Abstract
Patients with extremely low high-density lipoprotein-cholesterol (HDL-C) pose distinct challenges to clinical diagnosis and management. Confirmation of HDL-C levels below 20 mg/dl in the absence of severe hypertriglyceridemia should be followed by evaluation for secondary causes, such as androgen use, malignancy, and primary monogenic disorders, namely, apolipoprotein A-I mutations, Tangier disease, and lecithin-cholesterol acyltransferase deficiency. Global cardiovascular risk assessment is a critical component of comprehensive evaluation, although the association between extremely low HDL-C levels and atherosclerosis remains unclear. Therapeutic interventions address reversible causes of low HDL-C, multiorgan abnormalities that may accompany primary disorders and cardiovascular risk modification when appropriate. Uncommon encounters with patients exhibiting extremely low HDL-C provide an opportunity to directly observe the role of HDL metabolism in atherosclerosis and beyond the vascular system.
Accreditation and Credit Designation Statements.
The Endocrine Society is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The Endocrine Society has achieved Accreditation with Commendation.
The Endocrine Society designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learning Objectives
Upon completion of this educational activity, participants should be able to:
Distinguish between artifactual, primary, and secondary causes of very low HDL-cholesterol.
Assess and manage cardiovascular risk and non-cardiovascular morbidity associated with very low HDL-cholesterol.
Target Audience
This Journal-based CME activity should be of substantial interest to endocrinologists.
Disclosure Policy
Authors, editors, and Endocrine Society staff involved in planning this CME activity are required to disclose to The Endocrine Society and to learners any relevant financial relationship(s) of the individual or spouse/partner that have occurred within the last 12 months with any commercial interest(s) whose products or services are discussed in the CME content. The Endocrine Society has reviewed all disclosures and resolved or managed all identified conflicts of interest, as applicable. Disclosures for JCEM Editors are found at http://www.endo-society.org/journals/Other/faculty_jcem.cfm.
Daniel J. Rader, M.D., Emil M. deGoma, M.D., and Editor-in-Chief, Leonard Wartofsky, M.D., reported no relevant financial relationships.
Endocrine Society staff associated with the development of content for this activity reported no relevant financial relationships.
Acknowledgement of Commercial Support
This activity is not supported by grants, other funds, or in-kind contributions from commercial supporters.
Privacy and Confidentiality Statement
The Endocrine Society will record learner's personal information as provided on CME evaluations to allow for issuance and tracking of CME certificates. No individual performance data or any other personal information collected from evaluations will be shared with third parties.
Method of Participation
This Journal-based CME activity is available in print and online as full text HTML and as a PDF that can be viewed and/or printed using Adobe Acrobat Reader. To receive a maximum of 1 AMA PRA Category 1 Credit™ participants should review the learning objectives and disclosure information; read the article and reflect on its content; then go to http://jcem.endojournals.org and find the article, click on CME for Readers, and follow the instructions to access and complete the post-activity test questions and evaluation achieving a minimum score of 70%. If learners do not achieve a passing score of 70%, they have the option to change their answers and make additional attempts to achieve a passing score. Learners also have the option to clear all answers and start over.
To complete this activity, participants must:
Have access to a computer with an internet connection.
Use a major web browser, such as Internet Explorer 7+, Firefox 2+, Safari, Opera, or Google Chrome; in addition, cookies and Javascript must be enabled in the browser's options.
The estimated time to complete this activity, including review of material, is 1 hour. If you have questions about this CME activity, please direct them to education@endo-society.org.
Activity release date: October 2012
Activity expiration date: October 2014
The Case
A 22-yr-old South Asian male college student was referred to our lipid clinic for a high-density lipoprotein-cholesterol (HDL-C) of 6 mg/dl observed by homogeneous assay. The remainder of the lipid panel showed a total cholesterol of 92 mg/dl, triglycerides of 184 mg/dl, and a low-density lipoprotein-cholesterol (LDL-C) of 49 mg/dl. He had no prior assessment of his lipids. He was asymptomatic and described no prior medical or surgical history. He reported a family history of mildly reduced HDL-C, with his mother and father demonstrating levels of 26 and 22 mg/dl, respectively. He had no family history of cardiovascular disease. He denied taking prescription medications or over-the-counter supplements. Physical examination revealed enlarged orange tonsils, no xanthomas or corneal opacification, and normal sensation and reflexes. β-Quantification confirmed extremely low levels of HDL-C (3 mg/dl) and apolipoprotein A-I (apoA-I) (8 mg/dl). Two-dimensional gel electrophoresis followed by immunoblotting with anti-apoA-I antibodies demonstrated only a preβ-HDL band; α-HDL subpopulations were absent. Carotid ultrasonography showed no plaque and a lower risk carotid intima-media thickness. He was diagnosed with Tangier disease and will follow up for surveillance of peripheral neuropathy and serial assessment of atherosclerotic risk.
