Abstract
Context:
Dual-energy x-ray absorptiometry-derived bone mineral density (BMD) does not explain interracial differences in fracture risk; thus, BMD-based fracture risk assessment requires patient race/ethnicity information and ethnicity-specific BMD reference databases.
Objective:
The objective of the study was to investigate whether composite femoral neck strength indices, which integrate dual-energy x-ray absorptiometry-derived femoral neck size, femoral neck BMD, and body size, will allow fracture risk assessment without requiring race/ethnicity information.
Design:
This was a prospective cohort study.
Setting and Participants:
A total of 1940 community-dwelling women aged 42–53 yr from four race/ethnicity groups (968 Caucasian, 512 African-American, 239 Japanese, and 221 Chinese) were followed up for 9 yr.
Outcome Measurements:
Self-reported, nondigital, noncraniofacial fractures were measured.
Results:
Two hundred and two women (10.4%) sustained fractures and 82 (4.3%) had minimum-trauma fractures. Each sd increment in any of the strength indices was associated with a 34–41% reduction in fracture hazard over 9 yr (each P < 0.001). Race/ethnicity predicted fracture hazard independent of BMD (P = 0.02) but did not predict fracture hazard independent of any of the composite indices (P = 0.11–0.22). Addition of race/ethnicity did not improve risk discrimination ability of the strength indices, but did significantly improve the discrimination ability of BMD. The discrimination ability of BMD with race/ethnicity was not statistically different from that of any of the strength indices without race/ethnicity.
Conclusions:
Composite strength indices of the femoral neck can predict fracture risk without race/ethnicity information as accurately as bone mineral density does in combination with race/ethnicity information and therefore would allow risk prediction in people of mixed race/ethnicity and in groups without a BMD reference database.
Osteoporotic fractures, especially hip fractures, constitute a major public health and cost burden and their incidence is expected to increase worldwide (1, 2). Therefore, it is imperative to identify people at increased fracture risk to optimally target preventive interventions.
Clinicians currently assess fracture risk based on bone mineral density (BMD) measurement by dual-energy x-ray absorptiometry (DXA), which provides two-dimensional-projected bone mass per unit area. BMD is an important contributor to bone strength, and low BMD is a major risk factor for fracture (3). However, low BMD does not explain interracial variation in fracture risks, and BMD fails to correctly stratify fracture risk across ethnic groups. For instance, Asian women, despite having lower BMD, have (nearly 50%) lower hip or all fracture rates compared with Caucasian women, even after adjusting for other important risk factors (4, 5).
This inability to capture interracial variation in fracture risk with BMD alone or in combination with other measured variables has meant that clinicians need to include race/ethnicity information to better predict their patient's fracture risk, using ethnicity-specific T scores and Z scores (6). However, race/ethnicity is a proxy for unmeasured factors that vary between groups but are not necessarily homogenous within groups (7), especially because diverse ethnic subgroups and persons with varying racial admixtures are frequently categorized under a single label (7). More importantly, fracture risk assessment using ethnicity-specific scoring is predicated on knowing the individual's race/ethnicity, a difficult proposition for individuals of mixed heritage, and on the availability of a BMD reference database for that race/ethnicity. Recognizing that satisfactory BMD reference databases are not available for all races and ethnic groups, the International Society for Clinical Densitometry had suggested that the Caucasian database be used uniformly in everyone (6, 8), but this leads to systematic over- or underestimation of fracture risk in some groups (9). The same drawbacks mentioned above may also apply to FRAX, a nation-specific and, within the United States, ethnic-specific web-based fracture risk calculator that integrates clinical risk factors and femoral neck BMD (http://www.shef.ac.uk/FRAX). There are many countries and ethnic groups for which a FRAX calculator is not available, and for such countries and groups, no specific recommendation is available other than to use a surrogate group for which the epidemiology of osteoporosis most closely approximates the index group (10).
Given the increasing number of minority groups and individuals of mixed heritage (11), accurate assessment of bone strength and fracture risk without race/ethnicity information is becoming increasingly important.
Body size and femoral neck geometry predict hip fracture risk independent of BMD and vary significantly by race/ethnicity (12–14). Composite indices of femoral neck strength integrate body size, femoral neck size, and femoral neck BMD to capture the structural contributions to bone strength relative to load. They are inversely associated with hip fracture incidence in community-dwelling Caucasian women (15), and (unlike BMD) race/ethnicity differences in the composite indices are consistent with documented race/ethnicity differences in fracture risk (16).
We postulate that race/ethnicity differences in fracture risk are mostly due to differences in bone strength relative to load and that the composite strength indices will capture all major bone strength and load factors that differ across race/ethnicity and contribute to fracture risk. Accordingly, we hypothesize that the composite strength indices will predict an individual's fracture risk without requiring race/ethnicity information and as accurately as BMD does in combination with race/ethnicity information. To test this hypothesis, we examined the ability of the femoral neck composite strength indices to predict fracture risk over 9 yr in a multiethnic cohort of women going through the menopause transition.
Materials and Methods
Study participants
The Study of Women's Health Across the Nation (SWAN) enrolled 3302 women in 1996–1997 who were 42–53 yr old, with intact uterus and at least one ovary, were not on sex steroid hormones, had at least one menstrual period in the previous 3 months, and self-identified as Caucasian, African-American, Hispanic, Chinese, or Japanese, at seven sites: Boston, MA; Chicago, IL; Detroit, MI; Pittsburgh, PA; Los Angeles, CA; Newark, NJ; and Oakland, CA (17). Each site enrolled Caucasians and one minority ethnic group. All 2413 women from five sites (Boston, Detroit, Pittsburgh, Los Angeles, and Oakland) were enrolled in the SWAN bone cohort, but 46 women weighed more than 136 kg (the scanner weight limit) and did not get DXA scans. The SWAN hip strength substudy measured femoral neck size from archived hip DXA scans from the 1960 women in the SWAN bone cohort who had a baseline and two or more follow-up scans by visit 10 (2006–2007); 20 women did not get either bone size or body size measurements at baseline, leaving 1940 women in the analytic sample (968 Caucasian, 512 African-American, 239 Japanese, 221 Chinese). The SWAN parent study and hip strength substudy protocols were approved by the institutional review board at each site, and all participants gave written informed consent.
