Abstract
We review the rationale for the use of synthetic oleanane triterpenoids (SOs) for prevention and treatment of disease, as well as extensive biological data on this topic resulting from both cell culture and in vivo studies. Emphasis is placed on understanding mechanisms of action. SOs are noncytotoxic drugs with an excellent safety profile. Several hundred SOs have now been synthesized and in vitro have been shown to: 1) suppress inflammation and oxidative stress and therefore be cytoprotective, especially at low nanomolar doses, 2) induce differentiation, and 3) block cell proliferation and induce apoptosis at higher micromolar doses. Animal data on the use of SOs in neurodegenerative diseases and in diseases of the eye, lung, cardiovascular system, liver, gastrointestinal tract, and kidney, as well as in cancer and in metabolic and inflammatory/autoimmune disorders, are reviewed. The importance of the cytoprotective Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1/nuclear factor (erythroid-derived 2)-like 2/antioxidant response element (Keap1/Nrf2/ARE) pathway as a mechanism of action is explained, but interactions with peroxisome proliferator-activated receptor γ (PARPγ), inhibitor of nuclear factor-κB kinase complex (IKK), janus tyrosine kinase/signal transducer and activator of transcription (JAK/STAT), human epidermal growth factor receptor 2 (HER2)/ErbB2/neu, phosphatase and tensin homolog (PTEN), the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway, mammalian target of rapamycin (mTOR), and the thiol proteome are also described. In these interactions, Michael addition of SOs to reactive cysteine residues in specific molecular targets triggers biological activity. Ultimately, SOs are multifunctional drugs that regulate the activity of entire networks. Recent progress in the earliest clinical trials with 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) methyl ester (bardoxolone methyl) is also summarized.
I. Introduction
Disease inflicts great pain and suffering. Current understanding of the natural history of the mechanisms and processes that cause most common chronic diseases now offers the possibility to prevent or alleviate much of that pain and suffering. Based on such mechanistic understanding, we can now design new preventive drugs to modify the disease process to make it less aggressive, less malignant, and less virulent, to allow a new approach to preventive medicine. This article will review the pharmacological basis for the use of one such class of preventive drugs, the synthetic pentacyclic oleanane triterpenoids (SOs1), in contemporary medicine. Both the inflammatory process and oxidative stress are at the pathogenetic core of so many chronic diseases (Glass et al., 2010; Grivennikov et al., 2010; Nathan and Ding, 2010), including cardiovascular, diabetic, pulmonary, arthritic, gastrointestinal, hepatic, cancerous, renal, or neurodegenerative diseases, and SOs have uniquely potent and safe ability to control inflammation and oxidative stress in almost every part of the body. Therefore, these agents now have the potential to alter patterns of medical practice to a more preventive orientation. This is indeed critically needed, because increasing costs of treating end-stage illness impose increasingly unsustainable economic burdens on society.
In this article, we will first provide a brief historical perspective on the inflammatory process and oxidative stress, as well as the use of natural pentacyclic triterpenoids to control these processes. We will then provide an updated summary (previously reviewed in Liby et al., 2007b; Petronelli et al., 2009a) on the development of SOs, from both the perspectives of synthetic organic chemistry and their molecular and cellular mechanisms of action, for prevention of disease in both experimental animals and in the clinic. Finally, we will discuss issues that need to be addressed for these important new agents to have their optimal use for human benefit.
II. Inflammation and Oxidative Stress
The importance of the inflammatory process for the pathogenesis of human disease has long been recognized, if only because of its readily observable four cardinal signs of pain, swelling, redness, and heat in superficial lesions. The pioneering studies of Virchow, Metchnikoff, and others, more than a century ago, began to focus on the cellular basis of inflammation, and it was Virchow's genius to include cancer as an inflammatory disease based on his microscopic observations of large numbers of macrophages in malignant tumors (Virchow, 1867). By the early 20th century, Virchow's perspective was largely ignored because of an increasing new emphasis on the cytogenetic aspects of cancer cells (which still dominates contemporary thinking), but approaches other than genetics have had a profound comeback, and it is now realized that the tumor microenvironment plays a critical role during the process of carcinogenesis (Kessenbrock et al., 2010; Dvorak et al., 2011). Inflammatory cells, such as macrophages, neutrophils, and lymphocytes, are not only important for tumor promotion but also have potent ability to generate molecules that directly damage DNA, such as highly reactive species of oxygen, nitrogen, and chlorine, which are clearly mutagenic (Marnett et al., 2003).
Thus, the old sequential paradigm of “tumor initiation,” “tumor promotion,” and “tumor progression” no longer seems to be adequate for a modern framework; all three processes can occur simultaneously in a person. Likewise, inflammatory cells contribute to the process of angiogenesis, which is another critical component of carcinogenesis. Although we have just described the above phenomena in the context of carcinogenesis, they can be equally relevant to many other chronic diseases, whether they be classic “inflammatory” diseases, such as rheumatoid arthritis or multiple sclerosis, or diseases for which a key role of inflammation has only been more recently recognized, such as atherosclerosis, diabetic nephropathy, or various forms of neurodegeneration in which microglia play an important role. In all of these situations, the opportunity to control the inflammatory process offers an opportunity to control pathogenesis.
It also needs to be realized that the hitherto separate processes of inflammation and oxidative stress are really two sides of the same coin, at least within the context of cells and tissues in vivo. Thus, inflammatory cells make reactive species of oxygen (ROS) and nitrogen, which in turn cause oxidative stress in nearby parenchymal cells, which in turn recruit more inflammatory cells to their microenvironment (Shiao et al., 2011; Balkwill and Mantovani, 2012). Furthermore, the production of mitochondrial reactive oxygen species (Rigoulet et al., 2011) in parenchymal cells in turn drives the production and activation of proinflammatory cytokines, further enhancing this vicious circle. There are many different cytokines and peptide growth factors involved in this scenario, whether they be classic cytokines such as the interleukins (there are now more than 30 molecules so named), the interferons, tumor necrosis factor, or a “growth factor” such as TGF-β, which has profound paracrine effects on almost every type of inflammatory cell as well as autocrine effects on the parenchymal cells that may synthesize it. Thus, there is by now an immense literature on the entire tissue network of inflammation and oxidative stress, further enhanced by the more recent concept of the “inflammasome” (Schroder and Tschopp, 2010; Schroder et al., 2010).
All of this new information is relevant to the potential use of SOs to prevent chronic human diseases. It is intuitively obvious for conditions such as rheumatoid arthritis or multiple sclerosis, in which there is no question of the importance of inflammatory cells, not only in the original genesis of disease but also in the maintenance of the diseased state. Consequently, there are opportunities in such instances for treatment of existing disease as well as for its prevention. More problematic are those inflammatory diseases in which it may be much more difficult to reverse severe pathologic tissue conditions, especially if irreversible cell death has occurred. The brilliant studies of Russell Ross on the inflammatory pathogenesis of atherosclerosis (Ross, 1999) opened up an entire field of cardiovascular investigation. Research on the mechanisms of atherogenesis then led to the clinical development of statins and antiplatelet agents, with important clinical results. These studies and their ensuing results serve as a model for emphasizing prevention at the earliest possible time during pathogenesis. This approach now needs to be extended to the prevention of the neurodegenerative diseases, in which inflammatory and oxidative stress seem to be so critical.
Central to these considerations is the role of the transcription factor Nrf2 and its inhibitor Keap1 in regulating the homeostatic response of the organism to many types of stresses, whether they are inflammatory, oxidative, electrophilic, or metabolic. Nrf2 controls the expression of a large number of genes that enable a coordinated protective response to stress. The Keap1/Nrf2 module is a prime, albeit not exclusive, target of the SOs, and the nature of the chemical and physical interactions between SOs and Keap1/Nrf2, and the downstream results of this process, are discussed in detail in this review. The general topic of Keap1/Nrf2 is of major current interest (more than 2000 citations in PubMed from 2006 to 2011) and has been the subject of several excellent reviews (Hayes et al., 2010; Taguchi et al., 2011).
It should also be emphasized that not all beneficial effects of the SOs are necessarily mediated by their anti-inflammatory or antioxidative actions. Thus, in an important review (Sheng and Sun, 2011), the ability of multitargeted triterpenoids to regulate glucose and lipid metabolism have been described. This then results in useful hypoglycemic and hypolipidemic effects that are of potential preventive and therapeutic benefit for diabetes and cardiovascular disease. Nrf2 is known to regulate transcription of many genes involved in lipid metabolism (Shin et al., 2007), but other proteins regulated by the SOs may also play a role in this regard. The importance of the multifunctional aspects of pentacyclic triterpenoids, and the molecular basis of their actions, are emphasized in our discussion in section VI. The SOs are most definitely not “magic bullets,” and they do not fit the single-target paradigm that dominates so much of present-day pharmaceutical research. The single-target paradigm has been unusually unsuccessful in developing new drugs for prevention and treatment of chronic disease, and it is now essential that we move beyond this to a more realistic approach as to how cells, tissues, and organs actually function. We have allowed ourselves to be mesmerized by the “magic” of imatinib (Gleevec), which turns out to be not a single-target drug but a multikinase inhibitor (Hasinoff and Patel, 2010). The media and publicity blitz that attended the great success of the introduction of this important drug in the treatment of chronic myelogenous leukemia has not been particularly helpful in furthering support for research on multifunctional drugs such as triterpenoids, and it is time to realize how critical multifunctionality may be, in terms of both direct drug action and lessening the development of drug resistance. To quote Richard Feynman, “Reality must take precedence over public relations, for nature cannot be fooled.” We turn our attention to the use of new SOs for prevention of disease, after providing a brief introduction to the topic of natural triterpenoids, how they exist in nature, and how they have been used, especially in Asian cultures, for medicinal purposes.
III. Naturally Occurring Pentacyclic Triterpenoids
Pentacyclic triterpenoids are ancient molecules. Triterpenoid hopanoid molecules have been identified in Archaea from prehistoric geological sediments. The fundamental isopentenyl pyrophosphate pathway plays a central role in the biological generation of all triterpenoids, as well as for monoterpenes, sesquiterpenes, diterpenes, and carotenoids, including the retinoids, which are critical metabolites of carotenoids. Although the carotenoids are biosynthesized from isoprenoid geranyl pyrophosphate, farnesyl pyrophosphate, and geranylgeranyl pyrophosphate precursors, the cyclic triterpenoids are biosynthesized from other linear isoprenoid 30-carbon precursors: either squalene (in the case of hopanoids) or oxidosqualene (in the case of most other cyclic triterpenoids) (Phillips et al., 2006). The basic reaction patterns for the biosynthesis of triterpenoids were elucidated more than 50 years ago by Ruzicka and colleagues, and a biogenetic “isoprene rule” was proposed. This then led to classic synthetic work by the groups of Corey, Ireland, Johnson, Stork, Van Tamelen, and others (for review and specific references, see Sheng and Sun, 2011), which further confirmed the structures of many naturally occurring pentacyclic triterpenoids. It is now possible to achieve total chemical synthesis of many naturally occurring triterpenoids, but plants are usually much more practical sources because very high amounts of triterpenoids are found in many plants and are easily extracted. Thus, crystalline ursolic acid (UA) of high purity is easily obtained in 20% yield by methanol extraction of rosemary leaf; the lupane alcohol betulin accounts for up to 20% of the dry weight of the bark from many species of common birch trees; and oleanolic acid (OA) can be easily obtained in high yield from olive pulp remaining after the oil is pressed from the olive fruit, as well as from olive leaves that are usually discarded after the trees are pruned (Jäger et al., 2009). These natural products, cheaply obtainable, can then serve as scaffolds for further modification by the organic chemist to achieve marked enhancement of the biological activity originally present.
There are almost 100 different patterns into which either squalene or oxidosqualene can be folded in nature to generate cyclic triterpenoids. Unique cyclase enzymes catalyze the different folding patterns (Phillips et al., 2006; Siedenburg and Jendrossek, 2011). From the further metabolism of these various skeletons, plants have generated some 20,000 different molecules. Many of them have been used medicinally in Asia for centuries. The classic monograph of Tang and Eisenbrand (1992) on “Chinese Drugs of Plant Origin” has 124 chapters, ranging from Acanthopanax senticosus to Ziziphus jujube. Triterpenoid structures are shown in 40 of those chapters, and anti-inflammatory actions of the constituent triterpenoids are described in 31 of the chapters.
The biological significance and meaning of this great diversity of structures in nature is still not well understood. Many of the triterpenoids, such as oleananes, ursanes, and friedelanes (found in the bark of the cork tree) have antibacterial or antifungal actions that are clearly of direct survival benefit to the plant itself or to its fruit (Tang and Eisenbrand, 1992). Benefits to animals that feed on plants containing triterpenoids are less clear. Many of the plants that biosynthesize triterpenoids are readily edible by both wild animals and humans, indicating that the natural molecules are relatively nontoxic and can be ingested safely for long periods of time. Thus, the synthetic organic chemist has relatively safe triterpenoid scaffolds, proven to be without harm in both animals and humans for centuries, on which to conduct further semisynthesis. Furthermore, the known anti-inflammatory activity of some of the natural platforms, such as OA or UA, already serves to target further new drug synthesis toward a desired pharmacological goal.
Moreover, the biological diversity of natural structures indicates that there are fundamental differences between different natural triterpenoids with respect to their interactions with protein targets in an animal cell. Thus, OA and UA differ from each other solely by the location of two methyl groups on their E-ring (both at C-20 on oleanolic acid, one at C-19 and one at C-20 on ursolic acid), but they do not have identical activities; such a small difference conveys unique stereospecificity. Likewise, the stereochemistry of the –OH groups on either OA or UA has important physiological implications; the less common 3-α-OH isomers of both OA and UC have distinct biological activities not shared by the more common 3-β-OH isomers. These steric properties of the exocyclic methyl and other R groups on natural triterpenoids are important determinants of both their activity and their safety, particularly the safety of their derivatives, in a pharmacological setting. This is especially true when the organic chemist adds functional groups that convey the property of Michael addition to the basic scaffold, as by inserting ene-one functions, and electron-withdrawing groups such as –CN. Ultimately, the steric hindrance conveyed on the new molecule by the exocyclic methyl groups can prevent a new derivative from being a random alkylating agent and allows the new drug to act more selectively at desired molecular targets (Sporn et al., 2007).
It is important to emphasize that the practical development of pentacyclic triterpenoids as useful pharmacological agents, particularly in contemporary American medical practice, will undoubtedly involve further semisynthetic modification of natural agents. Most of the pentacyclic triterpenoids, as they occur in nature, do not have enough potency to allow them to be used as successful drugs. To demonstrate activity of natural triterpenoid molecules in many cell culture experiments, one usually needs to employ concentrations as high as 20 μM or above, whereas many of the SOs are highly active at nanomolar levels and, in a few assays, even picomolar levels. The practicalities of dosing humans at milligram rather than gram amounts of drug are obvious.
IV. Biological Activities In Vitro
A. Anti-Inflammatory and Cytoprotective Properties
As described above, the importance of inflammation and oxidative stress in the pathogenesis of so many chronic diseases, including cancer, was the original rationale for making a series of SOs. More than 300 derivatives of OA have been made and screened for their ability to inhibit the de novo synthesis of the inflammatory enzyme inducible nitric-oxide synthase (iNOS). The rationale for choosing OA, a summary of the most important chemical modifications, and biochemical insights into the biological activities of the SOs are summarized by Sporn et al. (2011). The most active and useful SO derivatives (shown in Fig. 1) include several modifications at the C17 position of CDDO (2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid): methyl ester (CDDO-Me; bardoxolone methyl), imidazolide (CDDO-Im), di-CDDO (nitrile at C17 position of CDDO; TP-225) and various amides (methyl amide, CDDO-MA; ethyl amide, CDDO-EA; or trifluoroethyl amide, CDDO-TFEA). All of these compounds are active at low nanomolar concentrations for inhibiting the induction of iNOS in primary macrophages or in RAW264.7 macrophage-like cells stimulated with inflammatory cytokines. The SOs are equally effective at suppressing NO (nitric oxide) secretion if the cells are stimulated with interferon γ, alone or in combination with both TNFα and IL1β, or with lipopolysaccharide (LPS) (Honda et al., 1998; Suh et al., 1999; Place et al., 2003; Liby et al., 2007a,b). The SOs also block production of a variety of inflammatory cytokines and chemokines from immune or tumor cells (Chauhan et al., 2004; Thimmulappa et al., 2006b; Liby et al., 2009; Nichols et al., 2009; Auletta et al., 2010; Liby et al., 2010; Saha et al., 2010; Segal et al., 2010; Hogan et al., 2011), as summarized in Table 1.
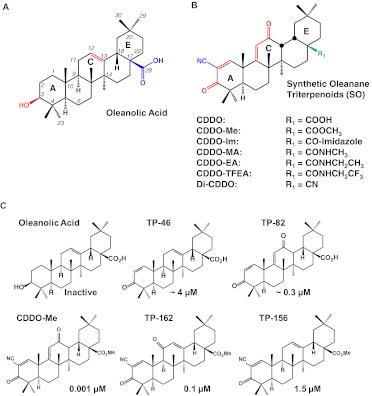
Fig. 1.
Triterpenoid structures. A, structure of oleanolic acid, the starting material for the synthetic oleanane triterpenoids (SOs), showing important positions and functionalities that have been modified to enhance the potency of the SOs: the C-28 carboxyl group (blue), the double bond at C-12/C-13 (red), and the hydroxyl group at C-3 (red). B, structures of the most useful SOs, the biological activities of which are summarized in the text. The numbering system and ring structure for all of the SOs are the same as for oleanolic acid. In CDDO, the A ring has been activated by formation of an enone function at C-1, C-2, and C-3 (red) and the insertion of a strong electron-withdrawing –CN group at C-2 (blue), which facilitates Michael addition at C-1; the C ring has been activated by the inclusion of another enone function at C-9, C-11, and C-12 (red). The rest of the SOs shown can be considered analogs of CDDO. Additional modification at C-28 (green) has been useful for generating biologically useful molecules with different pharmacokinetic properties. [A and B adapted from Liby KT, Yore MM, and Sporn MB (2007) Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7:357–369. Copyright © 2007 Nature Publishing Group. Used with permission.] C, the importance of the presence and location of the enone function in both the A and C rings for enhancing potency is shown by the CD (concentration required to double the specific activity) values for the induction of NQO1 enzyme activity; NQO1 is a classic Nrf2 target gene. [Adapted from Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, and Talalay P (2005) Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA 102:4584–4589. Copyright © National Academies of Science, USA. Used with permission.]
Table 1.
SOs inhibit the production of inflammatory mediators in various cells
| Triterpenoid | Reduced Inflammatory Mediators | Cell Type | Reference |
|---|---|---|---|
| CDDO | NO, iNOS, COX-2 | Peritoneal macrophages, human colon myofibroblasts | Honda et al., 1998; Suh et al., 1999 |
| CDDO | MMP-1, MMP-13 | Chondrosarcoma cells, human chondrocytes | Elliott et al., 2003; Mix et al., 2001 |
| CDDO-Im | IL-6 | Multiple myeloma cells | Chauhan et al., 2004 |
| CDDO-Me | VEGF, COX-2, MMP-9 | Human leukemia cells | Shishodia et al., 2006 |
| CDDO-Im | TNFα, IL-6, Mcp1, Mip2 | Neutrophils | Thimmulappa et al., 2006b |
| CDDO CDDO-Me |
IL-6, TNFα | Human PBMCs | Thimmulappa et al., 2007 |
| CDDO-Im | Serum IFNγ, TNFα | N.A. | Osburn et al., 2008 |
| CDDO-Me | TNFα, IL-1β, Mip1α, IL-6, IL-12, IL-10 | Microglia and peritoneal macrophages | Tran et al., 2008 |
| CDDO | IL-8, IL-1β, IL-6, TNFα, MIP-2, KC | Primary airway epithelia and bronchoalveolar lavage | Nichols et al., 2009 |
| CDDO-Im | TNFα, IL-17, GCSF, IL-23, LIX | Bronchoalveolar lavage fluid | Segal et al., 2010 |
| CDDO | TNFα | Donor T cells | Li et al., 2010 |
| CDDO-Me | IL-12p70, IFN-γ, IL-6, IL-17, IL-23 | Splenocytes | Auletta et al., 2010 |
| CDDO-Me | IL-6 | Pancreatic cancer cells | Liby et al., 2010 |
| CDDO | IL-6, MCP-1, COX-2, PGE2 | Human lung fibroblasts | Hogan et al., 2011 |
| CDDO-Im | IL-6, IL-10, TGF-β | Primary orthotopic breast tumors | Liao et al., 2011 |
| CDDO-TFEA | IL-6, IL-17, IFNγ, TNFα, GM-CSF | Blood lymphocytes from mice with EAE | Pareek et al., 2011 |
| CDDO-Me | CXCL12, CCL2, MMP-9 | Primary tumor cells from PyMT mice | Tran et al., 2012 |
COX, cyclooxygenase; KC, keratinocyte-derived cytokine; GCSF, granulocyte–colony-stimulating factor; LIX, lipopolysaccharide-induced CXC chemokine; GM-CSF, granulocyte macrophage–colony-stimulating factor; EAE, experimental autoimmune encephalomyelitis; PyMT, polyoma middle T.
A series of microarray studies revealed that the same concentrations of SOs that inhibit iNOS and other inflammatory cytokines also up-regulate a family of cytoprotective genes regulated by the transcription factor Nrf2 (Liby et al., 2005). In its inactive state, Nrf2 is targeted for ubiquitination and proteasomal degradation through its interaction with the Keap1 protein. In response to oxidative or electrophilic stress or after direct interaction of a compound such as a SO with Keap1, Nrf2 dissociates from Keap1. Nrf2 then forms dimers with maf proteins or other members of the Cap'n'Collar/basic leucine zipper family of transcription factors and binds to the antioxidant response element (ARE) on a number of target genes (Giudice et al., 2010; Taguchi et al., 2011). These Nrf2-responsive genes include Quinone reductase 1, γ-Glutamylcysteine synthetase, Thioredoxin, Thioredoxin and glutathione reductase, Glutathione and UDP-glucuronyl transferases, Epoxide hydrolase, Superoxide dismutase, Catalase, and Heme oxygenase 1. The proteins encoded by these genes function as direct antioxidants, metabolize free radicals, conjugate and detoxify natural and xenobiotic electrophiles, promote glutathione homeostasis, regulate the proteasome and molecular chaperones, recognize DNA damage, and inhibit inflammation (Itoh et al., 2010; Slocum and Kensler, 2011). The coordinated up-regulation of this network of phase 2 responsive genes is able to inactivate the original insult. The SOs are some of the most potent activators of the Nrf2 pathway in vitro (Lapillonne et al., 2003; Dinkova-Kostova et al., 2005; Liby et al., 2005) and in vivo (Liby et al., 2005; Yates et al., 2006, 2007, 2009). As would be predicted by their activation of the Nrf2 pathway, pretreating cells with SOs blocks the production of ROS when challenged with oxidants such as tert-butyl hydroperoxide, but this reduction in ROS is absent in Nrf2(−/−) cells (Liby et al., 2005). The SOs also protect against cell death from photooxidation in retinal pigment epithelial cells treated with UVA radiation (Dinkova-Kostova et al., 2005). It is noteworthy that the ability of triterpenoids to suppress NO production is lost in Nrf2-knockout fibroblasts stimulated with inflammatory cytokines.
