Abstract
Context:
Lipodystrophy is a disease characterized by a paucity of adipose tissue and low circulating concentrations of adipocyte-derived leptin. Leptin-replacement therapy improves eating and metabolic disorders in patients with lipodystrophy.
Objective:
The aim of the study was to clarify the pathogenic mechanism of eating disorders in lipodystrophic patients and the action mechanism of leptin on appetite regulation.
Subjects and Interventions:
We investigated food-related neural activity using functional magnetic resonance imaging in lipodystrophic patients with or without leptin replacement therapy and in healthy controls. We also measured the subjective feelings of appetite.
Results:
Although there was little difference in the enhancement of neural activity by food stimuli between patients and controls under fasting, postprandial suppression of neural activity was insufficient in many regions of interest including amygdala, insula, nucleus accumbens, caudate, putamen, and globus pallidus in patients when compared with controls. Leptin treatment effectively suppressed postprandial neural activity in many of these regions of interest, whereas it showed little effect under fasting in patients. Consistent with these results, postprandial formation of satiety feeling was insufficient in patients when compared with controls, which was effectively reinforced by leptin treatment.
Conclusions:
This study demonstrated the insufficiency of postprandial suppression of food-related neural activity and formation of satiety feeling in lipodystrophic patients, which was effectively restored by leptin. The findings in this study emphasize the important pathological role of leptin in eating disorders in lipodystrophy and provide a clue to understanding the action mechanism of leptin in human, which may lead to development of novel strategies for prevention and treatment of obesity.
Lipodystrophy is a disease characterized by a paucity of adipose tissue due to genetic or acquired conditions that alter the ability to store triglyceride in adipose tissue (1–4). Patients with lipodystrophy have abnormally low circulating concentrations of adipocyte-derived leptin and frequently develop a wide range of metabolic disorders including insulin-resistant diabetes, hypertriglyceridemia, and fatty liver (1, 5, 6). Lipodystropic patients also exhibit eating disorders, which makes diet therapy difficult (7).
We and others have demonstrated that leptin-replacement therapy effectively improves metabolic disorders in patients with lipodystrophy (1, 8, 9). In this context, leptin was also shown to suppress appetite in lipodystrophic patients (7, 10). Leptin treatment decreased satiation time, i.e. the time to voluntary cessation of eating, and increased satiety time, i.e. the time to hunger sufficient to consume a full meal. However, there is no report on the comparison of eating behaviors between healthy subjects and patients with lipodystrophy. Therefore, the pathophysiological role of leptin in eating disorders in patients with lipodystrophy remains unclear.
Leptin is a hormone secreted by the adipocytes, which serves to communicate the status of body energy store to the central nervous system and controls eating behavior and energy expenditure (11–16). From experimental studies in human and animals, it has long been established that leptin suppresses energy intake mainly by acting on the hypothalamus (7, 17, 18). However, there is little information about how the neural networks including the hypothalamus are influenced by leptin signals. Recently the advent of functional neuroimaging techniques such as functional magnetic resonance imaging (fMRI) has been providing novel insights into homeostatic and hedonic aspects of human eating behavior. fMRI measurements of food-related neural activity in congenital leptin-deficient patients were reported (19–21). These studies revealed that leptin treatment modulates neural activity in reward- and food-related areas such as the ventral striatum and orbitofrontal cortex.
In the present study, to reveal the pathogenic mechanism of eating disorders in lipodystrophic patients, we measured food-related neural activity by fMRI scans and investigated subjective feelings of appetite under both fasting and postprandial conditions in patients and age- and sex-matched healthy subjects. In addition, we performed the same sequential analyses in the same patients with leptin-replacement therapy. Data from these experiments might provide useful notions to understand the pathological role of leptin in eating disorders associated with lipodystrophy and action mechanism of leptin on appetite regulation.
