Abstract
Context:
It is assumed that in individuals with type 2 diabetes mellitus (T2DM), blood pressure sensitivity to salt intake and the frequency of a low renin state are both increased compared with the nondiabetic population. However, studies supporting these assumptions may have been confounded by participant inclusion criteria, and study results may reflect target organ damage.
Objective:
The objective of this study was to examine in a cohort of T2DM 1) the frequency of salt sensitivity of blood pressure and 2) whether alterations of the renin-angiotensin-aldosterone system (RAAS) contribute to salt sensitivity in this population.
Design, Patients, and Methods:
Within participants of the HyperPATH cohort, four groups were analyzed: 1) T2DM with hypertension (HTN), n = 51; 2) T2DM without HTN, n = 30; 3) HTN only, n = 451; and 4) normotensive, n = 209. Phenotype studies were conducted after participants completed two dietary phases: liberal sodium (200 mmol/d) and low sodium (10 mmol/d) for 7 d each. Participants were admitted overnight to a clinical research center after each diet, and supine measurements of the RAAS before and after a 60-min angiotensin II infusion (3 ng/kg · min) were obtained.
Results:
Multivariate regression analysis demonstrated that T2DM status (all individuals with T2DM vs. individuals without T2DM) was not associated with the change in mean arterial pressure between the low and liberal sodium diets after accounting for age, gender, body mass index, race, and baseline blood pressure (T2DM status, P = 0.5). Furthermore, two intermediate phenotypes of altered RAAS, low renin, and nonmodulation (NMOD), were associated with salt-sensitive blood pressure but occurred at different frequencies in the T2DM-HTN and HTN groups (low renin, 12% T2DM-HTN vs. 29% HTN; NMOD, 41% T2DM-HTN vs. 27% HTN; P = 0.01).
Conclusion:
The frequency of NMOD in participants with T2DM was significantly higher compared with HTN, suggesting that the salt sensitivity often seen in T2DM is driven by NMOD.
Salt sensitivity of blood pressure occurs frequently in individuals with type 2 diabetes mellitus (T2DM) and is associated with an increased risk for left ventricular hypertrophy (LVH), renal disease, and cardiovascular death (1–4). The underlying cause of salt sensitivity in individuals with T2DM is unknown. Previous studies suggest that salt sensitivity in T2DM is associated with low renin (LR) levels (5–7). However, other proposed causes include hypertension (HTN), activated sympathetic nervous system (SNS), and hyperinsulinism (5, 8–13). The complex nature and heterogeneity of salt sensitivity have made it difficult to identify the condition early to establish targeted prevention and treatment plans.
Two heritable subgroups of hypertension, both with alterations in the renin-angiotensin-aldosterone system (RAAS), have been identified as salt sensitive (14, 15). The first, the LR phenotype, displays a diminished rise in plasma renin activity (PRA) in the setting of low sodium balance (4). The second, nonmodulation (NMOD), exists within individuals that are capable of mounting a normal physiological renin response to low sodium balance but display a blunted adrenal aldosterone secretion in response to exogenous angiotensin II (ANGII) stimulus (16–18). Additionally, individuals classified as NMOD fail to demonstrate an increase in renal blood flow with sodium loading that likely underlies the salt sensitivity associated with this phenotype (19). Other characteristics of NMOD include insulin resistance, dyslipidemia, and an increased family history of cardiovascular disease (20–22).
The frequency of the LR and NMOD phenotypes has not been examined in a population of controlled T2DM without kidney disease. Examining the role of these salt-sensitive HTN subgroups in T2DM may improve our understanding of how alterations in the RAAS contribute to the development of salt sensitivity in a population with well-controlled T2DM. Thus, the objectives of this study were to 1) examine the frequency of salt sensitivity of blood pressure in a population with well-controlled T2DM and 2) examine how the RAAS (PRA and aldosterone) and intermediate phenotypes of HTN (LR and NMOD) contribute to salt sensitivity of blood pressure in T2DM. We examined populations of T2DM, HTN, and normotensive (NTN) subjects from the International Hypertensive Pathotype (HyperPATH) study who were evaluated in a clinical research center with meticulous control for dietary sodium intake, posture, and diurnal rhythm.
Subjects and Methods
Participants were studied within the HyperPATH Protocol, a dataset consisting of individuals with and without mild HTN and T2DM. Six centers contributed to this dataset: Brigham and Women's Hospital (Boston, MA), University of Utah Medical Center (Salt Lake City, UT), Hospital Broussais (Paris, France), University of Virginia (Charlottesville, VA), University of Rome (Rome, Italy), and Vanderbilt University (Nashville, TN). Individuals included in this analysis were HyperPATH participants with a diagnosis of HTN or T2DM and complete data for intermediate phenotype classification. HTN was defined as a seated diastolic blood pressure of at least 90 mm Hg off antihypertensive medications, at least 80 mm Hg taking at least one medication, or treatment with at least two medications. Diabetes was defined per American Diabetes Association criteria: fasting blood glucose at least 126 mg/dl, random blood glucose at least 200 mg/dl, glycated hemoglobin (HbA1c) at least 6.5%, 2-h oral glucose tolerance test blood glucose at least 200 mg/dl, or a previous clinician-confirmed diagnosis of T2DM (23).
