Abstract
Context:
Obesity is associated with an altered inflammatory and extracellular matrix (ECM) profile. Tenascin C (TNC) is an ECM glycoprotein with proinflammatory effects.
Objective:
We aimed to explore the expression levels of TNC in adipose tissue analyzing the contribution of adipocytes and stromovascular fraction cells (SVFC) as well as its impact on inflammation and ECM regulation. We also analyzed the effect of the stimulation with TNF-α and lipopolysaccharide (LPS) on both SVFC and adipocytes.
Patients and Methods:
Samples obtained from 75 subjects were used in the study. Expression levels of TNC, TLR4, MMP2, and MMP9 were analyzed in visceral adipose tissue (VAT) as well as in both adipocytes and SVFC. In addition, Tnc expression was measured in two mice models of obesity.
Results:
We show, for the first time, that VAT expression levels of TNC are increased in normoglycemic and type 2 diabetic obese patients (P < 0.01) as well as in obese patients with nonalcoholic steatohepatitis (P < 0.01). Furthermore, expression levels of Tnc in epididymal adipose tissue from two different mice models of obesity were significantly increased (P < 0.01). TNC and TLR4 were mainly expressed by SVFC, and its expression was significantly enhanced (P < 0.01) by TNF-α treatment. LPS treatment also increased mRNA levels of TNC. Moreover, the addition of exogenous TNC induced (P < 0.05) TLR4 and CCL2 mRNA expression in human adipocyte cultures.
Conclusions:
These findings indicate that TNC is involved in the etiopathology of obesity via visceral adipose tissue inflammation representing a link with ECM remodeling.
Obesity, characterized by a prolonged positive energy balance, induces different changes in adipose tissue, including a dramatic alteration of shape and growth of adipocytes, the differentiation of preadipocytes into adipocytes, and an accumulation of inflammatory cells (1). These changes are related to the extracellular matrix (ECM) remodeling (2, 3) and give rise to functional alterations in adipose tissue and variations in its secretion profile of adipokines (4, 5). The degradation of components of the ECM and regulation of adipose tissue architecture is mediated by different systems, mainly the matrix metalloproteinase (MMP) family and the fibrinolytic plasminogen and plasmin system (6). In this sense, the adipose tissue of obese and insulin-resistant humans shows an increase in components of the ECM, including members of the collagen family, fibrin, and thrombospondin (7, 8).
Much attention has focused on the adipose tissue changes in ECM and inflammation linked to obesity, highlighting the interrelation of both processes in the adipose transcriptomic signature of obese subjects (9). During the obesity-associated chronic inflammatory state, the ECM may act as a scaffold for cell infiltration as well as a reservoir for adipokines and growth factors (10). In addition, some ECM molecules, a class of the so-called damage-associated molecular patterns, can also directly activate the inflammatory process being rapidly released upon tissular damage (11). Tenascin C (TNC) is a large, extracellular matrix glycoprotein that belongs to the damage-associated molecular patterns family (12). The expression pattern of TNC is dynamic. Little or no TNC is found in most healthy adult tissues, being specifically induced and tightly controlled during acute inflammation and persistently expressed in chronic inflammation (13, 14). TNC is also reportedly overexpressed in human preadipocytes after stimulation with secreted factors from activated macrophages (15). Toll-like receptors (TLR) represent a key molecular link between tissue injury and inflammation (11, 16). TNC exhibits proinflammatory effects mediated by the activation of the TLR-4, which in turn promotes innate and adaptive immune responses, including induction of proinflammatory cytokines and the matrix metalloproteinase family (17). It has been also reported that TLR4 activation with lipopolysaccharide (LPS) induces the release of critical proinflammatory cytokines (18) as well as the expression of procollagen type 1 and integrin 1β (19), suggesting that direct activation of TLR4 has the potential to induce massive inflammation and may play a role in ECM remodeling. TNC is frequently coexpressed with MMP (20), with the inhibition of MMP suppressing the TNC expression (21).
Recent studies have identified close links between adipose tissue inflammation and the ECM and expression of TNC could play a key role (9, 22, 23). To our knowledge, there are no available data on the expression of TNC in human visceral (VAT) and sc (SAT) adipose tissue or its possible involvement in adipose tissue inflammation. The aim of the present study was to determine expression levels of TNC and TLR4 in adipose tissue as well and to evaluate the effect of obesity and the obesity-related comorbidities type 2 diabetes (T2D) and nonalcoholic fatty liver disease (NAFLD). Furthermore, we aimed to analyze the expression levels of TNC in different models of obesity in mice during active adipose tissue expansion. The role of TNC in extracellular matrix regulation was also explored by analyzing its relation with circulating concentrations of different MMP as well as the expression levels of MMP2 and MMP9. To gain insight into the molecular mechanism involved, the effect of TNF-α and LPS on the expression levels of TNC and TLR4 in cultures of human adipocytes and stromovascular fraction cells (SVFC) was further explored. Finally, we also investigated whether TNC itself can activate the inflammatory response in human adipocytes.
Materials and Methods
For detailed Materials and Methods, see Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Patient selection
Adipose tissue samples from 75 subjects (18 males and 57 females) recruited from healthy volunteers and patients attending the Departments of Endocrinology and Nutrition and Surgery at the Clínica Universidad de Navarra were used. In addition, an intraoperative liver biopsy was performed in the obese patients during bariatric surgery to establish a histological diagnosis of the hepatic state as well as to analyze TNC gene expression levels. The samples were collected from patients undergoing either Nissen fundoplication [for hiatus hernia repair in lean (LN) volunteers] or Roux-en-Y gastric bypass [for morbid obesity treatment in obese (OB) subjects] at the Clínica Universidad de Navarra. Tissue samples were immediately frozen in liquid nitrogen and stored at −80 C for subsequent analyses. The study was approved, from an ethical and scientific standpoint, by the Hospital's Ethical Committee responsible for research, and the written informed consent of participants was obtained. Blood assays and multiplex studies performed in the study subjects were measured as previously described (4). The transcript levels for TNC, TLR4, MMP2, and MMP9 were quantified by real-time PCR (Ref. 24 and Supplemental Table 1) and protein levels were assessed by Western blot (25).
Cell culture
Human SVFC were isolated from the VAT of obese normoglycemic subjects and differentiated to adipocytes as previously described (25). Differentiated human visceral adipocytes and SVFC were serum-starved for 24 h and then treated with increasing concentrations of TNF-α (1, 10, and 100 ng/ml) (Sigma, St. Louis, MO), LPS (10, 100, and 1000 ng/ml) (Sigma), and TNC (1, 10, and 100 nmol/liter) (R&D Systems, Minneapolis, MN) for 24 h.