Although protection against oxidative stress is a well known function of Nrf2 activation, the anti-inflammatory effects of the Nrf2 pathway were not widely understood, and the SOs were the original compounds used to show that these two pathways are mechanistically linked. The potencies for the induction of NQO1 enzyme activity and the suppression of iNOS are highly correlated over 6 orders of magnitude for 18 different triterpenoids (Dinkova-Kostova et al., 2005). The decreased mRNA expression of the cytokines TNFα and IL-6 and the chemokines Mcp1 and Mip2 in neutrophils after treatment with CDDO-Im also requires expression of Nrf2 (Thimmulappa et al., 2006b). Numerous studies have revealed that activation of the Nrf2/ARE pathway protects cells from a variety of chemical or physical insults, including carcinogens and ROS. The added benefit of simultaneously inhibiting inflammation through the same pathway suggests that potent activators of this pathway could serve as “multiorgan protectors” (Lee et al., 2005) against a number of diseases in which inflammatory and/or oxidative stress contribute to disease pathogenesis.
B. Differentiation
At slightly higher concentrations than those required to inhibit inflammation and induce the Nrf2 pathway (< 0.01 μM), the SOs also induce differentiation of a variety of primary leukemic blasts or human leukemia cell lines, in addition to neuronal differentiation of PC12 cells, adipocytic differentiation of 3T3L1 fibroblasts, osteoblastic differentiation of Saos-2 osteosarcoma cells, and megakaryocytic differentiation of normal hemopoietic progenitor cells. Additive or synergistic effects are obtained when SOs are combined with the retinoid ATRA, the rexinoid 6-(1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopropyl)nicotinic acid (LG100268), and 1α,25(OH2) vitamin D3 or related deltanoids. These studies are summarized in Table 2.
Table 2.
SOs induce cell differentiation in vitro
| Cells Undergoing Differentiation | Triterpenoid | Combination(s) | References |
|---|---|---|---|
| U937, THP-1 l monocytic leukemia cells; LCDB, NB4 myelocytic leukemia cells | CDDO CDDO-Im |
Deltanoid ILX23-7553 Rexinoid LG100268 TGF-β |
Suh et al., 1999; Place et al., 2003 |
| HL-60 granulomonocytic leukemia cells and/or AML primary blasts | CDDO-Me CDDO-Im CDDO |
ATRA 1α,25(OH2)vitamin D3 TGF-β superfamily |
Konopleva et al., 2002; Ji et al., 2006; Koschmieder et al., 2007; Tsao et al., 2010 |
| NB4 and MR2 acute promyelocytic leukemia cells and primary APL cells | CDDO CDDO-Im |
ATRA | Ikeda et al., 2005; Tabe et al., 2007 |
| Neuronal differentiation of PC12 pheochromocytoma cells | CDDO | NGF | Suh et al., 1999 |
| Adipogenic differentiation of 3T3L1 fibroblasts | CDDO | LG100268 | Suh et al., 1999; Wang et al., 2000 |
| Saos-2 osteosarcoma cells | CDDO | None | Ito et al., 2001 |
| Megakaryocytic differentiation of normal hemopoietic progenitor cells and HEL and TF1 human erythroleukemic cell lines | CDDO-Im | None | Petronelli et al., 2011 |
| Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells | CDDO-Im CDDO-EA |
None | Suh et al., 2012 |
ILX23-7553, 1,25-dihydroxy-16-ene-23-yne vitamin D3.
Although the mechanisms responsible for induction of cell differentiation by the SOs are not completely understood, several clues have emerged. It is noteworthy that there are differences between SOs, especially during adipogenesis. CDDO releases nuclear receptor corepressor 1 and recruits the coactivator CBP, a cAMP response element-binding binding protein, from the PPARγ receptor to induce differentiation of 3T3L1 cells to adipocytes, but CDDO-Me does not regulate these transcriptional regulators or induce adipogenic differentiation. It is noteworthy that CDDO induces adipocyte differentiation when used at doses between 10 and 100 nM but inhibits differentiation induced by the PPARγ agonist rosiglitazone when used at 1 μM (Wang et al., 2000). Treatment with 25 nM CDDO-Im inhibits the differentiation of MEFs into adipocytes stimulated with rosiglitazone, but this inhibition is absent in MEFs from Nrf2(−/−) mice (Shin et al., 2007). As previously suggested, CDDO, but not CDDO-Me, binds to PPARγ (Wang et al., 2000), and the enhanced differentiation of APL cells with the combination of CDDO and ATRA follows enhanced transcription of the PPARγ and RARβ2 genes and acetylation of histone H3 in RARβ2. Both the N-(4-aminopyridyl-2-chloro-5-nitrobenzamide (T007) PPARγ antagonist and PPARγ small interfering RNA partially reduce APL differentiation (Tabe et al., 2007). CDDO also activates the PPARγ receptor in leukemia cells by recruiting the vitamin D-interacting protein 205 (DRIP205) coactivator. The induction of differentiation by CDDO is enhanced in HL-60 cells overexpressing DRIP205, but the PPARγ antagonist T007 blocks differentiation when these cells are treated with CDDO (Tsao et al., 2010).
In osteosarcoma cells overexpressing the extrinsic caspase-8 inhibitor cytokine response modifier A (CrmA), but not in cells overexpressing Bcl-XL, CDDO fails to induce alkaline phosphatase activity, suggesting that induction of differentiation in osteoblasts by CDDO requires caspase-8 (Ito et al., 2001). CDDO-Im enhances ATRA-induced differentiation of APL cells and down-regulates expression of the PML/RARα fusion protein, most likely through activation of caspases, because the decreased PML/RARα expression is blocked when the pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone is included (Ikeda et al., 2005). In HL-60 cells, CDDO-Im rapidly phosphorylates Erk1/2, enhances Smad phosphorylation, and up-regulates expression of CCAAT/enhancer-binding protein β (CEBPB). The Erk1 inhibitor 2′-amino-3′-methoxyflavone (PD98059), a neutralizing TGF-β antibody, and the BMP antagonist noggin partially reduce the HL-60 differentiation of these cells by CDDO-Im (Ji et al., 2006). Differentiation of both granulocytes and monocytes requires the CCAAT/enhancer-binding protein α (CEBPA) transcription factor, and inappropriate expression or function of this transcription factor occurs in many cases of AML. CDDO initiates transcription of the p42 isoform of CCAAT/enhancer-binding protein α and thus improves the ratio of the active p42 isoform to the inactive p30 isoform in HL-60 cells and in AML blasts (Koschmieder et al., 2007). Low doses (10–50 nM) of CDDO-Im also induce differentiation of normal erythroid cells and megakaryocytes by altering expression of key transcription factors. CDDO-Im enhances phosphorylation of Erk1/2, decreases GATA-1 expression, and enhances GATA-2 and Friend of GATA-1 (FOG-1) expression in these cells. Although the differentiation of megakaryocytes by CDDO-Im could be a beneficial therapy for improving platelet counts, even low doses of CDDO-Im may inhibit the proliferation and survival of erythroid cells (Petronelli et al., 2011). These results suggest that the effects of this drug on normal hematopoiesis should be monitored if used for cancer treatment (Sardina et al., 2011), but these adverse effects have not been observed in clinical trials with the SOs.
C. Antiproliferative and Proapoptotic Properties
As summarized in Table 3, the SOs inhibit proliferation and induce apoptosis in vitro in a wide variety of human and rodent cancer cells, including all of the common forms of epithelial cancers as well as myelomas, leukemias, and sarcomas. CDDO-Me and CDDO-Im are equipotent and approximately 1 log more active than CDDO itself. Effective concentrations for suppressing cell growth in vitro are approximately 0.1 to 1 μM for the most active analogs, with slightly higher concentrations (0.5–5 μM) required to induce apoptosis in vitro. Although even higher concentrations of SOs have been reported to induce apoptosis, these concentrations are not physiologically relevant, even for cell culture studies, because there is no evidence that these very high concentrations can be achieved in tissue in vivo. The ability of the SOs to inhibit proliferation and induce apoptosis is usually independent of the p53, PPARγ, or multidrug resistance status of the cell (Kim et al., 2002; Konopleva et al., 2002; Lapillonne et al., 2003; Place et al., 2003; Melichar et al., 2004; Chintharlapalli et al., 2005; Ray et al., 2006). It is noteworthy that several studies have reported that concentrations of SOs that kill malignant cells have no effect on normal lymphocytes harvested from the same cancer patient or from healthy volunteers (Suh et al., 2003b; Chauhan et al., 2004; Ikeda et al., 2004; Kress et al., 2007).
Table 3.
SOs inhibit proliferation and/or induce apoptosis of cancer cells in vitro
CML, chronic myelogenous leukemia; NSCLC, non–small-cell lung carcinoma.
Combining SOs with a variety of other drugs enhances the selective apoptosis of cancer cells compared with either agent alone: CDDO-Me and either ATRA or LG100268 in AML (Konopleva et al., 2002); CDDO + ATRA in APL (Tabe et al., 2007); CDDO-Im + arsenic trioxide in APL (Ikeda et al., 2005); CDDO + bexarotene in mycosis fungoides and Sézary syndrome cells (Zhang et al., 2004); CDDO-Im and bortezomib in multiple myeloma cells (Chauhan et al., 2004); CDDO and inhibitors of the NF-κB pathway [SN50, 3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile (BAY11-7082), and helenalin] in B-cell lymphoma (Ray et al., 2006); CDDO-Im and the fatty acid synthase inhibitor cerulenin in liposarcoma cells (Hughes et al., 2008); and various SOs + TNF-related apoptosis-inducing ligand (TRAIL) or TRAIL receptor antibodies in AML (Suh et al., 2003b; Riccioni et al., 2008), lung cancer cells (Zou et al., 2004; Zou et al., 2007), ovarian cancer cells (Petronelli et al., 2009b), and estrogen receptor-negative breast cancer cells (Hyer et al., 2005). CDDO-Me also increases the effectiveness of chemotherapeutic agents for killing cancer cells. The combination of SOs and 5-fluorouracil, paclitaxel (Taxol), and doxorubicin are effective in leukemias (Shishodia et al., 2006), SOs and midostaurin (PKC412, an Fms-like tyrosine kinase-3 inhibitor in clinical trials) in AML blasts (Ahmad et al., 2010), SOs and cisplatin or doxorubicin in chordomas (Yang et al., 2010), and SOs and doxorubicin in osteosarcoma cells (Ryu et al., 2010b).
D. Mechanisms of Growth Arrest and Apoptosis
The SOs affect multiple cell cycle proteins to inhibit cell proliferation. Although the SOs can bind to and transactivate the PPARγ receptor (Wang et al., 2000), thus decreasing levels of cyclin D1 (Lapillonne et al., 2003; Konopleva et al., 2006) or inducing caveolin-1 (Chintharlapalli et al., 2005; Konopleva et al., 2006), the SOs can also block cell proliferation by PPARγ-independent mechanisms (Place et al., 2003; Melichar et al., 2004; Zhang et al., 2004; Chintharlapalli et al., 2005; Ray et al., 2006). Other important growth regulatory proteins that are altered by the SOs include p21, p27, proliferating cell nuclear antigen, and myc (Lapillonne et al., 2003; Han et al., 2006; Shishodia et al., 2006; Liby et al., 2009).
There are some apparent discrepancies in the literature regarding how the SOs induce apoptosis, but these differences are understandable when the specific triterpenoid used and cell type studied are considered. In contrast to many of the other known biological properties of the triterpenoids, apoptosis seems to be induced through different pathways by different SOs (Liby et al., 2007b; Petronelli et al., 2009a). As reported by several different investigators in some of the earliest studies on SOs, CDDO and CDDO-Im activate extrinsic, death receptor-mediated apoptosis (Ito et al., 2000; Ito et al., 2001; Stadheim et al., 2002; Ikeda et al., 2004), whereas CDDO-Me induces apoptosis through the intrinsic, mitochondrial-dependent pathway (Kim et al., 2002; Konopleva et al., 2002; Samudio et al., 2006). The extrinsic apoptotic pathway is activated by members of the TNF family by recruitment of caspase-8 into the death receptor complex. Bcl-2 family members regulate the intrinsic mitochondrial pathway; overexpression of Bcl-2 or Bcl-XL blocks the release of cytochrome c from the mitochondria and prohibits apoptosis, whereas increasing the concentration of Bax promotes apoptosis. Defects in the intrinsic pathway are frequently found in chemoresistant leukemias.
In U937 cells overexpressing Bcl-XL, an inhibitor of the intrinsic apoptotic pathway, no loss of transmembrane potential is observed in cells treated with CDDO-Me, but the transmembrane potential is reduced in cells treated with CDDO-Im. Conversely, overexpression of CrmA, an inhibitor of the extrinsic apoptotic pathway, blocks the loss of transmembrane potential induced by CDDO-Im but not by CDDO-Me. The ability of CDDO-Im to induce apoptosis is eliminated by overexpression of CrmA, whereas overexpression of Bcl-XL prevents apoptosis in cells treated with CDDO-Me (Ikeda et al., 2003). CDDO also activates the extrinsic apoptotic pathway in myeloid leukemia and osteosarcoma cells (Ito et al., 2000; Ito et al., 2001; Stadheim et al., 2002). Although CDDO does induce the release of cytochrome c from the mitochondria, its release follows activation of caspase-8, the subsequent activation of caspase-3, and the capase-8-dependent cleavage of Bid. Overexpression of CrmA blocks the induction of apoptosis by CDDO, whereas Bcl-XL only partially reduces caspase activation and apoptosis (Ito et al., 2000; Ito et al., 2001). As further evidence supporting the importance of the extrinsic pathway, both CDDO and CDDO-Im increase the expression of death receptors (DR) 4 and 5, cell surface receptors for TRAIL, and decrease expression of the antiapoptotic protein cellular FLICE-like inhibitory protein (c-FLIP) (Hyer et al., 2005). CDDO-Me does not affect expression of DR 4 and 5, and knockdown of these DR receptors with small interfering RNA abrogates apoptosis induced by CDDO and CDDO-Im, but not by CDDO-Me, in prostate cancer cells (Hyer et al., 2008). AML blasts expressing low levels of caspase-8 and FADD and high levels of Bcl-XL are also resistant to apoptosis when treated with CDDO-Im (Riccioni et al., 2008).
In contrast, CDDO-Me induces apoptosis in AML cells by increasing levels of the proapoptotic Bax protein, a member of the Bcl-2 family, and inducing caspase-3 cleavage (Konopleva et al., 2002). In lung cancer cells, CDDO-Me causes cytochrome c release from the mitochondria and activation of procaspase-3; the caspase-3 inhibitor N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethyl ketone blocks the induction of apoptosis by CDDO-Me (Kim et al., 2002). CDDO-Me also may directly permeabilize the inner mitochondrial membrane, which would deplete mitochondrial glutathione and inhibit electron transport required for respiration (Samudio et al., 2006). Despite the abundance of data suggesting that different SOs signal through separate apoptotic pathways, some exceptions have been reported. In some cells, CDDO-Me can induce apoptosis by the extrinsic pathway by rapidly down-regulating expression of FLICE-like inhibitory protein (FLIP), an endogenous antagonist of caspase-8 (Suh et al., 2003b; Zou et al., 2007), or by activating jnk and C/EBP homologous protein transcription factor (CHOP), thus inducing expression of DR 5 and activation of caspase-8 (Zou et al., 2004; Zou et al., 2008). CDDO has also been reported to induce apoptosis by the intrinsic pathway (Inoue et al., 2004; Konopleva et al., 2004).
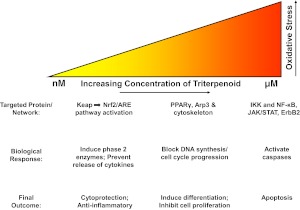
The same report describing the induction of different apoptotic pathways by CDDO-Im versus CDDO-Me suggests that CDDO, CDDO-Im, and CDDO-Me all activate the jnk and p38 stress pathways by increasing production of ROS and decreasing glutathione concentrations within the cancer cell (Ikeda et al., 2003). However, the induction of ROS by CDDO-Me in these studies is not as convincing as for CDDO and CDDO-Im. Other studies support the induction of ROS by CDDO and CDDO-Im (Ikeda et al., 2004; Liby et al., 2005; Brookes et al., 2007; Kim et al., 2011), but not by CDDO-Me (Samudio et al., 2006; Yue et al., 2006; Kim et al., 2011). It is noteworthy that the induction of ROS may be selective, because CDDO-Im induces ROS and activates DNA damage signaling pathways in breast cancer cells containing a defective BRCA1 gene but does not induce ROS in nonmalignant breast epithelial cells (Kim et al., 2011). A few exceptions regarding the inability of CDDO-Me to generate ROS have been reported, because high concentrations of this SO may induce apoptosis or autophagy in imatinib-resistant chronic myelogenous leukemia cells by increasing ROS, depleting glutathione and thus disrupting mitochondrial function (Samudio et al., 2008). CDDO-Me also stimulates the production of ROS in pancreatic cancer cells, thereby increasing levels of the ZBTB10 specificity protein repressor and decreasing expression of pro-growth, -survival, and -angiogenesis genes (Jutooru et al., 2010). It is also important to note that the effects of SOs on oxidative stress are bifunctional (Liby et al., 2007b). Higher concentrations (low micromolar) of SOs are required to induce ROS and apoptosis than the low nanomolar concentrations needed to activate the Nrf2/ARE pathway (Liby et al., 2005).
The event that initiates the apoptotic cascade after treatment with an SO has not been identified. Although disruption of the redox balance is one possible mechanism (Ikeda et al., 2003, 2004), ROS induction may be downstream of mitochondrial perturbation instead of an initiating event (Brookes et al., 2007). Other possible signals that may initiate apoptosis by the triterpenoids include altering calcium homeostasis (Hail et al., 2004), activating the jnk or p38 pathways (Ikeda et al., 2003; Zou et al., 2004; Konopleva et al., 2005), depleting mitochondrial glutathione (Samudio et al., 2005), inducing endoplasmic reticulum stress (Zou et al., 2008), inhibiting the NF-κB (Shishodia et al., 2006) and signal transducer and activator of transcription (STAT) pathways (Liby et al., 2006; Petronelli et al., 2009b), or targeting the Lon mitochondrial protease (Bernstein et al., 2012). In liposarcoma cells, CDDO-Im may induce apoptosis by targeting fatty acid synthase and thus decreasing the de novo fatty acid synthesis that is required for proliferation and survival of these cells (Hughes et al., 2008). One of the most intriguing hypotheses for the initial step in the apoptotic cascade is that the SOs directly interact with mitochondrial proteins and oxidize thiols, causing high-molecular-weight protein aggregates to form an unregulated permeability transition pore, resulting in the generation of ROS, the release of cytochrome c and apoptosis (Brookes et al., 2007).
V. Preclinical Biological Activities In Vivo
Activation of the Nrf2/ARE pathway by SOs should protect against a number of diseases driven by inflammatory and oxidative stress. Indeed, as summarized below and in Fig. 2, the triterpenoids are effective in a wide variety of preclinical disease models in all of the major organs that have been tested, including the brain, eye, lung, heart, liver, and kidney. The SOs also provide protection against radiation and chemical insults and help regulate the immune system and metabolism. If known, the contribution of Nrf2 activation by the SOs in the various disease models is discussed.
Fig. 2.
Activation of the Keap1/Nrf2/ARE/pathway by the SOs is cytoprotective. Nrf2 has been described previously (Lee et al., 2005) as a “multiorgan protector,” because this system can protect against diseases in a number of organs, including the brain, lungs, kidney, heart, liver, and eye. The SOs are among the most potent known inducers of the Nrf2 pathway, and these drugs not only activate Nrf2 in all of these organs but also protect the organs against a variety of diseases driven by inflammatory or oxidative stress. SOs that cross the blood-brain barrier are beneficial in experimental models of neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and ALS. These drugs also reduce damage to the eye from light, uveitis, or ischemia reperfusion and reduce cardiomyopathy induced by smoking and reduce the disease process in the lung in animal models of COPD, emphysema, asthma, and ALI/ARDS. In the liver and kidney, the SOs protect against toxicity from insults such as aflatoxin, ConA, acetaminophen, or cisplatin and against injury from ischemia reperfusion.
A. Neurodegenerative Diseases
The original biological characterization of CDDO included data indicating that this SO inhibits iNOS formation in primary microglial cultures and protects hippocampal neurons against death induced by β-amyloid (Suh et al., 1999). CDDO-Me also reduces the expression of inflammatory cytokines such as TNFα in microglia without blocking their phagocytic activity, thereby attenuating the production of ROS in dopaminergic neurons and blocking cell death (Tran et al., 2008). Other amide derivatives of CDDO increase activity of the phase 2 cytoprotective enzyme NQO1 and protect against toxicity from hydrogen peroxide in astrocytes and in neurons from rats (Graber et al., 2011). In astrocytes derived from human embryonic stem cells, CDDO-TFEA also activates the Nrf2 pathway, which induces glutamate-cysteine ligase expression and glutathione production and protects against H2O2 toxicity. However, no induction of Nrf2 target genes or neuroprotection is observed in neurons derived from the human embryonic stem cells, suggesting that astrocytes may be necessary for eliminating oxidative stress in the brain (Gupta et al., 2012). Taken together, these in vitro data suggest that if the SOs cross the blood-brain barrier, they might be useful for preventing neurodegenerative diseases.
Although many SOs do not gain access to the brain, several amide derivatives of CDDO can be detected in the brains of mice at concentrations that activate Nrf2-dependent genes in vitro and thus were tested in various animal models of neurodegenerative diseases. CDDO-MA blocks the depletion of striatal dopamine and the loss of tyrosine hydroxylase positive neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease and attenuates the lipid peroxidation and α-synuclein accumulation normally caused by treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. In a model of Huntington's disease, 3-nitropropionic acid causes striatal degeneration, but CDDO-MA reduces the volume of striatal loss by more than 70%. This reduction in neuronal death is accompanied by decreased oxidative damage to proteins, lipids, and DNA in the rats fed CDDO-MA (Yang et al., 2009). CDDO-MA also improves spatial memory and reduces the number and size of amyloid plaques in the hippocampus in a model of Alzheimer's disease in which mice carry two mutations in the human amyloid precursor protein (Dumont et al., 2009). Compared with CDDO-MA, higher drug levels of CDDO-EA and CDDO-TFEA can be obtained in the brain, and both of these amides prolong survival in transgenic mouse models of Huntington's disease (Stack et al., 2010) and ALS (Neymotin et al., 2011). Even when treatment is delayed until the onset of motor symptoms in G93A SOD1 mice, the SOs slow progression of ALS and extend the lifespan of these mice (Neymotin et al., 2011). In both of these studies, the SOs up-regulate the transcription of genes regulated by Nrf2, and activation of this pathway with drugs or genetic approaches are beneficial in various animal models of neurodegenerative diseases (Burton et al., 2006; Innamorato et al., 2008; Johnson et al., 2008; Calkins et al., 2009; Escartin and Brouillet, 2010). More recently, CDDO-TFEA has been shown to activate Nrf2-dependent genes, thereby suppressing IL-17 and other Th1 proinflammatory cytokines and attenuating disease progression in experimental autoimmune encephalomyelitis models of multiple sclerosis. Moreover, this triterpenoid also seemed to induce the maturation of oligodendrocytes and enhance the repair of myelin in these studies (Pareek et al., 2011).