All inclusion and exclusion criteria for the HyperPATH protocol have been described previously (24, 25). In brief, all participants received a screening examination with a medical history, physical examination, electrocardiogram, and laboratory evaluation. Participants with known or suspected secondary HTN, coronary artery disease, stroke, overt renal insufficiency [serum creatinine >1.5 mg/dl; estimated glomerular filtration rate (eGFR) <60 ml/min], psychiatric illness, current oral contraceptive use, current tobacco/illicit drug use, or moderate alcohol use were excluded. Participants with abnormal electrolyte or thyroid/liver function tests or electrocardiographic evidence of heart block, ischemia, or previous coronary events at the screening exam were excluded. Participants with diabetes and previously known retinopathy or neuropathy were excluded. When urine protein measurements were available at screening, participants were excluded if urine proteinuria was present. All participants were between the ages of 18 and 65 yr. Race was obtained via participant self-report.
The protocol was approved by the institutional review boards of each site, and informed consent was obtained before participant enrollment.
Protocol
Details of this protocol have been described previously (4, 24, 25). In brief, to control for the influence of medications on components of the RAAS, all angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers, or mineralocorticoid receptor antagonists were discontinued for 3 months and β-blockers and diuretics were discontinued for 1 month before the study. If necessary, participants were given amlodipine for blood pressure control. All antihypertensive medications were discontinued 2 wk before the start of the diet. Individuals with T2DM on sulfonylureas, insulin, biguanides, or HMG-CoA reductase inhibitors remained on these medications for the duration of the study. All participants with T2DM had fasting blood sugar values no higher than 160 mg/dl before the first in-patient study. Subjects were given sulfonylurea or insulin, within the 3-month antihypertensive medication washout period, to obtain this blood sugar if needed.
Participants completed two diets for 7 d each: liberal sodium [high sodium (HS)] (200 mmol/d) and low sodium (LS) (10 mmol/d) with each diet also containing 100 mmol/d potassium and 20 mmol/d calcium. On the morning of d 7 of the LS diet, participants were maintained in an upright posture position for 60 min at which time blood measurements for PRA and aldosterone were obtained to evaluate maximally stimulated RAAS activity (4, 22). On the evening of d 7, participants were admitted overnight to the clinical research center. Sodium balance was confirmed by 24-h urine collection (≥150 mmol sodium/24 h HS, ≤40 mmol sodium/24 h LS). An ANGII (Bachem AG, Bubendorf, Switzerland) infusion (3 ng/kg · min for 60 min) was administered to examine the adrenal response (aldosterone release) during a state of high ANGII stimulation. A p-aminohippuric acid (PAH) (Merck, Whitehouse Station, NJ) infusion of a bolus injection 8 mg/kg was followed by constant infusion 12 mg/min. Blood draws were conducted the following morning as previously described (17). Insulin, glucose, cholesterol, PRA, aldosterone, and blood pressure were measured between 0800 and 1000 h using standardized and validated methods as previously described (4, 22). Blood pressure was monitored every 2 min during the ANGII infusion. The eGFR was calculated using the modification of diet in renal disease formula (26).
Classification of intermediate phenotypes of salt sensitivity
Salt sensitivity of blood pressure was examined using a continuous variable: the change in supine mean arterial pressure (MAP) between the LS and HS diets (ΔMAP). Individuals with higher ΔMAP were considered more salt sensitive. Defining salt sensitivity of blood pressure using a continuous variable was chosen over the alternate dichotomized definition (ΔMAP ≥10 mm Hg are salt sensitive) to increase the power of our multivariate analyses. To examine the prevalence of salt sensitivity of blood pressure in our population, the dichotomized definition was used.
Renin status was determined by PRA measurement after 1 h of upright posture on the LS diet. LR status was designated if the PRA level was less than 2.5 ng/ml · h. Normal/high renin was designated if PRA levels were at least 2.5 ng/ml · h (4). Within the normal/high renin classification, NMOD was defined if individuals had a blunted increase in aldosterone (<15 ng/dl) in response to the 60-min 3-ng/kg · min infusion of ANGII on the LS diet. All other hypertensive individuals, with appropriate adrenal and PRA measurements were defined as modulators (17, 18, 20).
Statistical analyses
Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Population characteristics for the groups are represented as mean ± sd for normally distributed continuous variables and percentages for categorical variables. Nonnormally distributed continuous variables (glucose, triglycerides, and PRA) are shown as median values with the interquartile range. A χ2 analysis was used for comparison of categorical variables.
A multivariate linear model was performed to examine the effect of T2DM on the primary continuous outcome variable, ΔMAP, and accounted for baseline blood pressure (HS MAP), age, race, gender, and body mass index (BMI). We also examined the individual influence of each disease class (T2DM-HTN, T2DM-NTN, HTN, and NTN) on ΔMAP. A second multivariate linear model examined the effect of the intermediate phenotypes of HTN (LR, NMOD, and modulators) on ΔMAP and included the covariates of age, race, and BMI. Furthermore, a subgroup analysis was conducted in individuals matched for BMI (T2DM-HTN mean ± sd 30.2 ± 4.5; HTN 30.2 ± 3.9) and age (T2DM-HTN 51.6 ± 8.3; HTN 51.6 ± 7.9) when evaluating the frequency of NMOD by disease phenotypes. For our exploratory analyses, the effects of upright posture PRA and the LS aldosterone response to ANGII infusion were compared between individuals with T2DM-HTN and HTN using an unpaired t test. Furthermore, in a subset of the population (n = 28 for T2DM-HTN and n = 318 for HTN) the renal blood flow response to ANGII infusion on a HS diet measured by PAH (difference in PAH levels from baseline to ANGII infusion) was also tested using an unpaired t test. The natural-log-transformed PRA levels were used to meet normality assumptions for all tests. All statistical tests were two sided. Significance is indicated for P < 0.05.