Background
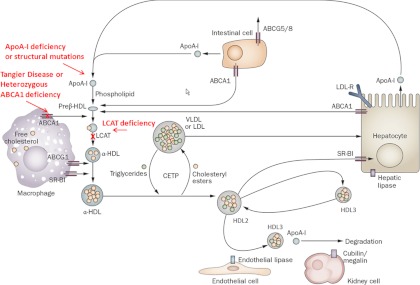
One of the first risk factors identified for coronary heart disease (CHD) (1), low HDL-C levels rank among the most common lipid abnormalities (2), particularly in patients with diabetes or CHD. Low HDL-C is currently defined as an HDL-C value below 40 mg/dl for men or below 50 mg/dl for women, corresponding to approximately the 50th percentile (3). Patients exhibiting extremely low HDL-C, defined in this review as levels below 20 mg/dl, often have severe hypertriglyceridemia (triglyceride >500 mg/dl). Patients with HDL-C less than 20 mg/dl in the absence of severe hypertriglyceridemia are infrequently encountered in clinical practice. Such extreme values well below the 5th percentile (3) arise from severe perturbations in the metabolic pathways of HDL (Fig. 1).
Fig. 1.
HDL metabolism and monogenic extremely low HDL-C disorders. ABC, ATP-binding cassette transporter; CETP, cholesteryl ester transfer protein; LDL-R, LDL receptor. [Reproduced from E. M. Degoma and D. J. Rader: Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol 8:266–277, 2011 (4) with permission. © Nature Publishing Group.]
In normal HDL metabolism, apoA-I is synthesized by the liver and the intestine (4). After secretion, apoA-I forms nascent preβ-HDL by acquiring phospholipid and unesterified cholesterol. These components are transferred to apoA-I from the liver and intestine via ATP-binding cassette transporter A1 (ABCA1) and from triglyceride-rich lipoproteins via plasma phospholipid transfer protein after lipolysis mediated by lipoprotein lipase. Preβ-HDL serves as the acceptor of free cholesterol efflux from peripheral cells, initiating the first step of reverse cholesterol transport. Lecithin-cholesterol acyltransferase (LCAT) esterifies the free cholesterol in HDL to form cholesteryl esters, which migrate to the core of the HDL particle, resulting in formation of α-HDL. These mature HDL particles acquire additional lipid via efflux mediated by ATP-binding cassette transporter G1 and potentially scavenger receptor class B type I (SR-BI). Cholesteryl ester transfer protein mediates exchange of cholesteryl esters for triglycerides with very low-density lipoprotein (VLDL) or LDL, effecting depletion in cholesteryl esters and enrichment in triglycerides of HDL. The resulting HDL particles can be modified by hepatic lipase (HL) and endothelial lipase. HL hydrolyzes triglycerides and phospholipids within HDL, converting lipid-rich large HDL2 to smaller, more dense HDL3, which is more rapidly catabolized. Endothelial lipase preferentially hydrolyzes HDL phospholipids, releasing lipid-poor apoA-I, which can be filtered by the glomeruli and degraded by cubilin/megalin in the proximal renal tubule. HDL-C can be taken up by the liver via SR-BI-mediated selective uptake or by a holoparticle uptake mechanism that is poorly understood. Although multiple processes can result in low HDL-C levels, extremely low HDL-C in the absence of severe hypertriglyceridemia is generally a result of impaired HDL biogenesis.
Differential diagnosis of extremely low HDL-C in the absence of hypertriglyceridemia
Extremely low HDL-C may be artifactual due to assay interference from paraproteinemia, secondary to drugs or malignancy, or primary resulting from monogenic disorders, namely apoA-I deficiency, Tangier disease, or LCAT deficiency (Table 1).
Table 1.
Differential diagnosis of extremely low HDL-C levels (<20 mg/dl)
| Artifactual cause |
| Paraproteinemia from immunoproliferative disorders such as multiple myeloma |
| Secondary causes |
| Anabolic androgenic steroids |
| Fibrates |
| TZD in combination with fibrates |
| Malignancy |
| Primary (monogenic) causes |
| ApoA-I deficiency |
| ApoA-I structural mutations |
| Tangier disease |
| Heterozygous deficiency of ABCA1 |
| LCAT deficiency |
Artifactual cause
Paraproteinemia
Many clinical laboratories currently use direct or homogeneous assays to quantify HDL-C. These techniques measure the cholesterol content of the HDL fraction without the need for labor-intensive pretreatment or separation, thereby permitting automated, high-throughput analysis (5). Paraproteinemia due to immunoproliferative disorders may interfere with the initial reagents used in several direct HDL-C assays that block detection of cholesterol in apolipoprotein-B-containing lipoproteins (6). The interaction, which remains poorly characterized, may result in artifactual extremely low and even undetectable HDL-C levels, and frequently low LDL-C as well. The hallmark of artifactual lipid abnormalities associated with multiple myeloma is normal lipid levels measured via ultracentrifugation, chemical precipitation, or electrophoresis, assays unaffected by the presence of excessive amounts of monoclonal gammaglobulin (6). Treatment of multiple myeloma results in normalization of the artifactual extremely low HDL-C observed with direct HDL-C assays (6).
Secondary causes
Androgenic anabolic steroids
Androgenic anabolic steroids used medically for catabolic diseases such as malignancy and AIDS or recreationally in weight-lifters may result in extremely low levels of HDL-C (7). Higher doses of steroids, 17α-alkylated compounds, and oral administration have been associated with profound HDL-C suppression exceeding 50%, as well as significant increases in LDL-C (7). The timing of HDL-C decline may occur within days of initiating these agents. Normalization of lipid parameters occurs after 1–3 months of steroid withdrawal. Androgens exert their HDL-lowering effects at least in part by enhancing the activity of HL, thereby accelerating the catabolic rate of HDL (7).