Materials and Methods
Subjects
Ten patients with lipodystrophy and 10 healthy subjects participated in the study. Among the 10 patients, six had congenital generalized lipodystrophy (CGL), two had acquired generalized lipodystrophy and the remaining two had Dunnigan-type partial lipodystrophy. Five of the six CGL patients were homozygous or compound heterozygous for mutations in the seipin gene (2). The etiology of the remaining CGL patient was unknown. One of the two patients with Dunnigan-type partial lipodystrophy was heterozygous for a mutation in the LMNA gene, whereas the other patient had an unknown etiology. For controls, age- and sex-matched healthy subjects with normal weight [body mass index (BMI) between 18.5 and 25.0 kg/m2] were recruited. None of the control subjects had a past or present history of psychiatric, neurological, endocrine, metabolic, gastrointestinal, or eating disorders, and none was taking medications at the time of study. For both patients and controls, individuals with contraindications for magnetic resonance imaging scanning including claustrophobia and the presence of a cardiac pacemaker or other metallic fragments in the body were excluded. All the subjects had been stable at their body weight for at least 3 months before recruitment. Characteristics of all the subjects are summarized in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. All the subjects were right-hand dominant according to the Edinburgh Handedness Inventory (22). The means of BMI and basal plasma leptin concentrations in patients were apparently lower than those in controls. All the patients had received leptin-replacement therapy as described below for more than 2 months. For patients, the entire study was conducted during their hospitalization period at Kyoto University Hospital. Study protocols were approved by the Ethical Committee of Kyoto University Graduate School of Medicine. After detailed explanation of the study design and any potential risks, written informed consent was obtained from all subjects before study initiation.
Leptin- replacement therapy
Recombinant methionyl human leptin (meterleptin) was provided by Amylin Pharmaceuticals, Inc. (San Diego, CA). Meterleptin was administered sc once a day at the physiological replacement dose on the basis of information provided by Amylin (1).
Study design
All the fMRI scans were performed at Kyoto University Hospital between 1300 and 1400 h under fasting and postprandial conditions on separate days (Supplemental Fig. 1A). For the fasting condition, subjects were prohibited from eating for 18 h from the night before the examination. For the postprandial condition, subjects ate a meal 1 h before the examination. In addition, fMRI scans were performed for patients with and without leptin treatment (leptin-on and leptin-off, respectively) under both fasting and postprandial conditions. For the leptin-off condition, leptin-replacement therapy was discontinued for more than 4 d. All the subjects were given practice trials outside the scanner and were familiarized with scanning procedures and safety regulations.
fMRI procedures
Blood oxygen level-dependent (BOLD) response to stimuli was measured by fMRI on a 3-Tesla Trio MRI scanner (Siemens, Erlangen, Germany). Whole-brain images were acquired in axial orientation using the following parameters: repetition time, 3000 msec; echo time, 30 msec; flip angle, 90°; voxel size, 3 × 3 × 3 mm; field of view, 192 × 192 mm; matrix size, 64 × 64; and number of slices, 48. The experiment was conducted in three separate sessions of 18 min, 42 sec each. In each session, 45 food and 30 nonfood pictures were presented randomly in an event-related design (Supplemental Fig. 1B). Food pictures were chosen to suit each subject's taste based on preliminary hearing investigations and included various kinds of food, such as warm meals, desserts, fruits, and vegetables (Supplemental Fig. 1C). Nonfood pictures contained scenery comprising naturally occurring objects, such as trees, bushes, grass, rocks, water, and flowers (Supplemental Fig. 1B). Each picture was presented for 5 sec, followed by 3 sec for the rating image (Supplemental Fig. 1C). Although subjects were presented with rating image, they were asked to rate how much they liked to eat each food or how much they liked each nonfood picture on a scale of 1 (not so appealing) to 4 (highly appealing) by pressing a button with their dominant hand. Next, a mosaic picture was presented for 7 sec as a resting baseline. All pictures were projected onto a screen in the scanner room using Presentation version 9.6 software (Neurobehavioral Systems, Albany, CA) and viewed through a mirror mounted on the head coil. Subjects were instructed to focus all their attention on the pictures.