Results
Characteristics
Baseline characteristics differed between the groups as expected (Table 1). The T2DM population was older, had higher BMI, and had a greater number of African-Americans (T2DM-HTN, 29%; T2DM-NTN, 28%; HTN, 14%). Individuals with both T2DM and HTN had the lowest estimated eGFR; however, no participant had moderate or severe kidney disease according to National Kidney Foundation criteria (27). Furthermore, fasting blood sugar was well controlled at the time of study evaluation in the T2DM-HTN and T2DM-NTN population per American Diabetes Association guidelines (23). In the subset of diabetic individuals with available HbA1c levels, all HbA1c values were less than 8.5% with a mean of 7.5%.
Table 1.
Clinical characteristics of the HyperPATH cohort by disease status
| Baseline characteristics | T2DM-HTN | T2DM-NTN | HTN | NTN |
|---|---|---|---|---|
| n | 51 | 30 | 451 | 209 |
| Age (yr) | 52.1 ± 8.3 | 49.4 ± 9.2 | 48.5 ± 8.9 | 39.2 ± 12.2 |
| Female gender (%) | 16 (27) | 13 (45) | 189 (42) | 105 (50) |
| Race (%) | ||||
| Caucasian | 34 (67) | 17 (59) | 381 (85) | 171 (82) |
| African-American | 15 (29) | 8 (28) | 54 (12) | 30 (14) |
| Other | 2 (4) | 4 (14) | 16 (3) | 6 (3) |
| BMI (kg/m2) | 30.2 ± 4.5 | 28.9 ± 4.5 | 28.1 ± 3.9 | 25.2 ± 3.6 |
| Fasting blood sugar (mg/dl) | 119 ± 48 | 109.5 ± 41 | 89 ± 13 | 86 ± 14 |
| Blood pressure (mm Hg) | ||||
| Systolic | 149.6 ± 20.6 | 122.4 ± 16.9 | 148.7 ± 19.4 | 111.0 ± 11.9 |
| Diastolic | 92.8 ± 19.3 | 75.0 ± 9.3 | 91.6 ± 15.5 | 67.2 ± 8.7 |
| MAP | 111.7 ± 17.2 | 90.8 ± 10.8 | 109.5 ± 13.6 | 81.3 ± 8.8 |
| eGFR (MDRD) | 87.6 ± 23 | 100.6 ± 19.5 | 93.2 ± 26.0 | 104.0 ± 26.4 |
| Urinary sodium HS | 236.6 ± 71.9 | 228.7 ± 68.7 | 231.1 ± 63.8 | 241.2 ± 70.6 |
| Urinary sodium LS | 14.4 ± 7.3 | 15.9 ± 8.4 | 13.4 ± 8.0 | 9.8 ± 7.5 |
| Total cholesterol (mg/dl) | 210.0 ± 44.9 | 174.6 ± 43.7 | 197.0 ± 39.5 | 164.5 ± 37.2 |
| LDL cholesterol (mg/dl) | 107.7 ± 31.6 | 98.5 ± 22.4 | 120.9 ± 34.3 | 95.8 ± 30.2 |
| HDL cholesterol (mg/dl) | 39.5 ± 11.7 | 49.6 ± 25.8 | 42.1 ± 13.4 | 50.2 ± 30.2 |
| Triglycerides (mg/dl)** | 133 ± 105 | 97 ± 137.5 | 125.0 ± 84 | 89 ± 63 |
| Medications (%) | ||||
| Sulfonyurea use | 7 (14) | 7 (23) | ||
| Metformin use | 7 (14) | 6 (20) | ||
| Insulin use | 3 (5) | 2 (6) | ||
| Statin use | 3 (5) | 6 (20) |
Results are shown as mean ± sd for normally distributed continuous variables, median ± interquartile range for nonnormal continuous variables (fasting glucose and triglycerides), and percentages for categorical variables. HDL, High-density lipoprotein; LDL, low-density lipoprotein; MDRD, modification of diet in renal disease formula.
Data represent median ± interquartile range.
Relationship between disease status and salt sensitivity of blood pressure
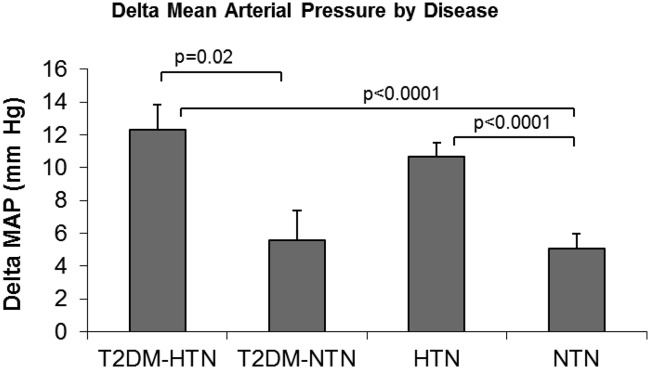
Because T2DM and high blood pressure has been reported to affect salt sensitivity of blood pressure (11), we examined the relationship between T2DM disease status (T2DM-HTN and T2DM-NTN vs. HTN and NTN) and salt sensitivity of blood pressure (ΔMAP between HS and LS diet) in the HyperPATH study. As expected, multivariate analysis demonstrated that baseline MAP was a significant predictor of ΔMAP; however, having T2DM was not associated with higher change in diastolic blood pressure (Table 2). When analyzing the influence of each disease class on ΔMAP, only HTN classes (T2DM-HTN and HTN) significantly associated with ΔMAP (P < 0.0001), suggesting that individuals with both T2DM and HTN tended to have salt sensitivity of blood pressure compared with individuals with T2DM and no HTN (Fig. 1).