Paradoxical response to peroxisome proliferator-activated receptor (PPAR) agonists
Treatment with either fibrates (PPARα agonists) or thiazolidinediones (TZD; PPARγ agonists) generally yields an average increase in HDL-C of 5–20% (8) or 5–10% (9), respectively. Paradoxical and severe decreases in HDL-C to below 20 mg/dl have been reported after fibrate administration or in the setting of concurrent fibrate and TZD use (10, 11). The prevalence of this idiosyncratic response remains unknown. A review of 54,000 patients followed in the primary care setting observed reductions in HDL-C to below 17 mg/dl in 0.02% of patients treated with fibrates alone and 1.39% of patients treated with the combination of fibrate and TZD (10). A referral lipid clinic observed a higher prevalence (7.4%) of transient low HDL-C below 20 mg/dl among patients receiving fibrates (11). The reported onset of HDL-C depression varies from weeks to years after initiation of fibrate or fibrate-TZD therapy; on the other hand, resolution after withdrawal of the offending medication more consistently occurred within 1 month. Significant reductions in HDL-C do not appear to be a class effect. Not only are gemfibrozil and pioglitazone rarely implicated in extremely low HDL-C, but case reports describe resolution of severe reductions in HDL-C after switching from other fibrates or TZD to these drugs (12). The mechanism of the paradoxical, reversible HDL-C depression remains uncertain. One kinetic study in a patient who exhibited extreme lowering of HDL-C with ciprofibrate suggested increased clearance of apoA-I as a contributing factor (13).
Malignancy
Case reports have described extremely low HDL-C levels in the setting of lymphoma (14). A sudden, dramatic decline in HDL-C may precede overt clinical manifestations of hematological malignancy. The mechanism underlying HDL-C suppression remains unknown, and the phenomenon does not appear to be related to paraproteinemia. A recent study identified antibodies specific for LCAT as the cause of a significant decrease in HDL-C in a patient with lymphoma (15). Successful treatment of the lymphoma resulted in clearance of the antibody and correction of HDL-C levels.
Primary causes
Monogenic disorders associated with low HDL-C, although rare in the overall population (16), are more frequently observed among individuals with extremely low HDL-C. An analysis of the Dallas Heart Study identified monogenic low HDL-C disorders in one of six black and white men and women with HDL-C below the 5th percentile, approximately 30 mg/dl (3). The extremely low HDL-C group is undoubtedly further enriched for variants in three key genes involved in HDL metabolism, as described below.
ApoA-I deficiency or structural mutations
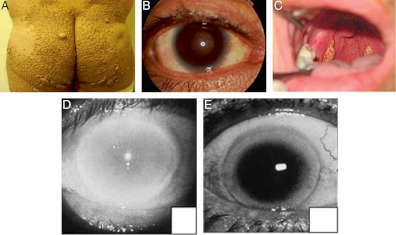
The major protein constituent of HDL, apoA-I, provides structure to the HDL particle, mediates the initial required step in HDL assembly, lipidation by ABCA1, and promotes activation of LCAT. ApoA-I deficiency is due to deletions of the apoA-I gene (17) or nonsense mutations early in the coding portion of the gene (18) in both apoA-I alleles. As a result, the apoA-I protein is not synthesized or secreted, leading to absent apoA-I in plasma and very low levels of HDL-C. Patients with apoA-I deficiency may present with cutaneous xanthomas or mild corneal opacification due to accumulation of cholesterol from impaired peripheral cellular efflux (Fig. 2) (19). Determining the cardiovascular risk associated with monogenic extremely low HDL-C disorders is complicated by the rarity of cases, referral bias, and the reliance in most kindred analyses on clinical events, which in younger individuals may not capture extensive premature atherosclerosis. With these caveats, the weight of evidence supports a high risk of early-onset CHD among patients homozygous or compound heterozygous for apoA-I deficiency. The association, however, is not invariant. A notable case of a 69-yr-old woman with apoA-I deficiency, HDL-C level of 5 mg/dl, LDL-C level of 196 mg/dl, hypertension, and impaired fasting glucose demonstrated a striking absence of significant atherosclerotic disease, with only mild plaque observed on carotid ultrasonography and an unremarkable exercise electrocardiogram (20). Similarly, a 39-yr-old man with apoA-I deficiency, HDL-C of 6 mg/dl, and LDL-C of 175 mg/dl exhibited no subclinical plaque on coronary iv ultrasound (21). Among apoA-I-deficient patients without clinical cardiovascular disease, lower LDL-C has been suggested as a possible protective trait (22). The aforementioned cases suggest additional mechanisms underlying the variable vascular phenotype in patients with extremely low HDL-C from apoA-I deficiency.
Fig. 2.
Physical examination findings in monogenic extremely low HDL-C disorders. Patients with apoA-I deficiency may manifest xanthomas (A) and mild corneal clouding (B). [Reproduced from E. J. Schaefer et al.: Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol 21:289, 2010 (19), with permission. © Lippincott Williams & Wilkins.] The hallmark physical findings in Tangier disease are enlarged yellow-orange tonsils (C). [Reproduced from T. Sampietro et al.: Images in cardiovascular medicine. Tangier disease in severely progressive coronary and peripheral artery disease. Circulation 119:2741, 2009 (26), with permission. © American Heart Association.] Patients with LCAT deficiency exhibit age-dependent corneal opacification. Eyes from a 32-yr-old patient (D) and a 67-yr-old (E) patient are shown. [Reproduced from A. von Eckardstein: Differential diagnosis of familial high density lipoprotein deficiency syndromes. Atherosclerosis 186:231, 2006 (16), with permission. © Elsevier.]