B. Diseases of the Eye
The SOs also reduce damage to the eye in models of smoking, light damage, uveitis, ischemia/reperfusion, and corneal scarring. Smoking induces oxidative stress in many organs, including the eye, and preliminary studies suggest that CDDO-Im can protect against the inflammatory and oxidative damage caused by smoking in retinal pigment epithelial cells in vivo by activating the Nrf2 cytoprotective pathway (Cano et al., 2010). CDDO-TFEA also induces expression of the Nrf2-target genes NQO1 and GLCL in the retinas of BALB/c mice and protects against retinal degeneration caused by light damage (Pitha-Rowe et al., 2009). Moreover, in a mouse model of uveitis, LPS increases the production of inflammatory cytokines and ROS in both the retina and the iris-ciliary body and also increases the adherence of leukocytes to retinal endothelium. Pretreatment with CDDO-Im increases expression of Nrf2-responsive genes in the retina in wild-type Nrf2 mice and reduces all of the adverse changes induced by LPS, but the protective effects of CDDO-Im against uveitis are lost in Nrf2(−/−) mice (Nagai et al., 2009). Ischemia-reperfusion in the retina also raises levels of superoxide, proinflammatory cytokines, and the infiltration of leukocytes, resulting in neuronal loss and capillary degeneration, and all of these changes are exacerbated in the retinas of Nrf2(−/−) mice. However, CDDO-Me reduces the levels of superoxide and subsequent capillary degeneration after retinal ischemia-reperfusion injury in Nrf2(+/+) mice but not in Nrf2-knockout mice (Wei et al., 2011). Wounds to the cornea, the second most common cause of blindness, initiate the release of cytokines such as TGF-β that induce keratocytes to differentiate into myofibroblasts. CDDO-Me inhibits the differentiation of primary corneal fibroblasts treated with TGF-β into myofibroblasts, as measured by the complete suppression of the in vitro production of α-smooth-muscle actin, collagen, and fibronectin (Kuriyan et al., 2012).
C. Diseases of the Lung
In many lung diseases, a number of stimuli can trigger an inappropriately robust inflammatory response. Genetic inactivation of the Nrf2 pathway exacerbates inflammation, chronic obstructive pulmonary disease (COPD), acute lung injury/acute respiratory distress syndrome (ALI/ARDS), emphysema, asthma, and fibrosis in the airways of Nrf2(−/−) mice (Cho et al., 2002, 2004; Rangasamy et al., 2004, 2005; Goven et al., 2008; Malhotra et al., 2008; Suzuki et al., 2008; Reddy et al., 2009a; Cho and Kleeberger, 2010). The SOs have been shown to be effective in preclinical animal models for all of these diseases, although activation of the Nrf2 pathway is only one of the mechanisms that explain these results, because in many of these studies, treatment with the SOs began after the initiating event.
Smoking is the primary risk factor for COPD, and elevated oxidative stress resulting from reduced activation of the Nrf2 system drives inflammation and apoptosis in the lungs of patients with COPD. Exposure to 6 months of cigarette smoking causes significant enlargement of the alveolar airspace and emphysema in mice, but concomitant treatment with CDDO-Im for the duration of the smoking exposure reduces the alveolar destruction. CDDO-Im also elevates expression of Nrf2 target genes, lowers oxidative stress, and reduces apoptosis in the lungs of the mice exposed to cigarette smoke (Sussan et al., 2009). The up-regulation of Nrf2-regulated genes in the lung by CDDO-Im also protects against hemorrhage, proteinaceous edema, and leukocytic infiltration, all hallmarks of ALI/ARDS, induced by 72 h of hyperoxia. Unexpectedly, the protection against ALI only occurs when CDDO-Im is administered during hyperoxic conditions and not when administered before the injury from oxygen (Reddy et al., 2009b). In both the COPD and ALI/ARDS studies, cytoprotection induced by up-regulation of Nrf2-inducible genes in the lung is observed only in Nrf2(+/+) mice but is lost in Nrf2(−/−) mice.
In lung diseases driven by genetic abnormalities, such as cystic fibrosis and chronic granulomatous disease (CGD), the anti-inflammatory and cytoprotective properties of the SOs are useful for suppressing the disease process. In human airway epithelial cells with a defective Cystic fibrosis transmembrane conductance regulator (CFTR) gene, SOs activate the Nrf2 pathway and inhibit activation of NF-κB. Mutations in this gene prevent normal regulation of chloride and sodium ion transport and cause cystic fibrosis. When mice with a R117H CFTR mutation are pretreated with CDDO and then challenged with LPS or flagellin, the infiltration of neutrophils and the release of the pro-inflammatory cytokines IL-1β, IL-6, macrophage inflammatory protein 2, and keratinocyte-derived cytokine are significantly reduced (Nichols et al., 2009). In CGD, phagocytes do not generate appropriate levels of superoxide or ROS because of inherited defects in NADPH oxidase. Patients with this disease suffer from recurring bacterial and fungal infections that are characterized by inappropriate and excessive inflammation. It is noteworthy that Nrf2 activity is reduced whereas NF-κB activity is activated in PBMCs from patients with CGD. CDDO-Im, however, activates the Nrf2 pathway independently of NADPH oxidase and thereby reduces lung injury caused by zymosan, a pro-inflammatory component of the cell wall in yeast, in a mouse model of CGD. CDDO-Im not only blocks neutrophil infiltration and the release of the pro-inflammatory cytokines TNFα, IL-17, IL-23, lipopolysaccharide-induced CXC chemokine, and granulocyte colony-stimulating factor when given before or after the zymosan challenge but also induces apoptosis in the infiltrating cells (Segal et al., 2010).
The pathophysiology of lung diseases such as idiopathic pulmonary fibrosis, sarcoidosis and even asthma involves fibrotic remodeling and scarring of the lung. Although activation of the Nrf2 pathway can protect against pulmonary fibrosis (Cho et al., 2004), SOs may also block experimental fibrosis through other mechanisms. When wound healing is not properly regulated, TGF-β drives fibroblasts to produce excessive amounts of collagen and fibronectin, which interfere with normal lung function. CDDO prevents the in vitro differentiation of human lung fibroblasts into myofibroblasts induced by TGF-β and the subsequent production of extracellular matrix components such as α-smooth-muscle actin, calponin, fibronectin, and collagen by suppressing acetylation of CBP/p300 (Ferguson et al., 2009a) and phosphorylation of Akt (Kulkarni et al., 2011). Although CDDO up-regulates HO-1 (Ferguson et al., 2009b) and suppresses the production of IL-6, prostaglandin E2, cyclooxygenase-2, and monocyte chemotactic protein-1 and inhibits NF-кB transcriptional activity (Hogan et al., 2011) in human fibroblasts at concentrations similar to those required to inhibit fibrosis, its antifibrotic effects seem to be independent of HO-1 induction (Ferguson et al., 2009b). These last four studies also found that 15-deoxy-Δ12,14-PGJ2 or PGA1 are active in these assays. However, removal of the electrophilic center in these ligands eliminates protection against fibrosis, suggesting the importance of an α,β-unsaturated carbonyl moiety for bioactivity. This topic will be explored in greater detail when describing molecular targets and mechanisms of the SOs.
D. Cardiovascular and Circulatory Diseases
Activation of the Nrf2 pathway is also useful for maintaining homeostasis in the cardiovascular system. Oxidative stress is a primary contributor to the development and progression of cardiovascular diseases (Li et al., 2009; Koenitzer and Freeman, 2010), but the SOs eliminate the damage of ROS in various models of cardiomyopathy. Dihydro CDDO-TFEA, in which the double bond in the C ring is removed, binds to Keap1, allowing Nrf2 translocation to the nucleus and transcription of Nrf2 target genes in cardiomyocytes in vitro and in vivo. The reduction by this SO in the production of ROS/reactive nitrogen species, which is induced by angiotensin II activation of NADPH oxidase, in these cells is eliminated with knockdown of Nrf2 (Ichikawa et al., 2009). Moreover, by inducing expression of HO-1, CDDO-Im increases the availability of NO and decreases levels of ROS and endothelial NOS in naive or stressed endothelial cells, thus mediating endothelial NOS coupling and vascular homeostasis (Heiss et al., 2009). Although cigarette smoking triggers cardiac dysfunction in the right ventricle that is worse in Nrf2(−/−) mice than in Nrf2(+/+) mice, CDDO-Im prevents the cardiac damage from smoking. Changes to end-systolic pressure, ejection fraction, and isovolumetric relaxation time after 6 months of cigarette smoke are eliminated in mice when CDDO-Im is administered concurrently with cigarette smoke, but the cardioprotective effects of CDDO-Im in this model are mostly absent in Nrf2(−/−) mice (Sussan et al., 2009).
E. Liver Diseases and Other Diseases of the Digestive System
A variety of toxins, chemicals, viruses, and even alcohol can induce inflammation and subsequent cell death in the liver. The resulting activation of the immune system can cause acute liver injury as well as more chronic conditions such as cirrhosis or fibrosis. Induction of phase 2 enzymes regulated by Nrf2 in the liver allows metabolism and elimination of these insults and is protective in both animals and humans (Klaassen and Reisman, 2010), and SOs protect the liver against toxicity from aflatoxin, concanavalin A (ConA), and acetaminophen. By up-regulating the Nrf2/ARE pathway in rats, CDDO-Im significantly blocks the formation of aflatoxin- DNA adducts and reduces the volume of aflatoxin-induced preneoplastic lesions in the liver (Yates et al., 2006). The formation of DNA-aflatoxin adducts is a well recognized risk factor for developing liver cancer, and the chemoprevetive agent oltipraz effectively reduces DNA-aflatoxin adducts in humans (Wang et al., 1999). In comparison, CDDO-Im is 30- and 100-fold more potent than 1,2-dithiole-3-thione and oltipraz, respectively, in this assay. During the pathogenesis of hepatitis, ROS released from activated immune cells destroys normal hepatocytes, and this necrosis amplifies the inflammation, forming a destructive feedback loop. The injection of the lectin ConA mimics this process in mice by activating T cells, inflammation, and the death of hepatocytes. Treatment with CDDO-Im before ConA challenge activates the Nrf2 pathway in the liver, which blocks T-cell activation and prevents hepatic necrosis and the accompanying elevated serum ALT levels. In Nrf2-knockout mice, however, CDDO-Im does not reduce the hepatoxicity that follows treatment with ConA even though it partially reduces levels of early phase proinflammatory cytokines (Osburn et al., 2008). These results suggest that the anti-inflammatory effects of the SOs alone are not sufficient to block liver injury. Pretreatment with CDDO-Im also lowers hepatotoxicity induced by acetaminophen by activating the Nrf2/ARE pathway, but the protection is lost in Nrf2(−/−) mice (Reisman et al., 2009).
Although the SOs have not been tested in animal models of many other digestive diseases, CDDO can prevent ileitis induced by infection with Toxoplasma gondii in a mouse model of inflammatory bowel disease (Minns et al., 2004). Because activation of the Nrf2 pathway is cytoprotective in the colon (Khor et al., 2006; Theiss et al., 2009) and CDDO-Im induces transcription of Nrf2-target genes such as NQO1 and GCLC in the stomach, small intestine, and colon (Yates et al., 2007), the SOs should also be effective against conditions such as Crohn's disease, inflammatory bowel disease, and ulcerative colitis. In these diseases, oxidative stress and inflammation can drive pathogenesis (Iborra et al., 2011; Strober and Fuss, 2011).
F. Renal Diseases
The kidneys are involved in the elimination of various chemicals and toxins, so excessive free radicals or oxidative stress can cause renal damage. Deficiency of Nrf2 induces an autoimmune nephritis in aged female mice (Yoh et al., 2001), and activation of the Nrf2 pathway can protect kidneys against ischemia-reperfusion injuries and damage from chemotherapeutic agents (Leonard et al., 2006; Tanaka et al., 2007; Liu et al., 2009). Activation of the Nrf2 pathway may also protect against the oxidative stress implicated as a cause of diabetic neuropathy (Negi et al., 2011). CDDO-Im induces gene transcription of the Nrf2 target genes NQO1 and GCLC in the kidneys, and administration of CDDO-Im 24 to 48 h before treatment with ferric nitrilotriacetate prevents the increased serum urea nitrogen and creatinine levels associated with kidney damage (Tanaka et al., 2008). CDDO-Im also protects against cisplatin nephrotoxicity, a dose-limiting side effect frequently associated with the use of cisplatin chemotherapy, because blood urea nitrogen levels and damage to the proximal tubules are reduced in mice treated with CDDO-Im before cisplatin treatment (Aleksunes et al., 2010). As expected, renal damage is worse in Nrf2(−/−) mice challenged with either ferric nitrilotriacetate or cisplatin than in wildtype Nrf2(+/+) mice, and the protection against cisplatin toxicity by CDDO-Im is absent in the Nrf2-knockout mice (Aleksunes et al., 2010). By selectively activating transcription of Nrf2 and PPARγ on glomerular endothelia and HO-1 in renal tubules and leukocytes, CDDO-Me improves both renal function and histopathology in a model of ischemic acute kidney injury; again, however, only when it is administered before the ischemic injury (Wu et al., 2011b).
G. Metabolic Disorders
Both genetic activation of Nrf2 signaling and pharmacological activation with CDDO-Im induce a number of genes involved in lipid metabolism (Yates et al., 2009), and the SOs are beneficial in preliminary animal studies of obesity and diabetes. Nrf2 can bind to an ARE on the promoter of the aryl hydrocarbon receptor, which then inhibits adipocyte differentiation. By activating Nrf2, CDDO-Im induces Ahr transcription and blocks lipid accumulation in Nrf2(+/+) MEFs but not in Nrf2(−/−) MEFs in vitro (Shin et al., 2007). When given orally to mice, CDDO-Im reduces the weight gain, adipose levels, and lipid accumulation in the liver that accompany a high-fat diet but has no effect on weight gain or energy balance in mice fed a normal diet. The reduction in obesity in the group treated with CDDO-Im is accompanied by increased energy expenditure and down-regulation of pathways regulating fatty acid synthesis in the liver, but these effects are lost in Nrf2-deficient mice (Shin et al., 2009). In mice fed a high-fat diet or in mice with a defective leptin receptor (Leprdb/db), CDDO-Me not only improves glucose tolerance and insulin sensitivity but also lowers levels of free fatty acids and plasma triglycerides. Although CDDO-Me reduces total body fat and suppresses production of the proinflammatory cytokines IL-1, IL-6, and TNFα in mice fed a high-fat diet, the antidiabetic effects of this SO may be mediated by stimulating phosphorylation of LKB1 and AMPK in muscle and liver, as knockdown of AMPK reduces glucose uptake in cells treated with CDDO-Me (Saha et al., 2010).
H. Inflammatory/Autoimmune Disorders
SOs have important activities in macrophages (Honda et al., 1998, 1999, 2000; Tran et al., 2012), human PBMCs and neutrophils (Thimmulappa et al., 2006b; Thimmulappa et al., 2007), B and T lymphocytes (Pedersen et al., 2002; Han et al., 2006; Kress et al., 2007; Sun et al., 2007; Elsawa et al., 2008; Gao et al., 2008), and myeloid-derived suppressor cells (MDSCs) (Nagaraj et al., 2010). In addition to their direct effects on immune cells themselves, SOs regulate the production and activity of inflammatory cytokines in a variety of diseases. CDDO blocks the induction of matrix metalloproteinase (MMP)-1 and MMP-13 in chondrosarcoma cells and in primary chondrocytes stimulated with IL-1β and TNF-α, and it inhibits cell invasion through collagen (Mix et al., 2001; Elliott et al., 2003; Mix et al., 2004). These MMPs induce the degradation of cartilage, so inhibiting their production may prevent osteoarthritis or rheumatoid arthritis, and Nrf2 deficiency exacerbates arthritis in mice (Maicas et al., 2011; Wruck et al., 2011). It is noteworthy that CDDO also blocks viral replication in macrophages infected with HIV but not through modulation of cytokines (Vázquez et al., 2005).
Inappropriate amplification of the innate immune response can result in sepsis, which can be fatal. LPS from bacterial infections can induce the secretion of proinflammatory cytokines and chemokines from macrophages and neutrophils, and elimination of Nrf2 in vivo exacerbates the lethality from sepsis induced by LPS, cecal ligation, or puncture injury (Thimmulappa et al., 2006a). CDDO-Im also reduces lethality after LPS challenge in mice by activating Nrf2-dependent genes such as HO-1, NQO1, GCLC, and GCLM and by blocking the transcription of TNFα, IL-6, Mcp1, and Mip2 inflammatory mediators, but the protective effect of CDDO-Im is eliminated in Nrf2-knockout mice or in Nrf2-deficient cells (Thimmulappa et al., 2006b). It is noteworthy that activation of these same Nrf2 target genes and suppression of TNFα and IL-6 are detected in human neutrophils and PBMCs, suggesting that SOs might be effective in septic shock and in other disorders characterized by excessive inflammation (Thimmulappa et al., 2007). Indeed, CDDO-Me extends the survival of mice challenged with LPS and decreases circulating levels of IL-6, IL-12, IL-17, IL-23, and interferon γ without altering the numbers or subtypes of immune cells (Auletta et al., 2010).
Because CDDO, CDDO-Me, and CDDO-Im all inhibit T-cell proliferation induced by mitogens or alloantigens in vitro and in vivo (Sun et al., 2007; Gao et al., 2008), they might be useful in graft-versus-host disease (GVHD) or organ transplantation. In a mouse model of GVHD, CDDO prolongs survival and decreases inflammatory-mediated damage in the liver but not in the gastrointestinal tract. It is noteworthy that CDDO does not interfere with engraftment of the donor cells or recovery of myeloid counts (Sun et al., 2007). CDDO-Me is more potent than CDDO, and it inhibits T-cell proliferation, reduces TNFα levels, and delays GVHD without interfering with the treatment for leukemia (Li et al., 2010). In these studies, SOs are administered immediately after transplantation instead of before transplantation. CDDO-Me also can promote the formation and expansion of hematopoietic lineages in both the spleen and bone marrow of mice. This response is dose-dependent, as high concentrations of the drug inhibit proliferation of myeloid cells. Moreover, in mice pretreated with CDDO-Me, myelopoiesis is accelerated after sublethal total-body irradiation or syngeneic bone marrow transplantation (Ames et al., 2012).
I. Other Diseases
Alkylating agents such as sulfur mustard have been used for chemical warfare since World War I and cause debilitating blisters and damage to the skin, cornea, and lungs. CDDO-Im and CDDO-Me can protect human keratinocytes against toxicity from the sulfur mustard analog 2-chloroethyl ethyl sulfide by inducing the synthesis of glutathione, which is depleted by sulfur mustard (Abel et al., 2011). Protection against ionizing radiation is important in the event of a nuclear incident or for treating cancer. Pretreating human colon epithelial cells with CDDO-Me protects them from cell death or transformation induced by radiation (Eskiocak et al., 2010). Pre-exposure to CDDO-TFEA also reduces whole organism apoptosis and aberrant development, restores metabolism and renal function, and increases survival of lethally irradiated zebrafish embryos. It is noteworthy that this SO also mitigates damage when administered 1 to 2 h after exposure to radiation (Daroczi et al., 2009).
J. Cancer
1. Prevention.
The SOs were originally developed as novel chemopreventive agents (Suh et al., 1999), and more studies have been published on triterpenoids and cancer than any other disease. As summarized in Table 4, the SOs are effective for preventing or treating cancer in a variety of preclinical animal models. Unlike most of the other diseases described previously, there is little evidence that Nrf2 activation is required for much of the anticancer activity of the SOs. Because they are not conventional cytotoxic agents, the SOs are more effective when used to intervene early in the process of carcinogenesis. Aflatoxin ingestion increases the risk of hepatocellular carcinoma in humans, and when administered to rats a week before an aflatoxin challenge, low doses of oral CDDO-Im (1–30 μmol/kg body weight) reduce preneoplastic hepatic foci by 85 to 99% (Yates et al., 2006). The SOs are also active when given after initiation with a UV-damaging or chemical insult. In SKH-1 hairless mice exposed to chronic low-level UBV radiation for 17 weeks and then treated topically with di-CDDO (TP-225), skin tumor multiplicity is 50% lower and total tumor burden is 5-fold lower in the mice treated with diCDDO compared with the controls. This SO also increases NQO1 and HO-1 cytoprotective enzyme activity in the skin of these “high risk” hairless mice (Dinkova-Kostova et al., 2008). The SOs are also potent inhibitors of lung carcinogenesis in A/J mice. When fed in the diet, starting 1 week after initiation with vinyl carbamate, CDDO-Me, CDDO-EA, and CDDO-MA all significantly decrease the number, size, and severity of lung adenocarcinomas (Liby et al., 2007a, 2008a, 2009). When fed in the diet, CDDO-Me and CDDO-EA are especially effective in this model and reduce total tumor burden in the lung by 86 to 98% compared with controls.
Table 4.
SOs are effective for prevention and treatment of cancer in vivo
| Triterpenoid | Cancer Model | Treatment or Prevention | Combination | Reference |
|---|---|---|---|---|
| CDDO-Im | Syngeneic L1210 leukemia and B16 melanoma models | Treatment | None | Place et al., 2003 |
| CDDO | Xenograft of MDA-MB-435 ER-breast cancer cells | Treatment | None | Lapillonne et al., 2003 |
| CDDO-Im | Xenograft of MDA-MB-468 ER-breast cancer cells | Treatment | TRAIL | Hyer et al., 2005 |
| CDDO-Im | Liver carcinogenesis induced by aflatoxin in rats | Prevention | None | Yates et al., 2006 |
| Liposomal CDDO |
Xenograft of MCF7 ER + breast cancer cells stably overexpressing HER2 | Treatment | None | Konopleva et al., 2006 |
| CDDO-Im | Plasmacytomas in iMycEμ transgenic mice | Treatment | None | Han et al., 2006 |
| CDDO-Me CDDO-Ea CDDO-Ma |
Lung carcinogenesis induced by vinyl carbamate in A/J mice | Prevention | None | Liby et al., 2007a, 2008a |
| Liposomal CDDO-Me |
4T1/BALB/c model of metastatic breast cancer | Treatment | None | Ling et al., 2007 |
| Liposomal CDDO CDDO-Im |
TRAF2DN/Bcl-2 transgenic mouse model of CLL and small B-cell lymphoma | Treatment | None | Kress et al., 2007 |
| CDDO-Me | Xenograft of Kaposi's sarcoma cells | Treatment | None | Vannini et al., 2007 |
| Liposomal CDDO-Me |
APL cells from hMRP8-PML/RARα transgenic mice propagated into syngenic FVB-N mice | Treatment | ATRA | Tabe et al., 2007 |
| di-CDDO (TP-225) | Skin carcinogenesis induced by UV radiation in “high-risk” hairless mice | Prevention | None | Dinkova-Kostova et al., 2008 |
| CDDO-Me | Xenograft of Dul45 prostate cancer cells in nude rats | Treatment | None | Hyer et al., 2008 |
| CDDO-Me | MMTV-neu model of ER- breast cancer | Both | LG100268 | Liby et al., 2008b |
| CDDO-Me | Xenograft of PC-3 prostate cancer cells | Treatment | None | Deeb et al., 2009 |
| CDDO-Me CDDO-EA |
Lung carcinogenesis induced by vinyl carbamate in A/J mice | Both | LG100268 and NRX194204 | Liby et al., 2009 |
| CDDO-Me | Xenograft of MC38 colon cancer cells | Treatment | Tumor-associated antigen vaccine | Nagaraj et al., 2010 |
| CDDO-Me | Xenograft of L3.6pL human pancreatic cancer cells | Treatment | None | Jutooru et al., 2010 |
| CDDO-Me CDDO-EA |
LSL-KrasG12D/+;LSL-Trp53R127H/+;Pdx-1-Cre mouse model of pancreatic cancer | Prevention | LG100268 | Liby et al., 2010 |
| CDDO-Im | Experimental metastasis model –B16F1 melanoma and HT-29 colon cancer cells injected into the mesenteric vein metastasize to the liver | Treatment | None | Townson et al., 2011 |
| CDDO | TRAMP model | Prevention | None | Deeb et al., 2011 |
| CDDO-Im nanoparticles | Orthotopic injection of primary MMTV-Neu or 4TO7 tumor cells into BALB/c or FVB/NJ mice | Treatment | HER-2 DNA vaccine | Liao et al., 2011 |
| CDDO-Me | Brca1Co/Co;MMTV-Cre;p53+/− transgenic mouse model of BRCA1-mutated breast cancer | Prevention | None | Kim et al., 2012 |
| CDDO-Me | PyMT model of ER- breast cancer | Prevention | None | Tran et al., 2012 |
MMTV, mouse mammary tumor virus; PyMT, polyoma middle T.