Study in animals
In the first animal model, 12-wk-old male C57BL/6 mice were maintained during 20 wk on a commercial high-fat diet (n = 10) to induce obesity or on a normal diet (n = 8) to serve as control. In the second murine model, 10-wk-old male wild-type (C57BL/6J) (n = 9) and obese ob/ob mice (C57BL/6J) (n = 8) were used to examine the effects in genetically based obesity. Both diets were isoproteic and contained a similar amount of sodium and phytates (26). Body weight was recorded on a regular basis to monitor progression of the diet-induced and genetically obese mice. The epididymal adipose tissue depot was carefully dissected out, frozen in liquid nitrogen, and stored at −80 C. All experimental procedures conformed to the European Guidelines for the Care and Use of Laboratory Animals (directive 86/609), and the study was approved by the Ethical Committee for Animal Experimentation of the University of Navarra.
Statistical analysis
Data are presented as mean ± sd. Differences in the proportion of subjects within groups regarding gender were assessed by using a contingency test (χ2 test). Differences between groups were assessed by one-way ANOVA followed by Tukey's post hoc tests, two-tailed unpaired Student's t test, and Mann-Whitney U pairwise comparisons as appropriate. Differences between groups adjusted for age were analyzed by analysis of covariance. Pearson's correlation coefficients (r) were used to analyze the association between variables.
Results
Expression of TNC in adipose tissue is increased in obesity and obesity-associated T2D
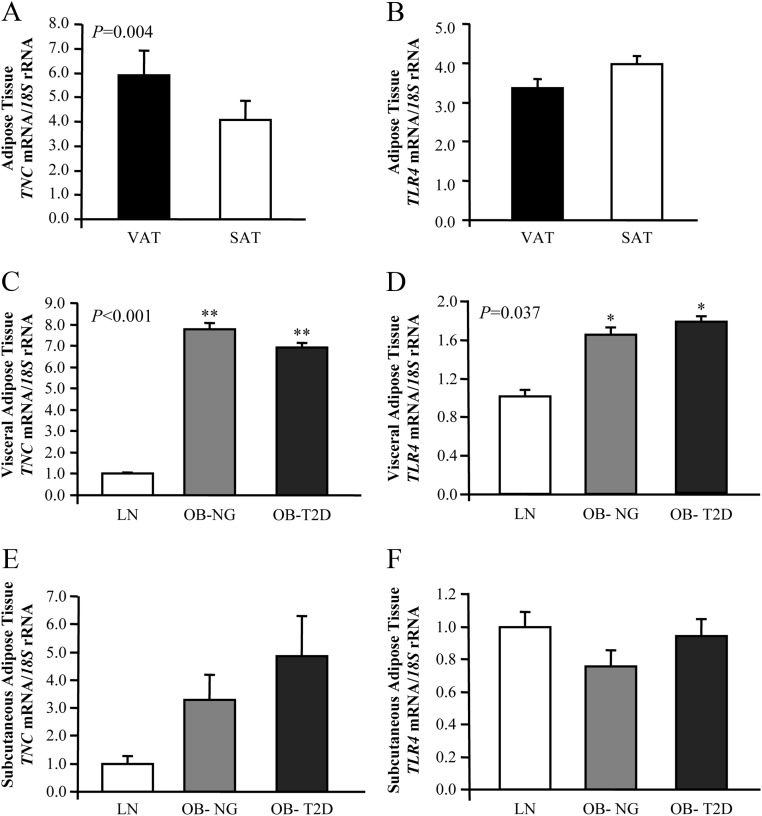
The biochemical and hormonal characteristics of the subjects included in the study are shown in Table 1. No differences in gender distribution between groups was found (P = 0.150). In light of the divergent pathological consequences of adipose tissue distribution, we assessed first the gene expression levels of TNC and TLR4 in paired samples of VAT and SAT. The mRNA levels of TNC in VAT were significantly increased (P = 0.004) compared with SAT, whereas no differences in gene expression levels of TLR4 were found (Fig. 1, A and B).
Table 1.
Anthropometric and biochemical characteristics of subjects included in the study
| Lean | Obese NG | Obese T2D | |
|---|---|---|---|
| n (male, female) | 13 (5, 8) | 32 (7, 25) | 30 (6, 24) |
| Age (yr) | 36 ± 13 | 38 ± 14 | 42 ± 12 |
| BMI (kg/m2) | 22.1 ± 3.0 | 42.2 ± 4.3a | 45.6 ± 7.4a |
| BF (%) | 22.4 ± 7.2 | 52.8 ± 4.8a | 52.2 ± 7.2a |
| Waist (cm) | 75.3 ± 9.7 | 120.3 ± 12.8a | 127.8 ± 12.9a,b |
| Waist to hip ratio | 0.80 ± 0.07 | 0.93 ± 0.09a | 0.96 ± 0.08a |
| SBP (mm Hg) | 105 ± 6 | 121 ± 16a | 134 ± 15a,c |
| DBP (mm Hg) | 66 ± 7 | 75 ± 7a | 84 ± 8a,c |
| Fasting glucose (mg/dl) | 87.8 ± 14.8 | 89.6 ± 10.9 | 125.4 ± 27.0a,c |
| 2 h OGTT glucose (mg/dl) | 114.4 ± 15.4 | 184.5 ± 45.4 | |
| Fasting insulin (μU/ml) | 6.8 ± 2.9 | 17.2 ± 16.3 | 20.2 ± 11.7d |
| 2-h OGTT insulin (μU/ml) | 88.1 ± 51.1 | 147.2 ± 85.0 | |
| HOMA | 1.5 ± 0.8 | 3.9 ± 2.8 | 5.6 ± 3.1a |
| QUICKI | 0.371 ± 0.037 | 0.329 ± 0.038a | 0.306 ± 0.024a,b |
| Triglycerides (mg/dl) | 67 ± 25 | 96 ± 38 | 139 ± 68a,c |
| Cholesterol (mg/dl) | 176 ± 24 | 188 ± 41 | 196 ± 37 |
| LDL-cholesterol (mg/dl) | 103 ± 25 | 115 ± 32 | 118 ± 32 |
| HDL-cholesterol (mg/dl) | 64 ± 12 | 53 ± 18 | 47 ± 12d |
| Leptin (ng/ml) | 8.1 ± 4.5 | 56.6 ± 20.9a | 48.2 ± 27.2a |
| Uric acid (mg/dl) | 4.2 ± 0.7 | 5.7 ± 1.1a | 5.5 ± 1.2a |
| Creatinine (mg/dl) | 0.80 ± 0.06 | 0.80 ± 0.13 | 0.76 ± 0.14 |
| CRP (mg/liter) | 1.0 ± 0.7 | 9.9 ± 6.7a | 7.3 ± 5.1d |
| Fibrinogen (mg/dl) | 215 ± 68 | 398 ± 72a | 349 ± 87a |
| von Willebrand factor (%) | 56 ± 25 | 131 ± 58a | 131 ± 49a |
| Homocysteine (μmol/liter) | 6.8 ± 1.5 | 9.2 ± 2.7d | 9.6 ± 2.6a |
| AST (UI/liter) | 13 ± 4 | 17 ± 13 | 15 ± 6 |
| ALT (UI/liter) | 10 ± 7 | 22 ± 15d | 24 ± 12a |
| ALP (UI/liter) | 93 ± 30 | 96 ± 26 | 93 ± 30 |
| γ-GT (UI/liter) | 11 ± 6 | 20 ± 11 | 29 ± 17d |
| MMP-1 (pg/ml) | 153.1 ± 102.7 | 241.9 ± 189.2 | 213.5 ± 115.5 |
| MMP-2 (ng/ml) | 21.84 ± 6.19 | 19.56 ± 6.36 | 19.89 ± 5.25 |
| MMP-7 (ng/ml) | 2.78 ± 1.53 | 3.36 ± 2.27 | 4.06 ± 1.72d |
| MMP-9 (ng/ml) | 0.22 ± 0.05 | 3.31 ± 0.07a | 2.27 ± 0.04a |
| MMP-10 (pg/ml) | 371.3 ± 199.2 | 218.4 ± 176.5 | 236.3 ± 106.72 |
Data are mean ± sd. Differences between groups were analyzed by one-way ANOVA followed by Tukey's post hoc tests. MMP levels were logarithmically transformed for statistical analysis due to their non-normal distribution. ALP, Alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; DBP, diastolic blood pressure; HOMA, homeostatic model assessment; OGTT, oral glucose tolerance test; SBP, systolic blood pressure.