In contrast, apoA-I structural mutations are caused by missense mutations in the apoA-I that result in the insertion of an alternate amino acid. This affects the structure of the apoA-I protein, often leading to impaired function and/or increased catabolism. The first apoA-I structural variant described was apoA-I Milano (23), and many others have since been described (19). Patients are almost always heterozygous for the mutation, and thus have one normal apoA-I allele. Like apoA-I Milano, most (but not all) apoA-I structural variants are not associated with increased cardiovascular risk, despite causing reduced HDL-C levels that are often below 20 mg/dl. A subset of apoA-I structural variants cause hereditary amyloidosis due to deposition of the mutant apoA-I in amyloid plaque (19).
Tangier disease
Tangier disease bears the name of the small island residence of the two siblings first identified with extremely low HDL-C, subsequently shown to be due to mutations in both alleles of the ABCA1 gene (24). Subsequent case reports have described the rare autosomal recessive monogenic disorder in a number of races throughout the world (16). The ABCA1 protein transports cellular cholesterol and phospholipids to the extracellular acceptor free apoA-I to form nascent HDL. Tangier patients carry two defective ABCA1 alleles resulting in very low or undetectable cellular ABCA1 activity. Levels of apoA-I are extremely low due to rapid catabolism of the apolipoprotein as a result of being poorly lipidated. Up to 50% lower LDL-C levels may be observed in Tangier patients due to increased clearance via up-regulated LDL-receptor expression (25). Preclinical data point to enhanced VLDL production as the cause of elevated triglyceride that may be seen in Tangier disease (25).
Hyperplastic foam cells are a characteristic feature of Tangier disease, resulting from the inability to efflux cholesterol via ABCA1. Lipid-laden cells of the reticuloendothelial system manifest as tonsillar enlargement and hepatosplenomegaly. Yellow-orange discoloration of the tonsils and rectal mucosa results from intracellular accumulation of lipophilic retinyl esters and carotenoids (Fig. 2) (26).
Peripheral neuropathy, observed in over 50% of Tangier patients (16), represents the most frequent reason for clinical presentation. Nerve dysfunction more commonly manifests as a remitting-relapsing multifocal neuropathy with sensory abnormalities. Lipid accumulation in the paranodal regions of myelinating Schwann cells has been implicated in the pathogenesis of nerve conduction delay or block (27). Rarely, Tangier disease has been associated with a debilitating syringomyelia-like neuropathy in which muscle wasting and anesthesia involve the face and the upper limbs before extending downward to the trunk and lower extremities. The mechanism remains uncertain but appears to involve primary neuronopathy of the dorsal roots (27).
The association of premature atherosclerosis with Tangier disease is surprisingly tenuous despite the defect in cellular cholesterol efflux and the very low levels of HDL-C and apoA-I. Early CHD has been observed in some kindreds but not in others (28). The incongruous cardiovascular risk in Tangier patients with extremely low HDL-C has previously been attributed to concurrently low LDL-C. However, high LDL-C levels have been observed in Tangier patients in the absence of clinical or subclinical cardiovascular disease, implicating additional mechanisms for the apparent paradox. One hypothesis is that the absence of hepatic ABCA1 may be atheroprotective, counterbalancing the impaired peripheral cholesterol efflux due to macrophage ABCA1 deficiency (29). Hepatic ABCA1 diverts cholesterol from the terminal step of reverse cholesterol transport, excretion into bile, by transporting cholesterol back into the circulation.
Individuals who are heterozygous for ABCA1 mutations have low levels of HDL-C that can be below 20 mg/dl. Indeed, in a family in which there appears to be dominant transmission of a low HDL-C trait, an ABCA1 mutation should be considered in the differential (30). As with Tangier disease, heterozygous ABCA1 deficiency is not uniformly associated with increased cardiovascular risk, and population-based studies have suggested that a low HDL-C due to ABCA1 mutations does not confer increased cardiovascular risk (31).
LCAT deficiency
After apoA-I acquires free cholesterol from ABCA1-mediated efflux, the next obligate step in HDL maturation requires LCAT. LCAT catalyzes conversion of free cholesterol to cholesteryl esters that migrate into the hydrophobic core of HDL, forming spherical α-HDL. Genetic deficiency of LCAT presents as one of two related autosomal recessive disorders, both exhibiting extremely low HDL-C. Patients with familial LCAT deficiency (FLD), characterized by essentially complete LCAT deficiency, develop corneal opacification, anemia, and progressive renal disease (32). Patients with fish-eye disease (FED) present with corneal opacification but are spared the anemia and renal disease and have evidence of some LCAT activity in the blood, generally associated with apoB-containing lipoproteins (33). Both disorders, however, are due to loss-of-function mutations in both LCAT alleles; FED can simply be thought of as a milder form of LCAT deficiency. In FLD and FED, apoB-containing lipoproteins may be elevated and are highly abnormal. VLDL is typically enriched for free cholesterol and relatively devoid of protein. The low-density fraction may contain normal LDL as well as lipoproteins predominantly consisting of free cholesterol, phospholipid, and apolipoprotein C, resembling the lipoprotein X observed in biliary obstruction (16).
