Abstract
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes that is associated with axonal atrophy, demyelination, blunted regenerative potential, and loss of peripheral nerve fibers. The development and progression of DPN is due in large part to hyperglycemia but is also affected by insulin deficiency and dyslipidemia. Although numerous biochemical mechanisms contribute to DPN, increased oxidative/nitrosative stress and mitochondrial dysfunction seem intimately associated with nerve dysfunction and diminished regenerative capacity. Despite advances in understanding the etiology of DPN, few approved therapies exist for the pharmacological management of painful or insensate DPN. Therefore, identifying novel therapeutic strategies remains paramount. Because DPN does not develop with either temporal or biochemical uniformity, its therapeutic management may benefit from a multifaceted approach that inhibits pathogenic mechanisms, manages inflammation, and increases cytoprotective responses. Finally, exercise has long been recognized as a part of the therapeutic management of diabetes, and exercise can delay and/or prevent the development of painful DPN. This review presents an overview of existing therapies that target both causal and symptomatic features of DPN and discusses the role of up-regulating cytoprotective pathways via modulating molecular chaperones. Overall, it may be unrealistic to expect that a single pharmacologic entity will suffice to ameliorate the multiple symptoms of human DPN. Thus, combinatorial therapies that target causal mechanisms and enhance endogenous reparative capacity may enhance nerve function and improve regeneration in DPN if they converge to decrease oxidative stress, improve mitochondrial bioenergetics, and increase response to trophic factors.
I. Introduction
DPN1 is the most prevalent complication of diabetes and often manifests as a distal, symmetric, sensorimotor neuropathy. In the United States, 26.8 million people are affected by diabetes; by the year 2030, that number is predicted to increase to approximately 35.9 million people (Shaw et al., 2010). A recent population-based study reported that more than half of patients who have type 1 or 2 diabetes develop DPN (Harati, 2007). Of these patients with DPN, 15 to 30% suffer from painful diabetic neuropathy, whereas the remainder experience a loss of sensation and numbness (Ramos et al., 2007). Clinical symptoms associated with DPN involve poor gait and balance associated with large sensory fibers and abnormal cold and/or heat sensation associated with small sensory fibers. Chronic pain associated with diabetes is represented by hyperalgesia, allodynia, paresthesias, and spontaneous pain (Gooch and Podwall, 2004; Edwards et al., 2008). Symptoms are described as tingling, “pins and needles,” burning, itching, and an abnormal sensation to pain and temperature. Over time, these symptoms may advance from the toes to the foot and up the leg, and these symptoms may occur in the fingers and hands (Zochodne, 2007).
DPN arises as a result of the degeneration of small, unmyelinated C fibers or thinly myelinated Aδ sensory fibers that mediate pain/temperature sensation. Diabetes-induced changes in C fibers lead to the development of small-fiber neuropathy, which often produces positive (painful) symptoms: allodynia and hyperesthesias. Progressive neurodegeneration may spontaneously resolve the neuropathic pain, but decreased response thresholds (Lennertz et al., 2011) and loss of epidermal innervation of C fibers in the feet (Beiswenger et al., 2008) can contribute to negative neuropathic symptoms such as thermal hypoalgesia. Likewise, degeneration of Aβ fibers leads to a loss of vibration and tactile sensation (Lennertz et al., 2011) with axon-myelin separation (Powell and Myers, 1983; Myers and Powell, 1984; Love et al., 1986) and eventual segmental demyelination in long-term DPN (Zochodne, 2007). Thus, deficits in both C and Aβ fiber function greatly affects the detection of noxious and non-noxious stimuli. Unfortunately, the complexity of the multiple and temporally nonuniform biochemical insults within endothelium, neurons, and Schwann cells that contribute to the sensory phenotypes associated with DPN has rendered the development of effective therapeutics difficult.
Many excellent reviews have been published that 1) describe the various biochemical insults that contribute to the pathogenesis of DPN and 2) outline treatment strategies directed at blocking either causal mechanisms or treating neuropathic pain (Leinninger et al., 2004; Vincent et al., 2004, 2009b; Pop-Busui et al., 2006; Calcutt and Backonja, 2007; Zochodne, 2007, 2008; Calcutt et al., 2008, 2009; Edwards et al., 2008; Tavakoli and Malik, 2008; Veves et al., 2008; Obrosova, 2009a; Fernyhough et al., 2010; Sivitz and Yorek, 2010; Malik et al., 2011). Thus, the intent of the current review is to briefly highlight and update many of these features and add to the discussion by proposing that both pharmacologic and nonpharmacologic approaches that help modulate the expression and activity of cytoprotective molecular chaperones may afford a complementary tactic toward improving the management of DPN.
II. Pathogenesis of Diabetic Peripheral Neuropathy
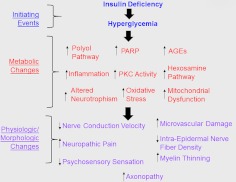
Results from the Diabetes Control and Complication Trial (DCCT) supported the hypothesis that DPN develops as result of increased blood glucose concentrations (hyperglycemia) (DCCT Research Group, 1988, 1993). However, more recent data seem to indicate that the development of DPN is not necessarily strictly glucocentric but also involves neuronal insulin deficiency/resistance (Kim and Feldman, 2012) and dyslipidemia (Wiggin et al., 2009). Nevertheless, the glucose-induced pathologic features of DPN are well characterized and include enhanced activity of the polyol pathway, the formation of advanced glycation end products (AGE), protein kinase C activation (PKC), increased poly(ADP-ribose) polymerase (PARP) activity, enhanced modification of proteins with N-acetyl glucosamine via the hexosamine pathway, increased inflammation, and a reduction in neurotrophic factors (Fig. 1). Many of these mechanisms contribute to increasing oxidative stress and mitochondrial dysfunction and have rightly served as focal targets in developing therapeutic interventions to improve or reverse DPN.
Fig. 1.
Overview of various pathogenetic components contributing to DPN.
A. Polyol Pathway
Under normoglycemic conditions, glucose is primarily metabolized through glycolysis, the tricarboxylic acid cycle, and oxidative phosphorylation (Yagihashi et al., 2007). However, under hyperglycemic conditions, the excess intracellular glucose can be shunted through the polyol pathway, where it is reduced to sorbitol by aldose reductase (AR), which requires oxidizing NADPH to NADP+. Sorbitol is then oxidized to fructose by sorbitol dehydrogenase, which reduces NAD+ to NADH (Duby et al., 2004).
The polyol pathway produces excess levels of sorbitol and fructose, which can decrease expression and uptake of myo-inositol (Kato et al., 1999) and blunt activation of the Na+/K+-ATPase (Kato et al., 1999; Nayak et al., 2011). In rats with streptozocin (STZ)-induced diabetes, impaired myo-inositol metabolism through phosphoinositide synthase invoked the slowing of nerve conduction velocity, an early indicator of DPN (Greene and Mackway, 1986).
NADPH is a coenzyme that is required by glutathione reductase to convert GSSG to GSH. Because the reduction of glucose to sorbitol via AR depletes NADPH, this compromises the recycling of GSSG to GSH. Decreased GSH limits the ability of glutathione peroxidase to reduce hydrogen peroxide to water, thus increasing oxidative stress. It is noteworthy that deletion of AR prevented GSH depletion, superoxide production, and nerve conduction velocity deceleration (Ho et al., 2006). Activation of the polyol pathway is likely to be localized in peripheral nerve because AR is not very abundant in sensory neurons but preferentially localizes to Schwann cells (Jiang et al., 2006). Despite this localization, inhibiting sorbitol dehydrogenase decreased oxidative stress in cultured adult sensory neurons obtained from diabetic rats (Akude et al., 2011).
B. Advanced Glycation End-Products
AGEs are formed when amino groups of proteins react nonenzymatically with reducing sugars (Bansal et al., 2012). Dicarbonyls are AGE precursors that are formed through the oxidation of glucose to glyoxal, the breakdown of Amadori products to 3-deoxyglucosone, and methylglyoxal formation from dihydroxyacetone phosphate (Negre-Salvayre et al., 2009).
There are three general mechanisms by which the production of AGEs damages cells. First, AGEs modify the biological function of intracellular proteins and promote apoptosis (Sekido et al., 2004; Vincent et al., 2007). Second, AGEs can modify components of the extracellular matrix such as laminin and fibronectin and contribute to poor collateral sprouting and axonal regeneration associated with DPN (Duran-Jimenez et al., 2009). Third, AGEs modify plasma proteins, creating ligands that bind to the receptor for AGEs (RAGE) on macrophages, smooth muscle, vascular endothelial cells, and Schwann cells (Wada and Yagihashi, 2005). The binding of AGEs to RAGE increases the production of reactive oxygen species (ROS) and activates nuclear factor κB (NF-κB), causing multiple changes in gene expression that lead to enhanced endothelial permeability, smooth muscle cell and fibroblast proliferation, extracellular matrix degradation, cytokine secretion, procoagulant effects, and apoptosis (Bierhaus et al., 2004). These changes can result in accelerated atherosclerosis, arterial stiffening, stenosis, thrombosis, and microvascular complications (Negre-Salvayre et al., 2009). The importance of RAGE activation to DPN is underscored by observations that functional and structural abnormalities of experimental DPN, such as pain perception (Bierhaus et al., 2004), nerve conduction deficits, and axonal atrophy, are diminished in RAGE-null mice (Toth et al., 2008).
C. Protein Kinase C
PKC comprises a family of enzymes that phosphorylate numerous target proteins. The activity of many PKC family members depends on Ca2+ ions, phosphatidylserine, and diacylglycerol; chronic hyperglycemia elevates diacylglycerol levels. In general, the contributions of aberrant PKC activation to DPN seem to center on affecting nerve blood flow and improving nerve conduction velocity deficits (Obrosova, 2009a). In vitro, PKC activation can lead to NF-κB activation, overexpression of plasminogen activator inhibitor-1, the expression of vascular endothelial growth factor (Madonna and De Caterina, 2011), along with reductions in nitric oxide production in Zucker fatty rats (Bohlen, 2004). These pathological changes can alter vasoconstriction and capillary permeability (Edwards et al., 2008). PKCβ is also involved in the mechanism, leading to reduced Na+/K+-ATPase activity, resulting in decreased nerve conduction velocities and nerve regeneration (Lehning et al., 1994). Treatment with an inhibitor of PKCβ improved MNCV, normalized nerve blood flow, and restored Na+/K+-ATPase activity in STZ-induced diabetic rats (Cameron and Cotter, 2002).
