Abstract
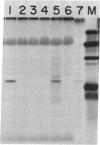
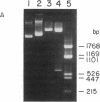
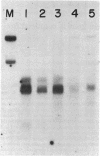
The gene locus for human cytoplasmic superoxide dismutase (SOD-1; superoxide:superoxide oxidoreductase, EC 1.15.1.1) is located in or near a region of chromosome 21 known to be involved in Down syndrome. To approach the molecular biology of this genetic disease we have constructed a SOD-1 cDNA clone. Poly(A)-containing RNA enriched for human SOD-1 mRNA was isolated, used to synthesize double-stranded cDNA, and inserted into the endonuclease Pst I site of the plasmid pBR322. The chimeric molecules were used to transform Escherichia coli. Two clones containing SOD-1 cDNA inserts were identified by their ability to hybridize specifically with mRNA coding for SOD-1. Each of these clones carries a 650-base-pair insert, as was determined by restriction enzyme digestion and electron microscopic heteroduplex analysis. Hybridization of labeled cloned cDNA to RNA blots revealed two distinct SOD-1 mRNA classes of 500 and 700 nucleotides. The data suggest that both are polyadenylylated and are coded by chromosome 21.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brack C. DNA electron microscopy. CRC Crit Rev Biochem. 1981;10(2):113–169. doi: 10.3109/10409238109114551. [DOI] [PubMed] [Google Scholar]
- Crosti N., Serra A., Rigo A., Viglino P. Dosage effect of SOD-A gene in 21-trisomic cells. Hum Genet. 1976 Feb 29;31(2):197–202. doi: 10.1007/BF00296146. [DOI] [PubMed] [Google Scholar]
- Feaster W. W., Kwok L. W., Epstein C. J. Dosage effects for superoxide dismutase-1 in nucleated cells aneuploid for chromosome 21. Am J Hum Genet. 1977 Nov;29(6):563–570. [PMC free article] [PubMed] [Google Scholar]
- Frants R. R., Eriksson A. W., Jongbloet P. H., Hamers A. J. Letter: Superoxide dismutase in Down syndrome. Lancet. 1975 Jul 5;2(7923):42–43. doi: 10.1016/s0140-6736(75)92996-7. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeijer A., Smit E. M. Partial trisomy 21. Further evidence that trisomy of band 21q22 is essential for Down's phenotype. Hum Genet. 1977 Aug 31;38(1):15–23. doi: 10.1007/BF00295803. [DOI] [PubMed] [Google Scholar]
- Hartz J. W., Deutsch H. F. Subunit structure of human superoxide dismutase. J Biol Chem. 1972 Nov 10;247(21):7043–7050. [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- Kurnit D. M. Down syndrome: gene dosage at the transcriptional level in skin fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2372–2375. doi: 10.1073/pnas.76.5.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEJEUNE J., GAUTIER M., TURPIN R. Etude des chromosomes somatiques de neuf enfants mongoliens. C R Hebd Seances Acad Sci. 1959 Mar 16;248(11):1721–1722. [PubMed] [Google Scholar]
- Lovett M. A., Guiney D. G., Helinski D. R. Relaxation complexes of plasmids ColE1 and ColE2: unique site of the nick in the open circular DNA of the relaxed complexes. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3854–3857. doi: 10.1073/pnas.71.10.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei J. F., Mattei M. G., Baeteman M. A., Giraud F. Trisomy 21 for the region 21q223: identification by high-resolution R-banding patterns. Hum Genet. 1981;56(3):409–411. doi: 10.1007/BF00274703. [DOI] [PubMed] [Google Scholar]
- Niebuhr E. Down's syndrome. The possibility of a pathogenetic segment on chromosome no. 21. Humangenetik. 1974 Jan 22;21(1):99–101. doi: 10.1007/BF00278575. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichitiu S., Sinet P. M., Lejeune J., Frézal J. Surdosage de la forme dimérique de l'indophénoloxydase dans la trisomie 21, secondaire au surdosage génique. Humangenetik. 1974 Jun 26;23(1):65–72. doi: 10.1007/BF00295684. [DOI] [PubMed] [Google Scholar]
- Sinet P. M., Couturier J., Dutrillaux B., Poissonnier M., Raoul O., Rethore M. O., Allard D., Lejeune J., Jerome H. Trisomie 21 et superoxyde dismutase-1 (IPO-A). Tentative de localisation sur la sous bande 21Q22.1. Exp Cell Res. 1976 Jan;97:47–55. doi: 10.1016/0014-4827(76)90653-4. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H., Swift M. R. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science. 1966 Sep 9;153(3741):1252–1254. doi: 10.1126/science.153.3741.1252. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Zeevi M., Landau T., Revel M. Identification of the translation products of human fibroblast interferon mRNA in reticulocyte lysates. Eur J Biochem. 1979 Jul;98(1):1–8. doi: 10.1111/j.1432-1033.1979.tb13153.x. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Williams J. D., Summitt R. L., Martens P. R., Kimbrell R. A. Familial Down syndrome due to t(10;21) translocation: evidence that the Down phenotype is related to trisomy of a specific segment of chromosome 21. Am J Hum Genet. 1975 Jul;27(4):478–485. [PMC free article] [PubMed] [Google Scholar]