Abstract
Context:
A high chromosomal abnormalities rate has been observed in human embryos derived from in vitro fertilization (IVF) treatments. The real incidence in natural cycles has been poorly studied, so whether this frequency may be induced by external factors, such as use of gonadotropins for ovarian stimulation, remains unknown.
Design:
We conducted a prospective cohort study in a University-affiliated private infertility clinic with a comparison between unstimulated and stimulated ovarian cycles in the same women. Preimplantation genetic screening by fluorescence in situ hybridization was performed in all viable d 3 embryos.
Objective:
The primary objective was to compare the incidence of embryo chromosomal abnormalities in an unstimulated cycle and in an ulterior moderate ovarian stimulated cycle. Secondary outcome measures were embryo quality, blastocyst rate of biopsied embryos, number of normal blastocysts per donor, type of chromosomal abnormalities, and clinical outcome.
Results:
One hundred eighty-five oocyte donors were initially recruited for the unstimulated cycle, and preimplantation genetic screening could be performed in 51 of them, showing 35.3% of embryo chromosomal abnormalities. Forty-six of them later completed a stimulated cycle. The sperm donor sample was the same for both cycles. The proportion of embryos displaying abnormalities in the unstimulated cycle was 34.8% (16 of 46), whereas it was 40.6% (123 of 303) in the stimulated cycle with risk difference = 5.8 [95% confidence interval (CI) = −20.6–9.0], and relative risk = 1.17 (95% CI = 0.77–1.77) (P = 0.45). When an intrasubject comparison was made, the abnormalities rate was 34.8% (95% CI = 20.5–49.1) in the unstimulated cycle and 38.2% (95% CI = 30.5–45.8) in the stimulated cycle [risk difference = 3.4 (95% CI = −17.9–11.2); P = 0.64]. No differences were observed for embryo quality and type of chromosomal abnormalities.
Conclusions:
Moderate ovarian stimulation in young normo-ovulatory women does not significantly increase the embryo aneuploidies rate in in vitro fertilization-derived human embryos as compared with an unstimulated cycle. Whether these results can be extrapolated to infertile patients is still unknown.
In vitro fertilization (IVF) treatment is a routine method to overcome infertility in humans. Despite having been developed more than three decades ago, IVF efficacy in terms of the live birth rate per started cycle is still lower than desired (1). This is due, in part, to the low fecundity of our species but can also be influenced by the potentially detrimental effects of the procedure, which has raised many questions in epidemiological studies on IVF babies (2, 3).
The low fecundity observed in humans, if compared with other mammalian species (4), could be due to embryo chromosomal abnormalities, which may lead to preimplantation embryonic death, implantation failure, and spontaneous miscarriages (5). In fact, 32–64% of IVF-derived embryos have chromosomal errors and aneuploidies in young good-prognosis women (6, 7).
It is still unknown whether this high frequency of embryo aneuploidy reflects a true picture in early human conceptuses under natural conditions or whether it is induced by external factors, such as in vitro culture conditions (8, 9) or use of gonadotropins for controlled ovarian stimulation (COS) (10). Obtaining data from naturally in vivo-fertilized human embryos would be ethically and technically unfeasible, but dissecting the different IVF steps to search for potential negative influences is also of paramount importance to reassure and improve IVF outcome.
The influence of COS is of particular interest. There is a trend toward less aggressive protocols to avoid clinical complications and to also reduce chromosomal abnormalities (11). In animals, ovarian stimulation seems to affect embryo development (12, 13) and to increase the frequency of chromosomal abnormalities (14, 15). In humans, data on the direct effect of COS are scarce. However, it has been recently suggested that use of gonadotropins may raise the chromosomal abnormality rate in a dose-dependent manner (11).
To investigate the influence of ovarian stimulation on chromosomal abnormalities, we need to establish the basal status in humans by analyzing the natural cycle in fertile women. The closest approach to date is based on 11 embryos derived from unstimulated cycles, which indicates that a chromosome aneuploidy is present in 36.4% of embryos (16).
Given this background, the current study has a dual purpose: first, to ascertain the actual incidence of chromosomal abnormalities in young fertile women to define a reference rate for human embryos fertilized in vitro after an unstimulated cycle, and second, to investigate to what extent COS could increase the chromosomal abnormalities rate in human embryos.
Subjects and Methods
This prospective cohort study was carried out in a University-affiliated infertility clinic between September 2006 and March 2010. Approval was obtained from the Institutional Review Board and the Institution's Ethics Committee before starting the study. The www.clinicaltrials.gov registration number is NCT00707525.
Study population
The study was performed within our oocyte donation program in couples for whom a sperm donation was also required. Therefore, only gametes from young subjects free of an infertility background were considered.
Inclusion criteria for both oocyte and sperm donors were aged between 18 and 34, had a normal physical examination, had no family history of hereditary or chromosomal diseases, tested negative in a screening for sexually transmitted diseases, and had a normal karyotype.