D. Poly(ADP-ribose) Polymerase Pathway
PARP is a ubiquitous nuclear DNA repair enzyme that cleaves NAD+ to produce ADP-ribose residues that can attach to proteins. PARP has been shown to cause and to be activated by oxidative stress (Obrosova et al., 2005). Enhanced PARP activity depletes NAD+, leading to energy failure, increased oxidative stress, and an inhibition of glyceraldehyde 3-phosphate dehydrogenase (Obrosova et al., 2005; Pacher and Szabo, 2008; Negi et al., 2010a,b). PARP has been implicated in nerve conduction velocity deficits, neurovascular dysfunction, thermal and mechanical hyper- and hypoalgesia, mechanical allodynia, and myelinated fiber loss (Obrosova et al., 2005, 2009a; Pacher and Szabo, 2008; Homs et al., 2011; Stavniichuk et al., 2011; Dieckmann et al., 2012). Moreover, an increase in poly(ADP-ribosylated) proteins has been reported after 4 weeks of diabetes in rat sciatic nerve and in cultured human Schwann cells (Obrosova et al., 2005). Although the identification of specific ADP-ribosylated proteins and their contribution to the progression of DPN remains unknown, the fact that PARP-1 knockout mice do not develop small-fiber neuropathy supports an important role of PARP activation in the development of DPN (Obrosova et al., 2004, 2008).
E. Hexosamine Pathway
During normal glucose metabolism, approximately 3% of total glucose is diverted into the hexosamine pathway via fructose-6 phosphate (F-6-P) (Marshall et al., 1991), and the magnitude of this shunting can increase in hyperglycemically stressed cells. Glutamine:F-6-P amidotransferase converts F-6-P to glucosamine-6 phosphate, which is a precursor for the formation of UDP-N-acetyl glucosamine (UDP-GlcNAc). O-GlcNAc transferase uses UDP-GlcNAc as the substrate to modify serine and threonine residues with an O-linked N-acetylglucosamine moiety.
Increased protein modification by O-GlcNAc can lead to diabetic vascular complications by inhibiting the transcription factor Sp1 (Yang et al., 2001, 2002). Sp1 mediates activation of many glucose-induced housekeeping genes, plasminogen activator inhibitor-1, and transforming growth factor-β1 (Du et al., 2000). The activation of these particular genes plays a role in the development of atherosclerosis by promoting vascular smooth muscle cell mitosis and endothelial fibrosis, increasing collagen matrix production, and decreasing proliferation in mesangial cells (Sayeski and Kudlow, 1996). Moreover, the cross-talk that occurs between O-GlcNAcylation and phosphorylation provides an additional level of regulation (Hart et al., 2011). For example, O-GlcNAc modification of eNOS can decrease eNOS serine phosphorylation at the Akt activation site, impairing vasodilation and accelerating atherosclerosis (Du et al., 2001). Thus, hyperglycemia-induced activation of the hexosamine pathway contributes to the pathogenesis of many diabetes-related complications as a result of changes in gene expression and protein function. However, any direct contribution of protein O-GlcNAcylation to the onset or progression of DPN has not been reported.
F. Oxidative/Nitrosative Stress and Mitochondrial Dysfunction
Chronic or acute hyperglycemia can induce oxidative stress by overloading the flow of reducing equivalents from glycolysis and the TCA cycle into oxidative phosphorylation. In hyperglycemically stressed endothelial cells, the shunting of excess glucose into glycolysis and the TCA pathway increases NADH and FADH2. This contributes to producing a hyperpolarized membrane potential as a result of the pumping of excess proteins across the inner mitochondrial membrane (Nishikawa et al., 2000). This slows electron transport and can increase the production of superoxide anion produced at complex I (Treberg et al., 2011) and complex III of the electron transport chain. Superoxide produced at complex I is directed primarily to the mitochondrial matrix, whereas that generated at complex III is more equally distributed between the mitochondrial matrix and intermembrane space (Sivitz and Yorek, 2010). Thus, both sites of superoxide production may contribute to oxidative damage of mitochondrial proteins.
It has been suggested that increased mitochondrial superoxide production may underlie the hyperglycemia-induced increases in polyol synthesis, PKC activation, protein GlcNAcylation, AGE formation, and the development of diabetic complications (Nishikawa et al., 2000). The elegant simplicity of the hypothesis is attractive and probably applicable to endothelium. For example, a complex I-dependent increase in mitochondrial superoxide was observed in epineurial arterioles of sciatic nerve from STZ-diabetic rats (Coppey et al., 2003). However, it is unlikely to account for all aspects of enhanced oxidative stress in diabetic nerve. In adult sensory neurons, prolonged diabetes (22 weeks) was associated with depolarization of the inner mitochondrial membrane and was not accompanied by an increase in superoxide production (Huang et al., 2003; Akude et al., 2011). Nonetheless, diabetic sensory neurons still exhibit enhanced levels of 4-hydroxy-2-nonenal, a product of lipid peroxidation (Akude et al., 2011). Regardless of whether enhanced superoxide generation is a central unifying mechanism in the pathophysiology of diabetic complications, its formation is critical to increasing protein nitration, via the formation of peroxynitrite (Ischiropoulos and Beckman, 2003). Although it is unclear whether peroxynitrite is actually produced within mitochondria, protein nitration is increased in sensory neuron cell bodies and peripheral nerve of diabetic animals (Obrosova et al., 2005; Vareniuk et al., 2007). It is noteworthy that the inducible form of nitric-oxide synthase seems more critical to the development of neuropathic symptoms than the neuronal isoform of this enzyme (Vareniuk et al., 2008, 2009). Although nitrosative stress contributes to DPN, it should be a priority to identify the role of specific nitrosylated proteins in the development and/or progression of DPN.
A recent review has extensively summarized the effect of diabetes-induced oxidative stress on mitochondrial function (Sivitz and Yorek, 2010). Numerous reports using adult sensory neurons or dorsal root ganglia isolated from diabetic rats support that prolonged diabetes can decrease inner mitochondrial membrane potential and respiratory chain activity (Srinivasan et al., 2000; Huang et al., 2003; Huang et al., 2005; Chowdhury et al., 2010, 2011). It is noteworthy that in-depth analysis of mitochondrial bioenergetics using intact adult sensory neurons isolated from diabetic rats (4 months of diabetes) (Chowdhury et al., 2012) or mice (6 months of diabetes) (Urban et al., 2012b) supports the theory that diabetes also decreases spare respiratory capacity. Because spare respiratory capacity is indicative of the bioenergetic limit at which a cell is functioning (Sansbury et al., 2011), a decrease in spare respiratory capacity limits the dynamic range available to respond to environmental challenges. This renders neurons more susceptible to stress because they cannot increase energy demand sufficiently to match environment needs (Nicholls et al., 2007; Choi et al., 2009). Not surprisingly, diabetes-induced increases in mitochondrial workload (Chowdhury et al., 2012; Zhang et al., 2012) also correlate with alterations in the mitochondrial proteome of sensory neurons. We recently demonstrated that a decrease in mitochondrial respiration of dorsal root ganglia isolated from diabetic rats (22 weeks of diabetes) correlated with a decrease in proteins linked to oxidative phosphorylation, ubiquinone biosynthesis, the TCA cycle, and antioxidant protection (i.e., MnSOD) (Akude et al., 2011; Chowdhury et al., 2011). It is noteworthy that insulin therapy reversed many of these changes in the mitochondrial proteome, indicating its highly dynamic nature (Akude et al., 2011). Although it is unclear how diabetes may directly alter the mitochondrial proteome (i.e., enhanced degradation versus decreased synthesis), quantitative proteomic analysis of protein translation indicated that hyperglycemic stress can negatively affect the translation of numerous mitochondrial proteins in primary cultures of embryonic sensory neurons (Zhang et al., 2012).
G. Inflammation, Lipid Mediators, and Dyslipidemia
Proinflammatory cytokines contribute to the pathogenesis and/or maintenance of neuropathic pain. Injury to peripheral nerves results in the production of cytokines that originate from resident and recruited lymphocytes, macrophages, neurons, and Schwann cells (Yasuda et al., 2003). Patients with both type 1 and 2 diabetes exhibit elevated blood levels of tumor necrosis factor-α (TNF-α), an inflammation promoting cytokine (Gonzalez-Clemente et al., 2005; Purwata, 2011). In these patients with diabetes, the plasma levels of TNF-α correlated with the severity of perceived pain (Purwata, 2011). The relevance of increased TNF-α levels to DPN is supported by both pharmacologic and genetic evidence. Infliximab is an FDA-approved monoclonal antibody that binds soluble TNF, and its administration decreased plasma TNF-α levels and attenuated nerve conduction deficits in diabetic mice (Yamakawa et al., 2011). Moreover, diabetic TNF-α knockout mice were resistant to developing DPN (Yamakawa et al., 2011). It is noteworthy that pharmacologically inhibiting cyclooxygenase 2 (COX-2) in diabetic mice prevented an increase in nerve levels of TNF-α and activation of NF-κB (Kellogg et al., 2007). Likewise, diabetic COX-2 knockout mice were resistant to developing DPN and showed no increase in nerve TNF-α levels (Kellogg and Pop-Busui, 2005; Kellogg et al., 2007). Inhibiting COX-2 also prevented spinally mediated hyperalgesia in diabetic rats and through inhibiting AR, blocked COX-2 activation, whether TNF-α levels were decreased was not examined (Ramos et al., 2007). These data would support that the production of TNF-α is downstream of arachidonic acid metabolism.
Finally, it should be noted that the role of TNF-α in the onset of DPN may vary with disease severity, duration, and/or species. For example, TNF-α levels and NF-κB signaling were decreased in dorsal root ganglia (DRG) obtained from rats that had a relatively moderate level of diabetes for 5 months (Saleh et al., 2011). Likewise, despite an increase in the expression of COX-2, TNF-α levels were decreased in the sciatic nerve of rats that manifested a tactile allodynia after 4 weeks of diabetes (Jolivalt et al., 2009). These data suggest that at least in the diabetic rat model, early sensory neuropathy is not associated with the enhanced production of TNF-α that is apparent in longer term and more severely diabetic animals. Indeed, numerous genes associated with the gene ontology category of inflammatory response were up-regulated in sural nerve biopsies from patients classified as having progressive DPN (Hur et al., 2011). How these mediators may contribute to ultrastructural changes in humans with minimal but progressive neuropathy remains unclear (Malik et al., 2005).
12/15-Lipoxygenases convert arachidonic acid to 12/15-hydroxyeicosatetraenoic (HETE) acids. These inflammatory lipid mediators are increased in diabetic nerve and spinal cord and enhance oxidative/nitrosative stress (Stavniichuk et al., 2010). It is noteworthy that inhibiting AR decreased formation of 12-HETE in sciatic nerve but not spinal cord (Stavniichuk et al., 2012), suggesting that the lipid metabolite may be produced preferentially in Schwann cells. Furthermore, neither p42/44 nor p38 MAPKs were activated in sciatic nerve of diabetic 12/15-lipoxygenase knockout mice. Given the localization of AR to Schwann cells and the role of aberrant p42/p44 MAPK in promoting demyelination (Harrisingh et al., 2004; Syed et al., 2010; Napoli et al., 2012), these data suggest that the coordinated actions of the polyol pathway and 12-HETE production may increase the degradation of myelin in Schwann cells via enhanced activation of p42/p44 MAPK. Consistent with this possibility, larger myelinated fibers in tibial nerve are spared from myelin thinning in diabetic 12/15-lipoxygenase-deficient mice, but the gene deletion did not prevent the loss of small unmyelinated fibers innervating the epidermis (Obrosova et al., 2010).