P < 0.01 vs. lean.
P < 0.05 vs. obese NG.
P < 0.01 vs. obese NG.
P < 0.05 vs. lean.
Fig. 1.
Impact of obesity and obesity-associated T2D on gene expression levels of TNC and TLR4 in adipose tissue. A and B, Analysis of mRNA levels of TNC and TLR4 in VAT and SAT (VAT: n = 75; SAT: n = 23). Bars represent the mean ± sd of the ratio between the gene expression to 18S rRNA. Differences between groups were analyzed by a two-tailed unpaired Student's t test. C and D, Gene expression levels of TNC and TRL4 in VAT of LN, obese NG, and obese T2D volunteers (LN: n = 13; OB-NG: n = 32; OB-T2D: n = 30). E and F, Gene expression levels of TNC and TRL4 in SAT of LN, obese NG, and obese T2D volunteers (LN: n = 7; OB-NG: n = 7; OB-T2D: n = 9). Bars represent the mean ± sd of the ratio between the gene expression to 18S rRNA. The expression level in LN subjects was assumed to be 1. Differences between groups were analyzed by one-way ANOVA followed by Tukey's tests. *, P < 0.05, **, P < 0.01 vs. LN.
Obesity and T2D were associated with an increased gene expression of TNC (P < 0.001) and TLR4 (P < 0.05) in VAT (Fig. 1, C and D). TNC protein expression in the visceral fat depot exhibited a similar pattern to that observed in the gene expression analysis (Supplemental Fig. 1A). No changes in TNC and TLR4 transcript levels were observed in SAT, although a tendency toward an increase in gene expression levels of TNC was shown (Fig. 1, E and F).
Although no differences between groups were detected regarding age, because the obese T2D group was on average slightly older, an analysis of covariance with age as covariable was performed to investigate the potential bias of age on mRNA levels of TNC and TLR4 in VAT. Similar results were obtained, with TNC and TLR4 expression being significantly increased (P < 0.01) in both obese normoglycemic (NG) and T2D patients compared with lean subjects. In this regard, mRNA TNC levels were positively associated (P < 0.05) with body mass index (BMI) and body fat (BF) as well as with fasting insulin concentrations and were negatively correlated with the quantitative insulin sensitivity check index (QUICKI) and high-density lipoprotein (HDL)-cholesterol concentrations (Table 2). A positive association was also found between mRNA levels of TNC and TLR4 in VAT (r = 0.26; P = 0.033). No sexual dimorphism was found in the gene expression levels of both adipokines (P = 0.547 for TNC and P = 0.296 for TLR4).
Table 2.
Univariate analysis of the correlation between mRNA expression levels of TNC and TLR4 in VAT with anthropometic and metabolic variables as well as with genes involved in extracellular matrix regulation
|
TNC mRNA expression |
TLR4 mRNA expression |
|||
|---|---|---|---|---|
| r | P | r | P | |
| TNC mRNA expression | — | — | 0.26 | 0.033 |
| TLR4 mRNA expression | 0.26 | 0.033 | — | — |
| MMP2 mRNA expression | 0.35 | 0.003 | 0.45 | <0.001 |
| MMP9 mRNA expression | 0.38 | <0.001 | 0.35 | 0.003 |
| BMI | 0.37 | 0.002 | 0.31 | 0.012 |
| BF | 0.26 | 0.038 | 0.22 | 0.085 |
| QUICKI | −0.33 | 0.011 | −0.04 | 0.791 |
| HDL-cholesterol | −0.32 | 0.016 | −0.06 | 0.651 |
Bold values denote statistically significant P values. —, The correlation of the TNC gene levels or TLR4 gene expression with itself is not performed/provided.
Based on the fact that TNC exhibited the highest gene expression in VAT and given the relevance of this depot in obesity-associated metabolic disturbances, the experiments thereafter were focused on TNC in this location.
Gene expression of Tnc in murine models of obesity
To corroborate the human findings regarding TNC and TLR4 and to further explore the potential role of both molecules in the development of obesity, two different murine models of obesity, diet-induced obesity and genetically obese leptin-deficient (ob/ob) mice, were used (27). After 12 wk on a high-fat diet, mice exhibited a higher final body weight than those on a chow diet (P < 0.001). The same was true for ob/ob mice compared with their wild-type littermates (P < 0.001). Both murine obesity models also exhibited significantly increased epididymal fat depot weights (P < 0.01). Tnc and Tlr4 expression was increased (P < 0.05) in the epididymal adipose tissue of both obesity models compared with those on a chow diet and with wild-type littermates, respectively (Supplemental Fig. 2).
