | 0.012 | 72.2 | 0.047 |
| High | |||||||||
| 1 | 172 | 6 | 1–9 | 286.1 | 5.3 | 70.2 | |||
| 2 | 166 | 14 | 9–19 | 299.1 | 5.5 | 71.7 | |||
| 3 | 169 | 28 | 20–43 | 292.2 | 5.3 | 71.1 | |||
| 4 | 168 | 81 | 44–1,605 | 313.4 | <0.001 | 5.6 | 0.001 | 73.5 | <0.001 |
Number of impacts according to g-band quartiles in males (n = 272) and females (n = 403), expressed as median number of counts (with range) per person per day (low = 0.5–2.1 g; medium = 2.1–4.2 g; high = 4.2 g+). P value for trend was calculated with linear regression.
Regression analyses of activity vs. pQCT
In subsequent regression analyses, high-impact activity was positively related to BAC after adjusting for age, gender, and height, in males and females combined, whereas little relationship was seen for medium or low impacts (Table 3, model 1). Similar results were observed for PC and BMCC [β for BMCC vs. high-impact activity, 0.049 (0.013, 0.084)]. High-impact activity was positively related to CT [β 0.044 (0.003, 0.086)], with evidence of a weak association with medium activity [0.027 (−0.020, 0.074)]. No relationship was observed between PA and BMDC (results not shown).
Table 3.
Association between PA and bone parameters
| Outcome | g-band | Sex | Model 1 |
Model 2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | β | 95% CI | P | p(int.) | R2 | β | 95% CI | P | p(int.) | |||||
| BAC (mm2) | Low | M | 0.09 | −0.061 | −0.155 | 0.034 | 0.206 | 0.29 | −0.071 | −0.154 | 0.013 | 0.098 | ||
| F | 0.13 | −0.008 | −0.077 | 0.060 | 0.818 | 0.31 | 0.008 | −0.054 | 0.071 | 0.793 | ||||
| All | 0.45 | −0.032 | −0.088 | 0.024 | 0.259 | 0.381 | 0.56 | −0.035 | −0.085 | 0.016 | 0.180 | 0.222 | ||
| Medium | M | 0.09 | 0.041 | −0.028 | 0.110 | 0.247 | 0.28 | 0.035 | −0.026 | 0.096 | 0.260 | |||
| F | 0.13 | 0.003 | −0.041 | 0.047 | 0.898 | 0.31 | −0.004 | −0.045 | 0.038 | 0.860 | ||||
| All | 0.45 | 0.018 | −0.020 | 0.056 | 0.354 | 0.345 | 0.56 | 0.008 | −0.027 | 0.042 | 0.663 | 0.160 | ||
| High | M | 0.10 | 0.067 | 0.004 | 0.129 | 0.036 | 0.29 | 0.050 | −0.005 | 0.106 | 0.076 | |||
| F | 0.14 | 0.034 | −0.004 | 0.072 | 0.079 | 0.31 | 0.020 | −0.015 | 0.055 | 0.260 | ||||
| All | 0.46 | 0.046 | 0.013 | 0.080 | 0.007 | 0.381 | 0.57 | 0.028 | −0.002 | 0.059 | 0.070 | 0.265 | ||
| CT (mm) | Low | M | 0.02 | −0.089 | −0.197 | 0.020 | 0.108 | 0.11 | −0.097 | −0.200 | 0.007 | 0.066 | ||
| F | 0.01 | 0.015 | −0.075 | 0.105 | 0.739 | 0.07 | 0.026 | −0.064 | 0.115 | 0.572 | ||||
| All | 0.17 | −0.033 | −0.102 | 0.036 | 0.347 | 0.143 | 0.24 | −0.037 | −0.103 | 0.030 | 0.283 | 0.104 | ||
| Medium | M | 0.01 | 0.027 | −0.053 | 0.106 | 0.507 | 0.10 | 0.022 | −0.054 | 0.098 | 0.563 | |||
| F | 0.01 | 0.028 | −0.030 | 0.086 | 0.351 | 0.07 | 0.023 | −0.036 | 0.082 | 0.450 | ||||
| All | 0.17 | 0.027 | −0.020 | 0.074 | 0.255 | 0.979 | 0.23 | 0.018 | −0.028 | 0.064 | 0.433 | 0.846 | ||
| High | M | 0.01 | 0.044 | −0.028 | 0.116 | 0.228 | 0.11 | 0.032 | −0.037 | 0.101 | 0.368 | |||