Table 2.
Relationship Between T2DM disease status and change in MAP with salt loading in the HyperPATH study
| Variables | T2DM status and Δ MAP with sodium loading |
||
|---|---|---|---|
| Effect estimates (B) | 95% CI | P value | |
| Disease status | |||
| T2DM status (yes) | 0.77 | −1.3–2.83 | 0.5 |
| MAP | 0.33 | 0.29–0.37 | <0.0001 |
| Age | 0.06 | −0.001–0.13 | 0.05 |
| Gender (male) | −1.8 | −3.08–−0.52 | 0.006 |
| BMI | −0.27 | −0.43–−0.12 | 0.0007 |
| Race | |||
| Caucasian | −0.77 | −4.1–2.6 | 0.7 |
| African-American | −0.55 | −4.2–3.1 | 0.8 |
| Other | |||
A multivariate regression model examining the relationship between Δ MAP (LS to HS diet) and T2DM disease status (T2DM-HTN and T2DM-NTN vs. HTN and NTN without T2DM), including the covariates of baseline MAP (HS), age, gender, BMI, and race. CI, Confidence interval.
Fig. 1.
Relationship between disease status and ΔMAP with salt loading in the HyperPATH cohort. The graph portrays the ΔMAP (change in MAP between LS and HS diet) least-square means value by HTN and T2DM. Covariates in the model include age, gender, BMI, and race.
The relationship between intermediate phenotypes of HTN and salt-sensitive blood pressure
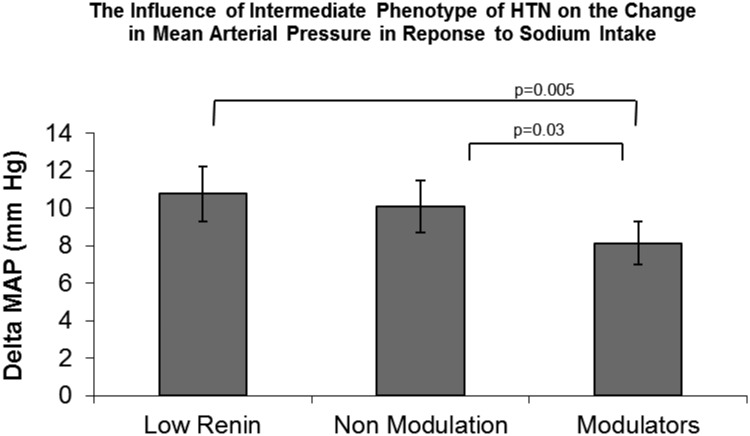
We examined the relationship between previously identified intermediate phenotypes of HTN: 1) LR status, 2) NMOD status, and 3) normal RAAS response (modulators) and ΔMAP in all individuals with HTN. Both LR and NMOD status had significantly higher ΔMAP (LS to HS diet) compared with modulators (P < 0.0001 LR vs. modulators; P < 0.02 NMOD vs. modulators) (Fig. 2). A significant relationship between LR and ΔMAP (P = 0.01) and NMOD and ΔMAP (P = 0.05) remained after accounting for the covariates of age, BMI, and race. As a group, individuals classified as modulators were not salt sensitive and did not demonstrate a greater than 10 mm Hg change in MAP with change in sodium diet (Fig. 2). These findings demonstrate that the LR and NMOD phenotypes were associated with greater salt sensitivity of blood pressure among all individuals with HTN.
Fig. 2.
The relationship between LR, NMOD, and modulator status and ΔMAP with salt loading in individuals with hypertension. Both LR and NMOD status had significantly higher ΔMAP (LS to HS diet) compared with modulators. A significant relationship between LR and NMOD and ΔMAP remained after accounting for the covariates of age, BMI, and race (P = 0.01 LR; P = 0.04 NMOD).
Frequency of salt-sensitive intermediate phenotypes by disease status
Because both the NMOD and LR phenotypes were associated with salt sensitivity of blood pressure and thus may contribute to the development of salt sensitivity demonstrated in the T2DM-HTN and HTN populations, we examined whether the frequency of the two salt-sensitive intermediate phenotypes differed in these two populations. Although the frequency of salt-sensitive blood pressure was similar in T2DM-HTN and HTN populations (48% T2DM-HTN vs. 49% HTN, P = 0.8), the frequency of NMOD in the T2DM-HTN cohort was significantly higher than in the HTN cohort (41 vs. 27%, P = 0.01) (Table 3). Surprisingly, we found the LR intermediate phenotype to be less frequent in the T2DM-HTN cohort compared with HTN cohort (12 vs. 29%) (Table 3), contrary to previous reports (5–7). This relationship was strengthened when T2DM-NTN were included (P = 0.01) and when the analysis was conducted in Caucasians only (P = 0.0001). Furthermore, to evaluate whether BMI or age influenced this finding, we conducted the same analysis in individuals matched on age and BMI. The results were the same. The NMOD phenotype was more frequent in the T2DM-HTN group compared with HTN (41 vs. 26%, P = 0.02), and LR was less frequent (11 vs. 34%).