TNC in adipose tissue in relation to extracellular matrix regulation and inflammation
Obese subjects with T2D exhibited higher circulating levels of MMP-7 (P < 0.05) and MMP-9 (P < 0.01) with the latter being also increased (P < 0.01) in obese NG patients (P < 0.01) compared with lean subjects (Table 1). Moreover, MMP-9 mRNA expression levels were significantly higher (P < 0.001) in both obese NG and obese with T2D subjects compared with lean volunteers. Gene expression levels of MMP2 followed a similar trend, although no statistically significant differences were detected (Supplemental Fig. 3). A positive correlation between the circulating concentrations and the expression levels in VAT of MMP-9 (r = 0.32; P = 0.022) was observed. Moreover, gene expression levels of TNC in VAT were positively correlated with the extracellular matrix regulatory genes MMP9 (P < 0.01) and MMP2 (P < 0.01) (Table 2).
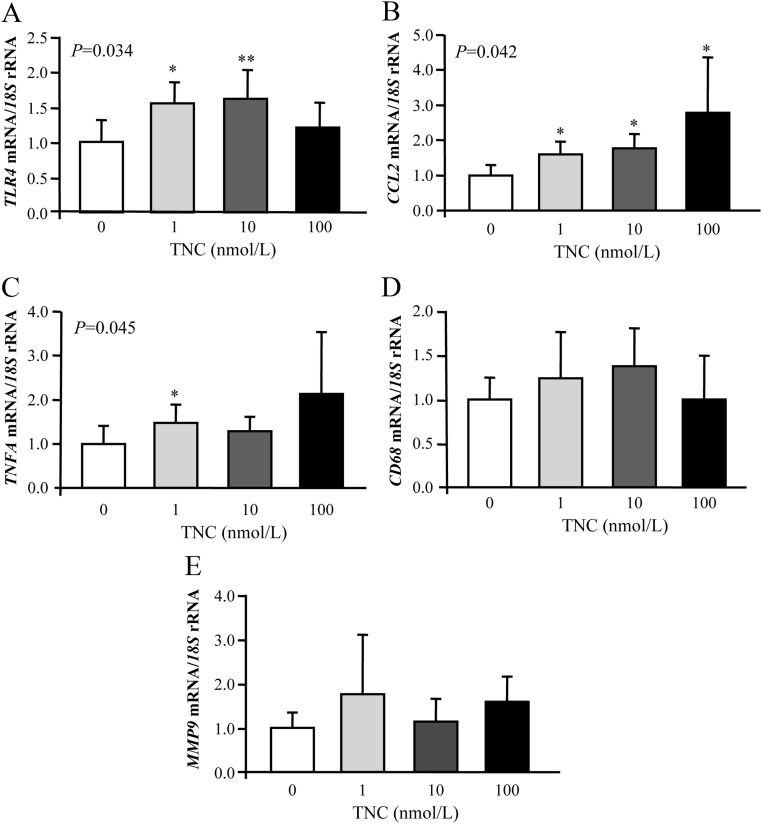
We next investigated whether TNC can activate the expression of TLR4 and genes involved in the inflammatory response in human adipocytes. Cells were stimulated with increasing concentrations of TNC for 24 h. As shown in Fig. 2, TNC treatment significantly enhanced (P < 0.05) the mRNA levels of TLR4 and CCL2 in adipocytes, whereas a slight increase of gene expression levels of TNFA was observed. We also detected an increased gene expression of CD68 and MMP9 after TNC treatment although the differences were not statistically significant.
Fig. 2.
Gene expression levels of TLR4 (A), chemokine (C-C motif) ligand 2 (CCL2) (B), TNFA (C), CD68 antigen (CD68) (D), and MMP9 (E) in human visceral adipocytes stimulated with recombinant TNC (1.0–100 nmol/liter) for 24 h. Gene expression levels in the unstimulated cells were assumed to be 1. Values are the mean ± sd (n = 6 per group). Differences between groups were analyzed by one-way ANOVA followed by Tukey's tests. *, P < 0.05 and **, P < 0.01 vs. unstimulated cells.
Effect of inflammatory factors on mRNA levels of TNC and TLR4 in human adipocytes and SVFC
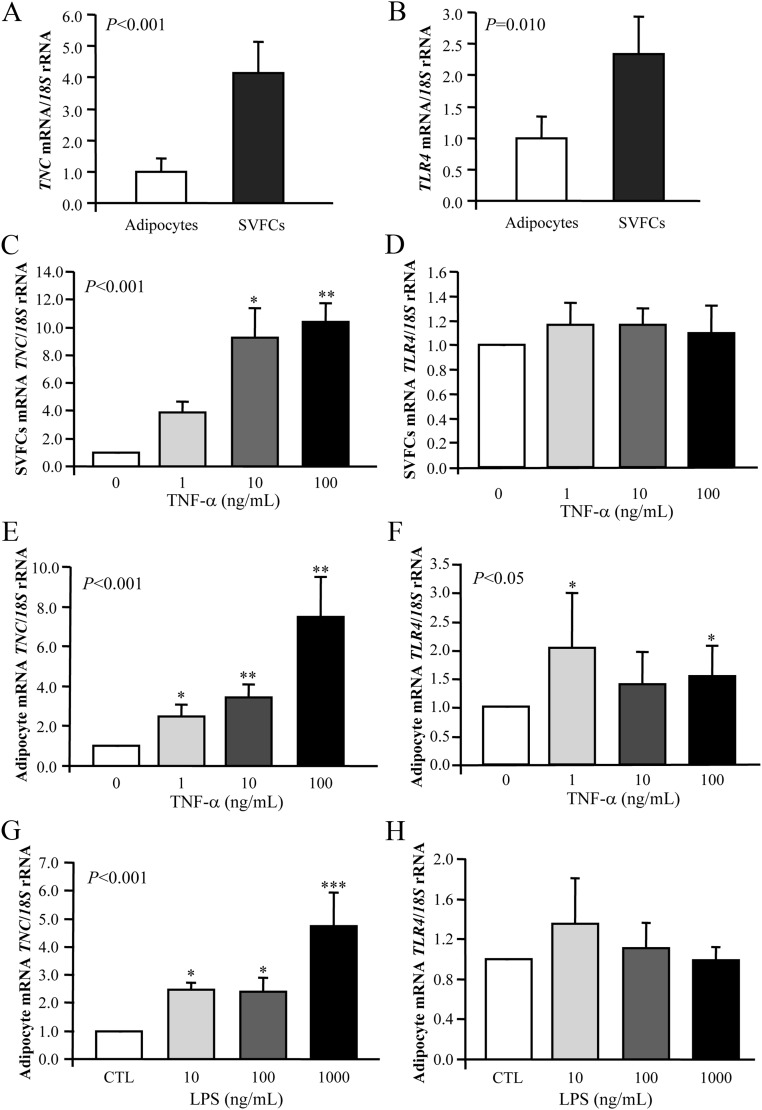
Because VAT of obese patients exhibited an increased macrophage infiltration (especially in those individuals with a high BMI), to identify which cell type preferentially contributed to the elevated TNC and TLR4 levels observed, adipocytes and SVFC were isolated from VAT samples obtained from 15 morbidly obese patients. Although both TNC and TLR4 expression were readily evident in mature adipocytes, gene expression levels were mainly detected in the SVFC (P < 0.01) (Fig. 3, A and B). Furthermore, the presence of TNC in sections of visceral adipose tissue was confirmed by immunohistochemistry (Supplemental Fig. 1B). Both adipocytes and SVFC were immunopositive for TNC, although a marked staining in SVFC was observed.