Specific inclusion criteria for oocyte donors were fertile (at least one child born and/or voluntary interrupted pregnancy), regular menstrual cycles (25–35 d); body mass index (BMI) between 18 and 25 kg/m2, no previous ovarian stimulation treatments, normal basal serum FSH (<10 IU/liter) and estradiol (<50 pg/ml) levels. Exclusion criteria were endometriosis, polycystic ovary syndrome, and recurrent miscarriage (at least three previous clinical miscarriages).
Specific inclusion criteria for sperm donors were more than 90 × 106 total motile progressive sperm and more than 14% of normal forms in the ejaculate and having proved to be fertile in our artificial insemination program (17).
Inclusion criteria for gametes recipients were under 45 yr old, BMI below 30 kg/m2, and no uterine or adnexa abnormalities assessed by vaginal ultrasound. Exclusion criteria were recurrent miscarriage, implantation failure, and organic or systemic diseases. All the donors and gametes recipients gave their written informed consent.
Study design
Procedures
Follicular phase.
Oocyte donors underwent two IVF cycles: first, an unstimulated cycle without medical intervention, except for 250 μg recombinant choriogonadotropin (rCG) (Ovitrelle; Merck-Serono, Geneva, Switzerland) administered when the spontaneous preovulatory follicle reached 18 mm in diameter. Second, a stimulated cycle in which ovarian stimulation was carried out by following a GnRH agonist long protocol, with a combination of 150 IU recombinant FSH (Gonal F; Merck-Serono) and 75 IU highly purified human menopausal gonadotropin (Menopur; Ferring Pharmaceuticals, Copenhagen, Denmark). When six or more follicles were more than 17 mm in diameter, rCG was administered for triggering ovulation. Doses were adjusted according to the ovarian response, as judged by serum estradiol concentrations and ultrasound scans every 2–3 d.
In both cycles, ultrasound-guided transvaginal oocyte retrieval was scheduled 36 h after triggering ovulation. Serum estradiol and progesterone were determined on the day of rCG administration. In the unstimulated cycle, the LH was also measured.
Lab procedures.
Intracytoplasmic sperm injection (ICSI) was performed in all cases by following our routine practice in preimplantation genetic screening (PGS) cycles to avoid contamination by extraneous DNA and to increase the number of fertilized embryos and, therefore, the potential number of embryos available for testing. Fertilization was assessed 17–20 h afterward, and embryo cleavage was recorded every 24 h. Embryos were grown in IVF/CCM medium (1:1 ratio) (Vitrolife, Göteborg, Sweden) until d 3 and were subsequently cultured in CCM medium (Vitrolife) with a monolayer of endometrial epithelial cells until d 5 (18).
An embryo biopsy was performed on d 3 using a noncontact laser system (OCTAX, Herbron, Germany). Only those embryos with at least five nucleated blastomeres of a similar size and a fragmentation degree of less than 20% were biopsied. (Fragmentation is a parameter related to embryo quality. It refers to the percentage of the total volume of the embryo with fragments resulting from the degeneration of one or more blastomeres.) A single blastomere was removed and a fluorescence in situ hybridization (FISH) analysis was performed for nine chromosomes in two consecutive hybridization rounds: first, MultiVysion PB panel for chromosomes 13, 16, 18, 21, and 22, and second, MultiVysion 4 Color Custom panel for chromosomes 15, 17, X, and Y (Vysis, Inc. Downers Grove, IL). Additional hybridization rounds for these chromosomes, using probes that bind to different loci, were conducted to rescue the nonconclusive results and to confirm certain aneuploidies, as previously described (19). Thirty-six randomly selected embryos, diagnosed as chromosomally abnormal on d 3, were reanalyzed on d 5 to validate their diagnosis.
Protocol
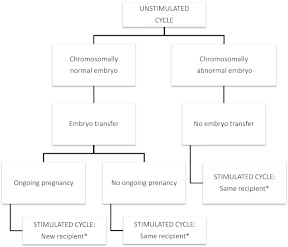
If the sole embryo from the unstimulated cycle was chromosomally normal, then an embryo transfer was performed. If ongoing pregnancy was accomplished, the embryos obtained in the stimulated cycle were donated to a new recipient. If after the first embryo transfer no ongoing pregnancy was achieved, the same recipient received embryos from the stimulated cycle (Fig. 1).
Fig. 1.
Study protocol. Oocyte donors underwent an unstimulated cycle and a subsequent stimulated cycle. *, Donated oocytes were always inseminated with the same sperm sample in a given couple of recipients.
If the embryo resulting from the unstimulated cycle was abnormal, the transfer was cancelled and the donor was submitted to the stimulated cycle. A maximum of two embryos were then transferred to the recipient. In all cases, the sperm donor was the same for both cycles to rule out male influence.
The time frame between both cycles was a maximum of 3 months. All the oocyte recipients received hormonal replacement therapy, as described elsewhere (20).