| F | 0.01 | 0.045 | −0.005 | 0.095 | 0.079 | 0.07 | 0.034 | −0.016 | 0.084 | 0.181 | ||||
| All | 0.17 | 0.044 | 0.003 | 0.086 | 0.036 | 0.963 | 0.24 | 0.030 | −0.010 | 0.070 | 0.144 | 0.960 | ||
| PC (mm) | Low | M | 0.17 | −0.018 | −0.098 | 0.062 | 0.658 | 0.36 | −0.026 | −0.097 | 0.044 | 0.462 | ||
| F | 0.23 | −0.023 | −0.092 | 0.045 | 0.507 | 0.41 | −0.005 | −0.066 | 0.056 | 0.873 | ||||
| All | 0.53 | −0.021 | −0.072 | 0.031 | 0.436 | 0.855 | 0.64 | −0.020 | −0.066 | 0.026 | 0.390 | 0.913 | ||
| Medium | M | 0.17 | 0.043 | −0.015 | 0.102 | 0.145 | 0.37 | 0.039 | −0.013 | 0.090 | 0.140 | |||
| F | 0.23 | −0.016 | −0.060 | 0.029 | 0.490 | 0.41 | −0.023 | −0.064 | 0.017 | 0.260 | ||||
| All | 0.53 | 0.008 | −0.028 | 0.043 | 0.667 | 0.107 | 0.63 | 0.000 | −0.032 | 0.031 | 0.982 | 0.033 | ||
| High | M | 0.19 | 0.068 | 0.015 | 0.121 | 0.011 | 0.37 | 0.054 | 0.007 | 0.100 | 0.024 | |||
| F | 0.23 | 0.022 | −0.016 | 0.060 | 0.262 | 0.41 | 0.007 | −0.028 | 0.041 | 0.707 | ||||
| All | 0.53 | 0.039 | 0.008 | 0.070 | 0.014 | 0.170 | 0.64 | 0.022 | −0.005 | 0.050 | 0.113 | 0.099 | ||
Results are shown for regression analysis between BAC, CT, PC, and PA within three bands of impact in 272 males and 403 females (low = 0.5–2.1 g; medium = 2.1–4.2 g; high = 4.2 g+). β-Coefficient represents sd change in outcome per doubling in activity. Model 1 is adjusted for age, gender, and height. Model 2 is additionally adjusted for fat mass and lean mass. p(int.) represents a P value for interaction of gender and activity. M, Male; F, female; CI, confidence interval.
Adjustment for fat and lean mass led to partial attenuation of these associations, as reflected by a decrease in β coefficients of approximately 30%, 95% confidence limits now overlapping with zero in all instances (Table 3, model 2). Similar relationships were observed between impacts and derived cortical bone strength to those seen for BAC and PC; in analyses based on model 1, high-impact activity was positively related to CSMI and SSI, whereas no relationship was seen with low or medium impacts, or for buckling ratio (Supplemental Table 2). Adjustment for fat and lean mass resulted in attenuation of associations such that all 95% confidence intervals overlapped zero (model 2).
Activity vs. pQCT: gender interactions
Based on model 1, β coefficients for the relationship between high-impact activity and BAC/PC were approximately twice as high in males as in females [e.g. for BAC, 0.067 (0.004, 0.129) and 0.034 (−0.004, 0.072) in males and females, respectively), although formal gender interaction tests were P > 0.1 (Table 3). There was weak evidence of a positive relationship between medium-impact activity and BAC/PC in males but not females [e.g. for medium impacts vs. BAC, β coefficients were 0.041 (−0.028, 0.110) and 0.003 (−0.041, 0.047) for males and females, respectively]. β coefficients for the relationship with CT were similar in males and females.