Fig. 3.
Gene expression levels of TNC and TLR4 in human visceral adipocytes and SVFC. A and B, Comparison of TNC and TLR4 gene expression in adipocytes and SVFC isolated from VAT of obese patients. Bars represent the mean ± sd of the ratio between the gene expression to 18S rRNA. The expression level in adipocytes was assumed to be 1 (adipocytes: n = 9; SVFC: n = 11). Statistical differences were assessed by a two-tailed unpaired Student's t test. *, P < 0.05, **, P < 0.01 vs. adipocytes. C and D, Effect of TNF-α. Bar graphs show the effect of TNF-α incubated for 24 h on the transcript levels of TNC and TLR4 in visceral SVFC. E and F, Transcript levels of TNC and TLR4 after 24 h incubation with TNF-α in visceral adipocytes. G and H, Gene expression levels of TNC and TLR4 in human visceral adipocytes after LPS treatment. The gene expression levels in the unstimulated cells were assumed to be 1. Values are the mean ± sd (n = 6 per group). Differences between groups were analyzed by one-way ANOVA followed by Tukey's tests. CTL, Control. *, P < 0.05; ***, P < 0.01 vs. unstimulated cells.
Based on the fact that TNF-α is linked to the inflammatory response in obesity being a well-known regulator of the production of certain adipokines, the effect of TNF-α on TNC and TLR4 expression in human visceral adipocytes and SVFC was examined. Cells were stimulated with increasing concentrations of TNF-α for 24 h. As shown in Fig. 3, C and E, TNF-α treatment significantly enhanced the mRNA levels of TNC in both SVFC and adipocytes. Although we also detected an increased gene expression of TLR4 after TNF-α treatment in adipocytes (P < 0.05), the stimulation was not dose dependent, and the huge variation found between samples made it difficult to conclude that TNF-α stimulates TLR4 expression. No statistically significant differences were found for the transcript levels of TLR4 in SVFC (Fig. 3, D and F).
Because bacterial LPS initiates acute inflammatory responses typical of the host reaction to tissue injury or infection, we also analyzed the effect of LPS on human visceral adipocytes. Gene expression levels of TNC were strongly induced by LPS (P < 0.001), whereas gene expression of TLR4 was increased, but the differences felt out of statistical significance (Fig. 3, G and H).
Increased expression of TNC in VAT is related to NAFLD
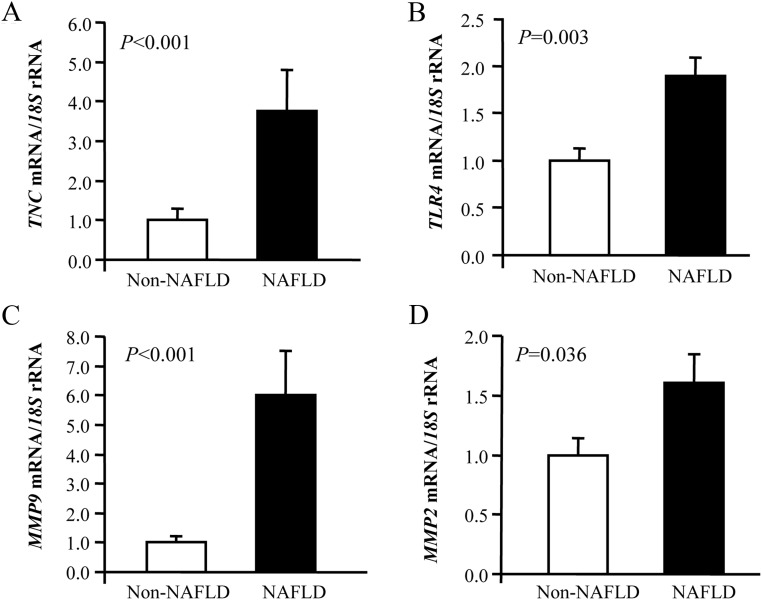
Because one of the best known hepatic derangements associated to obesity and diabetes is NAFLD, we aimed to investigate the regulation of TNC and TLR4 as well as MMP2 and MMP9 in this condition. Obese patients were classified according to the presence or absence of NAFLD and were matched by age and BMI to exclude the effect of obesity in gene expression levels (Supplemental Table 2). Circulating concentrations of the hepatic enzyme γ-glutamyltransferase (γ-GT) (P < 0.05) were increased in NAFLD patients, who also showed elevated circulating concentrations of glucose 2 h after an oral glucose tolerance test and triglycerides (P < 0.05). Real-time PCR analysis indicated that mRNA expression levels of TNC and TLR4 as well as those of both MMP in VAT were significantly higher (P < 0.05) in patients with NAFLD (Fig. 4). The gene expression levels of TNC were also assessed in hepatic biopsies in a subgroup of obese subjects, being higher in obese patients with T2D, although no statistically significant differences were reached (Supplemental Fig. 4A). We also explored the effect of NAFLD on TNC mRNA expression in the liver, and no differences between the groups were found (Supplemental Fig. 4B).
Fig. 4.
Gene expression levels of TNC (A), TLR4 (B), MMP9 (C), and MMP2 (D) in the VAT of obese patients with and without NAFLD. Bars represent the mean ± sd of the ratio between the gene expression to 18S rRNA. The expression level in non-NAFLD subjects was assumed to be 1 (non-NAFLD: n = 18; NAFLD: n = 25). Differences between groups were assessed by two-tailed unpaired Student's t test.