Image processing and statistical analysis of fMRI data
The fMRI data were preprocessed and statistically analyzed using SPM2 (Wellcome Department of Cognitive Neuroscience, University College London, London, UK) and MATLAB 6.5 (The Mathworks Inc., Natick, MA). Functional images were realigned to the first image and normalized into the Montreal Neurological Institute coordinate by an echo planar imaging template. Normalized images were then smoothed with a 6-mm full-width-at-half-maximum isotropic Gaussian kernel. The functional data were temporally filtered using an autoregressive model and a high pass filter with a cutoff of 128 sec. Five experimental conditions (food picture, nonfood picture, rating for food picture, rating for nonfood picture, and pressing button) were modeled by a function convolved with a hemodynamic response function in the general linear model, and an activation parameter was estimated at each voxel for each stimulus type. Significant signal changes were identified with a voxel-by-voxel analysis on the basis of a comparison of the mean signal amplitude during the periods of stimulation and those of resting baselines, as determined by t test comparisons. At the first level, a statistical parametric map for comparing brain activation to food greater than nonfood was generated for each subject and each condition. These contrast images were then entered into a second level random effect analysis. In the random effects analysis, one-sample t test resulted in images for within-group analysis. For between-group analysis, two-sample t tests created images for control vs. patient comparison, and paired t tests created images for leptin-on vs. leptin-off comparison. Finally, we transformed the t statistics into Z-scores and generated a Z-score map image. The Z-score maps were then superimposed onto the magnetic resonance images to allow visual inspection of the composite images. We set the significance threshold at P < 0.05, false discovery rate (FDR) corrected, for whole-brain analysis, and P < 0.005, uncorrected, for region of interest (ROI) analysis with a spatial extent of 10 contiguous voxels. For ROI analysis, brain regions known to be involved in energy homeostasis and appetite regulation were chosen on the basis of previous comparable fMRI studies (23–29). These regions included the hypothalamus, orbitofrontal cortex, amygdala, hippocampus, insula, nucleus accumbens, caudate, putamen, and globus pallidus. ROI were defined using the Wake Forest University Pickatlas (30) and the AAL Talairach Daemon atlas (Research Imaging Center, University of Texas Health Science Center, San Antonio, TX) (31). Regions that were unavailable in these libraries (e.g. nucleus accumbens) were drawn within the Wake Forest University Pickatlas using three-dimensional spheres centered at a voxel location determined based on a relevant fMRI study (23).
Measurement of subjective feelings
The participants were asked to provide subjective hunger ratings on a 100-mm visual analog scale (VAS) immediately before every scanning to assess their hunger feelings (32, 33). Higher scores indicated stronger hunger. In addition, appetite was also measured using the mean value of the rating scale for 135 food pictures while viewing them in the scanner. Higher values indicated stronger desire to eat the food in each picture.
Biochemical analyses
Blood samples were obtained in the fasting state. Plasma glucose concentrations were determined by a glucose oxidase method (Arkrey Marketing Inc., Tokyo, Japan), and plasma insulin concentrations were determined by use of an enzyme immunoassay method (TOSOH, Corp., Tokyo, Japan). Plasma leptin concentrations were determined by a competitive RIA method (Millipore Inc., Billerica, MA).
Statistical analysis
Differences between patients and controls in age, BMI, plasma leptin concentration, plasma glucose, and plasma insulin were determined using unpaired t tests. Differences between biochemical values under leptin-on and leptin-off conditions were determined by paired two-tailed t tests. Differences between patients and controls regarding VAS hunger scores and rating scores for food pictures were calculated using repeated measure ANOVA. P < 0.05 was considered statistically significant.
Results
Comparison of neural response to food-specific stimuli between healthy controls and patients with lipodystrophy
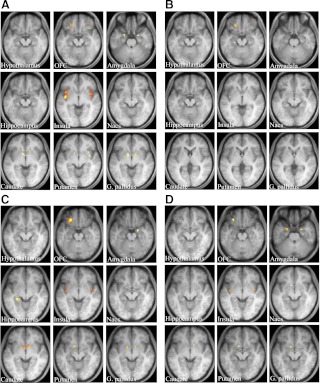
A within-group analysis of controls and patients for the contrast of food greater than nonfood revealed no significant activation in whole brain analysis at a significance level of P < 0.05 (FDR corrected). With a within-group ROI analysis for the contrast food greater than nonfood in healthy controls, significant activation was detected in the bilateral orbitofrontal cortex, amygdala, insula, caudate, putamen, and globus pallidus under the fasting conditions (Fig. 1A). However, significant activation was detected only in the bilateral orbitofrontal cortex and left insula under the postprandial conditions (Fig. 1B). On the other hand, in leptin-off patients, significant activation was detected in the left orbitofrontal cortex, right amygdala, left hippocampus, bilateral insula, bilateral caudate, left putamen, and bilateral globus pallidus under the fasting conditions (Fig. 1C). Significant activation was also detected in most of these areas under the postprandial conditions (Fig. 1D). Coordinates and maximum Z-scores in ROI areas under fasting and postprandial conditions in controls and patients are shown in Supplemental Table 2.
Fig. 1.
Neural response to food-specific stimuli in healthy controls and leptin-off patients. Food-specific activations in ROI in the brains of controls (A and B) and patients (C and D) under fasting (A and C) and postprandial (B and D) conditions. Activation is overlaid onto the group average T1-weighted anatomical axial images (right is right side of the brain). The brighter yellow color represents the higher Z-score. ROI areas are the hypothalamus, orbitofrontal cortex (OFC), amygdala, hippocampus, insula, nucleus accumbens (Nacs), caudate, putamen, and globus pallidus (G. pallidus).