A growing body of evidence supports expanding the focus on glucocentric mechanisms in the development and progression of DPN to also encompass dyslipidemia (Vincent et al., 2009b). Evidence supporting this premise has been gathered from human cohorts (Wiggin et al., 2009) and by feeding mice a high-fat diet, which promoted DPN even in the absence of elevating blood glucose level (Obrosova et al., 2007b; Vincent et al., 2009a). Remarkably, a recent report indicates that superimposing dyslipidemia on a diabetic background can differentially affect sensory modalities associated with DPN. Diabetic C57BL/6 mice given a standard chow exhibited a mechanical hypoalgesia (an insensate neuropathy), whereas diabetic mice placed on a high-fat diet exhibited a mechanical allodynia (a painful neuropathy) (Guilford et al., 2011). On the other hand, the high-fat diet did not significantly worsen the thermal hypoalgesia that was manifested in the diabetic mice. The effect of the high-fat diet on promoting a mechanical allodynia may be related to an increase in 12/15-lipoxygenase activity because an ethanolic extract from the plant Artemisia dracunculus L. helped normalize these measures (Watcho et al., 2010). These results suggest that a high-fat diet may differentially affect metabolic functions in myelinated versus unmyelinated fibers. Given the complex interactions between glucose and lipid metabolism, as well as nerve function and psychosensory behaviors, systems biology approaches will undoubtedly prove useful in dissecting how the up-/down-regulation of gene networks by diabetes and dyslipidemia may differentially contribute to the progression and severity of DPN (Wiggin et al., 2008; Hur et al., 2011; Pande et al., 2011).
H. Growth Factors
Neurotrophic factors are molecules that develop and maintain the nervous system by promoting the growth and/or survival of neurons. The neurotrophin family of growth factors includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3, and NT-4/5. Glial cell-derived neurotrophic factors (GDNF) form a second family and include GDNF, neurturin, artemin, and persephin. Each neurotrophic factor regulates neuronal function and supports the growth and survival of distinct groups of neurons (Boucher and McMahon, 2001). Likewise, members of the neuregulin family of growth factors are important for the survival of Schwann cells (Syroid et al., 1996) and regulate both the formation (Taveggia et al., 2005; Nave and Salzer, 2006; Syed et al., 2010) and degeneration (Zanazzi et al., 2001; Syed et al., 2010) of the myelin sheath.
A deficiency in NGF and NT-3 has long been characterized in both tissue and serum in DPN (Faradji and Sotelo, 1990; Hellweg and Hartung, 1990; Fernyhough et al., 1998). Diabetes also reduces anterograde and retrograde axonal transport of BDNF, NGF, and NT-3 in peripheral nerves (Hellweg et al., 1994; Fernyhough et al., 1998; Mizisin et al., 1999). Loss of neurotrophic support can affect fiber morphology because intrathecal delivery of NGF or NT-3 improved myelinated fiber innervation in the dermal footpad of diabetic mice (Christianson et al., 2007). Likewise, unmyelinated, nonpeptidergic fibers were increased after intrathecal administration of GDNF (Akkina et al., 2001), whereas intramuscular GDNF gene therapy improved myelination and neuropeptide levels in the sciatic nerve of diabetic rats (Liu et al., 2009). In addition, GDNF may be useful in ameliorating autonomic neuropathy associated with poor gastrointestinal motility (Anitha et al., 2006).
Although chronic hyperglycemia is considered the major trigger in the pathogenesis of DPN (DCCT Research Group, 1993), patients with impaired glucose tolerance (prediabetes) also develop DPN (Smith and Singleton, 2008). In an STZ rat model, altered mechanical sensitivity occurred before the development of hyperglycemia, when blood insulin levels fell below 2 ng/ml; the onset of hyperglycemic-induced DPN occurred when insulin levels decreased to less than 0.3 ng/ml (Romanovsky et al., 2006). Thus, impaired insulin signaling or insulin deficiency in peripheral neurons may also contribute to DPN. Insulin is important for general neuronal function (Kim and Feldman, 2012; Urban et al., 2012a), and insulin receptors are abundantly expressed in neuronal cell bodies in the DRG and peripheral axons innervating the epidermis (Sugimoto et al., 2000, 2002; Toth et al., 2006; Guo et al., 2011). It is noteworthy that neuronal insulin receptors are increased after physical injury of peripheral nerves (Toth et al., 2006) and in diabetes (Guo et al., 2011). At doses insufficient to alter hyperglycemia, local injections of insulin into the hindpaw footpad of diabetic mice improved nerve fiber density and mechanical sensation (Guo et al., 2011). Likewise, intranasal insulin administration reduced several physiological indices of DPN and increased sensory nerve fibers in the plantar footpads in the absence of correcting the systemic hyperglycemia (Francis et al., 2009).
Insulin deficiency may alter neuronal function in diabetic sensory neurons by decreasing mitochondrial respiration because insulin enhanced respiratory activity and inner mitochondrial membrane potential in a phosphatidylinositol-3-kinase-dependent manner (Huang et al., 2003, 2005; Chowdhury et al., 2010). It is also notable that insulin-dependent mitochondrial production of H2O2 can enhance insulin receptor phosphorylation in cerebellar granule neurons (Storozhevykh et al., 2007). However, diabetic sensory neurons are less responsive to producing ROS, even when using the potent superoxide inducer antimycin A (Zherebitskaya et al., 2009; Chowdhury et al., 2010). Although it remains unclear whether the increase in insulin receptor phosphorylation by H2O2 is critical for the efficacy of insulin, these results suggest that a point may exist at which insulin therapy may no longer improve mitochondrial function if insulin-induced H2O2 production is compromised (Cheng et al., 2010). Along this line, the electrophysiological deficits of DPN were less responsive to insulin as the disease progressed (Szilvássy et al., 2012).
The response of Schwann cells to growth factors may also be affected in diabetic nerve. Neuregulin-1 forms a family of several gliotrophic factors (Esper et al., 2006) that bind to Erb B2 receptors, which preferentially localize to Schwann cells (Grinspan et al., 1996). Neuregulins play a complex role in the regulation of myelination because they may both promote myelination and induce demyelination in a concentration-dependent manner (Syed et al., 2010). After axotomy, increases in neuregulin levels may contribute to Wallerian degeneration by activating Erb B2 receptors (Guertin et al., 2005). In cultured neonatal rat Schwann cells, hyperglycemia stimulated neuregulin-induced Erb B2 activation and increased thymidine uptake (Tan et al., 2003). On the other hand, a separate report found no effect of hyperglycemia on Erb B2 signaling and a decrease in neuregulin-induced mitogenesis (Gumy et al., 2008). Although the underlying reason for these discrepant findings is unclear, changes in Erb B2 activity may indeed contribute to altered nerve function in DPN. In diabetic mice, Erb B2 phosphorylation increased in sciatic nerve, and this was correlated with a decrease in MNCV and the induction of a sensory hypoalgesia (McGuire et al., 2009). It is noteworthy that treating diabetic C57BL/6 mice with an Erb B2 inhibitor, 4-phenethylamino-6-(yderoxyl)phenyl-7H-pyrrolo(2,3-d)pyrimidine (PKI-166) or erlotinib, attenuated the electrophysiological deficits and improved mechanical but not thermal hypoalgesia. Because thermal sensitivity is mediated primarily via unmyelinated C fibers (Julius and Basbaum, 2001), these data indicate that myelinated fibers may be more sensitive to pathological activation of Erb B2. In this regard, hyperglycemia increased the extent of neuregulin-induced demyelination in myelinated Schwann cell-sensory neuron cocultures (Yu et al., 2008). However, the effect of diabetes on the expression of various neuregulin-1 isoforms and whether they contribute to myelin thinning in peripheral nerve remains to be determined.
III. Management of Diabetic Peripheral Neuropathy
A. Targeting Hyperglycemia
Strict glycemic control is the only proven method available that prevents the development of DPN and/or slows the progression of this disease (Tesfaye et al., 2005, 2011). This is mainly achieved with the use of oral antidiabetics and insulin in conjunction with lifestyle changes in diet and exercise. Although modulating incretin expression is a new approach toward managing blood glucose levels, increasing incretin levels may directly improve DPN in type 1 diabetes, even without affecting blood glucose levels. Receptors for the incretin, glucagon-like peptide 1, localize to axons and Schwann cells of sciatic nerve (Jolivalt et al., 2011). Treating diabetic rats with exenatide, a glucagon-like peptide-1 mimetic, improved MNCV and intraepidermal nerve fiber density without improving plasma insulin and blood glucose (Jolivalt et al., 2011). Likewise, dipeptidyl peptidase IV inhibitors are a new class of antidiabetic agents that increase the half-life of endogenous incretins. Because a vildagliptin analog also improved DPN in diabetic rats without affecting blood glucose levels (Bianchi et al., 2012), incretins are likely to have extrapancreatic effects that are directly neurotrophic (Perry et al., 2002).
B. Targeting Casual Mechanisms
1. Aldose Reductase Inhibitors.
Due to the numerous negative effects of increased AR activity during chronic hyperglycemia, the inhibition of this enzyme remains an attractive strategy for treating DPN (Oates, 2008). Aldose reductase inhibitors inhibit tissue accumulation of sorbitol and fructose by reducing the flux of glucose through the polyol pathway and are represented by three chemical classes; acetic acid compounds, spirohydantoins, and a succinimide (Schemmel et al., 2010), although new agents also continue to be evaluated (Maccari et al., 2011; Rapposelli et al., 2011).
Alrestatin, epalrestat, ponalrestat, tolrestat, zenarestat, and zopolrestat represent the acetic acid compounds. Sorbinil and fiderestat are spirohydantoins, and ranirestat is a succinimide. Although all these agents have shown efficacy in animal models of DPN, epalrestat is the only AR inhibitor that has been approved to treat DPN and has been available in Japan since 1992 (Schemmel et al., 2010). In patients with diabetes treated with ranirestat for 12 weeks, the drug dose dependently inhibited sorbitol and fructose levels in the sural nerve (Bril and Buchanan, 2004), and a 48-week extension of this study revealed an improvement in SNCV by ≥1 m/s relative to baseline and compared with placebo (Bril and Buchanan, 2006). Unfortunately, in phase 3 testing, 52 weeks of ranirestat treatment (20 and 40 mg/day) failed to improve summed SNCV, possibly related to an unexpected improvement in SNCV in the placebo group (Bril et al., 2009). However significant improvements in MNCV were observed and the drug was well tolerated, suggesting that a more refined trial design may demonstrate efficacy in the primary endpoint of summed SNCV (Bril et al., 2009).