Statistical analysis
Outcome measures
The primary objective was to compare the proportion of embryos with chromosomal abnormalities resulting from an unstimulated IVF cycle to those obtained after ovarian stimulation in the same woman. Secondary outcomes were embryo quality in the cleavage stage (in terms of number of cells and fragmentation rate), blastocyst rate (of the biopsied embryos), number of normal blastocysts per donor, type of chromosomal abnormalities, and clinical outcome.
Sample size
Sample size was calculated to detect a difference of 22% in the aneuploidy incidence between both groups (from 33% in the unstimulated cycle to 55% after stimulation) in a two-sided test, with a statistical power of 80% (error β = 0.2) and a confidence level of 95% (error α = 0.05), by estimating an average of six embryos analyzed per stimulated cycle. This difference was expected according to not only the previously described aneuploid embryo incidences in oocyte donors under stimulated cycles (21, 22) but also the accepted normal embryos rate in fertile couples (23).
According to these criteria, 45 subjects were required for a comparison between both cycles in the same woman. A 15% dropout rate among the women who completed the unstimulated cycle and the stimulated cycle was considered. Therefore, women were recruited for the unstimulated cycle until 51 completed it.
Statistical methods
Student's t tests and χ2 were employed for the continuous and categorical quantitative variables, respectively, for comparisons to be made between both groups. A relative risk with its 95% confidence interval (CI) was calculated for all the comparisons between the categorical variables. For the nonparametric analysis, a Mann-Whitney U test was used. For the intrasubject comparison of the chromosomally abnormal embryos rate between both cycle types, a paired-samples t test was done.
Results
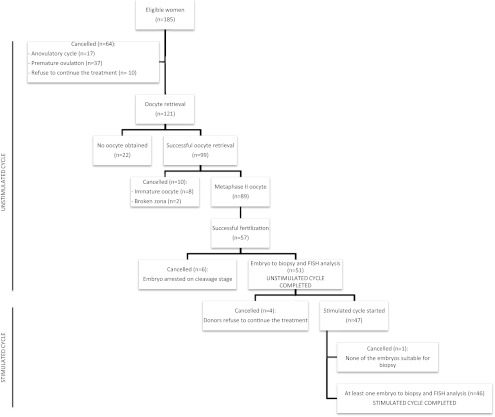
A summary of all the started cycles is shown in Fig. 2. A total of 185 eligible donors were enrolled. Sixty-four were excluded before oocyte retrieval because of premature ovulation (n = 37), anovulatory cycle (n = 17), or self-cancellation (n = 10). Therefore, 121 oocyte retrievals were planned (65.4%). Of these, an oocyte was obtained in 99 (81.8%), of whom 89 were metaphase II (89.9%). Fertilization occurred in 57 of them (64.0%). Finally, 51 of the 57 resulting embryos fulfilled all the criteria to be analyzed on d 3 of development. Of these 51 donors, 47 continued with the stimulated cycle, whereas four were cancelled because they refused to continue treatment. In one cycle with the 47 stimulated ones, none of the embryos was suitable for biopsy. In summary, 51 donors completed the unstimulated cycle, whereas 46 of them also completed the stimulated cycle. A completed protocol was considered when at least one embryo was suitable for biopsy, and PGS-FISH was performed in both cycles.
Fig. 2.
Flow chart of study population.
The mean age of the oocyte donors who completed the study was 25.4 ± 4.0 yr. Their BMI was 22.5 ± 2.8 kg/m2, and their basal FSH levels were 6.1 ± 2.1 IU/liter. The average age of the recipients population was 39.7 ± 3.5 yr, with a BMI of 25.6 ± 4.1 kg/m2.
The follicular phase and embryo development parameters of the completed cycles are outlined in Table 1. Although all 51 embryos from the unstimulated cycles were informative after the embryo biopsy, four of the 307 embryos from 46 stimulated cycles were not. Informative embryos were defined as embryos with a conclusive result after the FISH analysis. When the blastomere had two signals for all the analyzed chromosomes, the embryo was classified as normal with a conclusive result. However, an aneuploid embryo is when an abnormal number of signals for at least one analyzed chromosome is observed despite lack of information for another chromosome.
Table 1.