Table 3.
Intermediate phenotype class by disease status: the frequency of NMOD was significantly higher in individuals with T2DM-HTN vs. HTN
| Intermediate phenotype frequency by disease status |
|||
|---|---|---|---|
| T2DM-HTN | HTN | χ2 P value | |
| LR [n (%)] | 6 (12) | 133 (29) | 0.01a |
| NMOD [n (%)] | 21 (41) | 121 (27) | |
| Modulators [n (%)] | 24 (47) | 198 (44) | |
P values were obtained by χ2 analysis.
This relationship remained when T2DM-NTN were included (P = 0.01), and the analysis was conducted in Caucasians only (P = 0.0001). Furthermore, results were still significant (P = 0.02) when individuals between the two disease groups were matched on age and BMI.
RAAS response in individuals with T2DM-HTN vs. HTN
To confirm that the NMOD phenotype was more prevalent in individuals with T2DM-HTN compared with HTN alone, we examined the mean upright posture PRA and aldosterone response to ANGII on the LS diet, two parameters that define NMOD. As suggested by our findings above, PRA levels after upright posture on the LS diet were significantly higher in the T2DM-HTN compared with the HTN group [T2DM-HTN, 5.5 ng/kg · h (2.8); HTN, 3.8 ng/kg · h (3.1); P = 0.04] (Table 4). Furthermore, as expected, the aldosterone response to ANGII infusion on the LS diet was lower in individuals with T2DM-HTN [T2DM-HTN, 15.9 ng/dl (13.3); HTN, 20.2 (13.3) ng/dl; P = 0.05]. As demonstrated in other populations of T2DM (28), the renal blood flow response to ANGII infusion was blunted in individuals with T2DM-HTN compared with HTN [T2DM-HTN, 75.70 ng/liter · sec (50.8); HTN, 98.7 (50.6); P = 0.02)] (Table 4).
Table 4.
Evaluation of components of the RAAS by disease status
| T2DM-HTN |
HTN |
P value | |||
|---|---|---|---|---|---|
| Means | sd | Means | sd | ||
| Upright posture PRA (ng/kg · h) | 5.5 | 2.8 | 3.8 | 3.1 | 0.04 |
| HS ANGII Δ PAH (ng/liter · sec) | 75.70 | 50.80 | 98.70 | 50.60 | 0.02 |
| LS ALDO response to ANGII (ng/dl) | 15.9 | 13.3 | 20.2 | 13.3 | 0.05 |
Measurements of the RAAS were compared between groups. ALDO, Aldosterone.
Salt sensitivity has been associated with other alterations in the RAAS, including a smaller change in PRA levels with changes from LS to HS diet (ΔPRA) and a blunted blood pressure response to ANGII infusion on a LS diet (11, 29). Our analysis found no significant differences in ΔPRA levels or blood pressure response to ANGII between the T2DM-HTN and HTN groups with salt sensitivity, suggesting that these variables, unlike the intermediate phenotypes of NMOD and LR, could not differentiate the mechanism contributing to salt sensitivity in the T2DM-HTN vs. HTN groups.
Discussion
Our study demonstrates that salt sensitivity of blood pressure is not associated with controlled T2DM alone; elevated blood pressure was the strongest predictor of salt sensitivity of blood pressure regardless of T2DM status. Interestingly, although the occurrence of salt sensitivity is relatively the same in all individuals with HTN, the underlying mechanism differs between hypertensive individuals with and without T2DM. Our data suggest that contrary to previous reports (5–7), LR status is not the predominant mechanism for salt sensitivity of blood pressure in T2DM. We found that the salt-sensitive intermediate phenotype of NMOD was more frequent in T2DM, suggesting that salt sensitivity of blood pressure seen in T2DM may be driven by NMOD status.
Previous studies suggest that a positive relationship between T2DM and salt sensitivity exists, and this relationship is driven by low PRA levels and an increased frequency of LR status (5–7, 30). Our carefully controlled human study refutes these observations. We believe that clear differences in study design and participant inclusion criteria contribute to the conflicting results. First, many of the human studies that report a high frequency of LR status in individuals with T2DM were conducted in participants with T2DM and nephropathy, suggesting that kidney damage may contribute to altered renin secretion and hyporeninemia (5, 10). Animal and cellular studies also suggest a relationship between kidney damage and decreased renin release (31, 32). Importantly, a close examination of the study by Christlieb et al. (5) demonstrates that LR status was present only in individuals with T2DM and nephropathy, not in populations of individuals with T2DM-HTN or T2DM-NTN without kidney disease. Second, many of the human studies were done over 30 yr ago when treatment and control of T2DM were much different from current clinical guidelines. Thus, the high incidence of nephropathy and long duration of disease in the participants included in these studies likely contributed to the findings that LR status occurred more frequently in T2DM. Other, more recent studies agree with our finding reporting normal-high PRA levels in T2DM populations (33, 34). Thus, the assumption that LR status occurs frequently in T2DM may be incorrect.