2. Blocking Advanced Glycation End-Product Formation and Activation of the Receptor for Advanced Glycation End-Products.
Several experimental and/or clinical studies have examined agents that reduce/inhibit AGE formation, cleave AGEs, or block RAGE. Aminoguanidine was the first AGE inhibitor studied and prevents AGE formation by reacting with carbonyl groups of reduced sugars (Sugimoto et al., 2008). Double-blind, multidose, placebo-controlled, randomized clinical trials in patients with type 1 and 2 diabetes with diabetic nephropathy showed no reduction in the nephropathy progression (Freedman et al., 1999; Bolton et al., 2004). Although aminoguanidine did slow the development of retinopathy in these patients, clinical trials have been discontinued because of adverse effects, such as gastrointestinal disturbances and liver function test abnormalities (Bolton et al., 2004).
An alternative strategy for decreasing AGEs is to decrease methylglyoxal, a major dicarbonyl glucose metabolite that modifies arginine residues of proteins. In this regard, diabetic rats treated with thiamine or benfotiamine (also see section B.4) showed decreased levels of protein glycation (Karachalias et al., 2010). Likewise, Xue et al. (2011) suggest that up-regulating glyoxalase 1, which metabolizes methylglyoxal, can decrease methylglyoxal-derived protein adducts. Glyoxalase activity may also be a potential biomarker for susceptibility to DPN. BALB/cByJ mouse express a 10-fold greater level of glyoxalase 1 than BALB/cJ mice and were resistant to developing an insensate neuropathy and loss of intraepidermal nerve fibers compared with the diabetic BALB/cJ mice (Jack et al., 2012).
Another approach to modulate the effects of AGEs is to prevent their binding to RAGE. This is achieved with a soluble form of the extracellular ligand-binding domain of RAGE (sRAGE) and/or anti-RAGE antibodies. Similar to diabetic RAGE knockout mice, wild-type mice treated with sRAGE show a diminished extent of diabetes-related complications (Sakaguchi et al., 2003; Wendt et al., 2003). In diabetic rodents, sRAGE treatment normalized thermo-nociception and corrected neuronal deficits (Bierhaus et al., 2004). The presence of endogenous sRAGE has also been identified in the blood. In patients with diabetes who have DPN, plasma levels of sRAGE were decreased by 59 and 77% compared with patients with diabetes who did not have DPN and healthy patients without diabetes, respectively (El-Mesallamy et al., 2011). Although sRAGE is in clinical trials for other disorders, no current trials were listed for its use in DPN in early 2012 (http://www.clinicaltrials.gov).
3. Protein Kinase C Inhibitors.
Ruboxistaurin (RBX, also known as LY333531 or Arxxant) is a bisindolylmaleimide that selectively inhibits PKC-β1 and PKC-β2 (Geraldes and King, 2010). RBX has successfully reduced the development of diabetic retinopathy and nephropathy (Beckman et al., 2002; Tuttle et al., 2005; Aiello et al., 2006), but it has not been as successful in the setting of DPN (Vinik et al., 2005). However, in a subset of patients with less severe DPN, RBX did significantly improve neuropathic symptoms and nerve function compared with placebo. Nonetheless, the status for advancing the approval of RBX for use in DPN is unclear.
4. Hexosamine Pathway.
Currently, pharmacologic modulation of the hexosamine pathway is primarily indirect. Thiamine (vitamin B1) is a water-soluble vitamin that serves, after phosphorylation, as a coenzyme for transketolase. Transketolase plays a fundamental role in intracellular glucose metabolism by shifting excess F-6-P from glycolysis into the pentose phosphate pathway, thereby preventing activation of the hexosamine pathway and decreasing methylglyoxal formation as described above (Beltramo et al., 2008). Patients with diabetes show a high prevalence of thiamine and transketolase deficiency (Thornalley et al., 2007; Alam et al., 2011), and high doses of thiamine in combination with pyridoxine (vitamin B6) improved neuropathic symptoms in patients with diabetes who have DPN (Abbas and Swai, 1997).
Benfotiamine is a lipid-soluble thiamine derivative with improved bioavailability (Beltramo et al., 2008; Balakumar et al., 2010). In the Benfotiamine in Diabetic Polyneuropathy pilot study (Haupt et al., 2005), benfotiamine (400 mg/day for 3 weeks) improved painful neuropathic symptoms; similar results were seen in a follow-up phase 3 clinical trial, (600 mg/day benfotiamine for 6 weeks) (Stracke et al., 2008). Benfotiamine was well tolerated with no side effects in both clinical trials and is available as a dietary supplement in the United States. As mentioned above, any benefits of benfotiamine are likely to include modulation of AGEs as well.
5. Antioxidants, α-Lipoic Acid (Thiocytic Acid).
Increasing antioxidant defense is a logical therapeutic approach for treating DPN and α-lipoic acid (LA) is among the better studied antioxidants in humans. Oral administration of LA (minimum of 600 mg/day) reduced neuropathic symptoms associated with DPN by 50% from baseline in the SYDNEY 2 trial (Ziegler et al., 2006). However, compared with results from subjects in the control group, this reduction in symptoms falls below the clinically relevant threshold of 30% (Mijnhout et al., 2010). The recent Neurological Assessment of Thioctic Acid in Neuropathy (NATHAN) 1 trial was a 4-year treatment (600 mg/day) of patients with diabetes and mild-to-moderate DPN (Ziegler et al., 2011). LA did not significantly improve nerve conduction attributes but did improve some scores of small-fiber neuropathy and muscular function. It is important to note that the lack of efficacy in the NATHAN 1 trial seems to arise from the lack of further decline of nerve conduction deficits in the placebo-treated group during the study (Ziegler et al., 2011), an inherent challenge to trial design in DPN (Dyck et al., 2007). LA is approved for treating DPN in Germany (Gooch and Podwall, 2004) and is commercially available as a dietary supplement in the United States.
6. Growth Factors.
The use of growth factors in treating DPN has been extensively explored (Leinninger et al., 2004; Calcutt et al., 2008). NGF, BDNF, and NT-3 have been assessed in various levels of clinical trials of DPN with limited success. NGF therapy has advanced the furthest but showed no efficacy in a large, multicenter phase III trial (Apfel, 2002). In addition, a recent proof-of-concept trial examining the analgesic effects of tanezumab, a monoclonal antibody against NGF, was halted in 2010 for potential safety issues (http://www.clinicaltrials.gov/ct2/show/NCT01087203).
C. Targeting Painful Symptoms
Controlling pain is one of the most difficult management issues encountered when treating patients who have DPN. A number of drugs have shown efficacy at managing neuropathic pain in randomized clinical trials. Unfortunately, there are few FDA-approved treatments and no standardized algorithms for treating neuropathic pain associated with DPN.
1. Tricyclic Antidepressants.
Tricyclic antidepressants (TCAs) inhibit the reuptake of noradrenaline and/or serotonin, which modulates pain transmission within the central nervous system. Amitriptyline, imipramine, and desipramine are considered the first-line treatment for DPN, although large, controlled trials seem to be lacking (Veves et al., 2008). Nonetheless, a meta-analysis revealed that one in three patients with diabetes who have DPN that is treated with TCAs achieved a 50% reduction in neuropathic pain (Mendell and Sahenk, 2003). On the other hand, duloxetine (Cymbalta) is a serotonin-norepinephrine reuptake inhibitor that relieves pain by increasing synaptic availability of serotonin and norepinephrine in the descending inhibitory pathway (Smith and Nicholson, 2007). Duloxetine is the only antidepressant approved by the FDA for the treatment of DPN (Raskin et al., 2005).
2. Anticonvulsants.
Anticonvulsants were originally developed to prevent seizures, and because neuronal hyperexcitability is associated with neuropathic pain, it is believed they may be used for treating this debilitating symptom of DPN. Anticonvulsants regulate neuronal hyperexcitability by blocking calcium and/or sodium channels, through the enhancement of inhibitory GABAergic neurotransmission and inhibition of glutamatergic neurotransmission (Sullivan and Robinson, 2006).
Gabapentin (Neurontin) and pregabalin (Lyrica) are the two anticonvulsants used most frequently to treat neuropathic pain. These agents are structurally related compounds and are derivatives of the inhibitory neurotransmitter GABA; they do not have a direct pharmacological effect on GABA uptake or metabolism (Spruce et al., 2003). The α2-δ site of the voltage-gated calcium channel is the target of these agents. They reduce synaptic neurotransmitter release into hyperexcited neurons by diminishing calcium intake at nerve terminals (Finnerup et al., 2010).
Gabapentin at a dose of 1800 mg/day is effective in treating DPN (Backonja et al., 1998). Pregabalin is considered a successor to gabapentin and is more rapidly absorbed (1 versus 3–4 h) and has a higher bioavailability (90 versus 33–66%) than gabapentin (Piyapolrungroj et al., 2001). In the clinical setting, pregabalin (300–600 mg/day) effectively alleviates pain associated with DPN (Rosenstock et al., 2004; Tolle et al., 2008), and it is the only anticonvulsant approved for the treatment of DPN in the United States and Europe (Tzellos et al., 2010).
3. Opioids and Topical Analgesics.
Opioids are effective analgesics used for treating both acute and chronic pain. Several opiates, such as controlled and sustained-released oxycodone, tapentadol, and tramadol, have shown clinical efficacy in relieving painful DPN (Harati et al., 1998; Gimbel et al., 2003; Watson et al., 2003; Schwartz et al., 2011; Yao et al., 2012). The most common side effects that occur with opioid use are constipation, dizziness or vertigo, dry itchy skin, nausea, somnolence or drowsiness, and vomiting (Furlan et al., 2006). Although opioids may be effective in treating DPN, the development of tolerance with a potential for dependence may occur with repeated and prolonged exposure. Consequently, other therapeutic agents are prescribed first; however, when these therapies fail to provide sufficient pain relief, opioids may be the only choice.
Topical treatments offer several therapeutic advantages, including lack of drug interactions, minimal side effects, and no need for dose titration (Tesfaye et al., 2011). However, few randomized control trials have been designed that explore the use of topical treatments as a therapy for treating neuropathic pain in DPN. Topical lidocaine is a voltage-gated sodium channel blocker that has been recommended as a first line treatment for localized neuropathic pain. A systematic review reported that 5% lidocaine medicated patches are effective in reducing painful DPN, and these patients experience a greater improvement in quality of life (Wolff et al., 2010). Side effects include burning sensation, elevated aspartate aminotransferase levels and blood pressure, headache, muscle spasms, and tingling sensation. However, compared with other agents used to reduce symptoms associated with painful DPN, 5% lidocaine medicated patches are associated with fewer and less clinically significant adverse events (Wolff et al., 2010). The FDA has approved these patches.