Assessment of composite indices of femoral neck strength
DXA scans of the hip were acquired with Hologic QDR 4500 or QDR 2000 scanners (Hologic Inc., Waltham, MA) and OsteoDyne's hip positioner system (Osteodyne Inc., Research Triangle Park, NC), and the two-dimensional-projected (areal) BMD was recorded. Two size measurements were made on archived baseline scans using pixel dimensions provided by the manufacturer: femoral neck axis length (FNAL) and femoral neck width (FNW) (Fig. 1). Composite femoral neck strength indices were computed using height, weight, FNAL, FNW, and femoral neck BMD (Fig. 1) (15). Compression strength index (CSI) reflects the ability to withstand axial compressive loads, bending strength index (BSI) reflects ability to withstand bending forces, and impact strength index (ISI) reflects the ability to absorb the energy of impact in a fall from standing height.
Fig. 1.
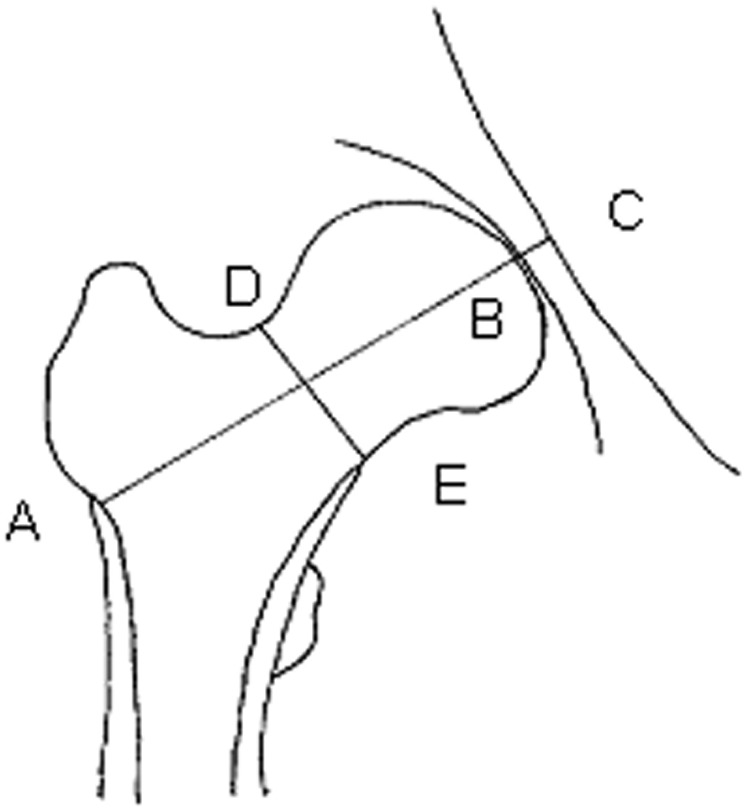
Femoral neck size measurements and formulae to compute composite femoral neck strength indices. AB is the FNAL, the distance from the base of the greater trochanter to the apex of the femoral head, and DE is the FNW, the smallest thickness of the femoral neck along any line perpendicular to the femoral neck axis. C is where the femoral neck axis meets the inner pelvic rim. Composite femoral neck strength indices were computed using the following formulae: 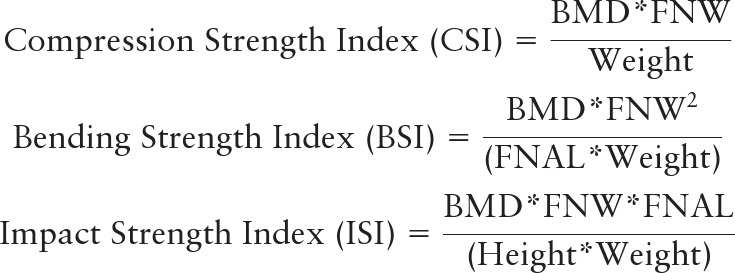 all three indices were recorded in units of grams per kilogram per meter. With BMD measured in grams per square centimeter, FNW and FNAL in centimeters, weight in kilograms, and height in meters, CSI and BSI were scaled by 100 to get values in units of grams per kilogram per meter.
all three indices were recorded in units of grams per kilogram per meter. With BMD measured in grams per square centimeter, FNW and FNAL in centimeters, weight in kilograms, and height in meters, CSI and BSI were scaled by 100 to get values in units of grams per kilogram per meter.
To examine reproducibility, 20 women volunteers were scanned twice after repositioning; intraclass correlation coefficient for each index was greater than 0.98.
Other measurements
Participants provided the following information at baseline: age (years); race/ethnicity; menopausal transition stage (premenopause vs. early perimenopause: no changes vs. some changes in regularity of menses); physical activity level [summary score of active living, home, and recreational physical activity from modified Baecke interview (18)]; prescription medications used; vitamin D and calcium supplement use; alcohol consumption [abstainer, infrequent (not abstainer but one or less drink per week), light (more than one drink per week but one or less per day), or heavy (more than one drink per day)]; and smoking history (current, ex-, or never smoking).
Height and weight were measured using a fixed stadiometer and a digital scale with the participant wearing light clothing and no shoes and used to compute body mass index (BMI). Serum glucose was measured after overnight 12-h fasting using a hexokinase-coupled reaction (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN). Women who reported use of diabetes medications or had fasting serum glucose of 126 mg/dl or greater were categorized as diabetic.
Incident fracture ascertainment and classification
During each of nine annual follow-up visits, fractures since the previous visit were reported by participants (using a standardized interviewer administered questionnaire), along with number of fractures, date(s), body site(s), and how fractures occurred. Dates of fractures were not collected in the first six follow-up visits and were imputed using the midpoint between the participant's last and index visits. Fractures were categorized as minimum-trauma fractures if they did not occur in the following conditions: 1) from a fall from height greater than 6 in.; 2) in a motor vehicle accident; 3) while moving fast (e.g. bicycling or skating); 4) while playing sports; or 5) from impact with heavy or fast-moving projectiles.