Corneal opacification, the hallmark physical finding in LCAT deficiency states, is more severe than the corneal cholesterol accumulation that may sporadically be observed in apoA-I deficiency and Tangier disease. FLD and FED patients develop slowly progressive corneal clouding accompanied by a white or gray ring in the corneal margin similar to arcus senilis (Fig. 2) (16). Despite the dramatic appearance, vision remains intact in most cases. Among FLD patients, the major cause of morbidity and mortality is renal failure. Proteinuria manifesting in childhood gradually progresses to nephrotic syndrome and end-stage chronic kidney disease (34). Why kidney function deteriorates in FLD remains unknown. Heterogenous glomerular pathology implicates several possible mechanisms including trapping of abnormal LDL and deposition of complement C3 (35). Mild normochromic, normocytic anemia to a hemoglobin of 10 g/dl has been attributed to increased erythrocyte fragility due to abnormal lipid composition in the membrane (36).
Limited data available from kindreds with FLD and FED reveal no clear evidence of increased risk of premature atherosclerotic disease (37). Assessment of carotid intima-media thickness, a surrogate imaging measure of cardiovascular risk, also suggests no significant worsening of vascular phenotype among individuals homozygous for LCAT mutations. Patients with FLD and FED exhibited comparable or even reduced carotid intima-media thickness compared with controls (38).
Diagnostic Testing
Initial evaluation of patients with extremely low HDL-C focuses on eliminating severe hypertriglyceridemia as the cause and then classifying the dyslipidemia as artifactual, secondary, or primary. Normalization of an extremely low HDL-C on β-quantification, electrophoresis, or precipitation techniques suggests artifactually low HDL-C from paraproteinemia. Confirmation requires identification of monoclonal gammaglobulin by serum or urine protein electrophoresis. Serial lipid values, if available, are helpful, with previously normal HDL-C levels excluding a primary monogenic etiology. A detailed family history, with a focus on HDL-C levels, is important in assessing the potential for a familial condition. A complete medication history, including over-the-counter therapies and nutritional supplements, should be elicited in an attempt to identify secondary culprit drugs. In the setting of a sudden decrease in HDL-C below 20 mg/dl, if the aforementioned assessment is unrevealing, it is prudent to conduct a comprehensive evaluation for occult malignancy. Physical examination findings, as described above, can suggest a particular monogenic etiology, with special attention to the skin, eyes, tonsils, and spleen.
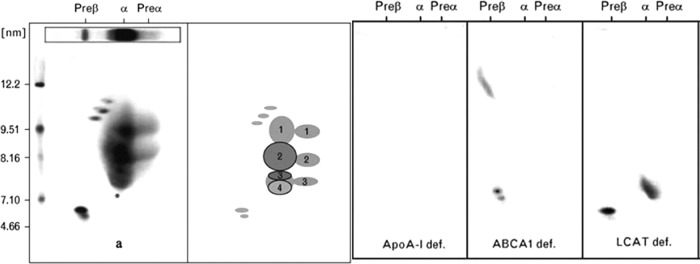
For suspected primary causes, workup involves additional biochemical and genetic assessment (Table 2). Plasma apoA-I levels should be obtained and can be useful diagnostically. Patients with apoA-I deficiency have undetectable plasma apoA-I, and Tangier disease patients have extremely low apoA-I levels (<5 mg/dl). In contrast, patients with LCAT deficiency, structural apoA-I mutations, and heterozygous ABCA1 deficiency have apoA-I levels that, although low compared with normal, are considerably higher. Measurement of plasma free (unesterified) cholesterol can be useful in that both forms of LCAT deficiency have a much higher ratio of free:total cholesterol in plasma due to the defect in cholesterol esterification. Specialized measurements of LCAT activity in plasma can be diagnostic for LCAT deficiency, although more definitive for FLD than for FED where residual LCAT activity can confound the interpretation. Two-dimensional gel electrophoresis of plasma followed by immunoblotting with antibodies specific for apoA-I separates lipid-poor preβ-HDL from lipid-rich α-HDL. In normal individuals, the former contributes a minor subfraction of the total apoA-I population. Plasma from Tangier patients, on the other hand, exhibits only preβ-HDL-migrating apoA-I; no α-HDL fraction is observed due to absent ABCA1-mediated conversion of preβ-HDL to α-HDL. Both FLD and FED have an absence of HDL particles larger than α-4 HDL, the farthest migrating α-HDL subspecies on two-dimensional gel electrophoresis (Fig. 3) (19). Examination of the peripheral blood smear in FLD may reveal target cells, anisopoikilocytosis, and evidence of hemolysis. The diagnosis of Tangier disease is generally made on clinical grounds, but rectal histological evaluation as well as skin fibroblast cholesterol efflux assays may provide confirmatory evidence. Biopsy of the rectum frequently reveals large lipid-laden foam cells in the mucosa and submucosa. Definitive molecular diagnosis of these conditions requires sequencing of the exons of the suspected gene to look for causal mutations, but the presumptive diagnoses can often be made on clinical grounds.