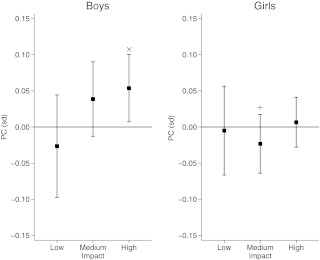
Adjustment for fat and lean mass led to partial attenuation of these relationships, and β coefficients now overlapped with zero, except for high-impact activity vs. PC in males [0.054 (0.007, 0.100)], whereas there was little evidence of an equivalent relationship in females [0.007 (−0.028, 0.041); P = 0.1 for gender interaction] (Table 3, model 2, and Fig. 2). There was also weak evidence of a positive relationship between medium-impact activity and PC in males but not females [β coefficients, 0.039 (−0.013, 0.090) and −0.023 (−0.064, 0.017) in males and females, respectively; P = 0.03 for gender interaction]. Adjustment for socioeconomic status as reflected by maternal social class led to a reduction in overall numbers (to 549), but β coefficients were virtually identical when comparing results between model 2 and a further model additionally adjusted for maternal social class (results not shown). CSMI and SSI showed equivalent results to those for BAC and PC; with model 2, high-impact activity was positively related to CSMI and SSI in males but not females (Supplemental Table 2). There was evidence of a weak positive relationship between medium impacts and CSMI and SSI in males but not females, with evidence of a gender interaction (P < 0.05).
Fig. 2.
The relationship of low (0.5–2.1 g), medium (2.1–4.2 g), and high (4.2 g+) impacts on PC adjusted for age, height, fat mass, and lean mass in males (n = 272) and females (n = 403). Data show β coefficient expressed as sd change in PC per doubling in impact variable. ×, P = 0.02 for activity association; +, P = 0.03 for gender interaction.
Activity vs. pQCT: interactions with fat and lean mass
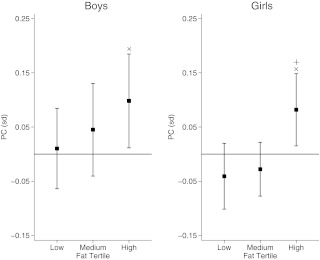
To explore possible interactions with fat mass, associations were compared between high-impact activity and PC/SSI in males and females according to tertile of height-adjusted fat mass, based on model 2. In males, the positive relationship between high-impact activity and PC was most marked in those in the upper tertile for fat mass adjusted for height (Fig. 3) (P > 0.1 for interaction with fat mass). In females, a positive association was seen between high-impact activity and PC in the highest fat tertile, whereas a similar relationship was not seen for the middle or lowest fat tertile (P = 0.01 for interaction with fat mass). Similar results were seen for CSMI and SSI (data not shown).
Fig. 3.
The relationship of high (4.2 g+) impact activity with PC stratified by fat mass tertiles adjusted for age, height, fat mass, and lean mass in males (n = 272) and females (n = 403). Data show β coefficient expressed as sd change in PC per doubling in impact variable. ×, P = 0.03 (boys) and P = 0.02 (girls); +, P = 0.01 for interaction between activity and fat mass.
Discussion
We examined the relationship between habitual exposure to PA graded according to level of impact, and cortical bone parameters as assessed by pQCT of the mid-tibia, in a population-based cohort of adolescents. Higher quartiles of medium-impact and particularly high-impact activity were associated with greater cortical bone mass and area, reflecting increases in both PC and CT. To examine whether these relationships persisted after adjustment for potential confounders, regression analyses were performed based on the number of counts for each individual within the three prespecified g-bands. After adjusting for sex, height, and age, high-impact activity was positively related to cortical bone mass, area, PC, and CT, whereas there was only very weak evidence of a positive relationship in the case of medium-impact activity. These findings are consistent with evidence from previous clinical and laboratory studies that stimulation of periosteal bone formation contributes to the adaptive response of the skeleton to increased mechanical strain (11, 12, 21). In further analyses where we adjusted for indirect associations via body composition, we only observed a positive relationship between high impacts and PC in males. Any suggestion that the response of the periosteal envelope to high-impact activity is attenuated in postpubertal females could conceivably contribute to their narrower long bones, which becomes more pronounced after puberty (7), and may contribute to the relatively high fracture risk in females in later life (1).