Follicular phase and embryo development parameters of the 51 unstimulated cycles and the 46 stimulated cycles in which PGS-FISH was successfully performed
| Parameter | Result |
|---|---|
| Unstimulated cycle (n = 51) | |
| Estradiol serum levels on rCG day (pg/ml) | 190.5 ± 76.5 |
| Progesterone serum levels on rCG day (ng/ml) | 1.1 ± 4.9 |
| LH serum levels on rCG day (IU/liter) | 10.54 ± 8.1 |
| No. of oocytes obtained | 99 (81.8% per oocyte retrieval) |
| No. of oocytes metaphase II | 89 (89.9% of the oocytes obtained) |
| No. of fertilized oocytes | 57 (64% of the metaphase II oocytes) |
| No. of embryos arrested on cleavage stage | 6 |
| No. of biopsied embryos | 51 |
| No. of informative embryosa | 51 |
| Stimulated cycle (n = 46) | |
| Length of stimulation (d) | 10.1 ± 2.3 |
| Total gonadotropin dose (IU) | 2230 ± 670 |
| Estradiol serum levels on rCG day (pg/ml) | 2707 ± 1135 |
| Progesterone serum levels on rCG day (ng/ml) | 0.8 ± 0.4 |
| No. of oocytes obtained | 820 (17.8 ± 7.7) |
| No. of oocytes metaphase II | 618 (13.4 ± 6.2) |
| No. of fertilized oocytes | 469 (10.2 ± 4.8) |
| No. of embryos arrested on cleavage stage | 162 |
| No. of biopsied embryos | 307 |
| No. of informative embryosa | 303 |
Plus-minus values are mean ± sd.
Informative embryos were defined as embryos with a conclusive result after the FISH analysis. A conclusive result for a normal embryo is two signals for all analyzed chromosomes. A conclusive result for an abnormal embryo is an abnormal number of signals for at least one analyzed chromosome despite the absence of information for another chromosome.
The proportion of embryo chromosomal abnormalities in the whole sample of 51 unstimulated cycles was 35.3% (18 of 51). In the group of 46 women who completed both cycles, this proportion was 34.8% (16 of 46), whereas it was 40.6% (123 of 303) in the stimulated cycle [risk difference = 5.8 (95% CI = −20.6–9.0), relative risk = 1.17 (0.77–1.77); P = 0.45]. No differences were observed between both cycles in embryo quality terms (Table 2).
Table 2.
Results of the PGS of 46 donors who fulfilled both the unstimulated and ovarian stimulated cycles
| Unstimulated cycle | Stimulated cycle | Relative risk (95% CI) | P value | |
|---|---|---|---|---|
| No. of biopsied embryos | 46 | 307 | ||
| No. of informative embryos | 46 | 303 | ||
| Blastomeres on d 3 (mean ± sd) | 7.4 ± 1.4 | 7.9 ± 0.9 | 0.07 | |
| Fragmentation on d 3 (mean ± sd) | 7.4 ± 6.2 | 9.1 ± 4.7 | 0.13 | |
| No. of chromosomally abnormal embryos/informative embryos [n (%)] | 16/46 (34.8%) (95% CI = 22.7–49.2) | 123/303 (40.6%) (95% CI = 35.2–46.2) | 1.17 (0.77–1.77) | 0.45 |
| Mean chromosomally abnormal embryos rate per subjecta (%) | 34.8 (95% CI = 20.5–49.1) | 38.2 (95% CI = 30.5–45.8) | 0.64 | |
| No. of blastocysts on d 5/biopsied embryo [n (%)] | 31/46 (67.4%) (95% CI = 53.0–79.1) | 238/307 (77.5%) (95% CI = 72.5–81.8) | 1.15 (0.93–1.42) | 0.13 |
Data are presented as mean ± sd or n (percent). P value <0.05 is considered statistically significant.
Paired-samples t test.
When an intrasubject comparison was made, the abnormalities rate was 34.8% (95% CI = 20.5–49.1) in the unstimulated cycle and 38.2% (95% CI = 30.5–45.8) in the stimulated cycle [risk difference = 3.4 (95% CI = −17.9–11.2) (P = 0.64).
If the embryo from the unstimulated cycle was abnormal, the aneuploidy rate in the stimulated cycle was 45.2%, whereas it was 35.2% if the embryo from the unstimulated cycle was euploid (P = 0.2).
The mean number of euploid embryos per woman was 3.9 (95% CI = 3.2–4.6) in the stimulated cycle, with a mean of cryopreserved embryos of 1.8 ± 2.2.
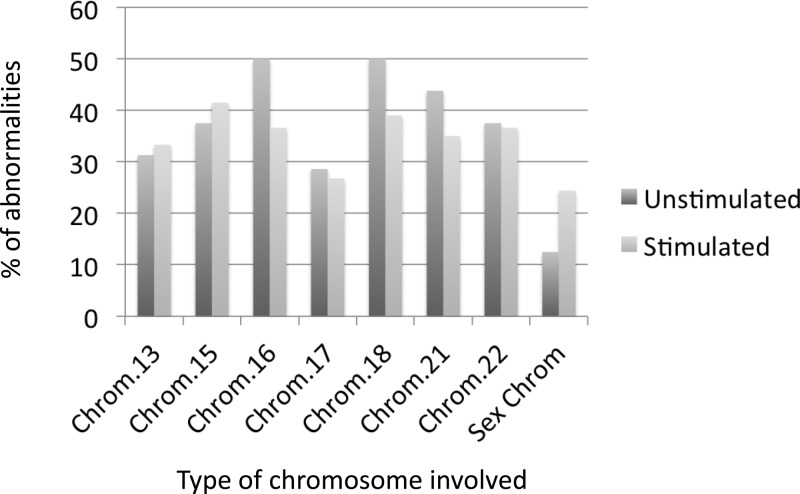
The distribution of chromosomal abnormalities was similar in both the unstimulated and the stimulated cycles (P = 0.87) and showed mainly monosomies (10 of 16 vs. 68 of 123), followed by trisomies (two of 16 vs. 30 of 123) and haploid embryos (three of 16 vs. five of 123), respectively. Moreover, there were no differences between both groups regarding the type of chromosome involved, as shown in Fig. 3.