We excluded fractures not typically associated with osteoporosis, in particular fractures of the face, skull, fingers, and toes (19).
To assess the reliability of self-reported fractures, fractures reported after visit 6 were confirmed by reviewing medical records. Medical records were available for 85% of fractures and of these, only four fractures (3.8%) could not be confirmed.
Statistical analysis
Two kinds of analyses (logistic and proportional hazards regression) were undertaken for each of two outcomes: all fractures and minimum-trauma fractures. In the first set of analyses, we used logistic regression to estimate the odds ratio for cumulative incidence of (one or more) fracture over 9 yr of follow-up, as a function of each of the four measures of femoral neck strength: areal BMD and the three composite strength indices. The model fit was verified by using Hosmer-Lemeshow test. The second analyses used Cox proportional hazards regression to determine the relative hazard of first incident fracture as a function of the same four strength measures. For proportional hazards regression with first minimum-trauma fracture as outcome, we censored women who reported a non-minimum-trauma fracture (competing risk) at the date of the fracture. We checked for interactions of the strength measures with time and verified the proportional hazards assumption.
We first adjusted only for study site and type of DXA scanner (Hologic QDR 2500 vs. 4000) to control for measurement variability by study site and scanner type. We then added controls for potential confounders of the association between bone strength and fracture risk: BMI, a surrogate for soft tissue mass that absorbs some fall-impact energy (continuous) and fall risk factors, namely age (continuous), use (any vs. none) of central nervous system active medications (antidepressants, antiepileptics, sedatives, soporifics), and alcohol consumption (abstainer, infrequent, light or heavy). Finally, we added controls for factors known to influence bone strength, namely menopause transition stage at baseline (premenopause vs. early perimenopause); physical activity (below median vs. above median of the summary score); body weight (continuous); smoking status (current, ex, or never); use (any vs. none) of bone-active medications (oral steroids, chemotherapy for breast cancer, aromatase inhibitors, antiepileptics); use (any vs. none) of vitamin D and calcium supplements; history of prior fracture after age 20 yr; and diabetes mellitus. Use of osteoporosis medications (bisphosphonates, selective estrogen receptor modulators, calcitonin, PTH, or vitamin D in pharmacological doses) at baseline was reported by only one participant and therefore not included in the model. In each of the three steps, we added race/ethnicity to the model to examine its independent contribution to fracture prediction, using the likelihood ratio test (20).
The ability of the four strength measures to correctly rank order women by fracture risk (discrimination ability) was assessed by the area under the receiver operator characteristic (ROC) curve for predicting 9-yr cumulative incidence risk and Harrel's c-index for predicting time to first fracture (21). The 95% confidence intervals (CI) for both discrimination measures (and for the change therein after inclusion of race/ethnicity as well as for differences therein between competing models) were computed using nonparametric bootstrapping (22). In addition, the improvement in accuracy of prediction by inclusion of race/ethnicity in each model was assessed by the integrated discrimination improvement (23).
Calibration formulas to convert the strength measures to 9-yr fracture risk were created using the regression coefficient estimates from the logistic regression models (with each strength measure as a sole predictor). Formulas for time-to-first-fracture estimates were created from the proportional hazards models. The mean predicted 9-yr fracture risk in each race/ethnicity group was then compared with the observed fracture risk in the group; 95% CI for the observed and predicted group risks were computed by Wilson's method (24) and nonparametric bootstrapping, respectively (22).
All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC) and R statistical software version 2.9.0 (R Foundation, Vienna, Austria). Two-sided P < 0.05 was considered statistically significant.
Results
The study sample (n = 1940) was representative of the complete SWAN bone cohort (n = 2413) with respect to clinical characteristics at baseline (Table 1). A total of 1727 (89.0%) remained in the study through follow-up visit 9. Over the follow-up period (median 9.0, interquartile range 8.9–9.1 yr), 202 women (10.4%) had fractures, with first fracture rate, 12.5 per 1000 person-years. Minimum-trauma fractures were sustained by 82 women (4.3%), with first fracture rate, 5.3 per 1000 person-years. Foot (nontoe) and ankle were the most common locations for first incident fracture. Only 89 women (4.6%) had one or more covariates missing and were excluded from the analysis. Excluded women were slightly younger and heavier and had higher BMD but did not differ in CSI, BSI, ISI, and the risk of fracture. Including them in the analysis (if possible) did not qualitatively alter the results.
Table 1.
Characteristics of study participants
| Characteristics | Study sample (n = 1940) | Bone cohort (n = 2413) |
|---|---|---|
| Age (yr)a | 45.9 (2.7) | 45.8 (2.7) |
| Height (cm)a | 162.3 (6.5) | 162.5 (6.6) |
| Weight (kg)a | 72.7 (19.4) | 74.3 (21.3) |
| BMI (kg/m2)a | 27.5 (6.9) | 28.0 (7.5) |
| Menopause status | ||
| Premenopausalb | 56.4 | 54.1 |
| Early perimenopausalb | 43.6 | 45.9 |
| Smoking status | ||
| Never smokedb | 59.7 | 57.8 |
| Ex-smokerb | 25.4 | 25.9 |
| Current smokerb | 14.9 | 16.4 |
| Alcohol consumption | ||
| Abstainerb | 51.5 | 51.8 |
| Infrequentb | 9.2 | 9.2 |
| Lightb | 25.7 | 25.3 |
| Heavyb | 13.6 | 13.8 |
| Physical activity | ||
| More activeb,c | 49.7 | 49.6 |
| Less activeb,c | 50.3 | 50.4 |
| Medications | ||
| Bone active medicationsb,d | 2.3 | 2.3 |
| Supplemental vitamin Db,d | 38.6 | 38.2 |
| Supplemental calciumb,d | 45.1 | 44.4 |
| Central nervous system active medicationsb,d | 10.5 | 10.9 |
| Diabetes mellitusb | 4.6 | 5.5 |
| FNAL (cm)a | 9.0 (0.5) | |
| FNW (cm)a | 2.8 (0.2) | |
| Femoral neck BMD (g/cm2)a | 0.84 (0.13) | 0.84 (0.13) |
| CSI (g/kg · m)a | 3.3 (0.6) | |
| BSI (g/kg · m)a | 1.02 (0.22) | |
| ISI (g/kg · m)a | 0.18 (0.04) | |
| Race/ethnicity | ||
| Caucasianb | 49.9 | 49.6 |
| African-Americanb | 26.4 | 28.4 |
| Chineseb | 11.4 | 10.4 |
| Japaneseb | 12.3 | 11.7 |
| Baseline history of fractures | ||
| All fracturesb | 18.3 | 19.0 |
| Spinal fracturesb | 1.0 | 1.0 |
| Foot fracturesb | 2.5 | 2.8 |
| Lower-arm fracturesb | 2.4 | 2.8 |
| Upper-arm fracturesb | 0.8 | 0.8 |
| Hip fracturesb | 0.3 | 0.3 |
| Lower-leg fracturesb | 5.0 | 4.9 |
Abbreviations: BMD, bone mineral density; BMI, body mass index; BSI, bending strength index; CSI, compression strength index; FNAL, femoral neck axis length; FNW, femoral neck width; ISI, impact strength index.