Discussion
Adipose tissue is a complex organ that regulates and coordinates metabolic homeostatic mechanisms. With the development of obesity, adipose tissue expands and exhibits an enhanced systemic low-grade inflammation profile involving macrophage infiltration together with an active adipokine production and release by adipocytes and other surrounding cells (2). To accommodate the changes, ECM remodeling takes place via the degradation of the existing and the production of new ECM components. The ECM is crucial for adipocyte development and function, thereby playing an important role in weight regulation, obesity and lipid metabolism (5).
A tight association between the ECM protein TNC and early inflammatory responses has been described (10). However, the clinical implications of TNC in obesity and adipose tissue remodeling remain largely unknown. Our findings provide evidence, for the first time, that TNC levels are increased in obese subjects in comparison with lean volunteers as well as in VAT compared with SAT. Consistently, we further show that the adipose tissue gene expression levels of Tnc are also increased in two murine models of obesity representative of the genetically based and exogenously induced disease. We also demonstrate that the increased transcript levels of TNC observed in obesity are further aggravated in the presence of NAFLD. Of interest, a correlation between gene expression levels of TNC and TLR4 as well as MMP2 and MMP9 in VAT was found. Finally, we show that the proinflammatory cytokine TNF-α elevates the mRNA levels of TNC in cultures of human visceral adipocytes and SVFC.
VAT and SAT display different morphological and functional features with the increased visceral adiposity being responsible of the metabolic abnormalities related to obesity (28). Our study is the first to show that obese patients exhibit an 8-fold increase in gene expression levels of TNC in VAT compared with lean individuals, whereas no differences are evident in SAT. The significant positive correlation found between TNC levels and BF indicates that TNC is related to the adipose tissue amount. Interestingly, the expression of TNC is highly regulated and shows a highly circumscribed pattern of expression to areas of active tissue reorganization and inflammation, suggesting a link between an increase of ECM remodeling in VAT and its inflammatory profile (15). In addition to cellular adhesion receptors, cells also express receptors for ECM proteins, such as TLR, whose activation lead to the up-regulation of intracellular signaling pathways resulting in the production of inflammatory cytokines. In this regard, we detected higher expression levels of TLR4 in both obese groups compared with lean volunteers as well as a positive association with the TNC expression levels. Recently it has been identified that the activation of TLR4 through TNC is required for maintaining joint inflammation but not for the initiation of inflammation (17). Moreover, Tnc-knockout mice have been shown to be protected from sustained and erosive joint inflammation. Induction of TNC is highly associated with a wide range of diseases related to inflammation, including diabetes, atherosclerosis, ulcerative colitis, inflammatory bowel disease, vasculitis, or lung inflammation (29). An early inflammatory response is generally associated with enhanced TNC levels both in plasma and tissue (13). In this sense, plasma levels of TNC constitute a well-known indicator for inflammatory bowel disease, chronic hepatitis C, or myocardial infarction (30, 31). It would be very interesting to evaluate circulating levels of TNC in light of its physiological consequences. The study of circulating levels of TNC may help to better understand the role of TNC in obesity and its associated comorbidities.
The higher expression levels of TNC and TLR4 in the SVFC of visceral fat tissue compared with adipocytes, observed in the present study indicate that different cell types such as mononuclear cells (monocytes, macrophages, and lymphocytes) among others, may produce this glycoprotein. Noteworthy, SVFC represent a source of inflammation-related molecules that exert a local action on adipose tissue biology, particularly within the enlarged adipose mass. It has been described that macrophages that accumulate within the enlarged adipose tissue acquire particular remodeling phenotypes, releasing MMP and/or stimulating the expression of inflammatory genes in human adipocytes (32, 33). In this context, the obese patients included in the study showed increased circulating levels of MMP-7 and MMP-9. Furthermore, previous studies have demonstrated that macrophage-secreted factors induce overexpression of ECM genes in inflammatory preadipocytes increasing the deposition of TNC (15). In this sense, we have shown increased gene expression levels of MMP9 in VAT from obese patients accompanied by a clear positive association between mRNA levels of TNC with MMP9 and MMP2, which is in line with previous studies reporting that TNC is cleaved by MMP (34, 35). Reciprocally, TNC increases MMP9 expression (36) and regulates vascular endothelial growth factor (37), highlighting a role in the control of angiogenesis. Recent studies have shown an alteration in the expression or activity of MMP in obesity underscoring their involvement in the pathophysiology of obesity-associated comorbidities (34).
To corroborate the human findings and to further explore the potential role of TNC in the development of obesity, gene expression levels of this protein were analyzed in two mice models of obesity differing in their underlying cause, namely genetic as opposed to diet-induced obesity. Increased expression levels of Tnc in epidydimal fat pads were observed in both experimental models of obesity. This is in agreement with previous studies describing that a high-fat diet increases the expression of procollagens and microfibril-forming components in gonadal adipose tissue, thereby pointing to an enhanced matrix turnover induced by high-fat feeding (38). It has been also described that n-3 polyunsaturated fatty acids (PUFA) inhibited the high-fat diet-induced up-regulation of genes involved in matrix remodeling and restored the adipocyte enlargement in adipose tissue of obese diabetic mice (38). Moreover, n-3 PUFA prevented adipose tissue inflammation induced by high-fat diet in obese diabetic mice, thereby suggesting that beneficial effects of n-3 PUFA on diabetes development could be mediated by their effect on adipose tissue inflammation (39). Furthermore, in the genetically obese ob/ob mice due to the lack of functional leptin, increased adipose tissue expression levels of Tnc have been also detected. In this regard, epidydimal adipose tissue of genetically obese db/db mice reportedly exhibit an overexpression of collagens with collagen VI-null ob/ob mice showing a decrease of inflammatory markers in adipose tissue (40).
Our in vitro studies provide, to our knowledge, the first evidence of an induction of the TNC expression levels in human visceral adipocytes and SVFC upon stimulation with the proinflammatory cytokine TNF-α. Although TGF-β1 was the first growth factor reported to up-regulate the mRNA of TNC (41), other inflammatory and growth factors such as epidermal growth factor or basic fibroblast growth factor have been shown to increase TNC production in adipose tissue-derived stem cells and macrophages (42). Because TNC is also stimulated by antiinflammatory cytokines, it has been suggested that this ECM protein may act as a mechanism to protect tissues during inflammation that is required to resolve inflammation rather than to trigger it. In accordance with this hypothesis, TNC has been shown to alter the adhesion properties of human monocytes to fibronectin (43). Adipocytes are capable of sensing the presence of LPS, a bacterial cell wall component. Interestingly, LPS administration has been shown to induce systemic inflammation signaling via TLR-4, without changes in its expression in 3T3-L1 adipocytes (44, 45). It has been also described that in response to stimulation with LPS, human monocytes significantly increased the expression of TNC. We detected for the first time that LPS induced the expression of TNC in human visceral adipocytes with the expression of TLR4 remaining unchanged. Moreover, we found that TNC stimulated mRNA expression levels of TLR4 and CCL2 in adipocytes, strengthening the role of TNC in the inflammatory response of adipose tissue.