Capsaicin is a natural product isolated from red chili peppers. Its topical application depletes substance P from sensory nerves and has shown promising effects on painful DPN (Zhang and Li Wan Po, 1994). The Capsaicin Study Group (1992) evaluated the efficacy of capsaicin for treating DPN in an 8-week, double-blind, placebo-controlled study. They concluded that topical capsaicin is effective for treating painful symptoms associated with DPN. In addition, patients exhibited improvements in daily activities and an overall enhancement in their quality of life (Capsaicin Study Group, 1992). Although a patch containing 8% capsaicin has yielded encouraging results in treating peripheral neuropathic pain (Peppin et al., 2011), a medical concern is that its application can result in denervation of the epidermis, which can be exacerbated in patients with diabetes and neuropathy (Polydefkis et al., 2004).
D. Chaperoning Diabetic Stress—Targeting Tolerance?
It is clear from the discussion above that the efficacy of many agents in preventing or reversing experimental neuropathy in rodents has not readily translated to aiding the management of human DPN. It is likely that this represents limitations of the animal models in serving as reliable surrogates for both functional and morphologic deficits seen in human DPN, but this is not necessarily the sole problem (Calcutt et al., 2009). Another confounding factor in identifying effective treatments is that the contribution of these targets/pathways to the progression of DPN differs between patients and does not occur with temporal and/or biochemical uniformity. Thus, with necessary attention to good glycemic control, combinatorial therapies targeting multiple glucose-mediated insults may prove the most beneficial. However, a parallel and/or complementary approach to help counteract glucotoxicity is up-regulating endogenous cytoprotective responses via pharmacologic modulation of molecular chaperones.
1. Controlling the Biological Outcome of Inhibiting the 90-kDa Heat-Shock Protein: Dissociating Cytotoxicity from Cytoprotection Is Paramount to Attain Specific Therapeutic Efficacies.
Molecular chaperones, such as the 70- and 90-kDA heat-shock proteins (Hsp70 and Hsp90), are essential for the folding of nascent polypeptides into their biologically active structures and for refolding aggregated and denatured proteins that may occur upon cell stress (Mayer and Bukau, 2005; Peterson and Blagg, 2009). Energy derived from hydrolysis of ATP by the intrinsic N-terminal ATPase activity of Hsp90 is important in the conformational maturation of “client proteins” that form a stabilized complex with homodimerized Hsp90. Inhibiting Hsp90 interferes with protein maturation, which leads to degradation of Hsp90 client proteins (Powers and Workman, 2007). Because many oncoproteins are Hsp90 clients, N-terminal Hsp90 inhibitors are attractive as potential chemotherapeutic agents because they can promote cytotoxicity via oncoprotein degradation (Bishop et al., 2007; Taldone et al., 2009). An important aspect of N-terminal Hsp90 inhibitors in cancer therapy is that the drugs selectively inhibit Hsp90 and induce client protein degradation in malignant versus normal cells (Chiosis et al., 2003; Kamal et al., 2003; Luo et al., 2008). Although this selectivity aids the clinical efficacy of N-terminal Hsp90 inhibitors, their use has been hampered because induction of client protein degradation and cytotoxicity can occur at drug concentrations that also activate an antagonistic aspect of Hsp90 biology, induction of the cytoprotective heat-shock response (HSR) (Fig. 2).
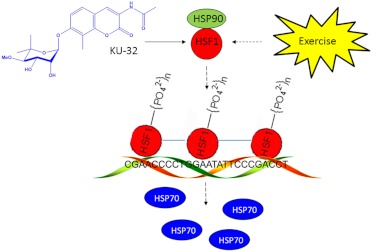
Fig. 2.
Activation of the heat-shock response by KU-32 and exercise. KU-32 is a C-terminal Hsp90 inhibitor that may increase the expression of Hsp70 by promoting the release of HSF1 from Hsp90. After trimerization, phosphorylation and translocation to the nucleus, HSF1 interacts with two heat-shock elements (HSE) on the Hsp70 promoter to increase the expression of Hsp70. Exercise may similarly increase Hsp70 expression but via indirect effects on Hsp90/HSF1. The binding of HSF1 to sequences within a portion of one HSE of the human Hsp70.1 promoter is shown (Calamini et al., 2012). Solid arrow, direct effect; dashed arrow, multiple steps.
Hsp90 binds the transcription factor heat-shock factor 1 (HSF1) and inhibits its transactivating capacity (Benarroch, 2011). Upon exposure to stress or Hsp90 inhibitors, HSF1 dissociates, trimerizes, undergoes phosphorylation, and translocates to the nucleus to up-regulate antioxidant genes and a variety of chaperones: the heat-shock response. Unfortunately, because chaperones can function as prosurvival factors that facilitate refolding of the oncoproteins, HSR induction antagonizes the desired cytotoxicity when treating malignancies. On the other hand, although induction of the HSR interferes with their chemotherapeutic potential, stimulating this aspect of Hsp90 biology has potential utility for treating neurodegenerative diseases associated with protein misfolding: HSR induction decreases protein aggregation.
N-terminal Hsp90 inhibitors have been shown to decrease τ protein aggregation in Alzheimer disease models (Dickey et al., 2007; Luo et al., 2007) and improve motor function in spinal and bulbar muscular atrophy (Waza et al., 2005). Although a similar selectivity exists for the use of N-terminal inhibitors in treating neurodegenerative diseases (Dickey et al., 2007), this selectivity does not circumvent the issue related to dissociating client protein degradation from induction of the HSR. Now, the inverse caveat exists; despite being neuroprotective, induction of client protein degradation may produce cytotoxicity. Thus, developing a highly effective Hsp90 inhibitor for treating neuronal decline requires establishing a sufficient therapeutic window that avoids client protein degradation, which antagonizes a cytoprotective HSR. Along this line, Hsp90 also contains a C-terminal ATP binding domain that binds the bacterial DNA gyrase inhibitor, novobiocin (Marcu et al., 2000a,b). Similar to N-terminal inhibitors, novobiocin can promote client protein degradation and induce an HSR. However, systematic modification of the novobiocin pharmacophore has identified N-{7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyl-tetrahydro-2H-pyran-2-yloxy]-8-methyl-2-oxo-2H-chromen-3-yl}acetamide (KU-32) as a lead neuroprotective compound (Ansar et al., 2007; Lu et al., 2009) that exhibits at least a 500-fold divergence of Hsp70 induction from client protein degradation (Urban et al., 2010). Because Hsp70 is critical for the neuroprotective efficacy of KU-32 (see section III.D.3), this divergence provides an excellent therapeutic window to promote neuroprotection and minimize potential off-target toxicity in treating DPN.
2. Heat-Shock Protein Expression and DPN—An Impaired Defense Against Stress??
Chronic hyperglycemia imposes ischemic, hypoxic, oxidative, and apoptotic stress leading to widespread damage to proteins, cells, and tissues (Tomlinson and Gardiner, 2008; Obrosova, 2009b). Although the etiology of DPN is unrelated to one specific misfolded protein aggregate, hyperglycemia can promote the oxidative modification of amino acids (Obrosova et al., 2007a; Akude et al., 2010) that may impair protein folding (Muchowski and Wacker, 2005), decrease mitochondrial protein import (Baseler et al., 2011), and promote mitochondrial dysfunction (Fernyhough et al., 2010; Sivitz and Yorek, 2010; Chowdhury et al., 2011). Postmitotic neurons may be especially vulnerable to stress-induced protein denaturation, because diabetic nerves that exhibit neuropathological changes can have a lower expression of molecular chaperones. For example, 10 months of hyperglycemia markedly reduced Hsp70 levels in DRG from spontaneously diabetic Bio-breeding/Worcester rats, a model of type 1 diabetes. This correlated with an advanced neuropathy characterized by loss of neurotrophic components and a decrease in both myelinated/unmyelinated fibers (Kamiya et al., 2006). On the other hand, after only 4 months of diabetes in the Bio-breeding/Worcester rat model, Hsp27 and Hsp70 were increased in DRG (Kamiya et al., 2005). After only 1 month of STZ-induced diabetes, Hsp expression was unaltered or enhanced by heat shock depending on the tissue (Najemnikova et al., 2007). Thus, chaperone expression is differentially affected as the degeneration progresses. It is conceivable that the level of endogenous chaperones may be sufficient to counter early glycemic insults, but as diabetes becomes more prolonged, increased chaperone expression may represent a cytoprotective response that is eventually overwhelmed by the excessive metabolic disturbances associated with chronic diabetes. That being said, the question remains whether impaired Hsp70 expression is an initiator or outcome of DPN. However, genetic knockout of the stress-inducible isoforms of Hsp70 (Hsp70.1 and Hsp70.3) caused no significant difference in the rate of onset or severity of a sensory hypoalgesia that developed in diabetic Hsp70 knockout mice (Urban et al., 2010). These data suggest that decreased Hsp70 expression is not an essential pathogenic component in DPN. Although there can be considerable functional redundancy between inducible (Hsp70) and constitutive (Hsc70) paralogs, we have noted that Hsc70 does not change in diabetic nerve, suggesting that its decline is also not a critical feature in the etiology of DPN. It is noteworthy that high anti-Hsp70 antibody levels, but not those of anti-Hsp60 antibody, were detected in an analysis of 531 patients with type 1 diabetes from the EURODIAB Study (Gruden et al., 2009). Independent of other risk factors and inflammation, a 50% lower likelihood of micro/macrovascular complications was associated with high anti-Hsp70 antibody levels (Gruden et al., 2009). Although the underlying origin of the antigen was not identified, these data suggest that elevated Hsp70 antibody levels may serve as a positive biomarker for patients less prone to developing diabetic complications (Gruden et al., 2009). It may be worthwhile to determine whether a relationship exists between Hsp70 antibody levels and the level of glyoxalase 1 expression (Jack et al., 2012) as a predictive biomarker pair for the development of DPN in humans (Rabbani and Thornalley, 2011).
3. 70-kDa Heat-Shock Protein and Neuroprotection in DPN.