Fig. 3.
Type of chromosome involved in the aneuploid embryos group. No statistically significant differences (P > 0.05) were observed in any of the analyzed chromosomes between the unstimulated and the stimulated cycle. Chrom., Chromosome.
The d 5 reanalysis by FISH of a sample of 36 embryos diagnosed as abnormal on d 3 produced a confirmation rate of 91.7%. An average of 32.9 ± 21.9 cells were studied per blastocyst. In the unstimulated cycle, we reanalyzed seven of the 18 chromosomally abnormal embryos with a confirmation rate of 100%. In the stimulated cycle, we reanalyzed 29 abnormal embryos and found three cases with false-positive diagnoses.
In the remaining 33 confirmed cases, 10 blastocysts were diagnosed as homogeneously aneuploid (>80% cells with the same abnormality) and 23 as abnormal mosaics (at least two cell lines, each with ≥20% of cells).
The means of the embryos transferred were significantly higher in the stimulated cycles (1.7 ± 0.6 vs. 0.7 ± 0.5; P < 0.001). Accordingly, the implantation rate (number of gestational sacs per embryos transferred) was the only reliable parameter to compare the outcome between both cycles. The implantation rate was 39.3 (11 of 28) vs. 32.5% (26 of 80) in the unstimulated vs. the stimulated cycles (P = 0.68). Each group included one biochemical and one ectopic pregnancy. The miscarriage rate was 33.3% (four of 12) vs. 13.3% (four of 30), respectively (P = 0.297). Live births per completed cycle were six of 46 (13%) in the unstimulated cycle and 21 of 46 (45.7%) in the stimulated one (P = 0.001).
Discussion
The current study shows that moderate ovarian stimulation does not significantly increase the chromosomally abnormal embryos rate obtained after IVF in a population of young women with no background of infertility or ovulation induction treatments. These findings are supported by previous data showing that the incidence of aneuploidies in aborted fetuses from pregnancies conceived with FSH stimulation did not increase when compared with spontaneous conceptions in infertile patients (24, 25). Nevertheless, we cannot rule out the possibility of minor increases in the chromosomal abnormality rate in stimulated vs. unstimulated cycles because our study sample size was calculated to detect a 22% difference between both cycles. A much higher sample of patients would be needed to detect smaller differences.
The impact of ovarian stimulation on embryo quality, and furthermore on the chromosomal constitution of the embryo, has been a matter of concern for many years. Some authors have reported a greater risk of major birth defects in ICSI and IVF cycles, (2, 3), suggesting that the medication used to induce ovulation, among other factors, could explain these findings. This was supported by the study of Baart et al. (11), which found a positive relationship between doses of gonadotropins and the chromosomal abnormalities rate. However, these studies were based on IVF-derived embryos from infertile patients after ovarian stimulation treatment, and no information for preimplantation embryos derived from natural conception is available for comparison.
Our study has overcome these difficulties using a novel model, which includes a comparison between the embryos from both the unstimulated and stimulated cycles in the same population, thus avoiding interpatient variability biases. In addition, because our study population was free of several confounding factors, such as maternal age, infertility, or severe male factors, we could analyze the net impact of gonadotropins on embryos.
The complex design of our study first enabled us to evaluate the chromosomal status of the human embryo in the absence of ovarian stimulation. We observed that embryo aneuploidies were still present even under this condition because approximately one of the three embryos was seen to be chromosomally abnormal. This is in good agreement with a shorter study that found an aneuploidy rate of 36.4% in 11 unstimulated cycles (16). The unstimulated IVF could be considered the closest scenario to the natural cycle, although we cannot rule out the potential impact of other factors, such as laboratory conditions (26), the use of rCG for triggering ovulation, or even the use of ICSI (27). Although we acknowledge these limitations, we included the largest series by exploring the basal human embryo chromosomal status without the use of ovarian stimulation.
It is worth noting the low efficacy of the natural cycle observed in our study because 175 women (10 of 185 refused to continue) had to be recruited to complete 51 unstimulated cycles.
Although natural IVF cycles are considered patient-friendly with low-risk protocols, their efficacy is hampered because of the high cancellation rates (28), thus reinforcing the need for ovarian stimulation to induce the development of several mature oocytes to improve chances of pregnancy in IVF treatments. Some reports have suggested the use of the natural cycle in poor-prognosis patients, mainly older women or low responders to COS (29). Our results suggest that chromosomal abnormalities are also present in the absence of stimulation with gonadotropins. Therefore, the use of a natural cycle in these patients is expected to give a poor outcome, which is in agreement with the higher aneuploidy rate observed in spontaneous pregnancies in older women (30).