For continuous variables, mean is shown with sd in parentheses.
Data are given as percentage of each group. Percentages may not add up to 100 because of rounding errors.
For physical activity, more active is defined as above median of the summary score, and less active is below median.
Use of medications and supplements indicates any use (vs. none).
Prediction of cumulative incidence of fracture
Femoral neck BMD and all three composite strength indices were inversely associated with the odds of fracture over the following 9 yr, adjusted only for study site and DXA scanner type (Table 2, model 1). Additional adjustment for fracture risk factors strengthened these associations (data not shown). Further adjustment for risk factors for low bone strength did not substantially attenuate these associations (Table 2, model 2). Each sd increment in each of the composite strength indices was associated with between 36 and 44% relative reduction in odds of fracture over 9 yr (Table 2, model 2).
Table 2.
Adjusted odds ratios for fracture over 9 yr follow-up per sd increment in measures of femoral neck strengtha
| Models without race/ethnicity |
Models with race/ethnicity |
|||||
|---|---|---|---|---|---|---|
| OR (95% CI) for strength measure | P for strength measure | Area under ROC (95% CI) | OR (95% CI) for strength measure | P for raceb | Increment in area under ROC (95% CI)c | |
| Outcome: any fracture | ||||||
| CSI | ||||||
| Model 1 | 0.69 (0.59, 0.80) | <0.001 | 0.608 (0.570, 0.645) | 0.72 (0.61, 0.85) | 0.11 | +0.012 (−0.006, 0.031) |
| Model 2 | 0.56 (0.44, 0.72) | <0.001 | 0.659 (0.622, 0.697) | 0.57 (0.45, 0.74) | 0.10 | +0.009 (−0.005, 0.024) |
| BSI | ||||||
| Model 1 | 0.68 (0.58, 0.80) | <0.001 | 0.611 (0.572, 0.649) | 0.71 (0.60, 0.85) | 0.14 | +0.012 (−0.005, 0.028) |
| Model 2 | 0.64 (0.51, 0.79) | <0.001 | 0.655 (0.615, 0.694) | 0.66 (0.53, 0.83) | 0.18 | +0.007 (−0.006, 0.019) |
| ISI | ||||||
| Model 1 | 0.71 (0.61, 0.83) | <0.001 | 0.600 (0.559, 0.642) | 0.75 (0.63, 0.88) | 0.09 | +0.014 (−0.004, 0.031) |
| Model 2 | 0.60 (0.47, 0.76) | <0.001 | 0.652 (0.615, 0.689) | 0.61 (0.47, 0.78) | 0.07 | +0.012 (−0.003, 0.027) |
| BMD | ||||||
| Model 1 | 0.78 (0.67, 0.91) | <0.001 | 0.572 (0.534, 0.611) | 0.68 (0.57, 0.81) | <0.001 | +0.054 (0.023, 0.085) |
| Model 2 | 0.61 (0.50, 0.76) | <0.001 | 0.659 (0.621, 0.698) | 0.58 (0.47, 0.73) | 0.01 | +0.018 (0.001, 0.035) |
| Outcome: minimum-trauma fracture | ||||||
| CSI | ||||||
| Model 1 | 0.64 (0.51, 0.81) | 0.002 | 0.643 (0.583, 0.703) | 0.67 (0.52, 0.86) | 0.23 | +0.017 (−0.012, 0.046) |
| Model 2 | 0.61 (0.42, 0.89) | 0.009 | 0.674 (0.626, 0.723) | 0.58 (0.39, 0.84) | 0.08 | +0.011 (−0.011, 0.033) |
| BSI | ||||||
| Model 1 | 0.59 (0.46, 0.75) | <0.001 | 0.661 (0.603, 0.720) | 0.61 (0.47, 0.80) | 0.35 | +0.008 (−0.014, 0.031) |
| Model 2 | 0.59 (0.42, 0.82) | 0.002 | 0.684 (0.629, 0.740) | 0.58 (0.41, 0.82) | 0.15 | +0.006 (−0.013, 0.026) |
| ISI | ||||||
| Model 1 | 0.67 (0.53, 0.85) | 0.001 | 0.638 (0.580, 0.696) | 0.70 (0.55, 0.91) | 0.18 | +0.019 (−0.013, 0.051) |
| Model 2 | 0.67 (0.46, 0.97) | 0.03 | 0.667 (0.613, 0.721) | 0.64 (0.43, 0.93) | 0.09 | +0.012 (−0.011, 0.035) |
| BMD | ||||||
| Model 1 | 0.98 (0.79, 1.22) | 0.87 | 0.573 (0.510, 0.636) | 0.81 (0.63, 1.05) | 0.01 | +0.067 (0.009, 0.125) |
| Model 2 | 0.78 (0.58, 1.05) | 0.10 | 0.659 (0.602, 0.716) | 0.68 (0.50, 0.93) | 0.03 | +0.022 (−0.006, 0.049) |
Model 1 was adjusted for study site and type of DXA scanner. Model 2 was adjusted for study site, type of DXA scanner, age, BMI, menopausal status, history of fracture since age 20 yr, smoking status, physical activity, body weight, use of medications that adversely affect bones, central nervous system active medications, alcohol consumption, supplements of vitamin D and calcium, and diabetes mellitus.