In our previous pQCT-based study in the same cohort performed 2 yr previously, there was only weak evidence to suggest that PA was associated with PC more strongly in males compared with females (15). The different methods of ascertaining PA exposure may have been responsible for this apparent discrepancy; conceivably, more precise estimates of high-impact loading as obtained in the present study, in contrast to use of an Actigraph device calibrated against oxygen consumption in our previous study, enabled a more accurate characterization of skeletal responses to PA. The present findings are consistent with a previous cross-sectional study in prepubertal and early pubertal children, which also found greater responsiveness to habitual PA in males compared with females as assessed by measurement of hip bone mineral content (22). In contrast, in a cross-sectional study of children aged 8–15 classified according to PA participation by questionnaire, greater PA participation was associated with higher bone mineral content to a similar extent in boys and girls, a relationship that persisted based on repeat DXA scans performed 15 yr later (23). Similarly, an earlier review of studies involving weight-bearing interventions in children and adolescents revealed some evidence that whereas BMD gains in response to PA are reduced after puberty, there was little evidence of a gender difference (24). Furthermore, in a recent school-based PA intervention, less BMD gains were observed after puberty, whereas no difference was observed according to gender (25).
A possible explanation for these apparently discrepant findings is that our present observations primarily relate to relationships between PA and bone size as reflected by PC. There was little evidence that other parameters more closely related to BMD as measured by DXA, such as CT and BMDC, showed an equivalent gender difference. Consistent with this conclusion, in our recent paper where we examined equivalent associations of PA with hip BMD, there was little evidence of a gender interaction (18). On the other hand, in studies of the osteogenic response of the upper limb to external loading based on comparisons between playing and nonplaying humeri of tennis players, whereas loading appeared to elicit a response in peripubertal boys but not girls, neither sex showed evidence of a skeletal response after puberty, including measures of overall bone size (26, 27). However, although this suggests that the gender difference in skeletal responsiveness that we observed may be confined to the lower limb, the small sample sizes involved in the latter study, particularly in the male postpubertal group (n = 8), make it difficult to draw definitive conclusions.
A previous study in rodents revealed that pretreatment with estrogen reduced the osteogenic response to an externally applied load (28), suggesting that attenuation of the skeletal response to high impacts in females may reflect a direct influence of sex steroids on bone. In addition, rodent studies demonstrate that estrogen and androgens respectively suppress and stimulate periosteal bone formation (8, 9), providing further evidence that distinct actions of sex steroids may underlie gender differences in periosteal bone responsiveness suggested by our results. Human experiments of nature based on studies of individuals with estrogen and androgen receptor mutations support this conclusion (29, 30). Alternatively, in view of the suggestion that the skeletal response to PA may diminish after puberty (24, 25), adolescent males may have shown a stronger response to PA given that they were closer to puberty than females of the same age. However, on comparing PC presented here with that measured 2 yr previously, little consistent change was evident in either boys or girls (15), suggesting that we have examined relationships with PA at a time when age- and growth-related changes have largely ceased, in the case of both boys and girls. The conclusion that periosteal expansion remains responsive to high impacts after the cessation of skeletal growth, at least in males, is consistent with the recent cross-sectional study of 833 males (mean age, 24 yr) where increased participation in sports activities over the previous 5 yr was associated with greater periosteal bone expansion as assessed by tibial pQCT (31).