Our study suggests an alternate mechanism contributes to the presence of salt sensitivity of blood pressure in individuals with T2DM. The intermediate phenotype, NMOD, defined as an altered aldosterone response to ANGII infusion under RAAS stimulation (LS diet), is associated with salt sensitivity and occurs more frequently in our T2DM-HTN population (17, 20, 35). Interestingly, the NMOD phenotype is characterized as being insulin resistant (22) and dyslipidemic (20), even in the presence of a normal BMI (21), and is associated with an increased family history of cardiovascular disease (20). Our data suggest that this intermediate phenotype may explain the presence of salt sensitivity and, potentially, increased cardiovascular risk in some but not all individuals with T2DM. Importantly, understanding and targeting altered RAAS physiology, such as NMOD, in individuals with T2DM may improve salt sensitivity and cardiovascular complications in those at greatest risk.
The mechanism underlying the association of NMOD status, salt sensitivity, and T2DM is unknown, although previous physiological studies examining NMOD in humans provide potential explanations. NMOD is characterized by 1) a decreased adrenal response to ANGII during a LS diet, 2) a decreased renal vascular response to ANGII during a HS diet, and 3) a decrease in renal blood flow response during a HS diet. It has been proposed that these characteristics are caused by an increase in local ANGII levels and altered ATR1 receptor function at the vascular level (17, 35). Studies support this hypothesis demonstrating that the renal and vascular defects in NMOD are reversed by an ACEI (10, 35), a pharmacological agent that lowers ANGII levels. Furthermore, genetic variants in the angiotensinogen (AGT) gene, known to be associated with AGT levels (36), are also associated with the development of NMOD (37). It is possible that an increase in local AGT levels and altered renal blood flow response, both seen with NMOD status, are contributing to the salt sensitivity observed in our T2DM-HTN population. Recent studies support this explanation, demonstrating a relationship between higher plasma and urinary AGT levels and greater salt sensitivity (38, 39). Additional studies are also warranted to determine whether blocking AGT levels via a pharmacological RAAS inhibitor improves the vascular dysfunction, altered renal blood flow response, and salt sensitivity in T2DM, similar to HTN (35).
This study has some limitations. First, this cross-sectional analysis prevents conclusions of causality or directionality of association. Our data cannot determine a cause-and-effect relationship between NMOD, salt sensitivity, and T2DM. Furthermore, we do not have complete renal blood flow data in the T2DM population to determine the influence of renal blood flow on salt sensitivity in T2DM. Although we can estimate the relative absence of significant nephropathy based on eGFR, we do not have measurements of microalbuminuria, which could assist in providing more specific qualification of nephropathy. Furthermore, we do not have comprehensive data to support that all participants with T2DM had optimal glycemic control beyond the time of study. We attempted to minimize the effects of hyperglycemia at the time of study by acutely controlling blood glucose in the peri-study period. It is possible that prolonged uncontrolled hyperglycemia could influence hemodynamic and hormonal evaluations despite acute control. Furthermore, a few of the study participants were on insulin and oral glycemic agents that may have influenced the results. We have yet to study whether ACEI corrects the RAAS alterations of NMOD described in this T2DM population. However, this well-controlled study design provides an opportunity to examine salt sensitivity and the RAAS in a current population of T2DM without end-stage renal disease.
Many believe that the association between salt sensitivity and T2DM is related to the increased frequency of LR status in T2DM. Our findings, conducted in a well-phenotyped population, refute this claim and suggest that salt sensitivity in individuals with T2DM is related to other alterations in the RAAS, specifically NMOD status. This finding has important clinical implications. First, individuals with NMOD have been shown to have an increased cardiovascular risk profile, and using this inherited phenotype as a marker for cardiovascular risk in T2DM may enable earlier detection and targeted therapy. Second, because ACEI has been found to correct the RAAS alterations in a population of individuals with HTN, ACEI may also prevent or improve the cardiovascular risk profile associated with salt sensitivity in T2DM. Overall, the identification of NMOD as a salt-sensitive intermediate phenotype in T2DM provides new avenues to explore improved and targeted prevention and treatment options in this high-risk population.
Acknowledgments
We thank all other investigators and staff of the HyperPATH, particularly Xavier Jeunemaitre and Nancy Brown, as well as the Center for Clinical Investigation Research staff and participants of all protocol sites.
The project described was supported in part by the following grants: U54LM008748 from the National Library of Medicine; UL1RR025758, Harvard Clinical and Translational Science Center, from the National Center for Research Resources; and M01-RR02635, Brigham & Women's Hospital, General Clinical Research Center, from the National Center for Research Resources. Other support came from National Institutes of Health Grants HL47651, HL59424, F32 NR013318 (to P.C.U.); T32HL007609 (to A.V., P.C.U., and B.C.); K23 HL084236 (to J.S.W.); K23 HL111771 (to A.V.); and Specialized Center of Research (SCOR) in Molecular Genetics of Hypertension P50HL055000.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACEI
- Angiotensin-converting enzyme inhibitors
- AGT
- angiotensinogen
- ANGII
- angiotensin II
- BMI
- body mass index
- eGFR
- estimated glomerular filtration rate
- HbA1c
- glycated hemoglobin
- HS
- high sodium
- HTN
- hypertension
- LR
- low renin
- LS
- low sodium
- LVH
- left ventricular hypertrophy
- MAP
- mean arterial pressure
- NMOD
- nonmodulation
- NTN
- normotensive
- PAH
- p-aminohippuric acid
- PRA
- plasma renin activity
- RAAS
- renin-angiotensin-aldosterone system
- SNS
- sympathetic nervous system
- T2DM
- type 2 diabetes mellitus.