The sd of each strength measure was calculated in the complete study sample. Over the follow-up period (median 9.0, interquartile range 8.9–9.1 yr), 202 women (10.4%) had fractures; minimum-trauma fractures were sustained by 82 women (4.3%).
P for race was computed using the likelihood ratio test for the logistic regression model.
Increase in area under ROC was computed using nonparametric bootstrapping.
Race/ethnicity did not predict fracture odds independent of the composite strength indices, but did so independent of femoral neck BMD (Table 2). Addition of race/ethnicity did not increase the discrimination ability (area under the ROC) of models with composite strength indices. However, addition of race/ethnicity did significantly improve the discrimination ability of the BMD-based model (Table 2, last column).
Prediction of time to first fracture
Findings were nearly identical in proportional hazards analyses: all four strength measures were strongly and inversely associated with fracture hazard (Table 3). Each sd increment in each of the composite strength indices was associated with 34–41% relative reduction in fracture hazard (Table 3, model 2).
Table 3.
Adjusted relative hazard of fracture per sd increment in measures of femoral neck strengtha
| Models without race/ethnicity |
Models with race/ethnicity |
|||||
|---|---|---|---|---|---|---|
| RH (95% CI) for strength measure | P for strength measure | c-index (95% CI) | RH (95% CI) for strength measure | P for raceb | Increment in c-index (95% CI)c | |
| Outcome: all fractures | ||||||
| CSI | ||||||
| Model 1 | 0.70 (0.61, 0.81) | <0.001 | 0.607 (0.568, 0.646) | 0.74 (0.63, 0.86) | 0.16 | 0.012 (−0.006, 0.029) |
| Model 2 | 0.59 (0.47, 0.75) | <0.001 | 0.651 (0.615, 0.686) | 0.60 (0.48, 0.76) | 0.13 | 0.010 (−0.010, 0.031) |
| BSI | ||||||
| Model 1 | 0.70 (0.60, 0.81) | <0.001 | 0.609 (0.571, 0.648) | 0.73 (0.62, 0.86) | 0.19 | 0.011 (−0.005, 0.027) |
| Model 2 | 0.66 (0.54, 0.81) | <0.001 | 0.648 (0.614, 0.683) | 0.69 (0.56, 0.84) | 0.22 | 0.007 (−0.004, 0.017) |
| ISI | ||||||
| Model 1 | 0.72 (0.62, 0.83) | <0.001 | 0.601 (0.562, 0.639) | 0.76 (0.65, 0.88) | 0.14 | 0.013 (−0.0006, 0.027) |
| Model 2 | 0.62 (0.50, 0.78) | <0.001 | 0.646 (0.610, 0.682) | 0.63 (0.50, 0.79) | 0.11 | 0.012 (−0.004, 0.027) |
| BMD | ||||||
| Model 1 | 0.80 (0.69, 0.93) | 0.003 | 0.561 (0.523, 0.600) | 0.70 (0.60, 0.83) | <0.001 | 0.055 (0.029, 0.081) |
| Model 2 | 0.65 (0.54, 0.78) | <0.001 | 0.651 (0.616, 0.686) | 0.62 (0.51, 0.75) | 0.02 | 0.017 (−0.001, 0.036) |
| Outcome: minimum-trauma fractures | ||||||
| CSI | ||||||
| Model 1 | 0.64 (0.51, 0.80) | <0.001 | 0.644 (0.584, 0.705) | 0.67 (0.52, 0.85) | 0.19 | 0.020 (−0.005, 0.045) |
| Model 2 | 0.61 (0.43, 0.88) | 0.007 | 0.671 (0.614, 0.728) | 0.57 (0.40, 0.83) | 0.05 | 0.016 (−0.012, 0.045) |
| BSI | ||||||
| Model 1 | 0.59 (0.46, 0.75) | <0.001 | 0.664 (0.604, 0.724) | 0.62 (0.48, 0.80) | 0.28 | 0.010 (−0.005, 0.025) |
| Model 2 | 0.59 (0.43, 0.82) | 0.002 | 0.682 (0.624, 0.739) | 0.59 (0.42, 0.82) | 0.11 | 0.010 (−0.007, 0.026) |
| ISI | ||||||
| Model 1 | 0.67 (0.53, 0.84) | <0.001 | 0.638 (0.577, 0.699) | 0.70 (0.55, 0.90) | 0.15 | 0.022 (−0.006, 0.051) |
| Model 2 | 0.67 (0.47, 0.96) | 0.03 | 0.664 (0.611, 0.717) | 0.63 (0.44, 0.91) | 0.06 | 0.017 (−0.010, 0.044) |
| BMD | ||||||
| Model 1 | 0.98 (0.79, 1.22) | 0.88 | 0.561 (0.501, 0.620) | 0.81 (0.64, 1.04) | 0.01 | 0.082 (0.037, 0.126) |
| Model 2 | 0.78 (0.59, 1.04) | 0.09 | 0.656 (0.598, 0.715) | 0.68 (0.50, 0.91) | 0.02 | 0.028 (0.04, 0.053)d |
Model 1 was adjusted for study site and type of DXA scanner. Model 2 was adjusted for study site, type of DXA scanner, age, BMI, menopausal status, history of fracture since age 20 yr, smoking status, physical activity, body weight, use of medications that adversely affect bones, central nervous system active medications, alcohol consumption, supplements of vitamin D and calcium, and diabetes mellitus. RH, Relative hazard (or hazard ratio).
The sd of each strength measure was calculated in the complete study sample. In the cohort, the rate of first fracture was 12.5 per 1000 person-years; for minimum-trauma fractures, the first fracture rate was 5.3 per 1000 person-years.
P for race was computed using the likelihood ratio test for the Cox proportional hazards regression model.
Increase in area under ROC was computed using nonparametric bootstrapping.