The analysis of human gametes has revealed that an embryo aneuploidy is primarily caused by an error-prone meiotic chromosome segregation mechanism in oocytes. If ovarian stimulation can exert any deleterious effect on the embryo, this may be because it induces meiotic errors on the oocyte, but not mitotic errors after fertilization, which commonly lead to mosaicism (31). The two well-known mechanisms for meiotic errors are nondisjunction and premature separation of sister chromatids (32), and they usually result in a uniformly aneuploid embryo (monosomic, trisomic, haploid, or polyploidy); hence, they are expressed in both the d 3 PGS analysis and the d 5 reanalysis.
In the current study, a single cell was biopsied per embryo on d 3. Although this could lead to misdiagnosis in cases with mosaicism, we observed an overall cytogenetic confirmation rate of 91.7%, which is in agreement with our previously obtained data (19). This low error rate cannot radically affect the results and conclusions of the present study. Moreover, all the d 5 mosaic embryos observed in our study after reanalysis had all cell lines aneuploid; thus, they would have been discarded after the d 3 diagnosis. In any case, according to the data hereby shown, ovarian stimulation might not affect the incidence of mosaicism. Therefore, it can be assumed that the cases of mosaicism would be equally distributed in both unstimulated and stimulated cycles.
In our study, arrested embryos were not analyzed because only developmentally good embryos were, which follows the well-established criteria in PGS-FISH cycles by considering that only evolutive embryos can implant, and therefore, the aneuploidy risk in offspring would be related only to embryos that could reach the blastocyst stage and implant.
In line with this, 89.5 and 65.5% of embryos were biopsied in the unstimulated cycle and the stimulated cycle, respectively. Our biopsied embryos rate in the stimulated cycles is in agreement with that observed in the study of Baart et al. (11) (67.8%), which followed similar criteria to ours. Moreover, our rate of informative results per fertilized embryo was 64.6%, whereas it was 58.7% in the study of Baart et al. (11).
One of the limitations of our study is that the technique used to analyze embryos was FISH. This was because it was the most accurate technique for this purpose when the study was designed. Recently, we introduced the array comparative genomic hybridization system into our PGS program to allow the analysis of all the chromosomes. Nevertheless, the confirmation rate we obtained with the FISH procedure was about 95% according to our results of the reanalysis of single blastomeres on d 4 using array comparative genomic hybridization (19). Moreover, it has been reported that for chromosomes 13, 15, 16, 18, 21, 22, X, and Y, FISH should identify 80% of the most common chromosomal anomalies in spontaneous abortions samples (33). Therefore, by bearing in mind the risk of aneuploidy offspring, all the chromosomes were covered by the FISH technique.
The chromosomal abnormality rate in our population after COS was lower than that previously described in oocyte donors (57.0%) (21, 22). In those studies, the number of embryos biopsied per cycle was higher (14.6 vs. 6.7 in our study) because more aggressive stimulation protocols were used. This difference in results suggests that higher doses of gonadotropins (in comparison with those used in our study) may increase the percentage of aneuploidies. Nevertheless, the basic fact is that the total number of euploid embryos is more relevant than the percentage of abnormalities. The advantage of the stimulated cycle is that a mean number of four euploid embryos was obtained in comparison with the unstimulated cycle, which offers a maximum of one euploid embryo. Although the implantation rate was absolutely comparable between both cycles, the difference in the total number of euploid embryos available for transfer explains the higher cumulative pregnancy rates observed in egg donation stimulated cycles. Finally, the live births rate was significantly higher in the stimulated cycle as more embryos were transferred per cycle, whereas an unexpected yet not statistically significantly higher miscarriage rate was observed in the unstimulated cycle. However, we should not draw conclusions because sample size was not calculated for this purpose.
In conclusion, the results of this study reveal that embryo chromosomal abnormalities are present even under ovarian physiological conditions, which may explain the relatively low fertility of the human species. The use of moderate doses of gonadotropins for ovarian stimulation during an IVF cycle does not significantly increase the abnormalities rate in young normo-ovulatory women. Whether these results can be applied to infertile patients is still unknown.
Acknowledgments
We thank the clinicians, IVF embryologist, and technicians for their cooperation in this study. Special thanks go to the staff involved in our oocyte and sperm donation program and also to all the PGD team of IVI-Valencia, specially to Amparo Mercader, Pilar Buendía, and Arantxa Delgado, the embryologists who performed the embryo biopsy, blastomere fixation, and d-5 embryo fixation.
Financial support for this study was provided by an unrestricted grant from Merck-Serono, Geneva, Switzerland.