Table 2.
Overview of findings in monogenic extremely low HDL-C disorders
| ApoA-I mutations |
ABCA1 mutations |
LCAT mutations |
||||
|---|---|---|---|---|---|---|
| ApoA-I deficiency | ApoA-I structural mutations | Tangier disease | Heterozygous ABCA1 deficiency | FLD | FED | |
| Physical examination | Xanthomas | Xanthomas | Enlarged, yellow-orange tonsils | None | Corneal opacification | Corneal opacification |
| Mild corneal opacification | Mild corneal opacification | Peripheral neuropathy | Hepatosplenomegaly | |||
| Hepatosplenomegaly | ||||||
| Yellow-orange studded rectal mucosa | ||||||
| Laboratory assessment | ||||||
| LDL-C | Normal | Normal | Low | Normal | May be high | May be high |
| LDL structurally resembles lipoprotein X | LDL structurally resembles lipoprotein X | |||||
| Triglycerides | Isolated apoA-I deficiency: normal | Normal | High | Normal | High | High |
| ApoA-I/C-III/A-IV deletion: low | ||||||
| ApoA-I | 0–1 mg/dl | 10–20 mg/dl | 0–5 mg/dl | 10–20 mg/dl | 30–50 mg/dl | 30–50 mg/dl |
| Two-dimensional gel electrophoresis | No apoA-I | Low apoA-I | Preβ-HDL: present | Preβ-HDL: present | Preβ-HDL: present | Preβ-HDL: present |
| Any α-HDL: absent | Any α-HDL: low | α4-HDL: present | α4-HDL: present | |||
| Larger α-HDL: absent | Larger α-HDL: absent | |||||
| Additional lipid- related testing | Fibroblast efflux of radiolabeled cholesterol:low | Fibroblast efflux of radiolabeled cholesterol: low | LCAT mass/activity: absent | LCAT mass/activity: low | ||
| Rectal biopsy: lipid-laden foam cells | Rectal biopsy: lipid- laden foam cells | Cholesterol ester/total cholesterol: very low | Cholesterol ester/total cholesterol: normal | |||
| Other | Proteinuria | |||||
| Reduced creatinine clearance | ||||||
| Anemia with target cells and evidence of normocytic, nonimmune hemolysis | ||||||
| Association with premature atherosclerotic disease | ++ | +/− | +/− | +/− | − | − |
Fig. 3.
Two-dimensional gel electrophoresis of monogenic extremely low HDL-C disorders. A composite is shown of the HDL gel patterns observed in a normal subject, a homozygote with apoA-I deficiency, a Tangier patient, and an individual with LCAT deficiency. [Reproduced from E. J. Schaefer et al.: Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol 21:289–297, 2010 (19) with permission. © Lippincott Williams & Wilkins.]
Therapeutic Strategies
Management of secondary causes of extremely low HDL-C involves removal of the offending drug or treatment of the underlying disease. Evaluation and management of cardiovascular risk plays a central role, particularly for monogenic extremely low HDL-C disorders. Manifest cardiovascular disease warrants comprehensive secondary prevention measures, including achieving an LDL-C well below 70 mg/dl with high-dose statin therapy. In the primary prevention setting, in addition to optimizing identifiable traditional risk factors, subclinical atherosclerosis imaging with coronary artery calcium scanning or carotid intima-media thickness assessment may be helpful to refine risk and establish targets for lipid-lowering therapy.
ApoA-I deficiency is generally associated with markedly increased atherosclerotic cardiovascular disease and should be managed with aggressive reduction of LDL-C and non-HDL-C. Therapies such as repeated apoA-I infusion are being developed and could be useful for this condition in the future. apoA-I structural variants are not generally associated with clinical sequelae requiring specific treatment. In Tangier patients, hyperplastic tonsils are usually asymptomatic but in children may cause pharyngeal obstruction requiring tonsillectomy. Neuropathy is the major clinical issue, but to date, no therapeutic intervention has proven effective in treating the neuropathy of Tangier disease. Rarely, severe corneal opacification in patients with FLD or FED may interfere with vision, necessitating corneal transplantation. Management of FLD centers on the preservation of renal function. Angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, and in some cases steroid therapy have demonstrated efficacy in treating proteinuria and slowing progression of renal failure (39). Patients with end-stage renal disease have undergone renal transplantation; however, disease has been reported to reoccur in the allograft (40). Repeated infusion of recombinant LCAT is being developed as a therapy for this condition, and even gene therapy is a possibility for the future.
Summary
Extremely low HDL-C below 20 mg/dl may be an artifact of homogeneous assays in the setting of multiple myeloma; secondary to androgenic anabolic steroids, fibrates, TZD, or malignancy; or primary resulting from genetic disorders. Each of the three known monogenic disorders in which patients manifest extremely low HDL-C—apoA-I deficiency or structural mutations, Tangier disease, and LCAT deficiency—presents with a unique constellation of physical and biochemical findings. The association between extremely low HDL-C and premature atherosclerosis is surprisingly inconsistent, an observation that remains incompletely explained. Evaluation and management of the risk for atherosclerotic cardiovascular diseases plays a central role in the care of these patients, as well as addressing extravascular manifestations of abnormal HDL metabolism.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute, National Human Genome Research Institute, and National Center for Advancing Translational Sciences.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ABCA1
- ATP-binding cassette transporter A1
- apoA-I
- apolipoprotein A-I
- CHD
- coronary heart disease
- FED
- fish-eye disease
- FLD
- familial LCAT deficiency
- HDL
- high-density lipoprotein
- HDL-C
- HDL-cholesterol
- HL
- hepatic lipase
- LCAT
- lecithin-cholesterol acyltransferase
- LDL
- low-density lipoprotein
- LDL-C
- LDL-cholesterol
- PPAR
- peroxisome proliferator-activated receptor
- SR-BI
- scavenger receptor class B type I
- TZD
- thiazolidinedione
- VLDL
- very low-density lipoprotein.