Next, we directly compared the contrast food greater than nonfood between controls and patients by a between-group ROI analysis (Table 1). Under the fasting conditions, a significant difference in activity was detected between controls and patients only in the left insula and left caudate. Activity was down-regulated in the left insula and up-regulated in the left caudate in patients compared with controls. On the other hand, under the postprandial conditions, a significant difference in activity was detected in many areas, including the right orbitofrontal cortex, right amygdala, left insula, left nucleus accumbens, bilateral caudate, left putamen, and left globus pallidus between controls and patients. Activity was up-regulated in all these areas except the right orbitofrontal cortex in patients.
Table 1.
Between-group (controls vs. leptin-off patients) comparison of brain activations for the contrast food greater than nonfood
| Contrast | ROI area | Fasting |
Postprandial |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinate |
Z-score | Coordinate |
Z-score | ||||||
| x | y | z | x | y | z | ||||
| Controls greater than patients (leptin-off) | Hypothalamus | ||||||||
| Orbitofrontal cortex | 36 | 44 | −12 | 3.36 | |||||
| Amygdala | |||||||||
| Hippocampus | |||||||||
| Insula | −42 | −6 | 0 | 3.35 | |||||
| Nucleus accumbens | |||||||||
| Caudate | |||||||||
| Putamen | |||||||||
| Globus pallidus | |||||||||
| Patients (leptin-off) greater than controls | Hypothalamus | ||||||||
| Orbitofrontal cortex | |||||||||
| Amygdala | 22 | −4 | −22 | 2.92 | |||||
| Hippocampus | |||||||||
| Insula | −46 | −12 | 12 | 3.10 | |||||
| Nucleus accumbens | −8 | 10 | −6 | 3.12 | |||||
| Caudate | 14 | 2 | 14 | 3.21 | |||||
| −6 | 10 | 14 | 3.46 | −8 | 8 | −6 | 3.50 | ||
| Putamen | −10 | 8 | −6 | 3.48 | |||||
| Globus pallidus | −10 | 8 | −4 | 3.41 | |||||
Coordinate indicates the highest activity voxel of the cluster by Montreal Neurological Institute systems. Negative x-axis coordinates indicate left hemisphere. Z-score represents level of significance.
These results indicate that the suppression of neuronal response to food-specific stimuli after a meal is attenuated in patients with lipodystrophy compared with healthy subjects.
Comparison of subjective feelings of appetite between healthy controls and patients with lipodystrophy
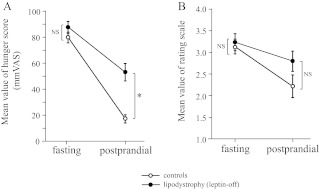
Subjective feelings of appetite were evaluated in healthy controls and leptin-off patients. Mean values of the self-reported hunger score on a 100-mm VAS were not significantly different between controls and patients under the fasting conditions (controls: 79.90 ± 4.11; patients: 87.50 ± 4.55) (Fig. 2A). In contrast, under the postprandial conditions, the score was significantly higher in patients than in controls (controls: 17.00 ± 3.09; patients: 53.0 ± 6.76). Consistent with the VAS results, mean values of rating scores for the 135 food pictures were also not different between controls and patients under the fasting conditions (controls: 3.11 ± 0.13; patients: 3.21 ± 0.20), but they tended to be higher in patients than in controls under the postprandial conditions (controls: 2.20 ± 0.24; patients: 2.78 ± 0.23) (Fig. 2B).
Fig. 2.
Subjective feelings of appetite under fasting and postprandial conditions in healthy controls and leptin-off patients. A, Hunger scores on the 100-mm VAS before fMRI scan. B, Mean value of rating scores for food pictures during the fMRI scan. Data are means ± sem (n = 10 in each group). *, P < 0.01 (repeated measure ANOVA).
These results indicate that the formation of a satiety feeling after a meal is attenuated in patients with lipodystrophy compared with healthy subjects.