In addition to their efficacy in chemical carcinogenesis models, the SOs also significantly delay tumor development in various transgenic mouse models of breast, prostate, and pancreatic cancer. In the mouse mammary tumor virus-neu transgenic model, targeted overexpression of the neu (ErbB2/HER2) receptor tyrosine kinase in the mammary gland drives tumorigenesis. When fed in the diet beginning at 10 weeks of age, CDDO-Me significantly delays tumor development in these mice by over 3 months. Tumor incidence of 50% is observed by 31 weeks of age in the control group but is not reached until 45 weeks in mice fed CDDO-Me (Liby et al., 2008b). In a mouse model in which deletion of the BRCA1 gene (breast cancer associated gene 1) is combined with a mutation in a single allele in the p53 tumor suppressor gene, CDDO-Me significantly delays tumor development. Germline mutations of the BRCA1 gene have been detected in ∼90% of familial breast and ovarian cancers, and patients with BRCA mutations have a 50 to 70% lifetime risk of developing ovarian or breast cancer. Tumor burden is also significantly lower in the BRCA1-deficient mice fed CDDO-Me in diet, and lifespan increases an average of 5 weeks compared with control mice. Unlike most cases of the human disease, pErbB2 is overexpressed in cell lines and tumors from these mice. CDDO-Me directly interacts with ErbB2 in vitro and inhibits its constitutive phosphorylation in vitro and in vivo (Kim et al., 2012). In a TRAMP model, oral gavage of CDDO (10 μmol/kg) for 20 weeks does not prevent the development of preneoplastic prostate lesions but does inhibit the pathological progression to adenocarcinomas and reduces metastasis. Several proteins involved in apoptosis, including Bcl-2, Bcl-XL, survivin, and cellular inhibitor of apoptosis protein, are lower in the prostates of the TRAMP mice treated with CDDO than in prostates from control mice (Deeb et al., 2011). In the KPC (LSL-KrasG12D/+;LSL-Trp53R127H/+;Pdx-1-Cre) model of pancreatic cancer, activating mutations in the Kras and p53 oncogenes are targeted to the pancreas, and these mice develop pancreatic lesions that rapidly progress to invasive and metastatic adenocarcinomas. CDDO-Me, alone or in combination with the rexinoid LG100268, extends survival in KPC mice by a relatively modest 3 to 4 weeks. In comparison, gemcitabine, the standard of care for pancreatic cancer, has no effect in this aggressive mouse model. In pancreatic cancer cell lines derived from KPC tumors, the SOs interact with STAT3 and IKK and block constitutive IL-6 secretion, STAT phosphorylation, and IKK activity (Liby et al., 2010). It is noteworthy that there is no evidence that long-term administration of CDDO for 20 weeks by oral gavage in the TRAMP model or CDDO-Me in diet for 35 to 45 weeks in the other transgenic mouse models enhances tumorigenesis.
In contrast to many other diseases, the ability of the SOs to prevent cancer in experimental animals may not be primarily through Nrf2 signaling. With the exception of the aflatoxin studies (Yates et al., 2006), the SOs were not administered until at least 1 week after challenge with a carcinogen, suggesting that the SOs were not simply preventing cancer by detoxifying the original insult before it could initiate DNA damage. The Nrf2/ARE pathway was important for prevention of carcinogenesis by the SOs in the liver (Yates et al., 2006) and skin (Dinkova-Kostova et al., 2008), but there is no evidence that this pathway is required for efficacy in the other prevention studies. In fact, several recent studies suggest that activation of the Nrf2/ARE pathway in cancer cells may be detrimental and contribute to resistance to chemotherapy or radiation (Taguchi et al., 2011). In several human cancer cells or tumors, mutations in Keap1 or Nrf2 result in constitutive activation of the pathway, causing an adaptive stress response and a survival advantage. Moreover, two recent studies used animal models in which mutations in the Kras protoncogene activate Nrf2 and drive tumorigenesis (Bauer et al., 2011; DeNicola et al., 2011); Kras mutations are thought to occur in human cancers at a higher rate than mutations in Keap1 or Nrf2. The emerging importance of Nrf2 and cancer is obviously relevant to SOs biology and has been reviewed extensively (Sporn and Liby, 2012). However, it is important to note that genetic activation of a pathway is far different from pharmacological activation in terms of the duration and amplitude of the response (Kensler and Wakabayashi, 2010). This fact has been verified experimentally; the global expression patterns vary significantly when comparing microarray results from the livers of mice treated with CDDO-Im versus mice in which Keap1 is deleted (Yates et al., 2009). Moreover, the SOs are useful for preventing and treating cancer in a variety of experimental models (Table 4), including models in which mutations in Kras drive carcinogenesis (Liby et al., 2007a, 2010).
2. Treatment.
In addition to their efficacy for prevention of cancer, the SOs have been used to treat established tumors in experimental models. As suggested by the number of published studies on SOs and leukemia (summarized in Table 1), leukemia cells seem to be especially sensitive to triterpenoids. When BDF1 mice injected with L1210 leukemia cells are treated with 100 μg/day CDDO or CDDO-Im, the number of leukemia cells decreases by 81 to 91% (Place et al., 2003), and similar antitumor effects are observed with B16 melanoma cells. The overexpression of Bcl-2 and mutations in TRAF2 are commonly found in human chronic lymphocytic leukemia (CLL), and when these alterations are introduced into B cells in mice, they develop small B-cell lymphoma and CLL. When liposomes containing CDDO or CDDO-Im are injected intravenously into TRAF2DN/Bcl-2 mice with active CLL, the SOs apoptose the malignant cells. CDDO-Im is more potent than CDDO and reduces tumor burden of circulating B cells by 60 to 90% (Kress et al., 2007). In a syngeneic mouse model of APL in which leukemic blasts from hMRP8-PML/RARα transgenic mice are injected into FBV-N mice, liposomal CDDO-Me enhances differentiation induced by a subtherapeutic dose of ATRA in established APL cells; the combination of ATRA and CDDO-Me also significantly extends survival (Tabe et al., 2007).
Despite concerns about the predictability and relevance of xenograft models, which lack appropriate immune and stromal components, the injection of human cancer cells into immunodeficient mice remains a standard model for testing cancer drugs. The SOs are active in xenograft models of breast cancer (Lapillonne et al., 2003; Konopleva et al., 2006; Liao et al., 2011), Kaposi's sarcoma (Vannini et al., 2007), prostate cancer (Hyer et al., 2008; Deeb et al., 2009), colon cancer (Nagaraj et al., 2010), and pancreatic cancer (Jutooru et al., 2010). However, significant antitumor activity against established tumors is more evident when a triterpenoid is combined with other drugs, such as TRAIL (Hyer et al., 2005), ATRA (Tabe et al., 2007), rexinoids (Liby et al., 2009), or vaccines (Nagaraj et al., 2010; Liao et al., 2011). In an immunocompetent mouse model of breast cancer, chemoresistant 4T1 cells, derived from a spontaneous mammary tumor, are injected back into BALB/c mice to study primary tumors as well as metastases. When treatment with CDDO-Me in liposomes is started 1 day after the injection of 4T1 cells, the drug completely blocks tumor formation and metastasis. Tumor size is also significantly smaller in the mice treated with CDDO-Me than in the control group, even when treatment is delayed until 5 days after inoculation of the aggressive 4T1 cells. Moreover, the population of mature dendritic cells in BALB/c mice with 4T1 tumors decreases by two thirds compared with BALB/c mice without tumors, but CDDO-Me helps maintain the number of mature dendritic cells in this model (Ling et al., 2007).
The triterpenoids may also prevent metastasis. In vitro, SOs inhibit cell migration by targeting cytoskeletal proteins (To et al., 2008, 2010). CDDO-Me not only inhibits the invasion of 4T1 breast cancer cells into a matrix but also eliminates metastasis to the lungs when these cells are injected into BALB/c mice (Ling et al., 2007). CDDO also reduces the incidence of metastasis to liver and lymph nodes in the TRAMP model of prostate cancer (Deeb et al., 2011). In both the 4T1 and TRAMP models, however, the SOs also suppress tumor growth, so it is possible that the antimetastatic effects of the SOs in these models are the result of a lower primary tumor burden, which reduces the number of cells available to metastasize. To address this issue, B16F1 melanoma cells and HT-29 colon cancer cells were directly injected into the mesenteric vein of mice. Once cells had colonized in the liver, the mice were fed a diet containing CDDO-Im for 8 weeks, and the treatment with the SOs reduced the metastatic burden of the HT-29 cells by 70%. CDDO-Im also lowered the tumor burden of the B16F1 melanoma cells in the liver by 50% but did not reduce the number of individual cells, suggesting that the drug inhibits proliferation of the malignant cells once colonized in the liver but did not induce apoptosis (Townson et al., 2011).
3. In Vivo Anticancer Mechanisms.
As mentioned previously, numerous mechanisms have been proposed to explain the growth inhibitory and apoptotic effects of the SOs for the treatment of cancer. However, these studies are based primarily on in vitro data, and there is little evidence that many of these mechanisms occur in vivo. Important biomarkers of anticancer efficacy for the SOs that have been validated in vivo include pSTAT3 (Ling et al., 2007; Liao et al., 2011), pHER2/pErbB2 (Konopleva et al., 2006; Kim et al., 2012), and pAKT (Deeb et al., 2011). The SOs decrease either the expression or the activity of all of these proteins, which are overexpressed or constitutively activated in many human cancers (Yu et al., 2009; Baselga, 2010; Grivennikov and Karin, 2010; Li et al., 2011). There are undoubtedly other targets and pathways that account for the effects of SOs on established cancers, but new mechanistic studies will require additional experiments and analysis in relevant animal models.
In addition to directly targeting tumor cells, the SOs have effects on a variety of stromal cells. Targeting tumor vasculature is an attractive strategy for treating cancer because angiogenesis is required for both tumor growth and metastases (Carmeliet and Jain, 2011; Weis and Cheresh, 2011), and CDDO-Me reduces the expression of MMP-9, VEGF, CXCL12, and CCL2 from tumor cells (Shishodia et al., 2006; Tran et al., 2012). This SO also inhibits the ability of human umbilical vein endothelial cells to proliferate and form capillary-like networks in vitro and is reportedly active at doses as low as 3 μg/kg of body weight for blocking angiogenesis induced by VEGF and TNFα in a Matrigel sponge assay (Vannini et al., 2007). CDDO-Me and CDDO also reduce the number of CD31-positive blood vessels in a Kaposi's xenograft model and in the TRAMP model, respectively (Vannini et al., 2007; Deeb et al., 2011).
Several recent studies also report that the SOs can target immune cells. Although maintaining levels of dendritic cells, as CDDO-Me does in the 4T1 breast cancer model (Ling et al., 2007), is beneficial in cancer therapy, tumors often hijack the immune system to secrete cytokines and growth factors that promote angiogenesis, tumor growth, and survival (Mantovani et al., 2008; Grivennikov et al., 2010; Qian and Pollard, 2010; Ruffell et al., 2010). MDSCs promote tumor angiogenesis and invasion and suppress T-cell mediated immunosuppression of cancer cells (Ostrand-Rosenberg, 2010; Youn and Gabrilovich, 2010). In an animal model and in MDSCs from patients treated with bardoxolone methyl, the drug blocks the activity of MDSCs, as measured by nitrotyrosine levels and T-cell responsiveness, but not the number of MDSCs. As a result, CDDO-Me may favorably improve antitumor immune function, especially when combined with a cancer vaccine (Nagaraj et al., 2010). MDSCs also suppress peptide binding to major histocompatibility complex proteins and thus prevent an antigen-specific CTL response to tumor cells. Although CDDO-Me does not affect peptide binding, it does eliminate the protective effect against a lethal CTL response and thus enhances killing of the tumor cells by T lymphocytes (Lu et al., 2011). In an orthotopic 4TO7 model of breast cancer, CDDO-Im increases expression of pSTAT1, IL-15, IL-12b, and granulocyte macrophage–colony-stimulating factor and decreases expression of IL-6, IL-16, and TGF-β, suggesting a Th1 response. This shift from a pro-tumor Th2 response to an antitumor Th1 phenotype is accompanied by the infiltration of activated CD8+ T cells, M1 macrophages, and dendritic cells. When CDDO-Im treatment is combined with a HER-2 vaccine, significant protection against tumor recurrence and an enhanced tumor-specific CTL response are observed (Liao et al., 2011).
The ability of SOs to modulate immune cells and other components of the tumor microenvironment suggest they should be tested in combination with immunomodulatory vaccines, antiangiogenic agents, or chemotherapeutic drugs. The triterpenoids are not conventional cytotoxic agents and frequently do not display potent apoptotic activity against established solid tumors, so their usefulness as cancer drugs might be enhanced if combined with radiation or chemotherapy (Shishodia et al., 2006). This approach could exploit the altered levels of oxidative stress in tumor cells versus normal cells for therapeutic benefit (Trachootham et al., 2006, 2009; Acharya et al., 2010; Montero and Jassem, 2011). Preliminary studies suggest that CDDO-Me can simultaneously enhance the antitumor effect of radiation against tumor xenografts and protect normal tissue against mucositis (Meyer et al., 2006). Ongoing studies are attempting to confirm and extend these provocative data. Moreover, additional work with the SOs may enable the exploitation of real differences between normal and cancer cells (Trachootham et al., 2009; Acharya et al., 2010). In this scenario, up-regulation of Nrf2 cytoprotective pathways by the SOs could protect normal cells and tissues without inhibiting the induction of oxidative stress by radiation or chemotherapeutic drugs required to apoptose cancer cells. The possibility that the SOs increase platelet production and myelopoiesis (Petronelli et al., 2011; Sardina et al., 2011; Ames et al., 2012) are especially germane to this hypothesis.
VI. Signaling Pathways and Mechanisms of Action
As described above, the SOs are multifunctional drugs that not only suppress inflammatory and oxidative stress, and are thus cytoprotective, but also inhibit cell proliferation and induce differentiation or apoptosis. These biological responses are dose-responsive, as summarized in Fig. 3. As would be expected based on their pleiotropic nature, pentacyclic triterpenoids, both natural and synthetic, interact with many different protein targets and signaling pathways. This is not surprising, given the evolutionary origin of these isoprenoid molecules. Their hydrophobic nature provides high overall affinity for membrane structures in the cell, as well as for individual hydrophobic side chains on specific target proteins. The natural terpenoids have been interacting with animal cells for millennia; now, in the case of the SOs, the chemist has added new mechanisms of interaction by introducing cyano-enone functions that provide specific mechanisms for formation of covalent bonds, either reversibly or irreversibly, with reactive cysteine residues on target proteins. However, despite this multifunctionality, these agents do not seem to be cytotoxic, as has been demonstrated amply in numerous studies in both animals and humans. As described in this section, many such targets have been identified by either cell biology or proteomic analysis.
Fig. 3.
Biological responses to SOs are dependent on dose. Low concentrations (nanomolar) of SOs target Keap1 and activate the Nrf2/ARE cytoprotective and anti-inflammatory response. As the concentration of SO increases, SOs target Arp3 and other components of the cytoskeleton to inhibit cell proliferation, whereas even higher concentrations (micromolar) of SOs can selectively induce apoptosis in cancer cells by targeting a number of key regulatory proteins and pathways that are frequently constitutively activated or overexpressed in cancer cells. [Adapted from Liby KT, Yore MM, and Sporn MB (2007) Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7:357–369. Copyright © 2007 Nature Publishing Group. Used with permission.]
Modifying OA by the addition of α,β-unsaturated carbonyl groups in the A and C rings and an electron-withdrawing nitrile group at C2 enhances the possibility of Michael addition to C1. Structure-activity analysis suggests that the maintenance of this Michael acceptor position is required for the potent anti-inflammatory activity of the SOs (Sporn et al., 2011). The presence of an enone group and its position in the C ring are also essential for induction of NQO1 enzyme activity. The introduction of a second enone functionality in the C ring (TP-82) enhances activity 10-fold compared with its inclusion in the A ring alone (TP-46) (Fig. 1C). Moreover, if this enone group in the C ring is moved from the 9(11)-en-12-one position, as found in CDDO-Me, to the 12-en-11-one position (TP-162), potency decreases 100 fold (Fig. 1C). The inclusion of a double bond between C12-C13 coupled with the complete elimination of the ketone group in the C ring (TP-156) is 1500-fold less active than CDDO-Me (Dinkova-Kostova et al., 2005). However, the SOs do not interact nonspecifically with all proteins containing cysteines, as discussed in greater detail in section VI.H. Instead, the nucleophilicity of the –SH group of cysteine is markedly influenced by the accessibility of a drug to a specific cysteine residue as well as the redox potential of the cell (Jones, 2010; Paulsen and Carroll, 2010).
A. Kelch-Like Erythroid cell-derived protein with CNC homology-Associated Protein 1 (Keap1)
The varied biological activities of the SOs are possible because of their ability to interact with cellular nucleophiles such as the –SH groups of cysteines on target proteins, as has been confirmed in a number of studies. When CDDO is incubated with nucleophiles such as dithiothreitol (DTT), cysteine or reduced glutathione, the resulting spectral shift and hypochromism indicate a direct interaction (Couch et al., 2005; Liby et al., 2007b).
A similar spectral shift is observed when di-CDDO (TP-225) is incubated with either DTT or Keap1, the inhibitor of Nrf2. Although mouse and human Keap1 contains 25 or 27 Cys residues, respectively, not all Cys residues have identical reactivities (Dinkova-Kostova et al., 2002; Yamamoto et al., 2008), and various Nrf2 inducers may interact with different cysteines and through different mechanisms (Kobayashi et al., 2009; Giudice et al., 2010; Kansanen et al., 2011). Three SOs also compete with [3H]Dexamethasone 21-mesylate for binding to Keap1, suggesting a direct interaction between SOs and Keap1 and that Keap1 acts as a molecular sensor for activation of the Nrf2 pathway by the SOs (Dinkova-Kostova et al., 2005).
The activation of the Nrf2 pathway remains a key target for understanding the mechanism of action and biology of the SOs. The elucidation of the specific cysteine residues in Keap1 that are binding sites for SOs is an active area of investigation, as is the possibility that SOs can activate Nrf2 by additional mechanisms beyond direct interaction with Keap1. However, it should also be noted that not all of the anti-inflammatory and cytoprotective properties of the SOs necessarily involve activation of the Nrf2 pathway. For example, the SOs are potent inducers of the HO-1 protein, and binding of Nrf2 to the ARE on the promoter region of this gene are only partially responsible for its induction by the SOs (Liby et al., 2005). The HO-1 enzyme and its downstream effectors are “druggable” targets (Mancuso and Barone, 2009), because induction of HO-1 is protective in many organs, including the cardiovascular system (Wu et al., 2011a), kidney (Abraham et al., 2009), lung (Raval and Lee, 2010), and brain (Jazwa and Cuadrado, 2010) against a variety of diseases. It is noteworthy that intracerebroventricular infusion of CDDO-Im enhances survival of neurons and decreases infarct volume in rats challenged by global or focal ischemia. Concomitant administration of tin protoporphyrin IX, a competitive inhibitor of the HO-1 enzyme, abrogates the protective effects of CDDO-Im, demonstrating the importance of HO-1 induction for its efficacy in these stroke models (Zhang et al., 2012). Moreover, the SOs can induce phosphorylation of Akt, which may be involved in protection of retinal pigment epithelial cells (Pitha-Rowe et al., 2009) and potentially other normal cells as well. It is also possible that the SOs inhibit Toll-like receptor signaling, which regulates inflammation and tissue repair after injury (Rakoff-Nahoum and Medzhitov, 2009), but this hypothesis has not been tested directly.
B. Peroxisome Proliferator-Activated Receptor γ (PPARγ)
Although Keap1 is an important direct target of the SOs, it was not the first molecular target to be identified. Because of its ability to induce differentiation of adipocytes, CDDO was evaluated as a ligand for PPARγ. In scintillation proximity assays, both CDDO and CDDO-Me compete with purified PPARγ for bound [3H]CDDO (Ki of 310 nM for CDDO versus 50 nM for the known PPARγ ligand rosiglitazone), but DTT interrupts this interaction. Although both CDDO and CDDO-Me are ligands for PPARγ, neither binds to PPARα (Wang et al., 2000); in contrast CDDO-Im binds to both receptors (Place et al., 2003). Although CDDO can transactivate both a wild-type PPARγ receptor as well as a chimeric PPARγ protein construct transfected into CV1 cells, CDDO-Me did not activate either of these constructs, apparently because of differences in the recruitment of the coactivator CBP and interactions with nuclear receptor corepressor 1 (Wang et al., 2000). However, in colon cancer cells, CDDO, CDDO-Me, and CDDO-Im all transactivate a PPARγ reporter construct (Chintharlapalli et al., 2005). The effects of the SOs on proliferation and apoptosis of these colon cancer cells were attributed to both PPARγ-dependent and -independent mechanisms, and several other investigators have suggested that SO-PPARγ interactions may decrease levels of cyclin D1 or induce caveolin-1 (Lapillonne et al., 2003; Konopleva et al., 2006). PPARγ has been suggested as a possible therapeutic target for diseases as diverse as diabetes, atherosclerosis, hypertension, chronic kidney disease, and cancer (Glass, 2006; Glass and Ogawa, 2006; Schmidt et al., 2010; Sugawara et al., 2010). It is noteworthy that 15-deoxy-Δ12,14-PGJ2 covalently binds to a cysteine residue in the ligand binding pocket of PPARγ through Michael addition (Shiraki et al., 2005); the ability of an SOs to interact with this Cys residue has not been confirmed.