We also investigated whether the suggestion of a reduced response of cortical bone to high impacts in females reflects their greater fat mass compared with males. Interestingly, we found evidence for an interaction between high-impact activity, PC, and fat mass, such that the response to PA was enhanced in the presence of greater fat mass. Given their higher fat mass, this interaction would be predicted to magnify rather than reduce the skeletal response in females. Although the basis for this interaction is unclear, one possible explanation is that higher fat mass results in a greater mechanical strain resulting from a given impact, leading to a greater osteogenic stimulus.
Because bone strength is strongly related to its cross-sectional area, the periosteal response to high-impact activity seen in males is likely to influence overall strength. Consistent with this interpretation, relationships between high-impact activity and PC closely mirrored those with strength indices, such as CSMI and SSI. Therefore, any deficiency in the response of cortical bone to high-impact activity in females may adversely affect bone strength.
Our observation that high-impact PA has a stronger association with PC compared with medium- or low-impact PA, at least in males, is consistent with previous observations based on this cohort where we used an Actigraph device to separate PA into low, moderate and vigorous activity, with a positive association observed for vigorous PA only, after adjusting for other activity types (15). A similar result was obtained in our recent study where we analyzed relationships between low-, medium-, and high-impact PA as defined here and hip BMD; whereas the strongest associations were evident with high-impact activity, there was some evidence of an association with medium-impact PA, but the latter was completely attenuated after adjusting for vigorous impact PA (18). This has important implications because it suggests that relatively high-impact PA is needed before a skeletal response to PA is observed [e.g. impacts >4.2 g are associated with jumping and with running more than 10 km/h (our unpublished observations)]. Although not presented here, there was no longer any evidence to suggest a positive association between medium-impact activity and PC in analyses adjusted for high-impact activity, in line with our previous findings. However, these adjusted analyses, which suggest that impacts below 4.2 g have little positive influence on the skeleton, need to be treated with caution because impacts within medium and high bands are closely correlated, and the apparent disappearance of effects may in part be due to issues related to near colinearity, which may exaggerate reciprocal changes in β coefficients after adjustment for closely correlated variables.
Limitations
One of the limitations of this study is its cross-sectional design. Although we adjusted for a range of potential confounders, including markers of socioeconomic status, other potential confounders were not adjusted for, such as dietary factors. Furthermore, although we have interpreted our findings as reflecting a response of cortical bone to high-impact activity, we are unable to exclude reverse causality whereby those with stronger bones are more likely to participate in high-impact PA. A further limitation was that compliance with home accelerometer recordings in this age group was relatively poor. To ensure that sufficient numbers were included, we used a threshold for accepting a valid recording of only 2 d. This may have been a particular problem in terms of obtaining representative values for rare high-impact events. However, this limitation is likely to have reduced the power and precision of the study, rather than introducing any bias. Although accelerometry provides an objective measure of PA exposure, one limitation is the failure to capture certain types of activity that could potentially be osteogenic, such as contact sports necessitating the removal of the device, which may particularly apply to boys. Participants involved in this study were a selected group that are not necessarily representative of ALSPAC as a whole. Evidence that participants were of higher social class than other ALSPAC participants who were not included is consistent with this view. Finally, in view of the age of participants, we were unable to examine relationships between PA and bone outcomes during skeletal growth, when the skeleton may be more responsive to exercise, leading us to underestimate the true impact of PA on bone accrual.
Conclusions
We related habitual PA exposure, assessed by partitioning outputs of an accelerometer into distinct impact bands, to cortical bone parameters as measured by pQCT in a population-based sample of adolescents. In analyses adjusted for body composition, we found that high-impact activity was positively related to PC and to estimated cortical strength as reflected by CSMI and SSI in males but not females. An interaction with fat mass was also observed, such that high-impact activity was positively related to PC in females whose fat mass was in the top tertile, whereas no association was present in those from the bottom and middle tertiles. We conclude that associations between high-impact activity and PC are attenuated by female gender and low body fat, suggesting that response of the skeleton to high-impact PA may be particularly reduced in females with low fat mass.