E.L. is the principal investigator of the study. She was involved with designing the study, participated in subject recruitment and care during the medical treatment, did the data analysis and interpretation, and wrote the manuscript. E.B. participated in the study design, patients recruitment, and statistical analysis and assisted with manuscript writing. P.A. participated in donors recruitment and care during the medical treatment. C.R. participated in the analysis of FISH signals on blastomeres and reanalysis of d-5 embryos and reviewed the final version of the manuscript. L.R. participated in the analysis of FISH signals on blastomeres and reanalysis of d-5 embryos. A.P. designed the study and reviewed multiple versions of the manuscript.
Disclosure Summary: We declare that we have no conflicts of interest.
Footnotes
- BMI
- Body mass index
- CI
- confidence interval
- COS
- controlled ovarian stimulation
- FISH
- fluorescence in situ hybridization
- ICSI
- intracytoplasmic sperm injection
- IVF
- in vitro fertilization
- PGS
- preimplantation genetic screening
- rCG
- recombinant choriogonadotropin.
References
- 1. Nygren KG, Sullivan E, Zegers-Hochschild F, Mansour R, Ishihara O, Adamson GD, de Mouzon J. 2011. International Committee for Monitoring Assisted Reproductive Technology (ICMART) world report: assisted reproductive technology 2003. Fertil Steril 95:2209–2222, 2222.e1–e17 [DOI] [PubMed] [Google Scholar]
- 2. Hansen M, Kurinczuk JJ, Bower C, Webb S. 2002. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med 346:725–730 [DOI] [PubMed] [Google Scholar]
- 3. Olson CK, Keppler-Noreuil KM, Romitti PA, Budelier WT, Ryan G, Sparks AE, Van Voorhis BJ. 2005. In vitro fertilization is associated with an increase in major birth defects. Fertil Steril 84:1308–1315 [DOI] [PubMed] [Google Scholar]
- 4. Wells D, Delhanty JD. 2000. Comprehensive chromosomal analysis of human preimplantation embryos using whole genome amplification and single cell comparative genomic hybridization. Mol Hum Reprod 6:1055–1062 [DOI] [PubMed] [Google Scholar]
- 5. Plachot M, Veiga A, Montagut J, de Grouchy J, Calderon G, Lepretre S, Junca AM, Santalo J, Carles E, Mandelbaum J, et al. 1988. Are clinical and biological IVF parameters correlated with chromosomal disorders in early life: a multicentric study. Hum Reprod 3:627–635 [DOI] [PubMed] [Google Scholar]
- 6. Baart EB, Martini E, van den Berg I, Macklon NS, Galjaard RJ, Fauser BC, Van Opstal D. 2006. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod 21:223–233 [DOI] [PubMed] [Google Scholar]
- 7. Meyer LR, Klipstein S, Hazlett WD, Nasta T, Mangan P, Karande VC. 2009. A prospective randomized controlled trial of preimplantation genetic screening in the “good prognosis” patient. Fertil Steril 91:1731–1738 [DOI] [PubMed] [Google Scholar]
- 8. Almeida PA, Bolton VN. 1995. The effect of temperature fluctuations on the cytoskeletal organisation and chromosomal constitution of the human oocyte. Zygote 3:357–365 [DOI] [PubMed] [Google Scholar]
- 9. Beyer CE, Osianlis T, Boekel K, Osborne E, Rombauts L, Catt J, Kralevski V, Aali BS, Gras L. 2009. Preimplantation genetic screening outcomes are associated with culture conditions. Hum Reprod 24:1212–1220 [DOI] [PubMed] [Google Scholar]
- 10. Munne S, Magli C, Adler A, Wright G, de Boer K, Mortimer D, Tucker M, Cohen J, Gianaroli L. 1997. Treatment-related chromosome abnormalities in human embryos. Hum Reprod 12:780–784 [DOI] [PubMed] [Google Scholar]
- 11. Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC. 2007. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod 22:980–988 [DOI] [PubMed] [Google Scholar]
- 12. Van der Auwera I, D'Hooghe T. 2001. Superovulation of female mice delays embryonic and fetal development. Hum Reprod 16:1237–1243 [DOI] [PubMed] [Google Scholar]
- 13. Lee ST, Kim TM, Cho MY, Moon SY, Han JY, Lim JM. 2005. Development of a hamster superovulation program and adverse effects of gonadotropins on microfilament formation during oocyte development. Fertil Steril 83:1264–1274 [DOI] [PubMed] [Google Scholar]
- 14. Vogel R, Spielmann H. 1992. Genotoxic and embryotoxic effects of gonadotropin-hyperstimulated ovulation of murine oocytes, preimplantation embryos, and term fetuses. Reprod Toxicol 6:329–333 [DOI] [PubMed] [Google Scholar]