References
- 1. Trevisan R, Bruttomesso D, Vedovato M, Brocco S, Pianta A, Mazzon C, Girardi C, Jori E, Semplicini A, Tiengo A, Del Prato S. 1998. Enhanced responsiveness of blood pressure to sodium intake and to angiotensin II is associated with insulin resistance in IDDM patients with microalbuminuria. Diabetes 47:1347–1353 [DOI] [PubMed] [Google Scholar]
- 2. Weinberger MH. 2001. Salt and blood pressure: what's new? Curr Hypertens Rep 3:271–272 [DOI] [PubMed] [Google Scholar]
- 3. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. 2001. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37:429–432 [DOI] [PubMed] [Google Scholar]
- 4. Williams JS, Williams GH, Jeunemaitre X, Hopkins PN, Conlin PR. 2005. Influence of dietary sodium on the renin-angiotensin-aldosterone system and prevalence of left ventricular hypertrophy by EKG criteria. J Hum Hypertens 19:133–138 [DOI] [PubMed] [Google Scholar]
- 5. Christlieb AR, Kaldany A, D'Elia JA. 1976. Plasma renin activity and hypertension in diabetes mellitus. Diabetes 25:969–974 [DOI] [PubMed] [Google Scholar]
- 6. de Châtel R, Weidmann P, Flammer J, Ziegler WH, Beretta-Piccoli C, Vetter W, Reubi FC. 1977. Sodium, renin, aldosterone, catecholamines, and blood pressure in diabetes mellitus. Kidney Int 12:412–421 [DOI] [PubMed] [Google Scholar]
- 7. Perez GO, Lespier L, Jacobi J, Oster JR, Katz FH, Vaamonde CA, Fishman LM. 1977. Hyporeninemia and hypoaldosteronism in diabetes mellitus. Arch Intern Med 137:852–855 [PubMed] [Google Scholar]
- 8. Bigazzi R, Bianchi S, Baldari G, Campese VM. 1996. Clustering of cardiovascular risk factors in salt-sensitive patients with essential hypertension: role of insulin. Am J Hypertens 9:24–32 [DOI] [PubMed] [Google Scholar]
- 9. Giner V, Coca A, de la Sierra A. 2001. Increased insulin resistance in salt sensitive essential hypertension. J Hum Hypertens 15:481–485 [DOI] [PubMed] [Google Scholar]
- 10. Price DA, Porter LE, Gordon M, Fisher ND, De'Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. 1999. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol 10:2382–2391 [DOI] [PubMed] [Google Scholar]
- 11. Tuck M, Corry D, Trujillo A. 1990. Salt-sensitive blood pressure and exaggerated vascular reactivity in the hypertension of diabetes mellitus. Am J Med 88:210–216 [DOI] [PubMed] [Google Scholar]
- 12. Vedovato M, Lepore G, Coracina A, Dodesini AR, Jori E, Tiengo A, Del Prato S, Trevisan R. 2004. Effect of sodium intake on blood pressure and albuminuria in Type 2 diabetic patients: the role of insulin resistance. Diabetologia 47:300–303 [DOI] [PubMed] [Google Scholar]
- 13. Yatabe MS, Yatabe J, Yoneda M, Watanabe T, Otsuki M, Felder RA, Jose PA, Sanada H. 2010. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am J Clin Nutr 92:77–82 [DOI] [PubMed] [Google Scholar]
- 14. Lifton RP, Hopkins PN, Williams RR, Hollenberg NK, Williams GH, Dluhy RG. 1989. Evidence for heritability of non-modulating essential hypertension. Hypertension 13:884–889 [DOI] [PubMed] [Google Scholar]
- 15. Williams RR, Hasstedt SJ, Hunt SC, Wu LL, Hopkins PN, Berry TD, Stults BM, Barlow GK, Kuida H. 1991. Genetic traits related to hypertension and electrolyte metabolism. Hypertension 17:I69–I73 [DOI] [PubMed] [Google Scholar]
- 16. Hollenberg NK, Williams GH. 1988. Sodium sensitive hypertension: renal and adrenal non-modulation in its pathogenesis. Kidney 21:13–18 [PubMed] [Google Scholar]
- 17. Shoback DM, Williams GH, Moore TJ, Dluhy RG, Podolsky S, Hollenberg NK. 1983. Defect in the sodium-modulated tissue responsiveness to angiotensin II in essential hypertension. J Clin Invest 72:2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams GH, Dluhy RG, Lifton RP, Moore TJ, Gleason R, Williams R, Hunt SC, Hopkins PN, Hollenberg NK. 1992. Non-modulation as an intermediate phenotype in essential hypertension. Hypertension 20:788–796 [DOI] [PubMed] [Google Scholar]
- 19. Hollenberg NK, Moore T, Shoback D, Redgrave J, Rabinowe S, Williams GH. 1986. Abnormal renal sodium handling in essential hypertension. Relation to failure of renal and adrenal modulation of responses to angiotensin II. Am J Med 81:412–418 [DOI] [PubMed] [Google Scholar]
- 20. Ferri C, Bellini C, Desideri G, Valenti M, De Mattia G, Santucci A, Hollenberg NK, Williams GH. 1999. Relationship between insulin resistance and nonmodulating hypertension: linkage of metabolic abnormalities and cardiovascular risk. Diabetes 48:1623–1630 [DOI] [PubMed] [Google Scholar]