Effects of the leptin-replacement therapy on neural response to food-specific stimuli in patients with lipodystrophy
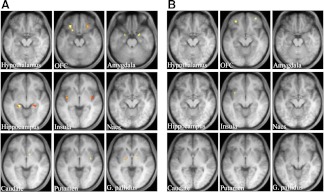
A within-group analysis of leptin-on patients for the contrast food greater than nonfood revealed no significant activation in whole brain analysis at a significance level of P < 0.05 (FDR corrected). With a within-group ROI analysis for the contrast food greater than nonfood under the fasting conditions, significant activation was detected in many brain areas, such as the bilateral orbitofrontal cortex, bilateral amygdala, bilateral hippocampus, bilateral insula, right caudate, right putamen, and bilateral globus pallidus, in leptin-on patients (Fig. 3A). In contrast, neural activity under the postprandial conditions was effectively reduced and significant activation was detected only in the bilateral orbitofrontal cortex and left insula in leptin-on patients (Fig. 3B). Coordinates and maximum Z-scores in ROI areas under the fasting and postprandial conditions in leptin-on patients are shown in Supplemental Table 3.
Fig. 3.
Neural response to food-specific stimuli in leptin-on patients. Food-specific activations in ROI in the brain under fasting (A) and postprandial (B) conditions. Activation is overlaid onto the group average T1-weighted anatomical axial images (right is right side of the brain). The brighter yellow color represents the higher Z-score. ROI areas are the same as described in Fig. 1.
Next, we directly compared the contrast food greater than nonfood between leptin-on and leptin-off patients by a between-group ROI analysis (Table 2). Under the fasting conditions, a significant difference in neural activity was detected between leptin-on and leptin-off patients only in the left caudate, in which the activity was down-regulated by leptin-replacement therapy in the patients. In contrast, a significant difference in activity was detected in many areas, including the right orbitofrontal cortex, left amygdala, left hippocampus, left insula, bilateral caudate, and left putamen, under the postprandial conditions. The activity was down-regulated in all these areas except the right orbitofrontal cortex by leptin-replacement therapy.
Table 2.
Between-group (leptin-on vs. leptin-off patients) comparison of brain activations for the contrast food greater than nonfood
| Contrast | ROI area | Fasting |
Postprandial |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinate |
Z-score | Coordinate |
Z-score | ||||||
| x | y | z | x | y | z | ||||
| Leptin-on greater than leptin-off | Hypothalamus | ||||||||
| Orbitofrontal cortex | 32 | 48 | −10 | 2.98 | |||||
| Amygdala | |||||||||
| Hippocampus | |||||||||
| Insula | |||||||||
| Nucleus accumbens | |||||||||
| Caudate | |||||||||
| Putamen | |||||||||
| Globus pallidus | |||||||||
| Leptin-off greater than leptin-on | Hypothalamus | ||||||||
| Orbitofrontal cortex | |||||||||
| Amygdala | −22 | 0 | −20 | 2.98 | |||||
| Hippocampus | −18 | −8 | −16 | 3.19 | |||||
| Insula | −42 | −16 | 10 | 4.26 | |||||
| Nucleus accumbens | |||||||||
| Caudate | 6 | 8 | −8 | 3.46 | |||||
| −4 | 6 | 0 | 3.02 | −4 | 6 | −8 | 3.34 | ||
| Putamen | −8 | 8 | −8 | 2.90 | |||||
| Globus pallidus | |||||||||
Coordinate indicates the highest activity voxel of the cluster by Montreal Neurological Institute systems. Negative x-axis coordinates indicate left hemisphere. Z-score represents level of significance.
These results indicate that leptin-replacement therapy enhances the suppression of neural response to food-specific stimuli after meal in patients with lipodystrophy.
Effects of the leptin-replacement therapy on subjective feelings of appetite in patients with lipodystophy
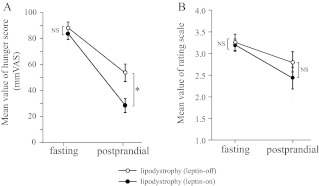
We compared subjective feelings of appetite between leptin-on and leptin-off patients. Although plasma leptin levels were significantly higher in leptin-on than in leptin-off patients, plasma glucose and insulin levels were not affected by the discontinuation of leptin-replacement therapy for approximately 4 d (Supplemental Table 4). Mean values of self-reported hunger score on a 100-mm VAS were not significantly different between leptin-on and leptin-off patients under the fasting conditions (leptin-on: 83.10 ± 4.40; leptin-off: 87.50 ± 4.55) (Fig. 4A). In contrast, the score was significantly higher in leptin-off than in leptin-on patients (leptin-on: 27.70 ± 5.39; leptin-off: 53.0 ± 6.76) under the postprandial conditions. Consistent with the VAS results, mean values of rating scores for the 135 food pictures were also not different between leptin-on and leptin-off patients under the fasting conditions (leptin-on: 3.17 ± 0.17; leptin-off: 3.21 ± 0.20), but they tended to be higher in the leptin-off than in the leptin-on patients under the postprandial conditions (leptin-on: 2.40 ± 0.26; leptin-off: 2.78 ± 0.23) (Fig. 4B).