Although most other Hsps are abundantly distributed in the nerve, the inducible form of Hsp70 is weakly expressed, and its induction typically represents a cellular protective program adopted by neurons and glia during stress response (Pavlik et al., 2003; Pavlik and Aneja, 2007). Thus, physically or pharmacologically increasing Hsp expression may confer protection against neuropathic changes associated with diabetes. For example, patients with type 2 diabetes experienced reductions in blood glucose concentration and symptomatic neuropathy after receiving regular hot-tub treatment (Hooper, 1999, 2003). In diabetic rats, the HSF1 activators bimoclomol and arimoclomol (BRX-220) improved diabetic wound healing and nerve conduction deficits, respectively (Vígh et al., 1997; Kürthy et al., 2002); direct HSF1 activators may provide another pharmacologic route to modulating genes that express chaperones and antioxidant proteins (Neef et al., 2011). The potential effectiveness of α-lipoic acid in treating DPN has already been noted and α-lipoic acid therapy has been normalized. Hsp70 levels were decreased in a cohort of patients with type 1 diabetes and DPN (Strokov et al., 2000). Overexpression of Hsp70 in muscle is sufficient to improve insulin resistance and glucose utilization (Chung et al., 2008), and this metabolic correction would be anticipated to also improve DPN. However, the therapeutic benefits of Hsp70 induction in DPN do not seem to hinge on a metabolic correction and may be relatively nerve-specific (Urban et al., 2010, 2012b). On the other hand, modulating chaperones may improve the trophic effects of impaired insulin signaling in DPN, because several components of the insulin signaling cascade rely on interactions with heat shock proteins (Urban et al., 2012a).
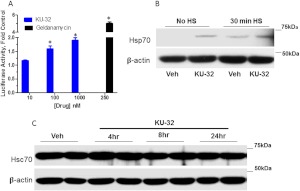
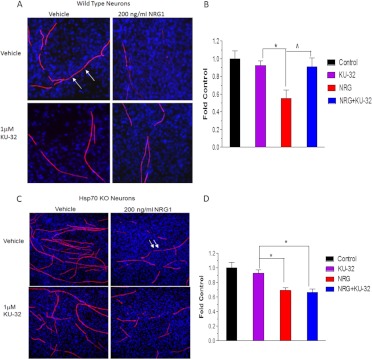
We have been exploring the potential of a novel class of Hsp90 inhibitors as a pharmacologic tool to modulate Hsp70 in DPN. KU-32 is a C-terminal, novobiocin-based Hsp90 inhibitor (Fig. 2) that protected against neuronal cell death (Ansar et al., 2007). Despite being only a weak activator of the Hsp70 promoter compared with the prototypical N-terminal Hsp90 inhibitor geldanamycin (Fig. 3A), KU-32 increased Hsp70 expression in explant cultures of neonatal mouse sensory neurons (Fig. 3B) without affecting the expression of Hsc70 (Fig. 3C). It is noteworthy that weekly treatment of diabetic mice with KU-32 reversed a pre-existing mechanical and thermal hypoalgesia and improved deficits in both sensory and motor nerve conduction velocities (Urban et al., 2010). Moreover, after 16 weeks of diabetes in Swiss-Webster mice, intraepidermal nerve fiber density was decreased by 30%, and this loss of fibers was reversed by 10 weeks of KU-32 therapy (Urban et al., 2012b). Because no obvious improvement in plasma glucose or insulin levels were observed in mice administered KU-32, the protection did not require a metabolic correction of glucose utilization and probably resulted from a direct effect on chaperone expression in peripheral nerve. Indeed, despite the modest induction of Hsp70 induced by KU-32, it is central to drug efficacy. For example, KU-32 protected against neuregulin-induced demyelination in myelinated Schwann cell-sensory neuron cocultures prepared from wild-type mice (Figs. 4, A and B), but this effect was lost in neurons isolated from Hsp70 knockout mice (Figs. 4, C and D). Likewise, although KU-32 reversed multiple indices of DPN in wild-type mice, the drug was ineffective at reversing an insensate neuropathy in diabetic Hsp70 KO mice (Figs. 5, A and B) (Urban et al., 2010). These data agree with other reports supporting Hsp70 as a key neuroprotective chaperone preventing neuronal apoptosis (Bienemann et al., 2008) and improving neurodegenerative diseases associated with protein misfolding (Dickey et al., 2007; Luo et al., 2007). Thus, modulating Hsp70 may increase the tolerance of neurons and glia to the fluxing metabolic stresses associated with diabetes (Calcutt, 2010).
Fig. 3.
Induction of Hsp70 but not Hsc70 by KU-32. A, 50B11 cells, an immortalized sensory neuron cell line (Chen et al., 2007) were transfected with a luciferase reporter linked to the human Hsp70.1 promoter, which contains two heat-shock response elements (Calamini et al., 2012). After 24 h, the transfected cells were seeded into a 96-well plate and maintained in culture for an additional 6 h before treatment with vehicle (0.1% dimethyl sulfoxide) or the indicated concentrations of KU-32 for 24 h. The cells were harvested, and luciferase activity was assessed and normalized to total protein per well. Some wells were treated with 250 nM geldanamycin as a positive control. Results are mean ± S.E.M. from 18 replicate wells in two experiments. B, primary neonatal mouse sensory neurons were isolated and grown in culture for 1 week (Urban et al., 2010). The cells were treated with vehicle or 1 μM KU-32 for 24 h in the absence or presence of heat shock (HS; 30 min at 42°C). Cell lysates were prepared, and the induction of Hsp70 was determined by immunoblot analysis. C, primary neonatal mouse sensory neurons were isolated, grown in culture for 1 week, and treated for the indicated time with vehicle or 1 μM KU-32. Cell lysates were prepared, and Hsc70 levels were determined by immunoblot analysis. The levels of β-actin served as a control for protein loading.
Fig. 4.
Hsp70 is required for KU-32 to protect against neuregulin-induced demyelination. Myelinated mouse SC-DRG neuron cocultures were prepared from wild-type (A and B) or Hsp70 knockout (KO; C and D) mice and treated overnight with vehicle or 1 μM KU-32. The cultures were treated with PBS or 200 ng/ml neuregulin-1 for 4 days, and myelin segments were visualized by staining the cultures for myelin basic protein. Total cell number was assessed by staining nuclei with 4,6-diamidino-2-phenylindole. Cell Profiler (http://www.cellprofiler.org) was used to calculate the total myelin segment area from six fields per six coverslips per treatment. The results are expressed as a fold of the untreated control and are an average of three experiments per genotype. *, p < 0.05 versus KU-32, ∧, p < 0.05 versus NRG. Arrows, examples of myelin internodes. [Modified from Urban MJ, Li C, Yu C, Lu Y, Krise JM, McIntosh MP, Rajewski RA, Blagg BS, and Dobrowsky RT (2010) Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro 2:e00040. Open Access from Portland Press Limited and the American Society for Neurochemistry.]
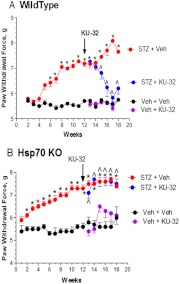
Fig. 5.
Hsp70 is required for the in vivo efficacy of KU-32 in reversing mechanical hypoalgesia. Wild-type (A) and Hsp70 knockout (KO; B) mice were rendered diabetic for 12 weeks and then treated with weekly doses of vehicle or 20 mg/kg KU-32 for 6 weeks. Beginning 2 weeks after the induction of diabetes, mechanical sensitivity was assessed weekly. Twelve weeks of diabetes produced a significant mechanical hypoalgesia, and weekly treatment with KU-32 induced a time-dependent improvement to near control levels in the wild-type (A) but not the Hsp70 KO (B) mice. *, p < 0.01 compared with time-matched untreated controls. ∧, p < 0.01 compared with time-matched Veh + KU-32. [Modified from Urban MJ, Li C, Yu C, Lu Y, Krise JM, McIntosh MP, Rajewski RA, Blagg BS, and Dobrowsky RT (2010) Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro 2:e00040. Open Access from Portland Press Limited and the American Society for Neurochemistry.]
4. 70-kDa Heat-Shock Protein Family Members and Mitochondrial Function.
Although it remains unclear how Hsp70 may antagonize the proneuropathic action of the established biochemical mediators of DPN, Hsp70 can improve oxidative stress and mitochondrial function. Hsp70 overexpression significantly potentiated the activities of glutathione peroxidase and glutathione reductase, leading to a higher GSH/GSSG ratio, attenuated ROS production, and improved cell survival under conditions of hypoxia and glucose deprivation (Guo et al., 2007). Likewise, overexpression of Hsp70 in astrocytes increased mitochondrial function and decreased neuronal injury after basal forebrain ischemia (Xu et al., 2010). Consistent with this result, KU-32 increased the translation of Hsp70, as well as other chaperones and MnSOD in hyperglycemically stressed sensory neurons. Increased expression of the molecular chaperones and MnSOD correlated with a decrease in mitochondrial superoxide levels and improved mitochondrial bioenergetics (Zhang et al., 2012). However, it remains to be determined whether chaperone induction improves mitochondrial bioenergetics to decrease oxidative stress or if enhanced expression of MnSOD and decreased superoxide production precedes improved respiratory function (Fig. 6). It is also worth noting that NADPH oxidases can serve as a potential source of superoxide in DPN (Coppey et al., 2003). It is noteworthy that down-regulation of Hsp70 with small interfering RNA increased NADPH-dependent ROS production in vascular smooth muscle cells (Madrigal-Matute et al., 2012), raising the possibility that increasing Hsp70 may also decrease extra-mitochondrial sources of superoxide via effects on NADPH oxidase.
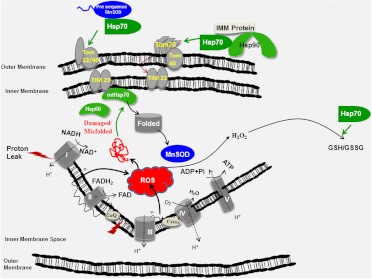
Fig. 6.
Potential mechanisms by which modulating chaperones may increase mitochondrial function in DPN. Diabetes-induced increases in mitochondrial ROS may promote formation of oxidatively modified proteins, which contribute to a decreased respiratory capacity. Increasing transport of proteins to mitochondria may aid in replacing damaged proteins. Hsp70 is involved in protein transport to mitochondria and contributes to the internalization of multispanning inner mitochondrial membrane proteins via TOM70. Although not required, Hsp70 may also facilitate the transfer of preproteins containing a mitochondrial targeting sequence, such as MnSOD, to TOM22. mtHsp70 is a requisite chaperone for internalization of preproteins before final proteolytic processing. Within the organelle, Hsp60 and mtHsp70 may aid the refolding of oxidatively damaged mitochondrial proteins. In the presence of sufficient levels of NADPH, Hsp70 may also increase the GSH/GSSG ratio in the cytoplasm by increasing the activity of glutathione peroxidase and reductase. TOM and TIM, translocases of the outer and inner mitochondrial membrane, respectively; Pi, inorganic phosphate; CoQ, coenzyme Q.