C. Inhibitor of Nuclear Factor-κB Kinase Complex (IKK) and the Nuclear Factor-κB Pathway
The NF-кB pathway is another important target of the SOs. This transcription factor activates a number of genes that promote inflammation, proliferation, and survival, and because it regulates the inflammatory microenvironment, this pathway can promote tumorigenesis (Karin, 2009; Grivennikov et al., 2010; Ben-Neriah and Karin, 2011). Although numerous studies report that the SOs inhibit this pathway (Stadheim et al., 2002; Ahmad et al., 2006; Ray et al., 2006; Shishodia et al., 2006; Yore et al., 2006; Nichols et al., 2009; Hogan et al., 2011), the direct target in the NF-кB pathway for the SOs was not known until biotinylated SOs were synthesized (Honda et al., 2004) and used to show direct interaction with protein targets. The SOs directly interact with Cys179 on IкB kinase β (IKKβ) and inhibit its kinase activity (Ahmad et al., 2006; Yore et al., 2006). IKKβ is the kinase that phosphorylates IкBα, causing its degradation and the subsequent translocation of NF-кB p65 to the nucleus. Although the NF-кB pathway is often proinflammatory, higher concentrations of SOs (0.3–1 μM) are typically needed to inhibit this pathway than to inhibit the production in vitro of the proinflammatory cytokines (0.001–0.1 μM) summarized in Table 1. As discussed previously, the anti-inflammatory activities of the SOs are more likely to be the result of activation of the Nrf2 pathway rather than direct inhibition of IKK and the NF-кB pathway itself. The inhibition of IкBα degradation by the SOs is more evident in cancer cells than in immune cells (Liby et al., 2008b), and inhibition of this pathway may be a useful marker of anticancer activity (Liby et al., 2009, 2010; Deeb et al., 2011) in cells with constitutive activation of NF-кB.
D. Janus Tyrosine Kinase/Signal Transducer and Activator of Transcription (JAK/STAT)
In addition to suppressing pro-survival signaling through the NF-кB pathway, the SOs also reduce phosphorylation of STAT proteins at similar concentrations needed to inhibit the NF-кB pathway (0.3–1 μM). STATs are transcription factors known to contribute to cellular transformation, proliferation, survival, invasion, and metastasis, and STAT3 is constitutively activated in many types of cancers (Yu et al., 2009; Grivennikov and Karin, 2010). After ligand binding to growth factor receptors, such as the IL-6 receptor, a Janus-activated kinase (JAK) phosphorylates the receptor, allowing recruitment and phosphorylation of a STAT. STATs then dimerize, translocate to the nucleus, and induce transcription of numerous STAT targets, including cyclin D1, myc, and survivin proteins. The SOs rapidly inhibit STAT3 or STAT5 phosphorylation, either constitutive or induced by IL-6 stimulation, in myelomas, osteosarcomas, and lung or breast cancer cells in vitro (Liby et al., 2006, 2007, 2008b; Ahmad et al., 2008; Ryu et al., 2010b) or in vivo (Liao et al., 2011). By directly interacting with Cys1077 on JAK1, CDDO-Me not only suppresses JAK1 phosphorylation but also inhibits its ability to phosphorylate STAT3 and induce STAT3 dimerization (Ahmad et al., 2008). Moreover, the SOs form adducts with STAT3 through interaction with Cys259, suggesting that the potent inhibition of the JAK-STAT pathway by the triterpenoids occurs because of direct inhibition of two different key proteins in the pathway. More recent studies suggest that the SOs reduce IL-6 secretion and inhibit STAT3 phosphorylation in ovarian (Duan et al., 2009) or pancreatic (Liby et al., 2010) cancer cells; the secreted IL-6 may act as a survival factor to drive constitutive activation of the JAK-STAT pathway in these cells (Neurath and Finotto, 2011). In addition to their direct inhibition of JAK and STAT, CDDO-Im also indirectly modulates this signaling pathway by up-regulating negative regulators of the STATs, such as SOCS1 (suppressor of cytokine signaling 1) and the tyrosine phosphatase SHP1 (Liby et al., 2006). Because STAT3 regulates a number of genes important in both wound healing and cancer (Dauer et al., 2005), it is possible that the SOs have different effects on JAK-STAT signaling at lower concentrations than used for the studies described above or in a physiological context of acute inflammation and healing rather than cancer. To date, these important studies have not been performed.
E. Human Epidermal Growth Factor Receptor 2 (HER2)/ErbB2/neu
Overexpression of HER2/ErbB2/neu is found in as many as 25 to 30% of breast cancers (Baselga, 2010; Gutierrez and Schiff, 2011). Because of the aggressiveness of this form of breast cancer, prognosis was poor until the introduction of drugs, such as trastuzumab (Herceptin), that inhibit this receptor tyrosine kinase. CDDO reduces phosphorylation of HER2 in breast cancer cells that overexpress this receptor and inhibits its kinase activity in vitro and in vivo (Konopleva et al., 2006). Although a direct effect on ErbB2 itself was not examined, CDDO-Me significantly delays tumor development and the growth of established tumors in mouse mammary tumor virus-neu mice in which neu overexpression drives tumorigenesis (Liby et al., 2008a). In a mouse model of BRCA-deficient breast cancer, ErbB2 expression increases over time in both the mammary gland and in tumors, and CDDO-Me not only directly interacts with this protein but also inhibits its phosphorylation (Kim et al., 2012).
F. Phosphatase and Tensin Homolog (PTEN) and the Phosphatidylinositol 3-Kinase/Protein Kinase B Pathway (PI3K/Akt)
The phosphatidylinositol 3-kinase (PI3K)/AKT pathway plays an important role in regulating cell division and death (Bunney and Katan, 2010; Vanhaesebroeck et al., 2010), and the loss of activity of the tyrosine phosphatase PTEN results in constitutive activation of the PI3K/Akt pathway (Hollander et al., 2011). Treating U937 leukemia cells with CDDO-Im induces a transient phosphorylation of Akt, but the PI3K inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) blocks the induction of HO-1 by the SOs in these cells (Liby et al., 2005). Likewise, CDDO-Im induces Akt phosphorylation in retinal pigment epithelial cells, but inhibition of Akt activation by LY294002 prevents the cytoprotective benefit of CDDO-Im for survival against oxidative stress induced by tBHP (Pitha-Rowe et al., 2009). A biotinylated SO directly interacts with PTEN, and this interaction is functionally significant because CDDO-Im suppresses, in a dose-dependent manner, the lipid phosphatase activity of PTEN. Moreover, the introduction of a Ser mutation at Cys124 interferes with the binding of the biotinylated SOs to PTEN, suggesting that SOs activate Akt by binding to Cys124 within the active site of PTEN and inhibiting its activity. It is interesting to note that induction of cytoprotective pathways by the SOs involves interacting with Keap or PTEN and the subsequent activation of Nrf2 and Akt, whereas an interaction with constitutively overexpressed IKK, ErbB2, or JAK/STAT by the SOs inhibits the pathway and thus reduces proliferation and/or induces apoptosis.
Although the activation of the Akt/PI3K pathway may be beneficial in normal retinal epithelial cells or in neurons to enhance survival, this pathway is often overexpressed or activated in cancer cells (Engelman, 2009), often through the loss of activity of its inhibitor PTEN (Hollander et al., 2011). Numerous inhibitors of the pathway are being evaluated in clinical trials for the treatment of cancer (Bunney and Katan, 2010; McNamara and Degterev, 2011; Shuttleworth et al., 2011). In cancer cells (Deeb et al., 2007, 2011; Ling et al., 2007; Liu et al., 2012) and in myofibroblasts stimulated with TGFβ (Kulkarni et al., 2011), the SOs inhibit Akt phosphorylation. The dose of SOs used in these experiments is important for explaining these opposing effects on Akt phosphorylation. Although 100 nM CDDO-Im enhances Akt phosphorylation (Liby et al., 2005; Pitha-Rowe et al., 2009), minimum concentrations of 0.5 μM (Ling et al., 2007; Kulkarni et al., 2011) or higher (Deeb et al., 2007, 2008; Liu et al., 2012) of CDDO or CDDO-Me are required to inhibit its phosphorylation. Similar high concentrations of SOs inhibit Akt kinase activity without affecting its upstream kinase PDK1 (Liu et al., 2012).
G. Mammalian Target of Rapamycin (mTOR)
In addition to the studies described above on the PI3K/Akt pathway, a proteomics analysis of potential SO binding partners found that several components of the PI3K/AKT/mTOR pathway are highly enriched, suggesting that mTOR may be a triterpenoid target (Yore et al., 2011). A biotinylated SO directly interacts with mTOR, and 250 nM CDDO-Im inhibits insulin-induced phosphorylation of S6 kinase, 4-EBP, P70S6 kinase, and mTOR without suppressing phosphorylation of Akt. Moreover, an in vitro kinase assay demonstrates that CDDO-Im inhibits the insulin-induced phosphorylation of mTOR. In prostate cancer cells in which the PI3K/Akt/mTOR pathway is constitutively expressed because of mutations in PTEN, CDDO-Im also inhibits phosphorylation of S6 and 4-EBP but not Akt. Because of its role as a sensor of energy balance, cellular stress, growth factors, and nutrient abundance, deregulation of the mTOR pathway contributes to diseases such as diabetes, aging, and cancer (Zoncu et al., 2011). Rapamycin, which targets the mTOR complex 1, is an FDA-approved drug for immunosuppression after organ transplantation and inhibits proliferation of smooth muscle cells after implantation of coronary stents, but several new mTOR inhibitors (Benjamin et al., 2011; Wander et al., 2011) are being developed for cancer and metabolic disorders such as diabetes.
H. Thiol Proteome
Based on the known biology of the triterpenoids, Keap1, PPARγ, IKK, JAK1, STAT3, ErbB2, PTEN, and mTOR were all shown to be direct, valid targets of the SOs. The response of these proteins to the SOs is rapid; changes can be detected within 15 to 60 min and thus before gene transcription and translation can occur. A biotinylated SO or binding studies with recombinant proteins have been used to show direct interactions, and biological effects on the proteins themselves, usually through kinase assays, or their respective downstream pathways confirm the relevance of the pathways to SO biology. It is noteworthy that all of these protein targets contain reactive cysteine residues, which play a critical role in the cellular function. On the basis of the structures of the SOs and the importance of two electrophilic Michael acceptor sites in the A and C rings for activity, it is anticipated that SOs should be able to interact with other proteins containing structurally available, redox-sensitive cysteine residues. It is also possible that a “cysteine code” allows for different reactivities of cysteine residues on target proteins that regulate unique downstream pathways (Kobayashi et al., 2009), potentially explaining the dose response observed with the SOs. To identify other putative protein targets of the SOs, a biotin-tagged triterpenoid was used to treat intact cells and then used as a probe to affinity-purify interacting proteins. More than 500 proteins were identified by liquid chromatography-tandem mass spectrometry in this unbiased proteomics snapshot (Yore et al., 2011), including the known SOs targets IKK, JAK1, and PTEN. When annotated by biological processes, these putative targets primarily function in processes of metabolism, transcription, cell cycle, and signal transduction. If the targets are categorized based on molecular function gene ontology identifiers, more than 75% of the proteins are involved in kinase or catalytic activity, transcription, or regulation of enzyme activity. Moreover, these proteins form a highly interconnected network, suggesting that the SOs do not interact nondiscriminately with random Cys residues in every protein in the cell.
A number of factors affect the reactivity of a Cys residue on a target protein and its ability to interact reversibly with a SO. Basic amino acids in close proximity to a Cys residue on a protein enhance its reactivity, and side chains or a bulky scaffold may limit access to a binding pocket on a protein. The pKa of the local subcellular compartment, hydrophobicity, electrostatic interactions, the redox status of the cell, and allosteric considerations are other factors that regulate Cys reactivity (Doulias et al., 2010; Vazquez-Torres, 2012). The relevance of these issues for SO biology has been reviewed recently (Sporn et al., 2011). Because of this complex biology, experiments in which thiol-containing reagents such as DTT, 2-mercaptoethanol, or N-acetyl cysteine are used to reverse the effects of the SOs must be interpreted with caution, because the resulting interaction between the triterpenoid and –SH group may simply prevent interaction with a cellular target instead of inhibiting the effect. The targeting of cysteine residues on proteins by a multifunctional compound (Na and Surh, 2006; Zhao et al., 2011) such as an SO is not without precedent; endogenous cyclopentenone prostaglandins (Kim and Surh, 2006; Surh et al., 2011) and multifunctional natural products such as sulforaphane and isothiocyanates (Clarke et al., 2008; Cheung and Kong, 2010; Brown and Hampton, 2011), avicins (Haridas et al., 2004; Haridas et al., 2005), and celastrol (Kannaiyan et al., 2011) also act through similar mechanisms. Indeed, intracellular redox signaling may be tightly regulated via a “thiolstat” to allow cells to respond reversibly to oxidative changes in a measured and appropriate manner (Janssen-Heininger et al., 2008; Paulsen and Carroll, 2010; Finkel, 2011; Jacob, 2011). Although drugs that covalently interact with proteins were avoided because of safety concerns early in the era of “targeted therapy,” the prevalence and apparent safety of useful covalent drugs has resulted in a resurgence in this field (Singh et al., 2011). Because of the importance of redox-sensing thiols in signaling and disease (Jones, 2010), the proteins that compose or regulate this signaling network are currently being targeted for drug development (Montero and Jassem, 2011).
A number of additional possible protein targets for the SOs have been reported using the biotinylated triterpenoid pulldown approach. These include the following proteins: protein kinase A, ataxia- and Rad-related-2, ataxia telangiectasia mutated, retinoid X receptor α, AMPK (Yore et al., 2011), cAMP response element-binding protein, CCAAT/enhancer-binding protein β, cyclin D1, and epidermal growth factor receptor (Liby et al., 2009, 2010; Tran et al., 2012). Mitochondrial glutathione (Samudio et al., 2005), the mitochondrial protease Lon (Bernstein et al., 2012), and a limited number of thiol-containing mitochondrial proteins (Brookes et al., 2007) have also been studied using a biotinylated triterpenoid. The SOs also directly interact with tubulin and actin (Couch et al., 2006; To et al., 2010), but the physiological consequences of these interactions are less apparent because very high concentrations of SOs (30–50 μM) are required to disrupt polymerization of these proteins. Moreover, mere interaction of a protein with a biotinylated SO does not ensure functionality. Protein complexes can be identified through proteomics analysis, and SOs can interact with proteins without changing any downstream signaling. The potential relevance of many of these proteins in diseases, however, suggests that additional studies are needed to determine the effects of the SOs on these proteins and pathways. The possibility that SOs interact with different targets in different cell types, that binding affinities to the SOs differ depending on the protein, and that protein abundance and SOs concentrations contribute to the biological complexity are still under investigation.
I. Transforming Growth Factor-β Pathway
In addition to the direct targets that have been identified, the SOs also affect other important signaling pathways. Like the multifunctional SOs, the TGF-β superfamily regulates physiological processes as diverse as inflammation and immunity, cell proliferation and migration, and differentiation and apoptosis (Bierie and Moses, 2010; Ikushima and Miyazono, 2010; Santibañez et al., 2011). CDDO and CDDO-Im increase mRNA expression of the type II TGFβ receptor and prolong Smad2 phosphorylation induced by TGF-β in leukemia cells (Suh et al., 2003a). The effects of the SOs on the TGF-β superfamily are most noticeable when coincubated with TGF-β, and this combination enhances promoter activity for TGF-β, activins, and BMPs. The prolonged Smad2 phosphorylation after treatment with CDDO-Im accompanies delays in the degradation of the TGF-β receptor, changes in microtubule dynamics, altered trafficking, and inhibition of TGF-β-dependent migration but is independent of Smad2 phosphatase activity (To et al., 2008). A biotinylated SO localizes to the leading edge polarity complex on migrating cells and alters the organization of microtubules and their attachment to the polarity complex by interfering with Clip-170, a microtubule-capping protein that regulates membrane association. By interacting with Arp3 and other proteins in the actin cytoskeletal complex, the SOs also disrupt the localization of these proteins and thus inhibit actin polymerization and migration (To et al., 2010). In Smad3(−/−) fibroblasts or after overexpression of Smad7, the ability of CDDO to inhibit MMP1 and MMP13 promoter activity is partially reversed (Mix et al., 2004). During the induction of monocytic differentiation by CDDO-Im, the triterpenoid activates phosphorylation of Erk1/2 and Smad1, -3, and -5 and increases mRNA expression of BMP-6, BMP receptor II, TGF-β2 and TGF-β receptor II (Ji et al., 2006). The mitogen-activated protein kinase kinase inhibitor 2′-amino-3′-methoxyflavone (PD98059), antibodies against TGF-β, and the BMP antagonist noggin all partially inhibit differentiation induced by CDDO-Im. Moreover, both CDDO-Im and CDDO-EA induce chondrogenesis in newborn mouse calvaria and up-regulate genes involved in this process, including SOX9, BMPs, Smads, and TIMPs (Suh et al., 2012). Context is again important, however, because the triterpenoids can also reduce the secretion of TGF-β (Liao et al., 2011) or inhibit its effects. In pulmonary fibrosis or in corneal scarring, TGF-β drives the differentiation of fibroblasts into myofibroblasts, but the SOs inhibit both the differentiation of these cells and the subsequent TGF-β-dependent production of collagen and other matrix components (Ferguson et al., 2009a; Kulkarni et al., 2011; Kuriyan et al., 2012). Despite these observations, no proximate direct interaction between a SO and any member of the TGF-β pathway has yet been identified.
J. Other Pathways
Although numerous microarray studies have revealed the importance of activation of Nrf2 by the triterpenoids (Lapillonne et al., 2003; Liby et al., 2005; Yates et al., 2006; Yates et al., 2009), other genes and signaling pathways are also affected and undoubtedly contribute to the mechanism of action of the SOs. A number of natural triterpenoids regulate glucose and lipid metabolism (Sheng and Sun, 2011), and the SOs also regulate a number of genes involved in metabolism (Yates et al., 2009). Single studies also suggest the possible importance of glycogen synthase kinase 3β (Venè et al., 2008), fatty acid synthase (Hughes et al., 2008), specificity proteins (Jutooru et al., 2010), and phospho-Chk1 and phospho-Chk2 (Kim et al., 2011) to SO activity, but additional work is needed to confirm the biological relevance of these proteins and to address whether they are direct targets or downstream of the targets discussed previously. Moreover, the triterpenoids clearly induce apoptosis of cancer cells, but neither the direct target of the SOs nor the initiating event for starting this cascade has been identified.
To add to the complexity of the biology, there is often cross talk between pathways targeted by the SOs. For example, components of the NF-кB pathway and STAT3 can physically interact, cooperate at enhancer or other promoter elements, and negatively regulate each other (Grivennikov and Karin, 2010; He and Karin, 2011). Mutations in Nrf2 can activate the mTOR pathway and induce phosphorylation of S6 by up-regulating Ras small GTPase D, a new activator of the mTOR pathway (Shibata et al., 2010). There are also several reports documenting interactions between the Nrf2/Keap1 pathway and IKK. Under physiological conditions, Keap1 ubiquitinates IKKβ, resulting in its degradation. If Keap1 is eliminated or the E3 ubiquitin ligase complex is disrupted, IKKβ accumulates and activates the NF-кB pathway to up-regulate numerous downstream angiogenic and proinflammatory cytokines and to enhance survival (Lee et al., 2009; Thu et al., 2011). Although Keap1 can negatively regulate the NF-кB pathway (Kim et al., 2010), the p65 subunit of NF-кB can also interact with Keap1 and suppress Nrf2-ARE signaling (Yu et al., 2011). Other pathways that interact with the Nrf2 pathway have been reviewed recently (Wakabayashi et al., 2010).
K. Summary
In summary, many of the targets of the SOs seem to be “hubs” in cellular networks that medicate anti-inflammatory, antioxidative, antiproliferative, differentiative, and proapoptotic activities and include proteins and signaling cascades as diverse as Keap1/Nrf2, the PPARγ receptor, the NF-κB pathway, the JAK-STAT pathway, the tumor suppressor PTEN, the growth factor receptor ErbB2, the mTOR complex, and the TGF-β pathway. Many of these targets are transcription factors or signaling pathways that regulate transcription factors; thus, there is an amplification effect on the expression of many downstream genes. The high affinity of SOs for the Keap1/Nrf2 pathway is undoubtedly of paramount importance, but somehow the activities of all of these different networks are integrated within the cell in a useful manner to maintain homeostasis. In addition to these direct protein targets, triterpenoid doses and differences in cell types and their redox status are also important considerations (Fig. 4). Clearly, the sum total and integration of all of these interactions are critical to an understanding of the overall physiology and pharmacology of the SOs.
Fig. 4.
The importance of context for SO biology. As summarized in the review, the SOs directly interact with a number of cellular targets, including Keap1, IKK, JAK1/STAT3, PPARγ, ErbB2, PTEN, mTOR, and Arp3; all of these are relevant targets because the SOs also affect downstream signaling pathways associated with each of these proteins. The molecular targets differ depending on cell type, because not all of these proteins are expressed in every cell. The SOs have been shown to regulate various biological outcomes such as inflammation in immune cells (macrophages, neutrophils, lymphocytes), angiogenesis in endothelial cells, and differentiation and cell proliferation/death in epithelial cells. Biological responses to the SOs also depend on dose, because low (nanomolar) concentrations induce anti-inflammatory and cytoprotective pathways, medium (mid-high nanomolar) concentrations affect cell growth and differentiation, and high (low micromolar) concentrations can induce apoptosis of cancer cells. It is noteworthy that the induction of apoptosis seems to depend on the mutational status of the cell, because the same concentrations of SOs that apoptose premalignant or malignant cancer cells have no effect on normal cells. Moreover, the pharmacokinetics (PK) of the SOs, including absorption, tissue distribution, metabolism, and excretion, differ depending on the specific SOs. For example, the amide derivatives of CDDO cross the blood brain-barrier better than other derivatives, and despite the exceptional potency of di-CDDO, its instability or rapid metabolism are not ideal for prolonged in vivo use. Thus, different protein targets within a cell, various cell types, the mutations within cells, the dose of the SOs, and the pharmacology of each unique derivative must be integrated and understood to determine the biological response to a SO and ideally to target specific compounds to the most relevant disease.
VII. Clinical Activity
The SOs were originally developed as anticancer agents, and the earliest clinical trials were in onocology. A first-in-man clinical trial evaluated CDDO (bardoxolone) in nine patients with refractory AML (Tsao et al., 2010). After treatment with 0.6 to 75 mg/m2 per hour for 5 days, differentiation of immature blasts increased in four of nine patients, as measured by increased expression of CD11b and CD14 and reduced expression of CD33 and CD34. Increased apoptosis was also detected as a loss of membrane potential in mitochondria in 3 patients. However, the maximum tolerated dose was not reached in this study, and differential counts did not change significantly. CDDO was also tested in a phase I dose-escalation study in patients with advanced solid tumors (http://clinicaltrials.gov/ct2/show/NCT00322140). CDDO was given to seven patients by continuous infusion (0.6–38.4 mg/m2 per hour) for 5 days in 28-day cycles to determine the maximum tolerated dose, toxicity, and pharmacokinetics and pharmacodynamics of the drug (Speranza et al., 2012). Plasma concentrations of CDDO above 1 μM, the target blood level determined in preclinical studies, were achieved in only a single patient. No antitumor activity or induction of apoptosis in PBMCs, as determined by poly (ADP-ribose) polymerase cleavage, was observed. This study was terminated because of adverse thrombotic events in four patients, although it was not possible to determine whether these adverse events were the result of CDDO or the well established high rate of thromboembolism in patients with metastatic disease.