Fig. 4.
Subjective feelings of appetite under fasting and postprandial conditions in patients with leptin-on and leptin-off. A, Hunger scores on the 100-mm VAS before the fMRI scan. B, Mean value of rating scores for food pictures during the fMRI scan. Data are means ± sem (n = 10 in each group). *, P < 0.01 (repeated measure ANOVA).
These results indicate that leptin-replacement therapy enhances the formation of satiety after meal in patients with lipodystrophy. These results were consistent with the results of fMRI analysis.
Discussion
This is the first report that demonstrates the difference in food-related neural activity between patients with lipodystrophy and healthy controls. A significant difference in food-related neural activity between patients and controls was detected in many brain areas under the postprandial conditions but in only a few brain areas under the fasting conditions (Table 1 and Supplemental Table 2 and Fig. 1). In addition, leptin-replacement therapy effectively restored neural activity in many brain areas under the postprandial conditions in patients with lipodystrophy (Table 2 and Supplemental Table 3 and Fig. 3).
The present study also indicates that leptin deficiency in patients accounts for a large part of the difference in postprandial neural activity in response to food stimuli between patients and controls. Indeed, in direct comparison between leptin-on patients and healthy controls (data not shown), a significant difference in food-related neural activity was detected only in the left globus pallidus, even under the postprandial condition. Alternatively, differences in neural activity in the globus pallidus may be due to factors other than leptin.
In the present study, we found that leptin treatment increased food-related neural activity in the orbitofrontal cortex, a region involved in satiety or the receipt of food reward (34–36), and suppressed activity in regions involved in hunger or the anticipation of food reward such as the amygdala, hippocampus, insula, caudate, and putamen (37–40) in patients under the postprandial conditions. In individuals with congenital leptin deficiency, leptin treatment also increased neural activity in the orbitofrontal cortex and reduced activity in the striatum, insula, amygdala, and substantia nigra/ventral tegmental area (19–21). Although results from the present study are not fully consistent with results from these previous reports on congenital leptin deficiency (19–21), they are consistent in that leptin enhances the neural activity in the regions involved in satiety and suppresses activity in regions involved in hunger (31). Furthermore, the present study demonstrates that leptin does not affect food-related neural activity in these regions under the fasting conditions.
This is also the first report that demonstrates the difference in appetite between patients with lipodystrophy and healthy controls. Consistent with neural activity, postprandial satiety was significantly reduced in patients compared with controls (Fig. 2), whereas there was no apparent difference in hunger under the fasting. Because leptin-replacement therapy effectively increased postprandial satiety and did not affect hunger under the fasting in patients (Fig. 4), leptin deficiency in patients accounts for a large part of the difference in postprandial satiety between patients and controls.
In the present study, to avoid the secondary effects of long-term leptin treatment such as changes in plasma glucose and insulin levels, fMRI scans and measurement of subjective feelings in leptin-off patients were performed within a short time after the discontinuation of leptin treatment. In patients who had been receiving leptin treatment for at least 2 months, no significant changes in glucose and insulin levels were observed after 4 d of discontinuation (Supplemental Table 4). Therefore, changes in food-related neural activity or feelings of appetite caused by leptin treatment were considered to be acute effects of leptin in this study.
The primary advantage of the present study lies in its imaging task methodology. First, the subjects were presented with 225 images during scanning, which was probably greater in numbers than those in any other previous studies. We also selected food pictures on the basis of an individual's food preference to maximize the saliency value of the food stimulus as a reinforcer for the subjects. Second, we used an event-related design in the imaging task to minimize habituation to each stimulus. Third, the subjects were instructed to press buttons to rate stimuli while viewing the rating images, not food or nonfood images. Thus, performance-related activation in the motor cortex (decision making, control mechanisms) was minimized during identification of neural activity elicited by the stimulus. Fourth, rating tasks were performed not only for food but also for nonfood stimuli. Therefore, the intensity of attention paid to stimuli was likely to have been comparable during food and nonfood picture presentation, which enabled us to disregard an effect arising from variance in attention while viewing, when we analyzed the contrast food greater than nonfood. We believe that these methodologies increased the reliability of obtained results.