Obesity is tightly linked to hepatic alterations, particularly NAFLD, mainly due to the state of chronic low-grade systemic inflammation. In this regard, IL-6 concentrations have been strongly associated with fatty liver (46). The expression of TNC detected in adipose tissue of obese patients was further enhanced by NAFLD. Moreover, gene expression levels of the remodeling metalloproteinases, MMP2 and MMP9, were increased in the NAFLD patients. Noteworthy, a sequential increase in the TNC deposition and expression in the liver of the wild-type mice after concanavalin A treatment has been found (47). However, no differences were found in the TNC mRNA levels in liver biopsies, suggesting an important role of adipose TNC in the regulation of inflammation. Interestingly, the increasing amounts of TNC in rare tumors of Ito cells, which are fat-storing cells, which represent approximately 20% of the hepatic sinusoidal cells (48), as well as in chronic hepatitis C (49) have been described. TNC may be involved in NAFLD by enhancing the inflammatory response or by its actions on ECM synthesis and assembly during tissue repair (29, 50). Surprisingly, patients with NAFLD presented normal levels of the hepatic enzymes, alanine aminotransferase, aspartate aminotransferase, and γ-GT, which may be due to the relatively low age of the selected patients because NAFLD worsens with advanced age (51).
In summary, our findings demonstrate that TNC levels are increased in VAT of obese subjects with or without T2D and in obese volunteers with NAFLD. The positive association with TLR4, MMP2, and MMP9 as well as its stimulation after TNF-α treatment in both adipocytes and SVFC suggests a role for this protein in maintaining the chronic inflammatory response associated to obesity, which may be related to an increase of extracellular matrix remodeling.
Supplementary Material
Acknowledgments
We gratefully acknowledge the valuable collaboration of all the members of the Multidisciplinary Obesity Team, Clinica Universidad de Navarra, Pamplona, Spain. Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición is an initiative of the Instituto de Salud Carlos III (Spain).
This work was supported by Fondo de Investigacion Sanitaria Grants PI09/02330, PI08/1146, and PI11/02681 from the Spanish Instituto de Salud Carlos III; the Department of Health (Grants 20/2005 and 3/2006) of the Gobierno de Navarra of Spain; and the Plan de Investigacion de la Universidad de Navarra (2009–2011).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BF
- Body fat
- BMI
- body mass index
- ECM
- extracellular matrix
- γ-GT
- γ-glutamyltransferase
- HDL
- high-density lipoprotein
- LN
- lean
- LPS
- lipopolysaccharide
- MMP
- matrix metalloproteinase
- NAFLD
- nonalcoholic fatty liver disease
- NG
- normoglycemic
- OB
- obese
- PUFA
- polyunsaturated fatty acids
- QUICKI
- quantitative insulin sensitivity check index
- SAT
- sc adipose tissue
- SVFC
- stromovascular fraction cell
- T2D
- type 2 diabetes
- TLR
- Toll-like receptor
- TNC
- tenascin C
- VAT
- visceral adipose tissue.
References
- 1. Rutkowski JM, Davis KE, Scherer PE. 2009. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J 276:5738–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mariman EC, Wang P. 2010. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci 67:1277–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krakoff J. 2012. The platform for adipose tissue expansion during positive energy balance. J Clin Endocrinol Metab 97:377–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gómez-Ambrosi J, Salvador J, Rotellar F, Silva C, Catalán V, Rodríguez A, Jésus Gil MJ, Frühbeck G. 2006. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obes Surg 16:262–269 [DOI] [PubMed] [Google Scholar]
- 5. Divoux A, Clément K. 2011. Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev 12:e494–e503 [DOI] [PubMed] [Google Scholar]
- 6. Christiaens V, Lijnen HR. 2006. Role of the fibrinolytic and matrix metalloproteinase systems in development of adipose tissue. Arch Physiol Biochem 112:254–259 [DOI] [PubMed] [Google Scholar]
- 7. Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. 2009. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab 94:5155–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. 2010. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab 299:E1016–EE1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, Langin D, Bedossa P, Zucker JD, Clement K. 2008. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 9:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS. 2010. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol 184:2655–2662 [DOI] [PubMed] [Google Scholar]
- 11. Piccinini AM, Midwood KS. 2010. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010:672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Midwood KS, Hussenet T, Langlois B, Orend G. 2011. Advances in tenascin-C biology. Cell Mol Life Sci 68:3175–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiquet-Ehrismann R, Chiquet M. 2003. Tenascins: regulation and putative functions during pathological stress. J Pathol 200:488–499 [DOI] [PubMed] [Google Scholar]
- 14. Udalova IA, Ruhmann M, Thomson SJ, Midwood KS. 2011. Expression and immune function of tenascin-C. Crit Rev Immunol 31:115–145 [DOI] [PubMed] [Google Scholar]
- 15. Keophiphath M, Achard V, Henegar C, Rouault C, Clément K, Lacasa D. 2009. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol 23:11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poulain-Godefroy O, Le Bacquer O, Plancq P, Lecoeur C, Pattou F, Frühbeck G, Froguel P. 2010. Inflammatory role of Toll-like receptors in human and murine adipose tissue. Mediators Inflamm 2010:823486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. 2009. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 15:774–780 [DOI] [PubMed] [Google Scholar]
- 18. Lu YC, Yeh WC, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151 [DOI] [PubMed] [Google Scholar]
- 19. He Z, Zhu Y, Jiang H. 2009. Toll-like receptor 4 mediates lipopolysaccharide-induced collagen secretion by phosphoinositide3-kinase-Akt pathway in fibroblasts during acute lung injury. J Recept Signal Transduct Res 29:119–125 [DOI] [PubMed] [Google Scholar]
- 20. Jones PL, Jones FS. 2000. Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol 19:581–596 [DOI] [PubMed] [Google Scholar]