Because of the lower potency of CDDO and the need to administer it intravenously, development efforts were switched to CDDO-Me, which is orally active and more potent. Interim results from dose-escalation studies with bardoxolone methyl (http://clinicaltrials.gov/ct2/show/NCT00508807) in 34 or 47 patients with advanced, refractory solid tumors or lymphoid malignancies have been presented (Dezube et al., 2007; Hong et al., 2008). The drug was administered orally for 21 of every 28 days at doses from 5 to 1300 mg/day. Modulation of the SO targets Nrf2, STAT3, and NFкB activity was confirmed in both studies. Bardoxolone methyl was well tolerated; 91% of patients had minimal toxicities (< grade 2) even when administered for up to 1 year, and a dose of 900 mg/day was set for phase 2 studies. Of 30 assessable patients in the second study, 40% achieved disease stabilization and two objective responses were seen (Hong et al., 2008). The results of this phase 1 study have been published (Hong et al., 2012).
In the phase 1 trials involving 81 patients with cancer, bardoxolone methyl unexpectedly reduced serum creatinine levels and increased the baseline estimated glomerular filtration rate (eGFR). To confirm these results, 20 patients with moderate to severe chronic kidney disease (CKD) and type 2 diabetes were treated with the drug (Pergola et al., 2011a). Patients received 25 mg of bardoxolone methyl for 28 days and then 75 mg of drug for another 28 days. Baseline eGFR increased an average of 7.2 ml/min per 1.73 m2; creatinine clearance increased whereas serum creatinine, blood urea nitrogen, and levels of circulating endothelial cells all declined. It is noteworthy that these significant improvements in kidney function were observed throughout the study in almost 90% of the patients, without any serious drug-related adverse events in this open-label, phase 2a study. In a subsequent phase 2, placebo-controlled, double-blind, randomized trial, 227 patients with stage 3 or 4 CKD were treated with 25, 75, or 150 mg of bardoxolone methyl (Pergola et al., 2011b). The primary objective in this BEAM trial (Bardoxolone Methyl Treatment: Renal Function in CKD/Type 2 Diabetes, NCT00811889) was a change in eGFR. This marker of kidney function increased within 4 weeks of treatment with bardoxolone methyl, peaked at 12 weeks, and was stable through 52 weeks. The primary outcome was achieved at 24 weeks, with a significant improvement from the baseline eGFR in all groups treated with bardoxolone methyl compared with the placebo group (mean differences of 8.2 ± 1.5, 11.4 ± 1.5, and 10.4 ± 1.5 ml/min per 1.73 m2 in the 25-, 75-, and 150-mg groups, respectively), and these significant changes were still maintained at 52 weeks. These changes in eGFR were accompanied by significant changes in blood chemistry, including declines in blood urea nitrogen, serum phosphorus, uric acid, and magnesium. Although the number of adverse events was more common in patients treated with bardoxolone methyl than in the placebo group, muscle spasms were the most common adverse event and frequently disappeared, whereas other adverse events were generally mild to moderate. Despite the potential utility of bardoxolone methyl for increasing kidney function in CKD, a disease for which there is no effective treatment, eGFR is only a surrogate marker for actual GFR and kidney function. Moreover, the confirmation of a long-term clinical benefit after these reported improvements is needed and will be evaluated in the BEACON study (Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes; http://clinicaltrials.gov/ct2/show/NCT01351675). This ongoing worldwide phase 3 study will evaluate the ability of bardoxolone methyl to slow the progression of end-stage renal disease or lessen cardiovascular death in approximately 1600 patients with stage 4 CKD and advanced type 2 diabetes.
In the clinical trials thus far with bardoxolone methyl, safety issues have been minimal (Pergola et al., 2011b). Likewise, many other SOs have been fed safely to both rats and mice for prolonged periods of time, sometimes for up to a year, with few untoward effects. Most of the data from animal toxicology studies remain unpublished, proprietary information. However, it is clear from the decision of the FDA to allow long-term clinical administration of bardoxolone methyl in a phase 3 trial that this agent seems to have a good safety profile.
VIII. Summary and Future Perspectives
The ultimate utility of SOs for prevention of disease rests on their ability to facilitate homeostatic responses to stress. Although their enhancement of Nrf2 activity is a critical aspect of such homeostasis, SOs have many other important molecular targets, which are connected through complex networks of feedback responses. In this article, we have reviewed additional interactions of SOs with regulatory networks such as NF-κB, PPAR-γ, JAK-STAT, HER2/ErbB2/neu, PTEN, PI3K/Akt, mTOR, TGF-β, and the thiol proteome, all of which are involved in response to stress. The concerted, integrated action of these various networks is ultimately what provides homeostasis and allows the development of pharmacologically useful drugs. Particularly because it is now realized from genetic studies that single-targeted enhancement of Nrf2 (as results from mutations of either Keap1 or Nrf2) can have undesirable cancer-enhancing activity (Kensler and Wakabayashi, 2010), it becomes all the more important to emphasize that SOs are not single-target drugs. Rather, they can interact with a wide range of molecular targets, and such interactions have desirable effects, including prevention of cancer, and many other diseases, in many different organs. However, SOs are not “magic bullets” for cures, and to be used most effectively, they should be used as early as possible in the pathogenesis of any disease, when a homeostatic agent is appropriate. Attempts to use SOs for treatment of end-stage inflammatory disease, when irreversible cell death and tissue damage have occurred, will most likely end in failure.
SOs are thus in the vanguard of development of new drugs designed to interact with entire networks (Barabási et al., 2011; Vidal et al., 2011) in the new science of “network pharmacology,” or “polypharmacology” (Hopkins, 2007, 2008). Ultimately, as noted by Barabási (2007),
… the fundamental question of where function lies within a cell is slowly shifting from a single-minded focus on genes to the understanding that behind each cellular function there is a discernible network module consisting of genes, transcription factors, RNAs, enzymes, and metabolites. This understanding forces us to view diseases as the breakdown of selected functional modules, rather than as single or small groups of genes.
There is thus a need to synthesize “a new generation of drugs that perturb biological networks rather than individual targets” (Hopkins, 2008). The appropriate use of multifunctional drugs, such as SOs, will depend on understanding the biological context in which they are to be used and, just as importantly, their appropriate dosages. It may be desirable to use a specific SO at a given dose level early in pathogenesis, but higher doses can provide opposite results and may not be appropriate for treatment of advanced inflammatory disease. However, the induction of apoptosis by high doses of SOs may be useful for treatment of invasive cancer, especially if the SOs are combined with chemotherapeutic or immunomodulatory drugs.
To account for this multifunctionality and contextuality of SOs, one need look only at the evolution of these molecules as drugs. To begin with, pentacyclic OA appeared in the evolution of plants as a consequence of a unique folding of the linear squalene molecule, driven by a stereospecific oxidosqualene cyclase. The stereochemistry of the five rings and the eight exocyclic carbons of OA provide specific recognition of protein motifs in cells. At the same time, this specific stereochemistry, especially that of the exocyclic carbon atoms, prevents random contact of OA with all cellular proteins (Sporn et al., 2007). Plants containing OA have been ingested by animals for millennia without harmful effects and with, some benefit undoubtedly resulting from the weak anti-inflammatory effect of OA. Thus, evolution has provided its own selective “wisdom” for the eventual design of SOs. The skill of the synthetic organic chemist has now been added to this evolutionary wisdom, to maximize the selective, contextual interactions of OA with target proteins by inserting new enone functions and electron-withdrawing substituents, such as –CN, to enhance Michael reactivity and create molecules thousands of times more active than OA itself but still relatively nontoxic. We have described some of these useful actions here. This paradigm of using synthetic organic chemistry to enhance pharmacological utility of a natural product now awaits further development with other stereochemically unique triterpenoid scaffolds, and it can again be predicted that multifunctional drugs will be the result.
Beyond the synthetic chemistry, the biological evaluation of the utility of such multifunctional drugs will need to be done in the context of their role in modulating the overall physiological behavior of complex, interactive cellular networks (Levy et al., 2010). This will require not only cell-based assays but also eventual validation in vivo. The ability of SOs to control both the inflammatory and the oxidative stress that drives so many human diseases is central to their potential clinical usefulness. We hope that synthetic triterpenoids will have a useful future in preventing or alleviating the pain and suffering caused by many chronic diseases. As summarized here, SOs should provide a useful model to assist in reaching that goal.
Acknowledgments
This work was supported by National Institutes of Health National Cancer Institute [Grant R01-CA78814]; the National Foundation for Cancer Research; the Breast Cancer Research Foundation; and Reata Pharmaceuticals. Many colleagues have contributed greatly to this work over the past 15 years. Special thanks are due to the following: for chemistry, G. Gribble, T. Honda, H. Finlay, L. Bore, B. Rounds, F. Favaloro, T. Janosik, J. Han, H. Yoshizawa, C. Sundararajan, E. David, M. Lajoie, E. Padegimas, X. Jiang, E. Anderson and M. Visnick. For biology, N. Suh, Y. Wang, A. Place, M. Rendi, E. Heiss, K. Mix, M. Yore, I. Pitha-Rowe, E. Kim, K. Tran, C. Williams, R. Risingsong, D. Royce, P. Talalay, A. Dinkova-Kostova, T. Kensler, M. Yates, S. Biswal, R. Thimmulappa, J. Letterio, J. Handa, E. Duh, F. Beal, A. Albini, M. Konopleva, M. Andreeff, J. Reed, C. Meyer, C. Wigley, D. Ferguson, S. Ruiz, and I. Dulubova. Special appreciation is owed to C. Nathan and the late A. Roberts for their inspiration and assistance in starting this project and to G. Gribble and T. Honda for their leadership with the synthetic chemistry. G. Tuson has provided helpful assistance with the manuscript and figures.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Liby and Sporn.
This article is available online at http://pharmrev.aspetjournals.org.
- AKT/Akt
- protein kinase B
- ALI
- acute lung injury
- ALS
- amyotrophic lateral sclerosis
- AML
- acute myeloid leukemia
- AMPK
- AMP-activated protein kinase
- APL
- acute promyelocytic leukemia
- ARDS
- acute respiratory distress syndrome
- ARE
- antioxidant response element
- ATRA
- all-trans-retinoic acid
- Bax
- Bcl-2–associated X protein
- BAY 11-7082
- 3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile
- Bcl-2
- B-cell lymphoma 2
- Bcl-xL
- B-cell lymphoma-extra large
- BMP
- bone morphogenetic protein
- BRCA
- breast cancer associated gene
- CBP
- cAMP response element-binding binding protein
- CDDO
- 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid
- CGD
- chronic granulomatous disease
- CKD
- chronic kidney disease
- CLL
- chronic lymphocytic leukemia
- ConA
- concanavalin A
- COPD
- chronic obstructive pulmonary disease
- CrmA
- cytokine response modifier A
- CTL
- cytotoxic T lymphocyte
- DR
- death receptor
- DTT
- dithiothreitol
- EA
- ethyl amide
- eGFR
- estimated glomerular filtration rate
- Erk
- extracellular signal-regulated kinase
- GVHD
- graft-versus-host disease
- Her2/ErbB2/neu
- human epidermal growth factor receptor 2
- HO-1
- heme oxygenase-1
- IKBα
- inhibitor of nuclear factor-κBα
- IKK
- IкB kinase
- IL
- interleukin
- Im
- imidazolide
- iNOS
- inducible nitric-oxide synthase
- JAK
- Janus kinase
- jnk
- c-Jun N-terminal kinase
- Keap1
- Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1
- LG100268
- 6-(1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopropyl)nicotinic acid
- LPS
- lipopolysaccharide
- LY294002
- 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
- MA
- methyl amide
- MDSC
- myeloid-derived suppressor cell
- MEF
- mouse embryonic fibroblast
- MMP
- matrix metalloproteinase
- mTOR
- mammalian target of rapamycin
- NF-κB
- nuclear factor-κB
- NOS
- nitric-oxide synthase
- NQO1
- NAD(P)H quinone oxidoreductase 1
- OA
- oleanolic acid
- p
- phospho
- PBMC
- peripheral blood mononuclear cell
- PD98059
- 2′-amino-3′-methoxyflavone
- PG
- prostaglandin
- PI3K
- phosphatidylinositol 3-kinase
- PKC412
- midostaurin
- PML
- promyelocytic leukemia
- PPAR
- peroxisome proliferator activated receptor
- PTEN
- phosphatase and tensin homolog
- RAR
- retinoic acid receptor
- ROS
- reactive oxygen species
- SN50
- Ala-Ala-Val-Ala-Leu-Leu-Pro-Ala-Val-Leu-Leu-Ala-Leu-Leu-Ala-Pro-Val-Gln-Arg-Lys-Arg-Gln-Lys-Leu-Met-Pro
- SO
- synthetic pentacyclic oleanane triterpenoid
- STAT
- signal transducer and activator of transcription
- T007
- N-(4-aminopyridyl-2-chloro-5-nitrobenzamide
- TFEA
- trifluoroethyl amide
- TGF
- transforming growth factor
- TNF
- tumor necrosis factor
- TRAIL
- TNF-related apoptosis-inducing ligand
- TRAMP
- transgenic adenocarcinoma of the mouse prostate
- UA
- ursolic acid
- VEGF
- vascular endothelial growth factor.
References
- Abel EL, Bubel JD, Simper MS, Powell L, McClellan SA, Andreeff M, MacLeod MC, DiGiovanni J. (2011) Protection against 2-chloroethyl ethyl sulfide (CEES)-induced cytotoxicity in human keratinocytes by an inducer of the glutathione detoxification pathway. Toxicol Appl Pharmacol 255:176–183 [DOI] [PubMed] [Google Scholar]
- Abraham NG, Cao J, Sacerdoti D, Li X, Drummond G. (2009) Heme oxygenase: the key to renal function regulation. Am J Physiol Renal Physiol 297:F1137–F1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya A, Das I, Chandhok D, Saha T. (2010) Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev 3:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad R, Liu S, Weisberg E, Nelson E, Galinsky I, Meyer C, Kufe D, Kharbanda S, Stone R. (2010) Combining the FLT3 inhibitor PKC412 and the triterpenoid CDDO-Me synergistically induces apoptosis in acute myeloid leukemia with the internal tandem duplication mutation. Mol Cancer Res 8:986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. (2006) Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem 281:35764–35769 [DOI] [PubMed] [Google Scholar]
- Ahmad R, Raina D, Meyer C, Kufe D. (2008) Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)–signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res 68:2920–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabran JL, Cheuk A, Liby K, Sporn M, Khan J, Letterio J, Leskov KS. (2008) Human neuroblastoma cells rapidly enter cell cycle arrest and apoptosis following exposure to C-28 derivatives of the synthetic triterpenoid CDDO. Cancer Biol Ther 7:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Goedken MJ, Rockwell CE, Thomale J, Manautou JE, Klaassen CD. (2010) Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther 335:2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames E, Harouna S, Meyer C, Welniak LA, Murphy WJ. (2012) The triterpenoid CDDO-Me promotes hematopoietic progenitor expansion and myelopoiesis in mice. Biol Blood Marrow Transplant 18:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auletta JJ, Alabran JL, Kim BG, Meyer CJ, Letterio JJ. (2010) The synthetic triterpenoid, CDDO-Me, modulates the proinflammatory response to in vivo lipopolysaccharide challenge. J Interferon Cytokine Res 30:497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill FR, Mantovani A. (2012) Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 22:33–40 [DOI] [PubMed] [Google Scholar]
- Barabási AL. (2007) Network medicine–from obesity to the “diseasome”. N Engl J Med 357:404–407 [DOI] [PubMed] [Google Scholar]
- Barabási AL, Gulbahce N, Loscalzo J. (2011) Network medicine: a network-based approach to human disease. Nat Rev Genet 12:56–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J. (2010) Treatment of HER2-overexpressing breast cancer. Ann Oncol 21 (Suppl 7):vii36–vii40 [DOI] [PubMed] [Google Scholar]
- Bauer AK, Cho HY, Miller-Degraff L, Walker C, Helms K, Fostel J, Yamamoto M, Kleeberger SR. (2011) Targeted deletion of Nrf2 reduces urethane-induced lung tumor development in mice. PLoS One 6:e26590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y, Karin M. (2011) Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol 12:715–723 [DOI] [PubMed] [Google Scholar]
- Benjamin D, Colombi M, Moroni C, Hall MN. (2011) Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov 10:868–880 [DOI] [PubMed] [Google Scholar]
- Bernstein SH, Venkatesh S, Li M, Lee J, Lu B, Hilchey SP, Morse KM, Metcalfe HM, Skalska J, Andreeff M, et al. (2012) The mitochondrial ATP-dependent Lon protease: a novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Blood 119:3321–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Moses HL. (2010) Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev 21:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Morse K, Ray D, Tompkins A, Young SM, Hilchey S, Salim S, Konopleva M, Andreeff M, Phipps R, et al. (2007) The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid and its derivatives elicit human lymphoid cell apoptosis through a novel pathway involving the unregulated mitochondrial permeability transition pore. Cancer Res 67:1793–1802 [DOI] [PubMed] [Google Scholar]
- Brown KK, Hampton MB. (2011) Biological targets of isothiocyanates. Biochim Biophys Acta 1810:888–894 [DOI] [PubMed] [Google Scholar]
- Bunney TD, Katan M. (2010) Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer 10:342–352 [DOI] [PubMed] [Google Scholar]
- Burton NC, Kensler TW, Guilarte TR. (2006) In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology 27:1094–1100 [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J, Johnson JA. (2009) The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal 11:497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M, Thimmalappula R, Fujihara M, Nagai N, Sporn M, Wang AL, Neufeld AH, Biswal S, Handa JT. (2010) Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Res 50:652–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10:417–427 [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Podar K, Hideshima T, Shringarpure R, Catley L, Mitsiades C, Munshi N, Tai YT, Suh N, et al. (2004) The bortezomib/proteasome inhibitor PS-341 and triterpenoid CDDO-Im induce synergistic anti-multiple myeloma (MM) activity and overcome bortezomib resistance. Blood 103:3158–3166 [DOI] [PubMed] [Google Scholar]
- Cheung KL, Kong AN. (2010) Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. Aaps J 12:87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Konopleva M, Andreef M, Samudio I, Safe S. (2005) 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol Pharmacol 68:119–128 [DOI] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. (2002) Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26:175–182 [DOI] [PubMed] [Google Scholar]
- Cho HY, Kleeberger SR. (2010) Nrf2 protects against airway disorders. Toxicol Appl Pharmacol 244:43–56 [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. (2004) The transcription factor NRF2 protects against pulmonary fibrosis. Faseb J 18:1258–1260 [DOI] [PubMed] [Google Scholar]
- Clarke JD, Dashwood RH, Ho E. (2008) Multi-targeted prevention of cancer by sulforaphane. Cancer Lett 269:291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RD, Browning RG, Honda T, Gribble GW, Wright DL, Sporn MB, Anderson AC. (2005) Studies on the reactivity of CDDO, a promising new chemopreventive and chemotherapeutic agent: implications for a molecular mechanism of action. Bioorg Med Chem Lett 15:2215–2219 [DOI] [PubMed] [Google Scholar]
- Couch RD, Ganem NJ, Zhou M, Popov VM, Honda T, Veenstra TD, Sporn MB, Anderson AC. (2006) 2-cyano-3,12-dioxooleana-1,9(11)-diene-28-oic acid disrupts microtubule polymerization: a possible mechanism contributing to apoptosis. Mol Pharmacol 69:1158–1165 [DOI] [PubMed] [Google Scholar]
- Daroczi B, Kari G, Ren Q, Dicker AP, Rodeck U. (2009) Nuclear factor kappaB inhibitors alleviate and the proteasome inhibitor PS-341 exacerbates radiation toxicity in zebrafish embryos. Mol Cancer Ther 8:2625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, Enkemann S, Jove R, Haura EB. (2005) Stat3 regulates genes common to both wound healing and cancer. Oncogene 24:3397–3408 [DOI] [PubMed] [Google Scholar]
- Deeb D, Gao X, Arbab AS, Barton K, Dulchavsky SA, Gautam SC. (2010a) CDDO-Me: a novel synthetic triterpenoid for the treatment of pancreatic cancer. Cancers 2:1779–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb D, Gao X, Dulchavsky SA, Gautam SC. (2007) CDDO-me induces apoptosis and inhibits Akt, mTOR and NF-kappaB signaling proteins in prostate cancer cells. Anticancer Res 27:3035–3044 [PubMed] [Google Scholar]
- Deeb D, Gao X, Dulchavsky SA, Gautam SC. (2008) CDDO-Me inhibits proliferation, induces apoptosis, down-regulates Akt, mTOR, NF-kappaB and NF-kappaB-regulated antiapoptotic and proangiogenic proteins in TRAMP prostate cancer cells. J Exp Ther Oncol 7:31–39 [PubMed] [Google Scholar]
- Deeb D, Gao X, Jiang H, Dulchavsky SA, Gautam SC. (2009) Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells by independently targeting pro-survival Akt and mTOR. Prostate 69:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb D, Gao X, Jiang H, Janic B, Arbab AS, Rojanasakul Y, Dulchavsky SA, Gautam SC. (2010b) Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem Pharmacol 79:350–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb D, Gao X, Liu Y, Jiang D, Divine GW, Arbab AS, Dulchavsky SA, Gautam SC. (2011) Synthetic triterpenoid CDDO prevents the progression and metastasis of prostate cancer in TRAMP mice by inhibiting survival signaling. Carcinogenesis 32:757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475:106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezube BJ, Kurzrock R, Eder JP, Supko JG, Meyer CJ, Camacho LH, Andreeff M, Konopleva M, Lescale-Matys L, Hong D. (2007) Interim results of a phase I trial with a novel orally administered synthetic triterpenoid RTA 402 (CDDO-Me) in patients with solid tumors and lymphoid malignancies (Abstract). J Clin Oncol 25 (18 Suppl):14101 [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA 99:11908–11913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Jenkins SN, Wehage SL, Huso DL, Benedict AL, Stephenson KK, Fahey JW, Liu H, Liby KT, Honda T, et al. (2008) A dicyanotriterpenoid induces cytoprotective enzymes and reduces multiplicity of skin tumors in UV-irradiated mice. Biochem Biophys Res Commun 367:859–865 [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, et al. (2005) Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA 102:4584–4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulias PT, Greene JL, Greco TM, Tenopoulou M, Seeholzer SH, Dunbrack RL, Ischiropoulos H. (2010) Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc Natl Acad Sci USA 107:16958–16963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Ames RY, Ryan M, Hornicek FJ, Mankin H, Seiden MV. (2009) CDDO-Me, a synthetic triterpenoid, inhibits expression of IL-6 and Stat3 phosphorylation in multi-drug resistant ovarian cancer cells. Cancer Chemother Pharmacol 63:681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Wille E, Calingasan NY, Tampellini D, Williams C, Gouras GK, Liby K, Sporn M, Nathan C, Flint Beal M, et al. (2009) Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer's disease. J Neurochem 109:502–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF, Weaver VM, Tlsty TD, Bergers G. (2011) Tumor microenvironment and progression. J Surg Oncol 103:468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S, Hays E, Mayor M, Sporn M, Vincenti M. (2003) The triterpenoid CDDO inhibits expression of matrix metalloproteinase-1, matrix metalloproteinase-13 and Bcl-3 in primary human chondrocytes. Arthritis Res Ther 5:R285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsawa SF, Novak AJ, Grote D, Konopleva M, Andreeff M, Witzig TE, Ansell SM. (2008) CDDO-imidazolide mediated inhibition of malignant cell growth in Waldenström macroglobulinemia. Leuk Res 32:1895–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA. (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9:550–562 [DOI] [PubMed] [Google Scholar]