Despite its many advantages, this study has some limitations. First, because of the relatively small sample size and genetic or phenotypic heterogeneity of the sample, statistical power was not sufficient. Second, we did not operate a diet and lifestyle standardization of the subjects sufficiently, which might affect their activity of reward systems. Our results need to be confirmed by further studies with a larger sample number and more homogeneous and standardized group of subjects. Furthermore, no significant blood oxygen level-dependent changes were observed in whole-brain analysis with a threshold of P < 0.05 (FDR corrected). Therefore, we used conservative analytic techniques and limited our investigation to ROI and possibly too liberal statistical thresholds. Besides our ROI, there must be many other brain regions, which are involved in feeding behaviors and are altered in patients with lipodystrophy. Additional whole-brain analysis with a larger sample number and more homogeneous and standardized group of subjects is required to accomplish this goal.
In conclusion, the present study using fMRI demonstrated the insufficiency of postprandial suppression of food-related neural activity and formation of satiety feeling in patients with lipodystrophy, which might be largely due to leptin deficiency. This study also demonstrated that leptin has little involvement in the regulation of neural activity and eating behavior under fasting, whereas leptin plays a significant role in these regulations under postprandial condition. The notion provided in the present study including information on ROI regulated by leptin might be useful for understanding the neural networks affected in obesity and eating disorder in leptin-deficient state and guiding the development of new pharmaceuticals for these conditions.
Supplementary Material
Acknowledgments
We thank Yoko Koyama for secretarial assistance. We also thank all the patients and healthy volunteers for participating in this study.
This work was supported by research grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology and the Japanese Ministry of Health, Labor, and Welfare.
Disclosure Summary: The authors report no conflicts of interest.
Footnotes
- BMI
- Body mass index
- CGL
- congenital generalized lipodystrophy
- FDR
- false discovery rate
- fMRI
- functional magnetic resonance imaging
- ROI
- region of interest
- VAS
- visual analog scale.
References
- 1. Ebihara K, Kusakabe T, Hirata M, Masuzaki H, Miyanaga F, Kobayashi N, Tanaka T, Chusho H, Miyazawa T, Hayashi T, Hosoda K, Ogawa Y, DePaoli AM, Fukushima M, Nakao K. 2007. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 92:532–541 [DOI] [PubMed] [Google Scholar]
- 2. Ebihara K, Kusakabe T, Masuzaki H, Kobayashi N, Tanaka T, Chusho H, Miyanaga F, Miyazawa T, Hayashi T, Hosoda K, Ogawa Y, Nakao K. 2004. Gene and phenotype analysis of congenital generalized lipodystrophy in Japanese: a novel homozygous nonsense mutation in seipin gene. J Clin Endocrinol Metab 89:2360–2364 [DOI] [PubMed] [Google Scholar]
- 3. Magré J, Delépine M, Khallouf E, Gedde-Dahl T, Jr, Van Maldergem L, Sobel E, Papp J, Meier M, Mégarbané A, Bachy A, Verloes A, d'Abronzo FH, Seemanova E, Assan R, Baudic N, Bourut C, Czernichow P, Huet F, Grigorescu F, de Kerdanet M, Lacombe D, Labrune P, Lanza M, Loret H, Matsuda F, Navarro J, Nivelon-Chevalier A, Polak M, Robert JJ, Tric P, Tubiana-Rufi N, Vigouroux C, Weissenbach J, Savasta S, Maassen JA, Trygstad O, Bogalho P, Freitas P, Medina JL, Bonnicci F, Joffe BI, Loyson G, Panz VR, Raal FJ, O'Rahilly S, Stephenson T, Kahn CR, Lathrop M, Capeau J; BSCL Working Group 2001. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28:365–370 [DOI] [PubMed] [Google Scholar]
- 4. Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O'Rahilly S, Trembath RC. 2000. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 24:153–156 [DOI] [PubMed] [Google Scholar]
- 5. Agarwal AK, Simha V, Oral EA, Moran SA, Gorden P, O'Rahilly S, Zaidi Z, Gurakan F, Arslanian SA, Klar A, Ricker A, White NH, Bindl L, Herbst K, Kennel K, Patel SB, Al-Gazali L, Garg A. 2003. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab 88:4840–4847 [DOI] [PubMed] [Google Scholar]
- 6. Van Maldergem L, Magré J, Khallouf TE, Gedde-Dahl T, Jr, Delépine M, Trygstad O, Seemanova E, Stephenson T, Albott CS, Bonnici F, Panz VR, Medina JL, Bogalho P, Huet F, Savasta S, Verloes A, Robert JJ, Loret H, De Kerdanet M, Tubiana-Rufi N, Mégarbané A, Maassen J, Polak M, Lacombe D, Kahn CR, Silveira EL, D'Abronzo FH, Grigorescu F, Lathrop M, Capeau J, O'Rahilly S. 2002. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet 39:722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDuffie JR, Riggs PA, Calis KA, Freedman RJ, Oral EA, DePaoli AM, Yanovski JA. 2004. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab 89:4258–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. 2002. Leptin-replacement therapy for lipodystrophy. N Engl J Med 346:570–578 [DOI] [PubMed] [Google Scholar]