The c-index for prediction of minimum-trauma fracture by the BMD+covariates+race/ethnicity model (0.684) was not statistically different from the c-index for any of the composite strength indices+covariates models withoutrace/ethnicity (0.671 for compression strength, 0.682 for bending strength, and 0.664 for impact strength).
Race/ethnicity did not predict fracture hazard independent of the composite strength indices, although it did predict fracture hazard independent of BMD (Table 3). Addition of race/ethnicity did not significantly increase the fracture hazard discrimination ability (Harrel's c-index) of models with composite strength indices but did significantly improve the discrimination ability of the BMD-based model (Table 3, last column).
Prediction of minimum trauma fracture
Nearly identical findings were seen when we examined the strength measures as predictors of minimum-trauma fractures: all four measures predicted minimum-trauma fractures as well as they predicted all fractures, and although race/ethnicity information improved BMD-based prediction, it did not improve prediction based on the composite strength indices (Tables 2 and 3). The discrimination ability of the model with femoral neck BMD, race/ethnicity, and covariates was not statistically different from that of the models with any of the composite strength indices and covariates, without race/ethnicity (Table 3, footnote b). Integrated discrimination improvements for each of composite strength indices with addition of race/ethnicity was not statistically significant (P = 0.44–0.78), whereas the integrated discrimination improvement for adding race/ethnicity to BMD was significant (0.005, 95% CI 0.002–0.007, P < 0.001).
Conversion of strength index to fracture risk
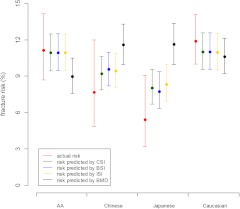
With CSI (in units of grams per kilogram per meter) as the sole predictor, the predicted 9-yr fracture risk is 0.71e−0.564CSI /(1 + 0.71e−0.564CSI). Similarly, the 9-yr fracture risk predicted by BSI (in units of grams per kilogram per meter) is 0.58e−1.62BSI/(1 + 0.58e−1.62BSI); by ISI (in units of grams per kilogram per meter) is 0.56e−8.80ISI/(1 + 0.56e−8.80ISI); and by BMD (in units of grams per square centimeter) is 0.47e−1.68BMD/(1 + 0.47e−1.68BMD). Under the constant hazard assumption, the CSI-predicted median time (in years) to first fracture is 9.79e0.538CSI. Similarly, the predicted median time by BSI is 12.1e1.54BSI, by ISI is 12.3e8.42ISI, and by BMD is 15.91.50BMD. BMD-based predictions of incident 9-yr fracture risk tended to be higher than the actual fracture risk in Chinese and Japanese women and lower in the other two race/ethnicity groups; however, the discordance was statistically significant in Japanese women only (Fig. 2). In contrast, the mean risk predicted by each of the composite strength indices for each race/ethnicity group was comparable with actual fracture risks (Fig. 2).
Fig. 2.
Actual vs. predicted fracture risk over 9 yr by race/ethnicity. AA, African-American. Error bars indicate 95% CI.
Discussion
Composite indices of femoral neck strength combine femoral neck BMD and size with body height and weight to quantify bone strength relative to the load that the femoral neck must bear. This longitudinal, multiethnic cohort study demonstrated that composite strength indices predict incident fracture risk without race/ethnicity information as accurately as BMD does with race/ethnicity. One-sd increment in each of the strength indices was associated with between 36 and 44% relative reduction in odds of fracture over 9 yr and between 34 and 41% relative reduction in rate (hazard) of first fracture. In the absence of race/ethnicity information, BMD (but not the composite indices) overpredicted the fracture risk in Japanese women. Addition of race/ethnicity information did not improve composite strength index-based risk prediction, whereas, in sharp contrast, it did significantly improve BMD-based risk prediction.
We replicated previous studies that have shown that areal BMD cannot correctly rank order women by fracture risk across race/ethnic groups (4, 5, 9, 25, 26). Therefore, uniform application of a Caucasian BMD reference database to women from other race/ethnic groups would over- or underestimate the fracture risk in some groups. This is an important error in calibration of BMD to fracture risk in some race/ethnicity groups and one that is likely to be of increasing relevance in the United States and elsewhere. In contrast, in this study, composite indices of femoral neck strength were able to correct for this calibration error and correctly rank order women by fracture risk across race/ethnic groups.
This study highlights the complex role played by body weight in fracture risk. Forces on bone increase with body weight (27); thus, bone mass that is adequate to resist fracture in a lighter person may be inadequate in a heavier individual. Hence, the need for composite indices that quantify how well the supply meets demand (15). On the other hand, the presence of more soft tissue around the hip in a heavier individual would act as padding to absorb some of the impact energy from a fall (27). Large weight and BMI (surrogate for soft tissue padding) differences between race/ethnicity groups may therefore hamper the ability of bone density to predict fracture risk across race/ethnicity groups. In this study, fracture prediction using BMD was improved by the inclusion of race/ethnicity information, even when the model included both body weight and BMI, yet fracture prediction using the composite strength indices (which incorporate body weight in a multiplicative fashion) was not improved by the inclusion of race/ethnicity information.
This study showed that composite indices designed to quantify bone strength in the femoral neck can index bone strength systematically and stratify individuals with respect to fragility fracture risk at any site. This is most likely because osteoporosis is a systemic condition that generally affects bone density and thus bone strength at all sites (28). BMD measured at specific sites such as the femoral neck, lumbar spine, or calcaneus predicts risk of fragility fracture at any site (3, 28–30). We hypothesize that, like bone density, other components of the composite indices that affect bone strength relative to load would also be correlated across various skeletal sites. Thus, for instance, bone dimensions at the hip and wrist have fracture prediction capacity across sites (31, 32). Specifically, femoral neck width is a measure of periosteal apposition, which compensates for endocortical bone loss (31), and periosteal apposition in the presence of increased endocortical resorption at the distal radius is associated with increased fracture risk at any site (even after adjusting for BMD) (32). Similarly, during a fall from standing height, body weight and height affect the degree of the load that any bone at the site of impact would be subject to (15). Finally, because of the consistently strong relationships between hip fracture risk and the risk of fragility fractures elsewhere (28, 33), a composite measure of bone strength in a select site such as the femoral neck should be able to predict the risk of osteoporotic fracture at any site.