Several lines of evidence indicate that cytosolic Hsp70 is positively involved in affecting mitochondrial bioenergetics. Rat hearts transfected with Hsp70 through viral infusion showed improved mitochondrial respiration and attenuated mitochondrial damage during ischemia/reperfusion injury (Suzuki et al., 2002). Transgenic overexpression of Hsp70 in mouse skeletal muscles increased citrate synthase and β-hydroxyacyl-CoA-dehydrogenase activities, suggesting an enhanced oxidative capacity (Chung et al., 2008). In contrast, lower Hsp70 mRNA expression correlated with reduced mitochondrial enzymatic activity in skeletal muscle of diabetic humans compared with healthy control subjects (Bruce et al., 2003). Overexpression of Hsp70 in glucose-deprived astrocytes also inhibited proton leak and ROS accumulation (Ouyang et al., 2006; Xu et al., 2010).
How Hsp70 may improve mitochondrial function is unresolved. Drp1 is a mitochondrial fission protein that may contribute to mitochondrial dysfunction in DPN (Vincent et al., 2010). A recent report indicates that inhibiting Drp1 translocation and phosphorylation in podocytes decreased oxidative stress and improved features of diabetic nephropathy (Wang et al., 2012). Given the ability of KU-32 to decrease mitochondrial superoxide levels in hyperglycemically stressed sensory neurons (Zhang et al., 2012), it will be important to determine whether this effect may be mediated via Hsp70-dependent interactions with Drp1 or upstream kinases in diabetic sensory neurons. Alternatively, oxidative modification of Drp1 leads to formation of tetramers and larger aggregates. Because modified Drp1 has enhanced GTPase activity, which can promote mitochondrial fission (Nakamura and Lipton, 2010), Hsp70 may decrease organellar fission by aiding Drp1 refolding. However, a folding-incompetent, ATPase-deficient Hsp70 mutant that maintained the ability to bind and aid the solubility of denatured proteins still improved mitochondrial function in cultured astrocytes (Ouyang et al., 2006). These data imply that the folding competency of Hsp70 may not be a key feature for protection. It is noteworthy that combining Hsp70 overexpression with inhibition of its ATPase activity by methylene blue increased the proteasomal degradation of τ (Jinwal et al., 2009). Although mitochondrial dysfunction in DPN is not necessarily associated with a single protein aggregate, it will be interesting to determine whether increasing protein clearance by combining an Hsp70 inducer with a selective ATPase inhibitor may prove beneficial in treating DPN, as has been suggested for cancer therapy (Koren et al., 2010). Finally, cytosolic Hsp70 and Hsp90 interact with TOMM70, a mitochondrial outer membrane translocase, to mediate import of nuclear-encoded proteins (Young et al., 2003). Because 99% of the mitochondrial proteins arise from nuclear genes and need to be imported into the organelle (Schmidt et al., 2010), Hsp70 may facilitate the replacement of oxidatively damaged mitochondrial proteins (Fig. 6).
Cytosolic Hsp70 may also have help from other Hsp70 family members in decreasing oxidative stress. Genetic overexpression of the mitochondrial paralog of Hsp70 (mortalin/Grp75/mtHsp70) is sufficient to protect cultured primary astrocytes from ischemia-induced oxidative damage and death (Voloboueva et al., 2008). Likewise, mtHsp70 overexpression in brain reduced oxidative stress markers and improved mitochondrial function induced by ischemia/reperfusion (Xu et al., 2009). Overexpression of mtHsp70 in cardiac myocytes also potentiated the activity of respiratory chain complexes III and IV and abrogated ROS generation and lipid peroxidation after hypoxia/reoxygenation (Williamson et al., 2008). Using an unbiased proteomic screen, we recently identified that KU-32 also increased translation of MnSOD and mtHsp70 in hyperglycemically stressed neurons (Zhang et al., 2012). Unfortunately, it is unclear how KU-32 may increase mtHsp70 expression, because it is not induced as part of the classic heat-shock response (Deocaris et al., 2006). Nevertheless, approaches that can increase both cytosolic and mitochondrial Hsp70 family members are likely to improve mitochondrial function in DPN via effects on decreasing oxidative stress, aiding the refolding of oxidatively damaged and improving protein import to replace damaged proteins (Fig. 6).
E. Exercise as a Nonpharmacological Therapy for DPN
Exercise has long been recognized as a part of the therapy for the management of diabetes. However, 31% of patients with type 2 diabetes fail to participate in basic physical activity, possibly because of secondary diabetic complications such as DPN (Nelson et al., 2002). Recent studies have shown that exercise has beneficial effects on painful symptoms in the setting of diabetes. In patients with type 1 or 2 diabetes who did not display any signs or symptoms of DPN, aerobic exercise (brisk walking on a treadmill) reduced the onset of both motor (0 versus 17%) and sensory (7 versus 30%) neuropathy compared with patients with diabetes who were sedentary (Balducci et al., 2006). As for patients with impaired glucose tolerance, lifestyle interventions (diet and exercise counseling) can also improve painful symptoms (Smith et al., 2006). The efficacy of exercise in exerting a similar effect in patients with diabetes who have signs and symptoms of DPN has yielded similar results. Patients with DPN who exercise regularly experience significant improvements in electrophysiological deficits and neuropathic symptoms, which is accompanied with increases in intraepidermal nerve fiber branching (Fisher et al., 2007; Hung et al., 2009; Kluding et al., 2012). In diabetic animals, exercise prevents myelin damage and attenuates changes in voltage-gated Ca2+ channel function, thereby ameliorating electrophysiological deficits that occur in DPN (Selagzi et al., 2008; Shankarappa et al., 2011). In addition, exercise can increase expression of Hsp70 (Paulsen et al., 2007; Ogata et al., 2009). Exercise leads to long-term activation of muscle fibers, which increases temperature, metabolic disturbances, and oxidative stress, thus activating a putative heat-shock response (Fig. 2) (Noble et al., 2008). In a transgenic mouse model of Alzheimer's disease, exercise repressed neuronal cell death, which correlated with an up-regulation of Hsp70 expression in the mice that exercised (Um et al., 2011). Likewise, aerobic exercise increased Hsp70 expression in diabetic rats, although the magnitude of this increase was less than that observed in nondiabetic animals (Atalay et al., 2004). These studies strongly suggest that exercise has beneficial actions on alleviating pain in patients with diabetes and provide a potential mechanism by which exercise beneficially maintains neural pain circuitry. It will be insightful to use the Hsp70 knockout mice to examine the contribution of Hsp70 in mediating the benefits of exercise in alleviating aspects of DPN.
It is tempting to speculate that exercise coupled with pharmacologic modulation of chaperone signaling may offer an alternative measure toward managing either painful or insensate DPN, assuming that patients are able to adequately exercise without risk. However, although exercise may be a cost-effective treatment, it may exacerbate complications or have unintended consequences because of pre-existing diabetic complications (Chipkin et al., 2001). Before starting an exercise program, patients with diabetes should be evaluated to minimize the risk of exacerbating existing macro- and microvascular complications. Patients with diabetes who experience peripheral neuropathy should consider only exercises they can tolerate without intensifying their painful symptoms. Most importantly, all patients should monitor blood glucose levels before, during, and after all bouts of exercise. For patients with diabetes, the overall benefits of exercise in preventing and reducing painful DPN are clearly significant (Balducci et al., 2006; Smith et al., 2006). Therefore, clinicians and patients must work together to maximize exercise-induced benefits while minimizing the adverse events to obtain a healthier lifestyle and improve overall quality of life (Chipkin et al., 2001).
IV. Conclusions
It is clear that oxidative stress and mitochondrial dysfunction contribute to the development and progression of DPN. The complex and inter-related mechanisms that lead to these pivotal deficits render it unlikely that the effective targeting of any one pathogenic pathway will offer comprehensive efficacy against DPN. This difficulty is further compounded by the fact that not all pathways may contribute to neuropathy in a temporally and/or biochemically uniform fashion, given the prolonged natural history of disease development (decades), nutritional influences, and genetic and phenotypic heterogeneity in humans. Although the idea of pharmacologically targeting multiple pathogenic pathways that contribute to DPN is not new, adding the modulation of endogenous, cytoprotective molecular chaperones to the mix is a novel supplement that does not rely on inhibiting any one particular mechanism. However, activating cytoprotective pathways is not necessarily a panacea and may succumb to similar limitations that have undermined the successful development of therapeutics that target causal mechanisms. Given that there are multiple pathogenic pathways, a logical and likely inversion is that multiple pathways also regulate neuronal recovery from and tolerance to recurrent glycemic damage. If cytoprotective pathways show a similar variability in efficacy between patients and over time, or poorly translate from the animal models, then emphasizing any one of these mechanisms may not yield a better outcome than our past emphasis on targeting individual pathogenic features of DPN. Although a valid concern, the bleak landscape of therapeutics for DPN would seem to necessitate broadening our paradigms toward disease treatment. It will no doubt remain important to always emphasize good glycemic control and to promote some level of adequate exercise. However, pharmacologic approaches that converge on decreasing inflammation, improving oxidative stress, and stimulating mitochondrial bioenergetics are likely to prove beneficial to the long-term management of DPN. This goal may be realized by targeting causation to limit glycemic damage while also enhancing chaperones to tolerate chronic diabetic stress.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grants NS054847, NS075311] (to R.T.D.); and the Juvenile Diabetes Research Foundation [Grants 1-2008-280, 17-2010-760].
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Farmer, Li, and Dobrowsky.
This article is available online at http://pharmrev.aspetjournals.org.
- AGE
- advanced glycation end product
- AR
- aldose reductase
- BDNF
- brain-derived neurotrophic factor
- COX
- cyclooxygenase
- DCCT
- Diabetes Control and Complication Trial
- DPN
- diabetic peripheral neuropathy
- DRG
- dorsal root ganglia
- F-6-P
- fructose-6 phosphate
- FDA
- U.S. Food and Drug Administration
- GDNF
- glial cell-derived neurotrophic factor
- GlcNAc
- N-acetyl glucosamine
- HETE
- hydroxyeicosatetraenoic
- HSF
- heat-shock factor
- Hsp
- heat-shock protein
- HSR
- heat-shock response
- KU-32
- N-{7-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyl-tetrahydro-2H-pyran-2-yloxy]-8-methyl-2-oxo-2H-chromen-3-yl}acetamide
- LA
- α-lipoic acid
- MAPK
- mitogen-activated protein kinase
- MNCV
- motor nerve conduction velocity
- MnSOD
- manganese superoxide dismutase
- mtHsp70
- mitochondrial paralog of Hsp70
- NF-κB
- nuclear factor κB
- NGF
- nerve growth factor
- NT
- neurotrophin
- PARP
- poly(ADP-ribose) polymerase
- PKC
- protein kinase C
- PKI-166
- 4-phenethylamino-6-(yderoxyl)phenyl-7H-pyrrolo(2,3-d)pyrimidine
- RAGE
- receptor for AGE
- RBX
- ruboxistaurin
- ROS
- reactive oxygen species
- SNCV
- sensory nerve conduction velocity
- sRAGE
- soluble RAGE
- STZ
- streptozotocin
- TCA
- tricyclic antidepressant
- TNF
- tumor necrosis factor.