References
- 1. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 62:707–714 [DOI] [PubMed] [Google Scholar]
- 2. Bruckert E. 2006. Epidemiology of low HDL-cholesterol: results of studies and surveys. Eur Heart J Suppl 8:F17–F22 [Google Scholar]
- 3. Schaefer EJ, Lamon-Fava S, Ordovas JM, Cohn SD, Schaefer MM, Castelli WP, Wilson PW. 1994. Factors associated with low and elevated plasma high density lipoprotein cholesterol and apolipoprotein A-I levels in the Framingham Offspring Study. J Lipid Res 35:871–882 [PubMed] [Google Scholar]
- 4. Degoma EM, Rader DJ. 2011. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol 8:266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Langlois MR, Blaton VH. 2006. Historical milestones in measurement of HDL-cholesterol: impact on clinical and laboratory practice. Clin Chim Acta 369:168–178 [DOI] [PubMed] [Google Scholar]
- 6. Tsai LY, Tsai SM, Lee SC, Liu SF. 2005. Falsely low LDL-cholesterol concentrations and artifactual undetectable HDL-cholesterol measured by direct methods in a patient with monoclonal paraprotein. Clin Chim Acta 358:192–195 [DOI] [PubMed] [Google Scholar]
- 7. Glazer G. 1991. Atherogenic effects of anabolic steroids on serum lipid levels. A literature review. Arch Intern Med 151:1925–1933 [PubMed] [Google Scholar]
- 8. McKenney JM. 2008. What is the best place for fibrate therapy in reducing cardiovascular risk? In: Toth PP, Sica DA, eds. Lipid disorders. Oxford, UK: Atlas Publishing [Google Scholar]
- 9. Goldberg RB. 2006. Impact of thiazolidenediones on serum lipoprotein levels. Curr Atheroscler Rep 8:397–404 [DOI] [PubMed] [Google Scholar]
- 10. Keidar S, Guttmann H, Stam T, Fishman I, Shapira C. 2007. High incidence of reduced plasma HDL cholesterol in diabetic patients treated with rosiglitazone and fibrate. Pharmacoepidemiol Drug Saf 16:1192–1194 [DOI] [PubMed] [Google Scholar]
- 11. Mymin D, Dembinski T, Friesen MH. 2009. Iatrogenic severe depression of high-density lipoprotein cholesterol. J Clin Pharmacol 49:865–871 [DOI] [PubMed] [Google Scholar]
- 12. Ebcioglu Z, Morgan J, Carey C, Capuzzi D. 2003. Paradoxical lowering of high-density lipoprotein cholesterol level in 2 patients receiving fenofibrate and a thiazolidinedione. Ann Intern Med 139:W80. [DOI] [PubMed] [Google Scholar]
- 13. Beghin L, Capps N, Duhal N, Davies J, Staels B, Luc G. 1999. Metabolism of apolipoproteins AI and AII in a patient with paradoxical reduction in high-density lipoprotein due to ciprofibrate. Ann Clin Biochem 36:523–525 [DOI] [PubMed] [Google Scholar]
- 14. Garg A, Hosfield EM, Brickner L. 2011. Disseminated intravascular large B cell lymphoma with slowly decreasing high-density lipoprotein cholesterol. South Med J 104:53–56 [DOI] [PubMed] [Google Scholar]
- 15. Simonelli S, Gianazza E, Mombelli G, Bondioli A, Ferraro G, Penco S, Sirtori CR, Franceschini G, Calabresi L. 2012. Severe high-density lipoprotein deficiency associated with autoantibodies against lecithin:cholesterol acyltransferase in non-Hodgkin lymphoma. Arch Intern Med 172:179–181 [DOI] [PubMed] [Google Scholar]
- 16. von Eckardstein A. 2006. Differential diagnosis of familial high density lipoprotein deficiency syndromes. Atherosclerosis 186:231–239 [DOI] [PubMed] [Google Scholar]
- 17. Schaefer EJ, Heaton WH, Wetzel MG, Brewer HB., Jr 1982. Plasma apolipoprotein A-1 absence associated with a marked reduction of high density lipoproteins and premature coronary artery disease. Arteriosclerosis 2:16–26 [DOI] [PubMed] [Google Scholar]
- 18. Ng DS, Leiter LA, Vezina C, Connelly PW, Hegele RA. 1994. Apolipoprotein A-I Q[-2]X causing isolated apolipoprotein A-I deficiency in a family with analphalipoproteinemia. J Clin Invest 93:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schaefer EJ, Santos RD, Asztalos BF. 2010. Marked HDL deficiency and premature coronary heart disease. Curr Opin Lipidol 21:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yokota H, Hashimoto Y, Okubo S, Yumoto M, Mashige F, Kawamura M, Kotani K, Usuki Y, Shimada S, Kitamura K, Nakahara K. 2002. Apolipoprotein A-I deficiency with accumulated risk for CHD but no symptoms of CHD. Atherosclerosis 162:399–407 [DOI] [PubMed] [Google Scholar]