Supplementary Material
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
The UK Medical Research Council (Grant ref 74882), the Wellcome Trust (Grant ref 076467), and the University of Bristol provide core support for ALSPAC. This specific project was funded by the Wellcome Trust (Grant ref 084632). This publication is the work of the authors, and J.T. will serve as guarantor for the contents of this paper.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALSPAC
- Avon Longitudinal Study of Parents and Children
- BAC
- cortical bone area
- BMCC
- cortical bone mineral content
- BMD
- bone mineral density
- BMDC
- cortical BMD
- BMI
- body mass index
- CSMI
- cross-sectional moment of inertia
- CT
- cortical thickness
- DXA
- dual-energy x-ray absorptiometry
- g
- gravitational force
- PA
- physical activity
- PC
- periosteal circumference
- pQCT
- peripheral quantitative computed tomography
- SSI
- strength strain index.
References
- 1. Seeman E. 2003. Periosteal bone formation - a neglected determinant of bone strength. N Engl J Med 349:320–323 [DOI] [PubMed] [Google Scholar]
- 2. Cooper C. 1989. Osteoporosis—an epidemiological perspective: a review. J R Soc Med 82:753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forwood MR, Bailey DA, Beck TJ, Mirwald RL, Baxter-Jones AD, Uusi-Rasi K. 2004. Sexual dimorphism of the femoral neck during the adolescent growth spurt: a structural analysis. Bone 35:973–981 [DOI] [PubMed] [Google Scholar]
- 4. Högler W, Blimkie CJ, Cowell CT, Kemp AF, Briody J, Wiebe P, Farpour-Lambert N, Duncan CS, Woodhead HJ. 2003. A comparison of bone geometry and cortical density at the mid-femur between prepuberty and young adulthood using magnetic resonance imaging. Bone 33:771–778 [DOI] [PubMed] [Google Scholar]
- 5. Kontulainen SA, Macdonald HM, Khan KM, McKay HA. 2005. Examining bone surfaces across puberty: a 20-month pQCT trial. J Bone Miner Res 20:1202–1207 [DOI] [PubMed] [Google Scholar]
- 6. Nieves JW, Formica C, Ruffing J, Zion M, Garrett P, Lindsay R, Cosman F. 2005. Males have larger skeletal size and bone mass than females, despite comparable body size. J Bone Miner Res 20:529–535 [DOI] [PubMed] [Google Scholar]
- 7. Sayers A, Marcus M, Rubin C, McGeehin MA, Tobias JH. 2010. Investigation of sex differences in hip structure in peripubertal children. J Clin Endocrinol Metab 95:3876–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tobias JH, Gallagher A, Chambers TJ. 1994. 5α-Dihydrotestosterone partially restores cancellous bone volume in osteopenic ovariectomised rats. Am J Physiol 267:E853–E859 [DOI] [PubMed] [Google Scholar]
- 9. Turner RT, Colvard DS, Spelsberg TC. 1990. Estrogen inhibition of periosteal bone formation in rat long bones: down-regulation of gene expression for bone matrix proteins. Endocrinology 127:1346–1351 [DOI] [PubMed] [Google Scholar]
- 10. Jagger CJ, Chow JW, Chambers TJ. 1996. Estrogen suppresses activation but enhances formation phase of osteogenic response to mechanical stimulation in rat bone. J Clin Invest 98:2351–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodship AE, Lanyon LE, McFie H. 1979. Functional adaptation of bone in increased stress. J Bone Joint Surg Am 61:539–546 [PubMed] [Google Scholar]
- 12. Rubin CT, Lanyon LE. 1985. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37:411–417 [DOI] [PubMed] [Google Scholar]
- 13. Duda GN, Kirchner H, Wilke HJ, Claes L. 1998. A method to determine the 3-D stiffness of fracture fixation devices and its application to predict inter-fragmentary movement. J Biomech 31:247–252 [DOI] [PubMed] [Google Scholar]