- 21. Gaboury CL, Hollenberg NK, Hopkins PN, Williams R, Williams GH. 1995. Metabolic derangements in nonmodulating hypertension. Am J Hypertens 8:870–875 [DOI] [PubMed] [Google Scholar]
- 22. Raji A, Williams GH, Jeunemaitre X, Hopkins PN, Hunt SC, Hollenberg NK, Seely EW. 2001. Insulin resistance in hypertensives: effect of salt sensitivity, renin status and sodium intake. J Hypertens 19:99–105 [DOI] [PubMed] [Google Scholar]
- 23. American Diabetes Association 2011. Standards of medical care in diabetes: 2011. Diabetes Care 34(Suppl 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chamarthi B, Williams JS, Williams GH. 2010. A mechanism for salt-sensitive hypertension: abnormal dietary sodium-mediated vascular response to angiotensin-II. J Hypertens 28:1020–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pojoga LH, Underwood PC, Goodarzi MO, Williams JS, Adler GK, Jeunemaitre X, Hopkins PN, Raby BA, Lasky-Su J, Sun B, Cui J, Guo X, Taylor KD, Chen YD, Xiang A, Raffel LJ, Buchanan TA, Rotter JI, Williams GH. 2011. Variants of the caveolin-1 gene: a translational investigation linking insulin resistance and hypertension. J Clin Endocrinol Metab 96:E1288–E1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vervoort G, Willems HL, Wetzels JF. 2002. Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new (MDRD) prediction equation. Nephrol Dial Transplant 17:1909–1913 [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. 2003. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139:137–147 [DOI] [PubMed] [Google Scholar]
- 28. Price DA, De'Oliveira JM, Fisher ND, Williams GH, Hollenberg NK. 1999. The state and responsiveness of the renin-angiotensin-aldosterone system in patients with type II diabetes mellitus. Am J Hypertens 12:348–355 [PubMed] [Google Scholar]
- 29. He FJ, Markandu ND, MacGregor GA. 2001. Importance of the renin system for determining blood pressure fall with acute salt restriction in hypertensive and normotensive whites. Hypertension 38:321–325 [DOI] [PubMed] [Google Scholar]
- 30. Trujillo A, Eggena P, Barrett J, Tuck M. 1989. Renin regulation in type II diabetes mellitus: influence of dietary sodium. Hypertension 13:200–205 [DOI] [PubMed] [Google Scholar]
- 31. Swales JD. 1975. Low-renin hypertension: nephrosclerosis? Lancet 1:75–77 [DOI] [PubMed] [Google Scholar]
- 32. Fray JC. 1991. Regulation of renin secretion by calcium and chemiosmotic forces: (patho) physiological considerations. Biochim Biophys Acta 1097:243–262 [DOI] [PubMed] [Google Scholar]
- 33. Alderman MH, Cohen HW, Sealey JE, Laragh JH. 2004. Plasma renin activity levels in hypertensive persons: their wide range and lack of suppression in diabetic and in most elderly patients. Am J Hypertens 17:1–7 [DOI] [PubMed] [Google Scholar]
- 34. Gordon MS, Price DA, Hollenberg NK. 2000. Blunted suppression of plasma renin activity in diabetes. J Renin Angiotensin Aldosterone Syst 1:252–256 [DOI] [PubMed] [Google Scholar]
- 35. Redgrave J, Rabinowe S, Hollenberg NK, Williams GH. 1985. Correction of abnormal renal blood flow response to angiotensin II by converting enzyme inhibition in essential hypertensives. J Clin Invest 75:1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watkins WS, Hunt SC, Williams GH, Tolpinrud W, Jeunemaitre X, Lalouel JM, Jorde LB. 2010. Genotype-phenotype analysis of angiotensinogen polymorphisms and essential hypertension: the importance of haplotypes. J Hypertens 28:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kosachunhanun N, Hunt SC, Hopkins PN, Williams RR, Jeunemaitre X, Corvol P, Ferri C, Mortensen RM, Hollenberg NK, Williams GH. 2003. Genetic determinants of nonmodulating hypertension. Hypertension 42:901–908 [DOI] [PubMed] [Google Scholar]
- 38. Gociman B, Rohrwasser A, Hillas E, Cheng T, Hunter G, Hunter J, Lott P, Monson S, Ying J, Lalouel JM. 2008. Response to genetic manipulations of liver angiotensinogen in the physiological range. J Hum Genet 53:775–788 [DOI] [PubMed] [Google Scholar]
- 39. Konishi Y, Nishiyama A, Morikawa T, Kitabayashi C, Shibata M, Hamada M, Kishida M, Hitomi H, Kiyomoto H, Miyashita T, Mori N, Urushihara M, Kobori H, Imanishi M. 2011. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension 58:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]