- 21. Takata K, Saku K, Ohta T, Takata M, Bai H, Jimi S, Liu R, Sato H, Kajiyama G, Arakawa K. 1995. A new case of apoA-I deficiency showing codon 8 nonsense mutation of the apoA-I gene without evidence of coronary heart disease. Arterioscler Thromb Vasc Biol 15:1866–1874 [DOI] [PubMed] [Google Scholar]
- 22. Pisciotta L, Miccoli R, Cantafora A, Calabresi L, Tarugi P, Alessandrini P, Bittolo Bon G, Franceschini G, Cortese C, Calandra S, Bertolini S. 2003. Recurrent mutations of the apolipoprotein A-I gene in three kindreds with severe HDL deficiency. Atherosclerosis 167:335–345 [DOI] [PubMed] [Google Scholar]
- 23. Franceschini G, Sirtori CR, Capurso A, 2nd, Weisgraber KH, Mahley RW. 1980. A-I Milano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest 66:892–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hobbs HH, Rader DJ. 1999. ABC1: connecting yellow tonsils, neuropathy, and very low HDL. J Clin Invest 104:1015–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chung S, Timmins JM, Duong M, Degirolamo C, Rong S, Sawyer JK, Singaraja RR, Hayden MR, Maeda N, Rudel LL, Shelness GS, Parks JS. 2010. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J Biol Chem 285:12197–12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sampietro T, Puntoni M, Bigazzi F, Pennato B, Sbrana F, Dal Pino B, Azzarà A, Magagnini E, Perossini P, Cei G, Bacci G. 2009. Images in cardiovascular medicine. Tangier disease in severely progressive coronary and peripheral artery disease. Circulation 119:2741–2742 [DOI] [PubMed] [Google Scholar]
- 27. Antoine JC, Tommasi M, Boucheron S, Convers P, Laurent B, Michel D. 1991. Pathology of roots, spinal cord and brainstem in syringomyelia-like syndrome of Tangier disease. J Neurol Sci 106:179–185 [DOI] [PubMed] [Google Scholar]
- 28. Frikke-Schmidt R. 2010. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis 208:305–316 [DOI] [PubMed] [Google Scholar]
- 29. Yamamoto S, Tanigawa H, Li X, Komaru Y, Billheimer JT, Rader DJ. 2011. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation 124:1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, Hobbs HH. 2004. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science 305:869–872 [DOI] [PubMed] [Google Scholar]
- 31. Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. 2008. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 299:2524–2532 [DOI] [PubMed] [Google Scholar]
- 32. Norum KR, Gjone E. 1967. Familial serum-cholesterol esterification failure. A new inborn error of metabolism. Biochim Biophys Acta 144:698–700 [DOI] [PubMed] [Google Scholar]
- 33. Carlson LA. 1979. A further case of fish-eye disease. Lancet 2:1376–1377 [DOI] [PubMed] [Google Scholar]
- 34. Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. 2009. Lecithin:cholesterol acyltransferase—from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes 16:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strøm EH, Sund S, Reier-Nilsen M, Dørje C, Leren TP. 2011. Lecithin:cholesterol acyltransferase (LCAT) deficiency: renal lesions with early graft recurrence. Ultrastruct Pathol 35:139–145 [DOI] [PubMed] [Google Scholar]
- 36. Suda T, Akamatsu A, Nakaya Y, Masuda Y, Desaki J. 2002. Alterations in erythrocyte membrane lipid and its fragility in a patient with familial lecithin:cholesterol acyltrasferase (LCAT) deficiency. J Med Invest 49:147–155 [PubMed] [Google Scholar]
- 37. Calabresi L, Simonelli S, Gomaraschi M, Franceschini G. 2012. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis 222:299–306 [DOI] [PubMed] [Google Scholar]
- 38. Calabresi L, Baldassarre D, Castelnuovo S, Conca P, Bocchi L, Candini C, Frigerio B, Amato M, Sirtori CR, Alessandrini P, Arca M, Boscutti G, Cattin L, Gesualdo L, Sampietro T, Vaudo G, Veglia F, Calandra S, Franceschini G. 2009. Functional lecithin:cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation 120:628–635 [DOI] [PubMed] [Google Scholar]
- 39. Miarka P, Idzior-Waluœ B, KuŸniewski M, Waluœ-Miarka M, Klupa T, Sułowicz W. 2011. Corticosteroid treatment of kidney disease in a patient with familial lecithin-cholesterol acyltransferase deficiency. Clin Exp Nephrol 15:424–429 [DOI] [PubMed] [Google Scholar]
- 40. Panescu V, Grignon Y, Hestin D, Rostoker G, Frimat L, Renoult E, Gamberoni J, Grignon G, Kessler M. 1997. Recurrence of lecithin cholesterol acyltransferase deficiency after kidney transplantation. Nephrol Dial Transplant 12:2430–2432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.