- 14. Rittweger J, Beller G, Ehrig J, Jung C, Koch U, Ramolla J, Schmidt F, Newitt D, Majumdar S, Schiessl H, Felsenberg D. 2000. Bone-muscle strength indices for the human lower leg. Bone 27:319–326 [DOI] [PubMed] [Google Scholar]
- 15. Sayers A, Mattocks C, Deere K, Ness A, Riddoch C, Tobias JH. 2011. Habitual levels of vigorous, but not moderate or light, physical activity is positively related to cortical bone mass in adolescents. J Clin Endocrinol Metab 96:E793–E802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vainionpää A, Korpelainen R, Vihriälä E, Rinta-Paavola A, Leppäluoto J, Jämsä T. 2006. Intensity of exercise is associated with bone density change in premenopausal women. Osteoporos Int 17:455–463 [DOI] [PubMed] [Google Scholar]
- 17. Golding J, Pembrey M, Jones R. 2001. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 15:74–87 [DOI] [PubMed] [Google Scholar]
- 18. Deere K, Sayers A, Rittweger J, Tobias J. 10 April 2012. Habitual levels of high, but not moderate or low, impact activity are positively related to hip BMD and geometry: results from a population-based study of adolescents. J Bone Miner Res doi: 10.1002/jbmr.1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deere K, Sayers A, Davey Smith G, Rittweger J, Tobias JH. 9 May 2012. High impact activity is related to lean but not fat mass: findings from a population-based study in adolescents. Int J Epidemiol doi:10.1093/ije/dys073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratec Medizintechnik GmbH. 2005 XCT 2000 Manual Version 6.66. [Google Scholar]
- 21. Specker B, Binkley T. 2003. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Miner Res 18:885–892 [DOI] [PubMed] [Google Scholar]
- 22. Kriemler S, Zahner L, Puder JJ, Braun-Fahrländer C, Schindler C, Farpour-Lambert NJ, Kränzlin M, Rizzoli R. 2008. Weight-bearing bones are more sensitive to physical exercise in boys than in girls during pre- and early puberty: a cross-sectional study. Osteoporos Int 19:1749–1758 [DOI] [PubMed] [Google Scholar]
- 23. Baxter-Jones AD, Kontulainen SA, Faulkner RA, Bailey DA. 2008. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone 43:1101–1107 [DOI] [PubMed] [Google Scholar]
- 24. Hind K, Burrows M. 2007. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 40:14–27 [DOI] [PubMed] [Google Scholar]
- 25. Meyer U, Romann M, Zahner L, Schindler C, Puder JJ, Kraenzlin M, Rizzoli R, Kriemler S. 2011. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. Bone 48:792–797 [DOI] [PubMed] [Google Scholar]
- 26. Ducher G, Daly RM, Bass SL. 2009. Effects of repetitive loading on bone mass and geometry in young male tennis players: a quantitative study using MRI. J Bone Miner Res 24:1686–1692 [DOI] [PubMed] [Google Scholar]
- 27. Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S. 2002. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res 17:2274–2280 [DOI] [PubMed] [Google Scholar]
- 28. Jagger CJ, Chow JW, Chambers TJ. 1996. Estrogen suppresses activation but enhances formation of osteogenic response to mechanical stimulation in rat bone. J Clin Invest 98:2351–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanderschueren D, Venken K, Ophoff J, Bouillon R, Boonen S. 2006. Clinical review: sex steroids and the periosteum—reconsidering the roles of androgens and estrogens in periosteal expansion. J Clin Endocrinol Metab 91:378–382 [DOI] [PubMed] [Google Scholar]
- 30. Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. 2010. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol 207:127–134 [DOI] [PubMed] [Google Scholar]
- 31. Nilsson M, Ohlsson C, Odén A, Mellström D, Lorentzon M. 2012. Increased physical activity is associated with enhanced development of peak bone mass in men: a five year longitudinal study. J Bone Miner Res 27:1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.