- Escartin C, Brouillet E. (2010) The Nrf2 pathway as a potential therapeutic target for Huntington disease A commentary on “Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington disease”. Free Radic Biol Med 49:144–146 [DOI] [PubMed] [Google Scholar]
- Eskiocak U, Kim SB, Roig AI, Kitten E, Batten K, Cornelius C, Zou YS, Wright WE, Shay JW. (2010) CDDO-Me protects against space radiation-induced transformation of human colon epithelial cells. Radiat Res 174:27–36 [DOI] [PubMed] [Google Scholar]
- Ferguson HE, Kulkarni A, Lehmann GM, Garcia-Bates TM, Thatcher TH, Huxlin KR, Phipps RP, Sime PJ. (2009a) Electrophilic peroxisome proliferator-activated receptor-gamma ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol 41:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson HE, Thatcher TH, Olsen KC, Garcia-Bates TM, Baglole CJ, Kottmann RM, Strong ER, Phipps RP, Sime PJ. (2009b) Peroxisome proliferator-activated receptor-gamma ligands induce heme oxygenase-1 in lung fibroblasts by a PPARgamma-independent, glutathione-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 297:L912–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Deeb D, Danyluk A, Media J, Liu Y, Dulchavsky SA, Gautam SC. (2008) Immunomodulatory activity of synthetic triterpenoids: inhibition of lymphocyte proliferation, cell-mediated cytotoxicity, and cytokine gene expression through suppression of NF-kappaB. Immunopharmacol Immunotoxicol 30:581–600 [DOI] [PubMed] [Google Scholar]
- Gao X, Deeb D, Hao J, Liu Y, Arbab AS, Dulchavsky SA, Gautam SC. (2010) Synthetic triterpenoids inhibit growth, induce apoptosis and suppress pro-survival Akt, mTOR and NF-{kappa}B signaling proteins in colorectal cancer cells. Anticancer Res 30:785–792 [PMC free article] [PubMed] [Google Scholar]
- Gao X, Deeb D, Jiang H, Liu Y, Dulchavsky SA, Gautam SC. (2007) Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-kappaB and Notch1 signaling. J Neurooncol 84:147–157 [DOI] [PubMed] [Google Scholar]
- Gao X, Deeb D, Liu P, Liu Y, Arbab-Ali S, Dulchavsky SA, Gautam SC. (2011a) Role of reactive oxygen species (ROS) in CDDO-Me-mediated growth inhibition and apoptosis in colorectal cancer cells. J Exp Ther Oncol 9:119–127 [PMC free article] [PubMed] [Google Scholar]
- Gao X, Liu Y, Deeb D, Arbab AS, Guo AM, Dulchavsky SA, Gautam SC. (2011b) Synthetic oleanane triterpenoid, CDDO-Me, induces apoptosis in ovarian cancer cells by inhibiting prosurvival AKT/NF-κB/mTOR signaling. Anticancer Res 31:3673–3681 [PMC free article] [PubMed] [Google Scholar]
- Giudice A, Arra C, Turco MC. (2010) Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol 647:37–74 [DOI] [PubMed] [Google Scholar]
- Glass CK. (2006) Going nuclear in metabolic and cardiovascular disease. J Clin Invest 116:556–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Ogawa S. (2006) Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol 6:44–55 [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goven D, Boutten A, Leçon-Malas V, Marchal-Sommé J, Amara N, Crestani B, Fournier M, Lesèche G, Soler P, Boczkowski J, et al. (2008) Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax 63:916–924 [DOI] [PubMed] [Google Scholar]
- Graber DJ, Park PJ, Hickey WF, Harris BT. (2011) Synthetic triterpenoid CDDO derivatives modulate cytoprotective or immunological properties in astrocytes, neurons, and microglia. J Neuroimmune Pharmacol 6:107–120 [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. (2010) Immunity, inflammation, and cancer. Cell 140:883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Karin M. (2010) Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 21:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Patani R, Baxter P, Serio A, Story D, Tsujita T, Hayes JD, Pedersen RA, Hardingham GE, Chandran S. (2012) Human embryonic stem cell derived astrocytes mediate non-cell-autonomous neuroprotection through endogenous and drug-induced mechanisms. Cell Death Differ 19:779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C, Schiff R. (2011) HER2: biology, detection, and clinical implications. Arch Pathol Lab Med 135:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hail N, Jr., Konopleva M, Sporn M, Lotan R, Andreeff M. (2004) Evidence supporting a role for calcium in apoptosis induction by the synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO). J Biol Chem 279:11179–11187 [DOI] [PubMed] [Google Scholar]
- Han SS, Peng L, Chung ST, DuBois W, Maeng SH, Shaffer AL, Sporn MB, Janz S. (2006) CDDO-Imidazolide inhibits growth and survival of c-Myc-induced mouse B cell and plasma cell neoplasms. Mol Cancer 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas V, Hanausek M, Nishimura G, Soehnge H, Gaikwad A, Narog M, Spears E, Zoltaszek R, Walaszek Z, Gutterman JU. (2004) Triterpenoid electrophiles (avicins) activate the innate stress response by redox regulation of a gene battery. J Clin Invest 113:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas V, Kim SO, Nishimura G, Hausladen A, Stamler JS, Gutterman JU. (2005) Avicinylation (thioesterification): a protein modification that can regulate the response to oxidative and nitrosative stress. Proc Natl Acad Sci USA 102:10088–10093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasinoff BB, Patel D. (2010) The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol Appl Pharmacol 249:132–139 [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. (2010) Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal 13:1713–1748 [DOI] [PubMed] [Google Scholar]
- He G, Karin M. (2011) NF-κB and STAT3—key players in liver inflammation and cancer. Cell Res 21:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss EH, Schachner D, Werner ER, Dirsch VM. (2009) Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem 284:31579–31586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan CM, Thatcher TH, Sapinoro RE, Gurell MN, Ferguson HE, Pollock SJ, Jones C, Phipps RP, Sime PJ. (2011) Electrophilic PPARγ ligands attenuate IL-1β and silica-induced inflammatory mediator production in human lung fibroblasts via a PPARγ-independent mechanism. PPAR Res 2011:318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander MC, Blumenthal GM, Dennis PA. (2011) PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 11:289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Janosik T, Honda Y, Han J, Liby KT, Williams CR, Couch RD, Anderson AC, Sporn MB, Gribble GW. (2004) Design, synthesis, and biological evaluation of biotin conjugates of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid for the isolation of the protein targets. J Med Chem 47:4923–4932 [DOI] [PubMed] [Google Scholar]
- Honda T, Rounds BV, Bore L, Favaloro FG, Jr., Gribble GW, Suh N, Wang Y, Sporn MB. (1999) Novel synthetic oleanane triterpenoids: a series of highly active inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 9:3429–3434 [DOI] [PubMed] [Google Scholar]
- Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG, Jr., Suh N, Wang Y, Sporn MB, Gribble GW. (2000) Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem 43:4233–4246 [DOI] [PubMed] [Google Scholar]
- Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB. (1998) Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 8:2711–2714 [DOI] [PubMed] [Google Scholar]
- Hong DS, Kurzrock R, Supko JG, He X, Naing A, Wheler J, Lawrence D, Eder JP, Meyer CJ, Ferguson DA, et al. (2012) A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin Cancer Res 18:3396–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Kurzrock R, Supko JG, Lawrence DP, Wheler JJ, Meyer CJ, Mier JW, Andreeff M, Shapiro GI, Dezube BJ. (2008) Phase I trial with a novel oral NF-{kappa}B/STAT3 inhibitor RTA 402 in patients with solid tumors and lymphoid malignancies. J Clin Oncol 26 (Suppl):351718541901 [Google Scholar]
- Hopkins AL. (2007) Network pharmacology. Nat Biotechnol 25:1110–1111 [DOI] [PubMed] [Google Scholar]
- Hopkins AL. (2008) Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 4:682–690 [DOI] [PubMed] [Google Scholar]
- Hughes DT, Martel PM, Kinlaw WB, Eisenberg BL. (2008) The synthetic triterpenoid CDDO-Im inhibits fatty acid synthase expression and has antiproliferative and proapoptotic effects in human liposarcoma cells. Cancer Invest 26:118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer ML, Croxton R, Krajewska M, Krajewski S, Kress CL, Lu M, Suh N, Sporn MB, Cryns VL, Zapata JM, et al. (2005) Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res 65:4799–4808 [DOI] [PubMed] [Google Scholar]
- Hyer ML, Shi R, Krajewska M, Meyer C, Lebedeva IV, Fisher PB, Reed JC. (2008) Apoptotic activity and mechanism of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic-acid and related synthetic triterpenoids in prostate cancer. Cancer Res 68:2927–2933 [DOI] [PubMed] [Google Scholar]
- Iborra M, Moret I, Rausell F, Bastida G, Aguas M, Cerrillo E, Nos P, Beltrán B. (2011) Role of oxidative stress and antioxidant enzymes in Crohn's disease. Biochem Soc Trans 39:1102–1106 [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. (2009) Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One 4:e8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Kimura F, Nakata Y, Sato K, Ogura K, Motoyoshi K, Sporn M, Kufe D. (2005) Triterpenoid CDDO-Im downregulates PML/RARalpha expression in acute promyelocytic leukemia cells. Cell Death Differ 12:523–531 [DOI] [PubMed] [Google Scholar]
- Ikeda T, Nakata Y, Kimura F, Sato K, Anderson K, Motoyoshi K, Sporn M, Kufe D. (2004) Induction of redox imbalance and apoptosis in multiple myeloma cells by the novel triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid. Mol Cancer Ther 3:39–45 [PubMed] [Google Scholar]
- Ikeda T, Sporn M, Honda T, Gribble GW, Kufe D. (2003) The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res 63:5551–5558 [PubMed] [Google Scholar]
- Ikushima H, Miyazono K. (2010) TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 10:415–424 [DOI] [PubMed] [Google Scholar]
- Innamorato NG, Rojo AI, García-Yagüe AJ, Yamamoto M, de Ceballos ML, Cuadrado A. (2008) The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 181:680–689 [DOI] [PubMed] [Google Scholar]
- Inoue S, Snowden RT, Dyer MJ, Cohen GM. (2004) CDDO induces apoptosis via the intrinsic pathway in lymphoid cells. Leukemia 18:948–952 [DOI] [PubMed] [Google Scholar]
- Ito Y, Pandey P, Place A, Sporn MB, Gribble GW, Honda T, Kharbanda S, Kufe D. (2000) The novel triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid induces apoptosis of human myeloid leukemia cells by a caspase-8-dependent mechanism. Cell Growth Differ 11:261–267 [PubMed] [Google Scholar]
- Ito Y, Pandey P, Sporn MB, Datta R, Kharbanda S, Kufe D. (2001) The novel triterpenoid CDDO induces apoptosis and differentiation of human osteosarcoma cells by a caspase-8 dependent mechanism. Mol Pharmacol 59:1094–1099 [DOI] [PubMed] [Google Scholar]
- Itoh K, Mimura J, Yamamoto M. (2010) Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal 13:1665–1678 [DOI] [PubMed] [Google Scholar]
- Jacob C. (2011) Redox signalling via the cellular thiolstat. Biochem Soc Trans 39:1247–1253 [DOI] [PubMed] [Google Scholar]
- Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. (2009) Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules 14:2016–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. (2008) Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwa A, Cuadrado A. (2010) Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr Drug Targets 11:1517–1531 [DOI] [PubMed] [Google Scholar]
- Ji Y, Lee HJ, Goodman C, Uskokovic M, Liby K, Sporn M, Suh N. (2006) The synthetic triterpenoid CDDO-imidazolide induces monocytic differentiation by activating the Smad and ERK signaling pathways in HL60 leukemia cells. Mol Cancer Ther 5:1452–1458 [DOI] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. (2008) The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci 1147:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. (2010) Redox sensing: orthogonal control in cell cycle and apoptosis signalling. J Intern Med 268:432–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutooru I, Chadalapaka G, Abdelrahim M, Basha MR, Samudio I, Konopleva M, Andreeff M, Safe S. (2010) Methyl 2-cyano-3,12-dioxooleana-1,9-dien-28-oate decreases specificity protein transcription factors and inhibits pancreatic tumor growth: role of microRNA-27a. Mol Pharmacol 78:226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, Liby KT, Williams CR, Yamamoto M, Kensler TW, Ratan RR, et al. (2012) Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson's disease. Antioxid Redox Signal http://dx.doi.org/10.1089/ars.2011.4491 [DOI] [PMC free article] [PubMed]
- Kannaiyan R, Shanmugam MK, Sethi G. (2011) Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 303:9–20 [DOI] [PubMed] [Google Scholar]
- Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Ylä-Herttuala S, et al. (2011) Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem 286:14019–14027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. (2009) NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1:a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N. (2010) Nrf2: friend or foe for chemoprevention? Carcinogenesis 31:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. (2006) Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66:11580–11584 [DOI] [PubMed] [Google Scholar]
- Kim EH, Deng C, Sporn MB, Royce DB, Risingsong R, Williams CR, Liby KT. (2012) CDDO-methyl ester delays breast cancer development in Brca1-mutated mice. Cancer Prev Res (Phila) 5:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EH, Deng CX, Sporn MB, Liby KT. (2011) CDDO-imidazolide induces DNA damage, G2/M arrest and apoptosis in BRCA1-mutated breast cancer cells. Cancer Prev Res (Phila) 4:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EH, Surh YJ. (2006) 15-deoxy-Delta12,14-prostaglandin J2 as a potential endogenous regulator of redox-sensitive transcription factors. Biochem Pharmacol 72:1516–1528 [DOI] [PubMed] [Google Scholar]
- Kim JE, You DJ, Lee C, Ahn C, Seong JY, Hwang JI. (2010) Suppression of NF-kappaB signaling by KEAP1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation. Cell Signal 22:1645–1654 [DOI] [PubMed] [Google Scholar]
- Kim KB, Lotan R, Yue P, Sporn MB, Suh N, Gribble GW, Honda T, Wu GS, Hong WK, Sun SY. (2002) Identification of a novel synthetic triterpenoid, methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate, that potently induces caspase-mediated apoptosis in human lung cancer cells. Mol Cancer Ther 1:177–184 [PubMed] [Google Scholar]
- Klaassen CD, Reisman SA. (2010) Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol 244:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. (2009) The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenitzer JR, Freeman BA. (2010) Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann NY Acad Sci 1203:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Kurinna SM, Chen W, Andreeff M, Ruvolo PP. (2005) The novel triterpenoid CDDO-Me suppresses MAPK pathways and promotes p38 activation in acute myeloid leukemia cells. Leukemia 19:1350–1354 [DOI] [PubMed] [Google Scholar]
- Konopleva M, Tsao T, Estrov Z, Lee RM, Wang RY, Jackson CE, McQueen T, Monaco G, Munsell M, Belmont J, et al. (2004) The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res 64:7927–7935 [DOI] [PubMed] [Google Scholar]
- Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, et al. (2002) Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood 99:326–335 [DOI] [PubMed] [Google Scholar]
- Konopleva M, Zhang W, Shi YX, McQueen T, Tsao T, Abdelrahim M, Munsell MF, Johansen M, Yu D, Madden T, et al. (2006) Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest in HER2-overexpressing breast cancer cells. Mol Cancer Ther 5:317–328 [DOI] [PubMed] [Google Scholar]
- Koschmieder S, D'Alò F, Radomska H, Schöneich C, Chang JS, Konopleva M, Kobayashi S, Levantini E, Suh N, Di Ruscio A, et al. (2007) CDDO induces granulocytic differentiation of myeloid leukemic blasts through translational up-regulation of p42 CCAAT enhancer binding protein alpha. Blood 110:3695–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress CL, Konopleva M, Martínez-García V, Krajewska M, Lefebvre S, Hyer ML, McQueen T, Andreeff M, Reed JC, Zapata JM. (2007) Triterpenoids display single agent anti-tumor activity in a transgenic mouse model of chronic lymphocytic leukemia and small B cell lymphoma. PLoS One 2:e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AA, Thatcher TH, Olsen KC, Maggirwar SB, Phipps RP, Sime PJ. (2011) PPAR-γ ligands repress TGFβ-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLoS One 6:e15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyan AE, Lehmann GM, Kulkarni AA, Woeller CF, Feldon SE, Hindman HB, Sime PJ, Huxlin KR, Phipps RP. (2012) Electrophilic PPARγ ligands inhibit corneal fibroblast to myofibroblast differentiation in vitro: A potentially novel therapy for corneal scarring. Exp Eye Res 94:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne H, Konopleva M, Tsao T, Gold D, McQueen T, Sutherland RL, Madden T, Andreeff M. (2003) Activation of peroxisome proliferator-activated receptor gamma by a novel synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res 63:5926–5939 [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, Shen J, Chen CT, Huo L, Hsu MC, et al. (2009) KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell 36:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. (2005) Nrf2, a multi-organ protector? Faseb J 19:1061–1066 [DOI] [PubMed] [Google Scholar]
- Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O'Farrelly C, Rabb H, Taylor CT. (2006) Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. Faseb J 20:2624–2626 [DOI] [PubMed] [Google Scholar]
- Levy ED, Landry CR, Michnick SW. (2010) Cell signaling. Signaling through cooperation. Science 328:983–984 [DOI] [PubMed] [Google Scholar]
- Li J, Ichikawa T, Janicki JS, Cui T. (2009) Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets 13:785–794 [DOI] [PubMed] [Google Scholar]
- Li M, Sun K, Redelman D, Welniak LA, Murphy WJ. (2010) The triterpenoid CDDO-Me delays murine acute graft-versus-host disease with the preservation of graft-versus-tumor effects after allogeneic bone marrow transplantation. Biol Blood Marrow Transplant 16:739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Grivennikov SI, Karin M. (2011) The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell 19:429–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Liu Z, Wrasidlo WJ, Luo Y, Nguyen G, Chen T, Xiang R, Reisfeld RA. (2011) Targeted therapeutic remodeling of the tumor microenvironment improves an HER-2 DNA vaccine and prevents recurrence in a murine breast cancer model. Cancer Res 71:5688–5696 [DOI] [PubMed] [Google Scholar]
- Liby K, Black CC, Royce DB, Williams CR, Risingsong R, Yore MM, Liu X, Honda T, Gribble GW, Lamph WW, et al. (2008a) The rexinoid LG100268 and the synthetic triterpenoid CDDO-methyl amide are more potent than erlotinib for prevention of mouse lung carcinogenesis. Mol Cancer Ther 7:1251–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, et al. (2005) The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res 65:4789–4798 [DOI] [PubMed] [Google Scholar]
- Liby K, Risingsong R, Royce DB, Williams CR, Ma T, Yore MM, Sporn MB. (2009) Triterpenoids CDDO-methyl ester or CDDO-ethyl amide and rexinoids LG100268 or NRX194204 for prevention and treatment of lung cancer in mice. Cancer Prev Res (Phila) 2:1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K, Risingsong R, Royce DB, Williams CR, Yore MM, Honda T, Gribble GW, Lamph WW, Vannini N, Sogno I, et al. (2008b) Prevention and treatment of experimental estrogen receptor-negative mammary carcinogenesis by the synthetic triterpenoid CDDO-methyl ester and the rexinoid LG100268. Clin Cancer Res 14:4556–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, Gribble GW, Dmitrovsky E, Sporn TA, Sporn MB. (2007a) The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res 67:2414–2419 [DOI] [PubMed] [Google Scholar]
- Liby K, Voong N, Williams CR, Risingsong R, Royce DB, Honda T, Gribble GW, Sporn MB, Letterio JJ. (2006) The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res 12:4288–4293 [DOI] [PubMed] [Google Scholar]
- Liby KT, Royce DB, Risingsong R, Williams CR, Maitra A, Hruban RH, Sporn MB. (2010) Synthetic triterpenoids prolong survival in a transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila) 3:1427–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. (2007b) Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7:357–369 [DOI] [PubMed] [Google Scholar]
- Ling X, Konopleva M, Zeng Z, Ruvolo V, Stephens LC, Schober W, McQueen T, Dietrich M, Madden TL, Andreeff M. (2007) The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res 67:4210–4218 [DOI] [PubMed] [Google Scholar]
- Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H. (2009) Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76:277–285 [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao X, Deeb D, Gautam SC. (2012) Oleanane triterpenoid CDDO-Me inhibits Akt activity without affecting PDK1 kinase or PP2A phosphatase activity in cancer cells. Biochem Biophys Res Commun 417:570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. (2011) Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 121:4015–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maicas N, Ferrándiz ML, Brines R, Ibáñez L, Cuadrado A, Koenders MI, van den Berg WB, Alcaraz MJ. (2011) Deficiency of Nrf2 accelerates the effector phase of arthritis and aggravates joint disease. Antioxid Redox Signal 15:889–901 [DOI] [PubMed] [Google Scholar]
- Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, et al. (2008) Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 178:592–604 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mancuso C, Barone E. (2009) The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab 10:579–594 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. (2008) Cancer-related inflammation. Nature 454:436–444 [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Riggins JN, West JD. (2003) Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J Clin Invest 111:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Degterev A. (2011) Small-molecule inhibitors of the PI3K signaling network. Future Med Chem 3:549–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melichar B, Konopleva M, Hu W, Melicharova K, Andreeff M, Freedman RS. (2004) Growth-inhibitory effect of a novel synthetic triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, on ovarian carcinoma cell lines not dependent on peroxisome proliferator-activated receptor-gamma expression. Gynecol Oncol 93:149–154 [DOI] [PubMed] [Google Scholar]
- Meyer C, Yore M, Ferguson D, Watkins B, Fey E, Sporn M, Huff J, Sonis S. (2006) RTA 402 suppresses tumor and treatment induced inflammation, sensitizing tumors to and protecting normal tissue from radiation (Abstract). Eur J Cancer Suppl 4:162 [Google Scholar]
- Minns LA, Buzoni-Gatel D, Ely KH, Rachinel N, Luangsay S, Kasper LH. (2004) A novel triterpenoid induces transforming growth factor beta production by intraepithelial lymphocytes to prevent ileitis. Gastroenterology 127:119–126 [DOI] [PubMed] [Google Scholar]
- Mix KS, Coon CI, Rosen ED, Suh N, Sporn MB, Brinckerhoff CE. (2004) Peroxisome proliferator-activated receptor-gamma-independent repression of collagenase gene expression by 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid and prostaglandin 15-deoxy-delta(12,14) J2: a role for Smad signaling. Mol Pharmacol 65:309–318 [DOI] [PubMed] [Google Scholar]
- Mix KS, Mengshol JA, Benbow U, Vincenti MP, Sporn MB, Brinckerhoff CE. (2001) A synthetic triterpenoid selectively inhibits the induction of matrix metalloproteinases 1 and 13 by inflammatory cytokines. Arthritis Rheum 44:1096–1104 [DOI] [PubMed] [Google Scholar]
- Montero AJ, Jassem J. (2011) Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs 71:1385–1396 [DOI] [PubMed] [Google Scholar]
- Na HK, Surh YJ. (2006) Transcriptional regulation via cysteine thiol modification: a novel molecular strategy for chemoprevention and cytoprotection. Mol Carcinog 45:368–380 [DOI] [PubMed] [Google Scholar]