- 21. Cowan KN, Jones PL, Rabinovitch M. 2000. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest 105:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, Kern PA. 2011. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 96:E1990–E1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun K, Kusminski CM, Scherer PE. 2011. Adipose tissue remodeling and obesity. J Clin Invest 121:2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Salvador J, Frühbeck G. 2011. Increased circulating and visceral adipose tissue expression levels of YKL-40 in obesity-associated type 2 diabetes are related to inflammation: impact of conventional weight loss and gastric bypass. J Clin Endocrinol Metab 96:200–209 [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez A, Gómez-Ambrosi J, Catalán V, Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Salvador J, Frühbeck G. 2009. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 33:541–552 [DOI] [PubMed] [Google Scholar]
- 26. Frühbeck G, Alonso R, Marzo F, Santidrián S. 1995. A modified method for the indirect quantitative analysis of phytate in foodstuffs. Anal Biochem 225:206–212 [DOI] [PubMed] [Google Scholar]
- 27. Frühbeck G, Gómez-Ambrosi J. 2003. Control of body weight: a physiologic and transgenic perspective. Diabetologia 46:143–172 [DOI] [PubMed] [Google Scholar]
- 28. Rodríguez A, Catalán V, Gómez-Ambrosi J, Frühbeck G. 2007. Visceral and subcutaneous adiposity: are both potential therapeutic targets for tackling the metabolic syndrome? Curr Pharm Des 13:2169–2175 [DOI] [PubMed] [Google Scholar]
- 29. Midwood KS, Orend G. 2009. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal 3:287–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka H, El-Karef A, Kaito M, Kinoshita N, Fujita N, Horiike S, Watanabe S, Yoshida T, Adachi Y. 2006. Circulating level of large splice variants of tenascin-C is a marker of piecemeal necrosis activity in patients with chronic hepatitis C. Liver Int 26:311–318 [DOI] [PubMed] [Google Scholar]
- 31. Celik A. 2011. The relationship between tenascin-C levels and the complexity of coronary lesion after myocardial infarction. J Atheroscler Thromb 18:693–697 [DOI] [PubMed] [Google Scholar]
- 32. O'Hara A, Lim FL, Mazzatti DJ, Trayhurn P. 2009. Microarray analysis identifies matrix metalloproteinases (MMPs) as key genes whose expression is up-regulated in human adipocytes by macrophage-conditioned medium. Pflugers Arch 458:1103–1114 [DOI] [PubMed] [Google Scholar]
- 33. O'Hara A, Lim FL, Mazzatti DJ, Trayhurn P. 2012. Stimulation of inflammatory gene expression in human preadipocytes by macrophage-conditioned medium: upregulation of IL-6 production by macrophage-derived IL-1β. Mol Cell Endocrinol 349:239–247 [DOI] [PubMed] [Google Scholar]
- 34. Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E, Tartare-Deckert S. 2003. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem 278:11888–11896 [DOI] [PubMed] [Google Scholar]
- 35. Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Silva C, Rotellar F, Gil MJ, Cienfuegos JA, Salvador J, Frühbeck G. 2009. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J Mol Med 87:803–813 [DOI] [PubMed] [Google Scholar]
- 36. Kalembeyi I, Inada H, Nishiura R, Imanaka-Yoshida K, Sakakura T, Yoshida T. 2003. Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: direct and synergistic effects with transforming growth factor β1. Int J Cancer 105:53–60 [DOI] [PubMed] [Google Scholar]
- 37. Tanaka K, Hiraiwa N, Hashimoto H, Yamazaki Y, Kusakabe M. 2004. Tenascin-C regulates angiogenesis in tumor through the regulation of vascular endothelial growth factor expression. Int J Cancer 108:31–40 [DOI] [PubMed] [Google Scholar]
- 38. Huber J, Löffler M, Bilban M, Reimers M, Kadl A, Todoric J, Zeyda M, Geyeregger R, Schreiner M, Weichhart T, Leitinger N, Waldhäusl W, Stulnig TM. 2007. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond) 31:1004–1013 [DOI] [PubMed] [Google Scholar]
- 39. Todoric J, Löffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhäusl W, Stulnig TM. 2006. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia 49:2109–2119 [DOI] [PubMed] [Google Scholar]
- 40. Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. 2009. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29:1575–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pearson CA, Pearson D, Shibahara S, Hofsteenge J, Chiquet-Ehrismann R. 1988. Tenascin: cDNA cloning and induction by TGF-β. EMBO J 7:2977–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eagan MJ, Zuk PA, Zhao KW, Bluth BE, Brinkmann EJ, Wu BM, McAllister DR. 22 September 2011. The suitability of human adipose-derived stem cells for the engineering of ligament tissue. J Tissue Eng Regen Med 10.1002/term.474 [DOI] [PubMed] [Google Scholar]
- 43. Rüegg CR, Chiquet-Ehrismann R, Alkan SS. 1989. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci USA 86:7437–7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin Y, Lee H, Berg AH, Lisanti MP, Shapiro L, Scherer PE. 2000. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem 275:24255–24263 [DOI] [PubMed] [Google Scholar]
- 45. Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. 2007. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 292:E740–E747 [DOI] [PubMed] [Google Scholar]
- 46. Tarantino G, Conca P, Pasanisi F, Ariello M, Mastrolia M, Arena A, Tarantino M, Scopacasa F, Vecchione R. 2009. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur J Gastroenterol Hepatol 21:504–511 [DOI] [PubMed] [Google Scholar]
- 47. El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. 2007. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol 211:86–94 [DOI] [PubMed] [Google Scholar]
- 48. Tillmann T, Kamino K, Dasenbrock C, Germann PG, Kohler M, Morawietz G, Campo E, Cardesa A, Tomatis L, Mohr U. 1999. Ito cell tumor: immunohistochemical investigations of a rare lesion in the liver of mice. Toxicol Pathol 27:364–336 [DOI] [PubMed] [Google Scholar]
- 49. El-Karef A, Kaito M, Tanaka H, Ikeda K, Nishioka T, Fujita N, Inada H, Adachi Y, Kawada N, Nakajima Y, Imanaka-Yoshida K, Yoshida T. 2007. Expression of large tenascin-C splice variants by hepatic stellate cells/myofibroblasts in chronic hepatitis C. J Hepatol 46:664–673 [DOI] [PubMed] [Google Scholar]
- 50. Tarantino G, Conca P, Riccio A, Tarantino M, Di Minno MN, Chianese D, Pasanisi F, Contaldo F, Scopacasa F, Capone D. 2008. Enhanced serum concentrations of transforming growth factor-β1 in simple fatty liver: is it really benign? J Transl Med 6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tarantino G, Scopacasa F, Colao A, Capone D, Tarantino M, Grimaldi E, Savastano S. 2011. Serum Bcl-2 concentrations in overweight-obese subjects with nonalcoholic fatty liver disease. World J Gastroenterol 17:5280–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.