This study also demonstrated that low bone strength, as assessed by the composite strength indices, is associated with high fracture risk, regardless of whether the fractures were minimum-trauma. The discrimination ability of the strength measures for fracture risk/hazard prediction was as good for predicting all fractures as it was for predicting minimum-trauma fractures. Previous studies have also found that low BMD is associated with increased risk of both minimum-trauma and non-minimum-trauma fractures in older adults (34).
The study has several limitations. First, the SWAN cohort is relatively young, and absolute fracture risks were lower than in older women. However, bone mass in young and middle adulthood is a major predictor of osteoporosis and fracture risk in later life (35, 36). Therefore, between-women fracture risk differences in this age group are likely to translate to similar fracture risk differences in older age. This contention is supported by the similarity of observed fracture risk differences in the SWAN cohort between race/ethnic groups and between diabetic and nondiabetic women (37) to fracture risk differences between these groups in older ages (38). Second, the composite strength indices do not take into account bone microstructural aspects (e.g. mineralization quality) (39) and some hip geometry characteristics (e.g. femoral neck shaft angle) (40). Despite this, the composite indices represent a major improvement over the current clinical standard for bone strength assessment and fracture risk stratification, DXA-based areal BMD. Third, we could not adjust for history of falls, an important risk factor for fracture. However, falls before baseline (when the women were 42–53 yr old) were likely rare in this cohort. Fourth, fractures were self-reported; however, the majority of fractures (all fractures reported after visit 6) were confirmed by medical records review. Also, the exact date of fracture was not available until follow-up visit 7. Finally, this cohort was limited to middle-aged women. Therefore, our findings need to be replicated by future studies in other populations, especially in men and in older women.
This study also has several strengths, including the high retention rate of the SWAN cohort, the diverse geographical areas represented, the large representation of minority women in the cohort, the use of bootstrapping to generate confidence intervals for discrimination statistics and change therein, and the robustness of the main findings to model covariate selection, exclusion of non-minimum-trauma fractures, and analytical approach.
In conclusion, composite indices of femoral neck strength predict incident fracture risk in middle-aged women without requiring race/ethnicity information and as accurately as bone mineral density does when supplemented with race/ethnicity information. The indices are able to correct a significant error in the calibration of conventional BMD to fracture risk in the race/ethnic groups included in this study. Thus, we propose that unlike BMD, composite femoral neck strength indices will permit fracture risk assessment in individuals of unclear/mixed ethnicity and in underrepresented race/ethnicity groups that do not have a bone density reference database. Our findings may inform a new clinical approach to fracture risk assessment because the composite strength indices can be easily computed from routine DXA measures and represent an important improvement over BMD.
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Institute of Nursing Research, Office of Research on Women's Health, Veterans Affairs, or the National Institutes of Health. Clinical centers included the following: University of Michigan, Ann Arbor, MaryFran Sowers, principal investigator (PI) 1994–2001; Siobán Harlow, PI 2011–present; Massachusetts General Hospital, Boston, Massachusetts, Joel Finkelstein, PI 1999 to present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, Illinois, Howard Kravitz, PI 2009 to present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser, Ellen Gold, PI; University of California, Los Angeles, Los Angeles, California, Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, New York, Carol Derby, PI 2011–present; Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry-New Jersey Medical School, Newark, New Jersey, Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, Pennsylvania, Karen Matthews, PI. The National Institutes of Health Program Office, National Institute on Aging, Bethesda, MD, Winifred Rossi 2012; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD, Project Officer. The central laboratory was the University of Michigan, Ann Arbor, Daniel McConnell (Central Ligand Assay Satellite Services). The coordinating center was University of Pittsburgh, Pittsburgh, Pennsylvania, Kim Sutton-Tyrrell, PI 2001 to present; Maria Mori Brooks, Co-PI 2012; New England Research Institutes, Watertown, Massachusetts, Sonja McKinlay, PI 1995–2001. The Steering Committee included Susan Johnson, current chair, and Chris Gallagher, former chair. We thank the study staff at each site and all the women who participated in the Study of Women's Health Across the Nation.
The Study of Women's Health Across the Nation (SWAN) was supported by grants from the National Institutes of Health (NIH), Department of Health and Human Resources, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research onWomen's Health (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495). The Hip Strength Through the Menopausal Transition Substudy has grant support from the National Institute on Aging (Grant AG026463). S.I. was supported by the Veterans Affairs Greater Los Angeles Healthcare System Geriatric Research, Education, and Clinical Center, and a Veterans Affairs Advanced Geriatrics Fellowship.
Disclosure Summary: There is no conflict of interest to be disclosed for any of the authors regarding this study.
Footnotes
- BMD
- Bone mineral density
- BMI
- body mass index
- BSI
- bending strength index
- CI
- confidence interval
- CSI
- compression strength index
- DXA
- dual-energy x-ray absorptiometry
- FNAL
- femoral neck axis length
- FNW
- femoral neck width
- ISI
- impact strength index
- ROC
- receiver operator characteristic
- SWAN
- Study of Women's Health Across the Nation.