- Nagai N, Thimmulappa RK, Cano M, Fujihara M, Izumi-Nagai K, Kong X, Sporn MB, Kensler TW, Biswal S, Handa JT. (2009) Nrf2 is a critical modulator of the innate immune response in a model of uveitis. Free Radic Biol Med 47:300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, et al. (2010) Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res 16:1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. (2010) Nonresolving inflammation. Cell 140:871–882 [DOI] [PubMed] [Google Scholar]
- Negi G, Kumar A, Joshi RP, Sharma SS. (2011) Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun 408:1–5 [DOI] [PubMed] [Google Scholar]
- Neurath MF, Finotto S. (2011) IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev 22: 83–89 [DOI] [PubMed] [Google Scholar]
- Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M, Liby KT, Risingsong R, Sporn M, Beal MF, et al. (2011) Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic Biol Med 51:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DP, Ziady AG, Shank SL, Eastman JF, Davis PB. (2009) The triterpenoid CDDO limits inflammation in preclinical models of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol 297:L828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen AM, Eisenberg BL, Kuemmerle NB, Flanagan AJ, Morganelli PM, Lombardo PS, Swinnen JV, Kinlaw WB. (2010) Fatty acid synthesis is a therapeutic target in human liposarcoma. Int J Oncol 36:1309–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, Taguchi K, Yamamoto M, Kensler TW. (2008) Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci 104:218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S. (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 59:1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek TK, Belkadi A, Kesavapany S, Zaremba A, Loh SL, Bai L, Cohen ML, Meyer C, Liby KT, Miller RH, et al. (2011) Triterpenoid modulation of IL-17 and Nrf-2 expression ameliorates neuroinflammation and promotes remyelination in autoimmune encephalomyelitis. Sci Rep 1:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen CE, Carroll KS. (2010) Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol 5:47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen IM, Kitada S, Schimmer A, Kim Y, Zapata JM, Charboneau L, Rassenti L, Andreeff M, Bennett F, Sporn MB, et al. (2002) The triterpenoid CDDO induces apoptosis in refractory CLL B cells. Blood 100:2965–2972 [DOI] [PubMed] [Google Scholar]
- Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, Warnock DG. (2011a) Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b-4 CKD. Am J Nephrol 33:469–476 [DOI] [PubMed] [Google Scholar]
- Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, et al. (2011b) Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365:327–336 [DOI] [PubMed] [Google Scholar]
- Petronelli A, Pannitteri G, Testa U. (2009a) Triterpenoids as new promising anticancer drugs. Anticancer Drugs 20:880–892 [DOI] [PubMed] [Google Scholar]
- Petronelli A, Pelosi E, Santoro S, Saulle E, Cerio AM, Mariani G, Labbaye C, Testa U. (2011) CDDO-Im is a stimulator of megakaryocytic differentiation. Leuk Res 35:534–544 [DOI] [PubMed] [Google Scholar]
- Petronelli A, Saulle E, Pasquini L, Petrucci E, Mariani G, Biffoni M, Ferretti G, Scambia G, Benedetti-Panici P, Greggi S, et al. (2009b) High sensitivity of ovarian cancer cells to the synthetic triterpenoid CDDO-Imidazolide. Cancer Lett 282:214–228 [DOI] [PubMed] [Google Scholar]
- Phillips DR, Rasbery JM, Bartel B, Matsuda SP. (2006) Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol 9:305–314 [DOI] [PubMed] [Google Scholar]
- Pitha-Rowe I, Liby K, Royce D, Sporn M. (2009) Synthetic triterpenoids attenuate cytotoxic retinal injury: cross-talk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Invest Ophthalmol Vis Sci 50:5339–5347 [DOI] [PubMed] [Google Scholar]
- Place AE, Suh N, Williams CR, Risingsong R, Honda T, Honda Y, Gribble GW, Leesnitzer LM, Stimmel JB, Willson TM, et al. (2003) The novel synthetic triterpenoid, CDDO-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin Cancer Res 9:2798–2806 [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. (2009) Toll-like receptors and cancer. Nat Rev Cancer 9:57–63 [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. (2004) Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114:1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et al. (2005) Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval CM, Lee PJ. (2010) Heme oxygenase-1 in lung disease. Curr Drug Targets 11:1532–1540 [DOI] [PubMed] [Google Scholar]
- Ray DM, Morse KM, Hilchey SP, Garcia TM, Felgar RE, Maggirwar SB, Phipps RP, Bernstein SH. (2006) The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) induces apoptosis of human diffuse large B-cell lymphoma cells through a peroxisome proliferator-activated receptor gamma-independent pathway. Exp Hematol 34:1202–1211 [DOI] [PubMed] [Google Scholar]
- Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. (2009a) Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol 182:7264–7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy NM, Suryanaraya V, Yates MS, Kleeberger SR, Hassoun PM, Yamamoto M, Liby KT, Sporn MB, Kensler TW, Reddy SP. (2009b) The triterpenoid CDDO-imidazolide confers potent protection against hyperoxic acute lung injury in mice. Am J Respir Crit Care Med 180:867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman SA, Buckley DB, Tanaka Y, Klaassen CD. (2009) CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol 236:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccioni R, Senese M, Diverio D, Riti V, Mariani G, Boe A, LoCoco F, Foà R, Peschle C, Sporn M, et al. (2008) Resistance of acute myeloid leukemic cells to the triterpenoid CDDO-Imidazolide is associated with low caspase-8 and FADD levels. Leuk Res 32:1244–1258 [DOI] [PubMed] [Google Scholar]
- Rigoulet M, Yoboue ED, Devin A. (2011) Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid Redox Signal 14:459–468 [DOI] [PubMed] [Google Scholar]
- Ross R. (1999) Atherosclerosis–an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- Ruffell B, DeNardo DG, Affara NI, Coussens LM. (2010) Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev 21:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu K, Choy E, Yang C, Susa M, Hornicek FJ, Mankin H, Duan Z. (2010a) Activation of signal transducer and activator of transcription 3 (Stat3) pathway in osteosarcoma cells and overexpression of phosphorylated-Stat3 correlates with poor prognosis. J Orthop Res 28:971–978 [DOI] [PubMed] [Google Scholar]
- Ryu K, Susa M, Choy E, Yang C, Hornicek FJ, Mankin HJ, Duan Z. (2010b) Oleanane triterpenoid CDDO-Me induces apoptosis in multidrug resistant osteosarcoma cells through inhibition of Stat3 pathway. BMC Cancer 10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. (2010) The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J Biol Chem 285:40581–40592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio I, Konopleva M, Hail N, Jr., Shi YX, McQueen T, Hsu T, Evans R, Honda T, Gribble GW, Sporn M, Gilbert HF, Safe S, Andreeff M. (2005) 2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide (CDDO-Im) directly targets mitochondrial glutathione to induce apoptosis in pancreatic cancer. J Biol Chem 280:36273–36282 [DOI] [PubMed] [Google Scholar]
- Samudio I, Konopleva M, Pelicano H, Huang P, Frolova O, Bornmann W, Ying Y, Evans R, Contractor R, Andreeff M. (2006) A novel mechanism of action of methyl-2-cyano-3,12 dioxoolean-1,9 diene-28-oate: direct permeabilization of the inner mitochondrial membrane to inhibit electron transport and induce apoptosis. Mol Pharmacol 69:1182–1193 [DOI] [PubMed] [Google Scholar]
- Samudio I, Kurinna S, Ruvolo P, Korchin B, Kantarjian H, Beran M, Dunner K, Jr., Kondo S, Andreeff M, Konopleva M. (2008) Inhibition of mitochondrial metabolism by methyl-2-cyano-3,12-dioxooleana-1,9-diene-28-oate induces apoptotic or autophagic cell death in chronic myeloid leukemia cells. Mol Cancer Ther 7:1130–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santibañez JF, Quintanilla M, Bernabeu C. (2011) TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci 121:233–251 [DOI] [PubMed] [Google Scholar]
- Sardina JL, López-Ruano G, Sánchez-Sánchez B, Hernández-Hernández A. (2011) CDDO-Im, an antitumor molecule that also improves platetet production. Leuk Res 35:427–428 [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Brüne B, von Knethen A. (2010) The nuclear hormone receptor PPARγ as a therapeutic target in major diseases. ScientificWorldJournal 10:2181–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. (2010) The inflammasomes. Cell 140:821–832 [DOI] [PubMed] [Google Scholar]
- Schroder K, Zhou R, Tschopp J. (2010) The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296–300 [DOI] [PubMed] [Google Scholar]
- Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, Feminella J, Dennis CG, Vethanayagam RR, Yull FE, Capitano M, et al. (2010) NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One 5:e9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Sun H. (2011) Synthesis, biology and clinical significance of pentacyclic triterpenes: a multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat Prod Rep 28:543–593 [DOI] [PubMed] [Google Scholar]
- Shiao SL, Ganesan AP, Rugo HS, Coussens LM. (2011) Immune microenvironments in solid tumors: new targets for therapy. Genes Dev 25:2559–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Saito S, Kokubu A, Suzuki T, Yamamoto M, Hirohashi S. (2010) Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res 70:9095–9105 [DOI] [PubMed] [Google Scholar]
- Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. (2009) Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol 620:138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. (2007) NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol 27:7188–7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki T, Kamiya N, Shiki S, Kodama TS, Kakizuka A, Jingami H. (2005) Alpha,beta-unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor gamma. J Biol Chem 280:14145–14153 [DOI] [PubMed] [Google Scholar]
- Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. (2006) A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res 12:1828–1838 [DOI] [PubMed] [Google Scholar]
- Shuttleworth SJ, Silva FA, Cecil AR, Tomassi CD, Hill TJ, Raynaud FI, Clarke PA, Workman P. (2011) Progress in the preclinical discovery and clinical development of class I and dual class I/IV phosphoinositide 3-kinase (PI3K) inhibitors. Curr Med Chem 18:2686–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedenburg G, Jendrossek D. (2011) Squalene-hopene cyclases. Appl Environ Microbiol 77:3905–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Petter RC, Baillie TA, Whitty A. (2011) The resurgence of covalent drugs. Nat Rev Drug Discov 10:307–317 [DOI] [PubMed] [Google Scholar]
- Slocum SL, Kensler TW. (2011) Nrf2: control of sensitivity to carcinogens. Arch Toxicol 85:273–284 [DOI] [PubMed] [Google Scholar]
- Speranza G, Gutierrez ME, Kummar S, Strong JM, Parker RJ, Collins J, Yu Y, Cao L, Murgo AJ, Doroshow JH, et al. (2012) Phase I study of the synthetic triterpenoid, 2-cyano-3, 12-dioxoolean-1, 9-dien-28-oic acid (CDDO), in advanced solid tumors. Cancer Chemother Pharmacol 69:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Liby KT. (2012) NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer 12:564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Liby K, Yore MM, Suh N, Albini A, Honda T, Sundararajan C, Gribble GW. (2007) Platforms and networks in triterpenoid pharmacology. Drug Dev Res 68:174–182 [Google Scholar]
- Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. (2011) New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod 74:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack C, Ho D, Wille E, Calingasan NY, Williams C, Liby K, Sporn M, Dumont M, Beal MF. (2010) Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free Radic Biol Med 49:147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadheim TA, Suh N, Ganju N, Sporn MB, Eastman A. (2002) The novel triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) potently enhances apoptosis induced by tumor necrosis factor in human leukemia cells. J Biol Chem 277:16448–16455 [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss IJ. (2011) Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140:1756–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara A, Uruno A, Kudo M, Matsuda K, Yang CW, Ito S. (2010) Effects of PPARγ on hypertension, atherosclerosis, and chronic kidney disease. Endocr J 57:847–852 [DOI] [PubMed] [Google Scholar]
- Suh N, Paul S, Lee HJ, Yoon T, Shah N, Son AI, Reddi AH, Medici D, Sporn MB. (2012) Synthetic triterpenoids, CDDO-Imidazolide and CDDO-Ethyl amide, induce chondrogenesis. Osteoarthritis Cartilage 20:446–450 [DOI] [PubMed] [Google Scholar]
- Suh N, Roberts AB, Birkey Reffey S, Miyazono K, Itoh S, ten Dijke P, Heiss EH, Place AE, Risingsong R, Williams CR, et al. (2003a) Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res 63:1371–1376 [PubMed] [Google Scholar]
- Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, et al. (1999) A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res 59:336–341 [PubMed] [Google Scholar]
- Suh WS, Kim YS, Schimmer AD, Kitada S, Minden M, Andreeff M, Suh N, Sporn M, Reed JC. (2003b) Synthetic triterpenoids activate a pathway for apoptosis in AML cells involving downregulation of FLIP and sensitization to TRAIL. Leukemia 17:2122–2129 [DOI] [PubMed] [Google Scholar]
- Sun K, Li M, Konopleva M, Konoplev S, Stephens LC, Kornblau SM, Frolova O, Wilkins DE, Ma W, Welniak LA, et al. (2007) The synthetic triterpenoid, CDDO, suppresses alloreactive T cell responses and reduces murine early acute graft-versus-host disease mortality. Biol Blood Marrow Transplant 13:521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Na HK, Park JM, Lee HN, Kim W, Yoon IS, Kim DD. (2011) 15-Deoxy-Δ12,14-prostaglandin J2, an electrophilic lipid mediator of anti-inflammatory and pro-resolving signaling Biochem Pharmacol 82:1335–1351 [DOI] [PubMed] [Google Scholar]
- Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, et al. (2009) Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA 106:250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Betsuyaku T, Nagai K, Fuke S, Nasuhara Y, Kaga K, Kondo S, Hamamura I, Hata J, Takahashi H, et al. (2008) Decreased airway expression of vascular endothelial growth factor in cigarette smoke-induced emphysema in mice and COPD patients. Inhal Toxicol 20:349–359 [DOI] [PubMed] [Google Scholar]
- Tabe Y, Konopleva M, Kondo Y, Contractor R, Tsao T, Konoplev S, Shi Y, Ling X, Watt JC, Tsutsumi-Ishii Y, et al. (2007) PPARgamma-active triterpenoid CDDO enhances ATRA-induced differentiation in APL. Cancer Biol Ther 6:1967–1977 [DOI] [PubMed] [Google Scholar]
- Taguchi K, Motohashi H, Yamamoto M. (2011) Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 16:123–140 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Goedken MJ, Chen C, Reisman SA, Manautou JE, Klaassen CD. (2008) Coordinated induction of Nrf2 target genes protects against iron nitrilotriacetate (FeNTA)-induced nephrotoxicity. Toxicol Appl Pharmacol 231:364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Maher JM, Chen C, Klaassen CD. (2007) Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Mol Pharmacol 71:817–825 [DOI] [PubMed] [Google Scholar]
- Tang W, Eisenbrand G. (1992) Chinese Drugs of Plant Origin, Springer-Verlag, Berlin [Google Scholar]
- Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. (2009) Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology 137:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Fuchs RJ, Malhotra D, Scollick C, Traore K, Bream JH, Trush MA, Liby KT, Sporn MB, Kensler TW, et al. (2007) Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxid Redox Signal 9:1963–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. (2006a) Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 116:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. (2006b) Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun 351:883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu KL, Pikor LA, Chari R, Wilson IM, Macaulay CE, English JC, Tsao MS, Gazdar AF, Lam S, Lam WL, et al. (2011) Genetic disruption of KEAP1/CUL3 E3 ubiquitin ligase complex components is a key mechanism of NF-kappaB pathway activation in lung cancer. J Thorac Oncol 6:1521–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To C, Kulkarni S, Pawson T, Honda T, Gribble GW, Sporn MB, Wrana JL, Di Guglielmo GM. (2008) The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid-imidazolide alters transforming growth factor beta-dependent signaling and cell migration by affecting the cytoskeleton and the polarity complex. J Biol Chem 283:11700–11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To C, Shilton BH, Di Guglielmo GM. (2010) Synthetic triterpenoids target the Arp2/3 complex and inhibit branched actin polymerization. J Biol Chem 285:27944–27957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townson JL, Macdonald IC, Liby KT, Mackenzie L, Dales DW, Hedley BD, Foster PJ, Sporn MB, Chambers AF. (2011) The synthetic triterpenoid CDDO-Imidazolide suppresses experimental liver metastasis. Clin Exp Metastasis 28:309–317 [DOI] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8:579–591 [DOI] [PubMed] [Google Scholar]
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, et al. (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10:241–252 [DOI] [PubMed] [Google Scholar]
- Tran K, Risingsong R, Royce D, Williams CR, Sporn MB, Liby K. (2012) The synthetic triterpenoid CDDO-methyl ester delays estrogen receptor-negative mammary carcinogenesis in polyoma middle T mice. Cancer Prev Res (Phila) 5:726–734 [DOI] [PubMed] [Google Scholar]
- Tran TA, McCoy MK, Sporn MB, Tansey MG. (2008) The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. J Neuroinflammation 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao T, Kornblau S, Safe S, Watt JC, Ruvolo V, Chen W, Qiu Y, Coombes KR, Ju Z, Abdelrahim M, et al. (2010) Role of peroxisome proliferator-activated receptor-gamma and its coactivator DRIP205 in cellular responses to CDDO (RTA-401) in acute myelogenous leukemia. Cancer Res 70:4949–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11:329–341 [DOI] [PubMed] [Google Scholar]
- Vannini N, Lorusso G, Cammarota R, Barberis M, Noonan DM, Sporn MB, Albini A. (2007) The synthetic oleanane triterpenoid, CDDO-methyl ester, is a potent antiangiogenic agent. Mol Cancer Ther 6:3139–3146 [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A. (2012) Redox active thiol sensors of oxidative and nitrosative stress. Antioxid Redox Signal http://dx.doi.org/10.1089/ars.2012.4522 [DOI] [PMC free article] [PubMed]
- Vázquez N, Greenwell-Wild T, Marinos NJ, Swaim WD, Nares S, Ott DE, Schubert U, Henklein P, Orenstein JM, Sporn MB, et al. (2005) Human immunodeficiency virus type 1-induced macrophage gene expression includes the p21 gene, a target for viral regulation. J Virol 79:4479–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venè R, Larghero P, Arena G, Sporn MB, Albini A, Tosetti F. (2008) Glycogen synthase kinase 3beta regulates cell death induced by synthetic triterpenoids. Cancer Res 68:6987–6996 [DOI] [PubMed] [Google Scholar]
- Vidal M, Cusick ME, Barabási AL. (2011) Interactome networks and human disease. Cell 144:986–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R. (1867) Die Krankhaften Geschwulste, Berlin, August Hirschwald [Google Scholar]
- Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. (2010) When NRF2 talks, who's listening? Antioxid Redox Signal 13:1649–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wander SA, Hennessy BT, Slingerland JM. (2011) Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest 121:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA, et al. (1999) Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J Natl Cancer Inst 91:347–354 [DOI] [PubMed] [Google Scholar]
- Wang Y, Porter WW, Suh N, Honda T, Gribble GW, Leesnitzer LM, Plunket KD, Mangelsdorf DJ, Blanchard SG, Willson TM, et al. (2000) A synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), is a ligand for the peroxisome proliferator-activated receptor gamma. Mol Endocrinol 14:1550–1556 [DOI] [PubMed] [Google Scholar]
- Wei Y, Gong J, Yoshida T, Eberhart CG, Xu Z, Kombairaju P, Sporn MB, Handa JT, Duh EJ. (2011) Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic Biol Med 51:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17:1359–1370 [DOI] [PubMed] [Google Scholar]
- Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, et al. (2011) Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis 70:844–850 [DOI] [PubMed] [Google Scholar]
- Wu ML, Ho YC, Yet SF. (2011a) A central role of heme oxygenase-1 in cardiovascular protection. Antioxid Redox Signal 15:1835–1846 [DOI] [PubMed] [Google Scholar]
- Wu QQ, Wang Y, Senitko M, Meyer C, Wigley WC, Ferguson DA, Grossman E, Chen J, Zhou XJ, Hartono J, et al. (2011b) Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol Renal Physiol 300:F1180–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. (2008) Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol 28:2758–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hornicek FJ, Wood KB, Schwab JH, Choy E, Mankin H, Duan Z. (2010) Blockage of Stat3 with CDDO-Me inhibits tumor cell growth in chordoma. Spine (Phila Pa 1976) 35:1668–1675 [DOI] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, et al. (2009) Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS One 4:e5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, et al. (2006) Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res 66:2488–2494 [DOI] [PubMed] [Google Scholar]
- Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, et al. (2007) Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther 6:154–162 [DOI] [PubMed] [Google Scholar]
- Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, et al. (2009) Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 30:1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. (2001) Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int 60:1343–1353 [DOI] [PubMed] [Google Scholar]
- Yore MM, Kettenbach AN, Sporn MB, Gerber SA, Liby KT. (2011) Proteomic analysis shows synthetic oleanane triterpenoid binds to mTOR. PLoS One 6:e22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. (2006) The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther 5:3232–3239 [DOI] [PubMed] [Google Scholar]
- Youn JI, Gabrilovich DI. (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40:2969–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Li H, Liu Q, Liu F, Tang L, Li C, Yuan Y, Zhan Y, Xu W, Li W, et al. (2011) Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal 23:883–892 [DOI] [PubMed] [Google Scholar]
- Yue P, Zhou Z, Khuri FR, Sun SY. (2006) Depletion of intracellular glutathione contributes to JNK-mediated death receptor 5 upregulation and apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me). Cancer Biol Ther 5:492–497 [DOI] [PubMed] [Google Scholar]
- Zhang C, Ni X, Konopleva M, Andreeff M, Duvic M. (2004) The novel synthetic oleanane triterpenoid CDDO (2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid) induces apoptosis in Mycosis fungoides/Sézary syndrome cells. J Invest Dermatol 123:380–387 [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Zhang M, Weng Z, Li P, Gan Y, Zhang L, Cao G, Gao Y, Leak RK, et al. (2012) Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke 43:1390–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Lee JY, Hwang DH. (2011) Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr Rev 69:310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Chen S, Liu X, Yue P, Sporn MB, Khuri FR, Sun SY. (2007) c-FLIP downregulation contributes to apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me) in human lung cancer cells. Cancer Biol Ther 6:1614–1620 [DOI] [PubMed] [Google Scholar]
- Zou W, Liu X, Yue P, Zhou Z, Sporn MB, Lotan R, Khuri FR, Sun SY. (2004) c-Jun NH2-terminal kinase-mediated up-regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1, 9-dien-28-oate in human lung cancer cells. Cancer Res 64:7570–7578 [DOI] [PubMed] [Google Scholar]
- Zou W, Yue P, Khuri FR, Sun SY. (2008) Coupling of endoplasmic reticulum stress to CDDO-Me-induced up-regulation of death receptor 5 via a CHOP-dependent mechanism involving JNK activation. Cancer Res 68:7484–7492 [DOI] [PMC free article] [PubMed] [Google Scholar]