- 9. Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. 2002. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109:1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moran SA, Patten N, Young JR, Cochran E, Sebring N, Reynolds J, Premkumar A, Depaoli AM, Skarulis MC, Oral EA, Gorden P. 2004. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism 53:513–519 [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 12. Ahima RS, Saper CB, Flier JS, Elmquist JK. 2000. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21:263–307 [DOI] [PubMed] [Google Scholar]
- 13. Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr 1996. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2:589–593 [DOI] [PubMed] [Google Scholar]
- 14. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. 1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- 15. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. 1995. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- 16. Golden PL, Maccagnan TJ, Pardridge WM. 1997. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Invest 99:14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. 1996. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98:1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Satoh N, Ogawa Y, Katsuura G, Tsuji T, Masuzaki H, Hiraoka J, Okazaki T, Tamaki M, Hayase M, Yoshimasa Y, Nishi S, Hosoda K, Nakao K. 1997. Pathophysiological significance of the obese gene product, leptin, in ventromedial hypothalamus (VMH)-lesioned rats: evidence for loss of its satiety effect in VMH-lesioned rats. Endocrinology 138:947–954 [DOI] [PubMed] [Google Scholar]
- 19. Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. 2007. Leptin regulates striatal regions and human eating behavior. Science 317:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. 2007. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA 104:18276–18279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frank S, Heni M, Moss A, von Schnurbein J, Fritsche A, Häring HU, Farooqi S, Preissl H, Wabitsch M. 2011. Leptin therapy in a congenital leptin-deficient patient leads to acute and long-term changes in homeostatic, reward, and food-related brain areas. J Clin Endocrinol Metab 96:E1283–E1287 [DOI] [PubMed] [Google Scholar]
- 22. Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- 23. Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd, Horwitz B. 2009. Effective connectivity of a reward network in obese women. Brain Res Bull 79:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. 2007. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37:410–421 [DOI] [PubMed] [Google Scholar]
- 25. Fuhrer D, Zysset S, Stumvoll M. 2008. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 16:945–950 [DOI] [PubMed] [Google Scholar]
- 26. Porubská K, Veit R, Preissl H, Fritsche A, Birbaumer N. 2006. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage 32:1273–1280 [DOI] [PubMed] [Google Scholar]
- 27. Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, Sammer G, Vaitl D. 2002. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport 13:2023–2026 [DOI] [PubMed] [Google Scholar]
- 28. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- 29. Stoeckel LE, Cox JE, Cook EW, 3rd, Weller RE. 2007. Motivational state modulates the hedonic value of food images differently in men and women. Appetite 48:139–144 [DOI] [PubMed] [Google Scholar]
- 30. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239 [DOI] [PubMed] [Google Scholar]
- 31. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. 2000. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. 2009. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res 198:149–158 [DOI] [PubMed] [Google Scholar]
- 33. Chaput JP, Gilbert JA, Gregersen NT, Pedersen SD, Sjödin AM. 2010. Comparison of 150-mm versus 100-mm visual analogue scales in free living adult subjects. Appetite 54:583–586 [DOI] [PubMed] [Google Scholar]
- 34. Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. 2007. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 450:106–109 [DOI] [PubMed] [Google Scholar]
- 35. Killgore WD, Yurgelun-Todd DA. 2006. Affect modulates appetite-related brain activity to images of food. Int J Eat Disord 39:357–363 [DOI] [PubMed] [Google Scholar]
- 36. Zald DH, Lee JT, Fluegel KW, Pardo JV. 1998. Aversive gustatory stimulation activates limbic circuits in humans. Brain 121:1143–1154 [DOI] [PubMed] [Google Scholar]
- 37. Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. 1999. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96:4569–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. 2004. Images of desire: food-craving activation during fMRI. Neuroimage 23:1486–1493 [DOI] [PubMed] [Google Scholar]
- 39. Morris JS, Dolan RJ. 2001. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci 21:5304–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. 2002. Neural responses during anticipation of a primary taste reward. Neuron 33:815–826 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.