References
- 1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. 2007. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- 2. Cheng SY, Levy AR, Lefaivre KA, Guy P, Kuramoto L, Sobolev B. 2011. Geographic trends in incidence of hip fractures: a comprehensive literature review. Osteoporos Int 22:2575–2586 [DOI] [PubMed] [Google Scholar]
- 3. Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, 3rd, O'Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. 2005. Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194 [DOI] [PubMed] [Google Scholar]
- 4. Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. 2001. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822 [DOI] [PubMed] [Google Scholar]
- 5. Robbins J, Aragaki AK, Kooperberg C, Watts N, Wactawski-Wende J, Jackson RD, LeBoff MS, Lewis CE, Chen Z, Stefanick ML, Cauley J. 2007. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA 298:2389–2398 [DOI] [PubMed] [Google Scholar]
- 6. Binkley NC, Schmeer P, Wasnich RD, Lenchik L. 2002. What are the criteria by which a densitometric diagnosis of osteoporosis can be made in males and non-Caucasians? J Clin Densitom 5(Suppl):S19–S27 [DOI] [PubMed] [Google Scholar]
- 7. Megyesi MS, Hunt LM, Brody H. 2011. A critical review of racial/ethnic variables in osteoporosis and bone density research. Osteoporos Int 22:1669–1679 [DOI] [PubMed] [Google Scholar]
- 8. The International Society for Clinical Densitometry Official positions (cited Dec. 6, 2011). Available from: http://www.iscd.org/Visitors/positions/OfficialPositionsText.cfm
- 9. Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. 2005. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA 293:2102–2108 [DOI] [PubMed] [Google Scholar]
- 10. Cauley JA, El-Hajj Fuleihan G, Arabi A, Fujiwara S, Ragi-Eis S, Calderon A, Chionh SB, Chen Z, Curtis JR, Danielson ME, Hanley DA, Kroger H, Kung AW, Lesnyak O, Nieves J, Pluskiewicz W, El Rassi R, Silverman S, Schott AM, Rizzoli R, Luckey M. 2011. Official Positions for FRAX(R) clinical regarding international differences from the Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(R). J Clin Densitom 14:240–262 [DOI] [PubMed] [Google Scholar]
- 11. U.S. Census Bureau The 2012 statistical abstract (cited Dec. 6, 2011). Available from: http://www.census.gov/compendia/statab/cats/population.html
- 12. Faulkner KG, Cummings SR, Black D, Palermo L, Glüer CC, Genant HK. 1993. Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. J Bone Miner Res 8:1211–1217 [DOI] [PubMed] [Google Scholar]
- 13. Nelson DA, Beck TJ, Wu G, Lewis CE, Bassford T, Cauley JA, LeBoff MS, Going SB, Chen Z. 2010. Ethnic differences in femur geometry in the women's health initiative observational study. Osteoporos Int 22:1377–1388 [DOI] [PubMed] [Google Scholar]
- 14. Alonso CG, Curiel MD, Carranza FH, Cano RP, Peréz AD. 2000. Femoral bone mineral density, neck-shaft angle and mean femoral neck width as predictors of hip fracture in men and women. Multicenter Project for Research in Osteoporosis. Osteoporos Int 11:714–720 [PubMed] [Google Scholar]
- 15. Karlamangla AS, Barrett-Connor E, Young J, Greendale GA. 2004. Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int 15:62–70 [DOI] [PubMed] [Google Scholar]
- 16. Ishii S, Cauley JA, Greendale GA, Danielson ME, Safaei Nili N, Karlamangla A. 2012. Ethnic differences in composite indices of femoral neck strength. Osteoporos Int 23:1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. 2000. Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community based cohort study of women and the menopausal transition. San Diego: Academic Press [Google Scholar]
- 18. Baecke JA, Burema J, Frijters JE. 1982. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36:936–942 [DOI] [PubMed] [Google Scholar]
- 19. Seeley DG, Browner WS, Nevitt MC, Genant HK, Scott JC, Cummings SR. 1991. Which fractures are associated with low appendicular bone mass in elderly women? The Study of Osteoporotic Fractures Research Group. Ann Intern Med 115:837–842 [DOI] [PubMed] [Google Scholar]
- 20. Hauck WW, Donner A. 1977. Wald's test as applied to hypotheses in logit analysis. J Am Stat Assoc 72:851–853 [Google Scholar]
- 21. Harrell FE, Jr, Lee KL, Mark DB. 1996. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387 [DOI] [PubMed] [Google Scholar]
- 22. Carpenter J, Bithell J. 2000. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 19:1141–1164 [DOI] [PubMed] [Google Scholar]
- 23. Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. 2008. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172 [DOI] [PubMed] [Google Scholar]
- 24. Agresti A, Coull BA. 1998. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52:119–128 [Google Scholar]
- 25. Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. 2005. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 20:185–194 [DOI] [PubMed] [Google Scholar]
- 26. Cauley JA. 2011. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res 469:1891–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. 2009. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women's health initiative-observational study. J Bone Miner Res 24:1369–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. 2003. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18:1947–1954 [DOI] [PubMed] [Google Scholar]
- 29. Marshall D, Johnell O, Wedel H. 1996. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanis JA, Johansson H, Oden A, McCloskey EV. 2009. Assessment of fracture risk. Eur J Radiol 71:392–397 [DOI] [PubMed] [Google Scholar]
- 31. Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. 2003. Bone loss and bone size after menopause. N Engl J Med 349:327–334 [DOI] [PubMed] [Google Scholar]
- 32. Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. 2006. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 21:1856–1863 [DOI] [PubMed] [Google Scholar]
- 33. Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. 1993. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 341:72–75 [DOI] [PubMed] [Google Scholar]
- 34. Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, Hillier TA, Lewis CE, Barrett-Connor E, Cummings SR. 2007. High-trauma fractures and low bone mineral density in older women and men. JAMA 298:2381–2388 [DOI] [PubMed] [Google Scholar]
- 35. Hansen MA, Overgaard K, Riis BJ, Christiansen C. 1991. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ 303:961–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riis BJ, Hansen MA, Jensen AM, Overgaard K, Christiansen C. 1996. Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture: a 15-year follow-up study. Bone 19:9–12 [DOI] [PubMed] [Google Scholar]
- 37. Khalil N, Sutton-Tyrrell K, Strotmeyer ES, Greendale GA, Vuga M, Selzer F, Crandall CJ, Cauley JA. 2011. Menopausal bone changes and incident fractures in diabetic women: a cohort study. Osteoporos Int 22:1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. 2005. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 165:1612–1617 [DOI] [PubMed] [Google Scholar]
- 39. Follet H, Boivin G, Rumelhart C, Meunier PJ. 2004. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone 34:783–789 [DOI] [PubMed] [Google Scholar]
- 40. Beck TJ. 2007. Extending DXA beyond bone mineral density: understanding hip structure analysis. Curr Osteoporos Rep 5:49–55 [DOI] [PubMed] [Google Scholar]