- 15. Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, Hardy K. 2005. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod 72:107–118 [DOI] [PubMed] [Google Scholar]
- 16. Verpoest W, Fauser BC, Papanikolaou E, Staessen C, Van Landuyt L, Donoso P, Tournaye H, Liebaers I, Devroey P. 2008. Chromosomal aneuploidy in embryos conceived with unstimulated cycle IVF. Hum Reprod 23:2369–2371 [DOI] [PubMed] [Google Scholar]
- 17. Garrido N, Zuzuarregui JL, Meseguer M, Simón C, Remohí J, Pellicer A. 2002. Sperm and oocyte donor selection and management: experience of a 10 year follow-up of more than 2100 candidates. Hum Reprod 17:3142–3148 [DOI] [PubMed] [Google Scholar]
- 18. Mercader A, Garcia-Velasco JA, Escudero E, Remohí J, Pellicer A, Simón C. 2003. Clinical experience and perinatal outcome of blastocyst transfer after coculture of human embryos with human endometrial epithelial cells: a 5-year follow-up study. Fertil Steril 80:1162–1168 [DOI] [PubMed] [Google Scholar]
- 19. Mir P, Rodrigo L, Mateu E, Peinado V, Milán M, Mercader A, Buendía P, Delgado A, Pellicer A, Remohí J, Rubio C. 2010. Improving FISH diagnosis for preimplantation genetic aneuploidy screening. Hum Reprod 25:1812–1817 [DOI] [PubMed] [Google Scholar]
- 20. Remohí J, Gutiérrez A, Cano F, Ruiz A, Simón C, Pellicer A. 1995. Long oestradiol replacement in an oocyte donation programme. Hum Reprod 10:1387–1391 [DOI] [PubMed] [Google Scholar]
- 21. Reis Soares S, Rubio C, Rodrigo L, Simón C, Remohí J, Pellicer A. 2003. High frequency of chromosomal abnormalities in embryos obtained from oocyte donation cycles. Fertil Steril 80:656–657 [DOI] [PubMed] [Google Scholar]
- 22. Munné S, Ary J, Zouves C, Escudero T, Barnes F, Cinioglu C, Ary B, Cohen J. 2006. Wide range of chromosome abnormalities in the embryos of young egg donors. Reprod Biomed Online 12:340–346 [DOI] [PubMed] [Google Scholar]
- 23. Rodrigo L, Peinado V, Mateu E, Remohí J, Pellicer A, Simón C, Gil-Salom M, Rubio C. 2010. Impact of different patterns of sperm chromosomal abnormalities on the chromosomal constitution of preimplantation embryos. Fertil Steril 94:1380–1386 [DOI] [PubMed] [Google Scholar]
- 24. Martínez MC, Méndez C, Ferro J, Nicolás M, Serra V, Landeras J. 2010. Cytogenetic analysis of early nonviable pregnancies after assisted reproduction treatment. Fertil Steril 93:289–292 [DOI] [PubMed] [Google Scholar]
- 25. Conway DA, Patel SS, Liem J, Fan KJ, Jalian R, Williams J, 3rd, Pisarska MD. 2011. The risk of cytogenetic abnormalities in the late first trimester of pregnancies conceived through assisted reproduction. Fertil Steril 95:503–506 [DOI] [PubMed] [Google Scholar]
- 26. Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, Land JA, Evers JL, Dumoulin JC. 2012. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod 27:1966–1976 [DOI] [PubMed] [Google Scholar]
- 27. Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A. 2012. Reproductive technologies and the risk of birth defects. N Engl J Med 366:1803–1813 [DOI] [PubMed] [Google Scholar]
- 28. Pelinck MJ, Hoek A, Simons AH, Heineman MJ. 2002. Efficacy of natural cycle IVF: a review of the literature. Hum Reprod Update 8:129–139 [DOI] [PubMed] [Google Scholar]
- 29. Schimberni M, Morgia F, Colabianchi J, Giallonardo A, Piscitelli C, Giannini P, Montigiani M, Sbracia M. 2009. Natural-cycle in vitro fertilization in poor responder patients: a survey of 500 consecutive cycles. Fertil Steril 92:1297–1301 [DOI] [PubMed] [Google Scholar]
- 30. Thum MY, Abdalla HI, Taylor D. 2008. Relationship between women's age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril 90:315–321 [DOI] [PubMed] [Google Scholar]
- 31. Frumkin T, Malcov M, Yaron Y, Ben-Yosef D. 2008. Elucidating the origin of chromosomal aberrations in IVF embryos by preimplantation genetic analysis. Mol Cell Endocrinol 282:112–119 [DOI] [PubMed] [Google Scholar]
- 32. Pellestor F, Andréo B, Arnal F, Humeau C, Demaille J. 2002. Mechanisms of non-disjunction in human female meiosis: the co-existence of two modes of malsegregation evidenced by the karyotyping of 1397 in-vitro unfertilized oocytes. Hum Reprod 17:2134–2145 [DOI] [PubMed] [Google Scholar]
- 33. Lathi RB, Westphal LM, Milki AA. 2008. Aneuploidy in the miscarriages of infertile women and the potential benefit of preimplanation genetic diagnosis. Fertil Steril 89:353–357 [DOI] [PubMed] [Google Scholar]