References
- Abbas ZG, Swai AB. (1997) Evaluation of the efficacy of thiamine and pyridoxine in the treatment of symptomatic diabetic peripheral neuropathy. East Afr Med J 74:803–808 [PubMed] [Google Scholar]
- PKC-DRS2 Group, Aiello LP, Davis MD, Girach A, Kles KA, Milton RC, Sheetz MJ, Vignati L, Zhi XE. (2006) Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology 113:2221–2230 [DOI] [PubMed] [Google Scholar]
- Akkina SK, Patterson CL, Wright DE. (2001) GDNF rescues nonpeptidergic unmyelinated primary afferents in streptozotocin-treated diabetic mice. Exp Neurol 167:173–182 [DOI] [PubMed] [Google Scholar]
- Akude E, Zherebitskaya E, Chowdhury SK, Smith DR, Dobrowsky RT, Fernyhough P. (2011) Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes 60:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akude E, Zherebitskaya E, Roy Chowdhury SK, Girling K, Fernyhough P. (2010) 4-Hydroxy-2-nonenal induces mitochondrial dysfunction and aberrant axonal outgrowth in adult sensory neurons that mimics features of diabetic neuropathy. Neurotox Res 17:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SS, Riaz S, Akhtar MW. (2011) Effect of high dose thiamine therapy on activity and molecular aspects of transketolase in type 2 diabetic patients. Afr J Biotech 10:17305–17316 [Google Scholar]
- Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. (2006) GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest 116:344–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar S, Burlison JA, Hadden MK, Yu XM, Desino KE, Bean J, Neckers L, Audus KL, Michaelis ML, Blagg BS. (2007) A non-toxic Hsp90 inhibitor protects neurons from Abeta-induced toxicity. Bioorg Med Chem Lett 17:1984–1990 [DOI] [PubMed] [Google Scholar]
- Apfel SC. (2002) Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol 50:393–413 [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hänninen O, Sen CK. (2004) Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol 97:605–611 [DOI] [PubMed] [Google Scholar]
- Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E. (1998) Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 280:1831–1836 [DOI] [PubMed] [Google Scholar]
- Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A. (2010) The multifaceted therapeutic potential of benfotiamine. Pharmacol Res 61:482–488 [DOI] [PubMed] [Google Scholar]
- Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. (2006) Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications 20:216–223 [DOI] [PubMed] [Google Scholar]
- Bansal S, Siddarth M, Chawla D, Banerjee BD, Madhu SV, Tripathi AK. (2012) Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol Cell Biochem 361:289–296 [DOI] [PubMed] [Google Scholar]
- Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, Hollander JM. (2011) Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol 300:R186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. (2002) Inhibition of protein kinase C beta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res 90:107–111 [DOI] [PubMed] [Google Scholar]
- Beiswenger KK, Calcutt NA, Mizisin AP. (2008) Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem 110:351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo E, Berrone E, Tarallo S, Porta M. (2008) Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta Diabetologica 45:131–141 [DOI] [PubMed] [Google Scholar]
- Benarroch EE. (2011) Heat shock proteins: multiple neuroprotective functions and implications for neurologic disease. Neurology 76:660–667 [DOI] [PubMed] [Google Scholar]
- Bianchi R, Cervellini I, Porretta-Serapiglia C, Oggioni N, Burkey B, Ghezzi P, Cavaletti G, Lauria G. (2012) Beneficial effects of PKF275–055, a novel, selective, orally bioavailable, long-acting dipeptidyl peptidase IV inhibitor in streptozotocin-induced diabetic peripheral neuropathy. J Pharmacol Exp Ther 340:64–72 [DOI] [PubMed] [Google Scholar]
- Bienemann AS, Lee YB, Howarth J, Uney JB. (2008) Hsp70 suppresses apoptosis in sympathetic neurones by preventing the activation of c-Jun. J Neurochem 104:271–278 [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, et al. (2004) Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest 114:1741–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SC, Burlison JA, Blagg BS. (2007) Hsp90: a novel target for the disruption of multiple signaling cascades. Curr Cancer Drug Targets 7:369–388 [DOI] [PubMed] [Google Scholar]
- Bohlen HG. (2004) Protein kinase betaII in Zucker obese rats compromises oxygen and flow-mediated regulation of nitric oxide formation. Am J Physiol Heart Circ Physiol 286:H492–H497 [DOI] [PubMed] [Google Scholar]
- Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, et al. (2004) Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol 24:32–40 [DOI] [PubMed] [Google Scholar]
- Boucher TJ, McMahon SB. (2001) Neurotrophic factors and neuropathic pain. Curr Opin Pharmacol 1:66–72 [DOI] [PubMed] [Google Scholar]
- Bril V, Buchanan RA. (2004) Aldose reductase inhibition by AS-3201 in sural nerve from patients with diabetic sensorimotor polyneuropathy. Diabetes Care 27:2369–2375 [DOI] [PubMed] [Google Scholar]
- Bril V, Buchanan RA. (2006) Long-term effects of ranirestat (AS-3201) on peripheral nerve function in patients with diabetic sensorimotor polyneuropathy. Diabetes Care 29:68–72 [DOI] [PubMed] [Google Scholar]
- Bril V, Hirose T, Tomioka S, Buchanan R, and Ranirestat Study Group (2009) Ranirestat for the management of diabetic sensorimotor polyneuropathy. Diabetes Care 32:1256–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. (2003) Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52:2338–2345 [DOI] [PubMed] [Google Scholar]
- Calamini B, Silva MC, Madoux F, Hutt DM, Khanna S, Chalfant MA, Saldanha SA, Hodder P, Tait BD, Garza D, et al. (2012) Small-molecule proteostasis regulators for protein conformational diseases. Nat Chem Biol 8:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt NA. (2010) Tolerating diabetes: an alternative therapeutic approach for diabetic neuropathy. ASN Neuro 2:e00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt NA, Backonja MM. (2007) Pathogenesis of pain in peripheral diabetic neuropathy. Curr Diab Rep 7:429–434 [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Cooper ME, Kern TS, Schmidt AM. (2009) Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov 8:417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt NA, Jolivalt CG, Fernyhough P. (2008) Growth factors as therapeutics for diabetic neuropathy. Curr Drug Targets 9:47–59 [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. (2002) Effects of protein kinase Cbeta inhibition on neurovascular dysfunction in diabetic rats: interaction with oxidative stress and essential fatty acid dysmetabolism. Diabetes Metab Res Rev 18:315–323 [DOI] [PubMed] [Google Scholar]
- Capsaicin Study Group (1992) Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Diabetes Care 15:159–165 [DOI] [PubMed] [Google Scholar]
- Chen W, Mi R, Haughey N, Oz M, Höke A. (2007) Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J Peripher Nerv Syst 12:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Tseng Y, White MF. (2010) Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab 21:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiosis G, Huezo H, Rosen N, Mimnaugh E, Whitesell L, Neckers L. (2003) 17AAG: low target binding affinity and potent cell activity–finding an explanation. Mol Cancer Ther 2:123–129 [PubMed] [Google Scholar]
- Chipkin SR, Klugh SA, Chasan-Taber L. (2001) Exercise and diabetes. Cardiol Clin 19:489–505 [DOI] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls DG. (2009) Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem 109:1179–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SK, Zherebitskaya E, Smith DR, Akude E, Chattopadhyay S, Jolivalt CG, Calcutt NA, Fernyhough P. (2010) Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes 59:1082–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SK, Dobrowsky RT, Fernyhough P. (2011) Nutrient excess and altered mitochondrial proteome and function contribute to neurodegeneration in diabetes. Mitochondrion 11:845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SK, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, Akude E, Morrow D, Calcutt NA, Fernyhough P. (2012) Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain 135:1751–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. (2007) Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience 145:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, et al. (2008) HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci 105:1739–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey LJ, Gellett JS, Davidson EP, Yorek MA. (2003) Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res 37:33–40 [DOI] [PubMed] [Google Scholar]
- Deocaris CC, Kaul SC, Wadhwa R. (2006) On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 11:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Patterson C, Dickson DW, Nahman NS, Jr., Hutton M, Burrows F, Petrucelli L. (2007) The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117:648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann A, Kriebel M, Andriambeloson E, Ziegler D, Elmlinger M. (2012) Treatment with Actovegin® improves sensory nerve function and pathology in streptozotocin-diabetic rats via mechanisms involving inhibition of PARP activation. Exp Clin Endocrinol Diabetes 120:132–138 [DOI] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci 97:12222–12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duby JJ, Campbell RK, Setter SM, White JR, Rasmussen KA. (2004) Diabetic neuropathy: an intensive review. Am J Health Syst Pharm 61:160–173 [DOI] [PubMed] [Google Scholar]
- Duran-Jimenez B, Dobler D, Moffatt S, Rabbani N, Streuli CH, Thornalley PJ, Tomlinson DR, Gardiner NJ. (2009) Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 58:2893–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, Norell JE, Tritschler H, Schuette K, Samigullin R, Ziegler D, Bastyr EJ, 3rd, Litchy WJ, O'Brien PC. (2007) Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care 30:2619–2625 [DOI] [PubMed] [Google Scholar]
- Edwards JL, Vincent AM, Cheng HT, Feldman EL. (2008) Diabetic neuropathy: mechanisms to management. Pharmacol Ther 120:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mesallamy HO, Hamdy NM, Ezzat OA, Reda AM. (2011) Levels of soluble advanced glycation end product-receptors and other soluble serum markers as indicators of diabetic neuropathy in the foot. J Investig Med 59:1233–1238 [DOI] [PubMed] [Google Scholar]
- Esper RM, Pankonin MS, Loeb JA. (2006) Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev 51:161–175 [DOI] [PubMed] [Google Scholar]
- Faradji V, Sotelo J. (1990) Low serum levels of nerve growth factor in diabetic neuropathy. Acta Neurol Scand 81:402–406 [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Diemel LT, Tomlinson DR. (1998) Target tissue production and axonal transport of neurotrophin-3 are reduced in streptozotocin-diabetic rats. Diabetologia 41:300–306 [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Roy Chowdhury SK, Schmidt RE. (2010) Mitochondrial stress and the pathogenesis of diabetic neuropathy. Expert Rev Endocrinol Metab 5:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup NB, Sindrup SH, Jensen TS. (2010) The evidence for pharmacological treatment of neuropathic pain. Pain 150:573–581 [DOI] [PubMed] [Google Scholar]
- Fisher MA, Langbein WE, Collins EG, Williams K, Corzine L. (2007) Physiological improvement with moderate exercise in type II diabetic neuropathy. Electromyogr Clin Neurophysiol 47:23–28 [PubMed] [Google Scholar]
- Francis G, Martinez J, Liu W, Nguyen T, Ayer A, Fine J, Zochodne D, Hanson LR, Frey WH, 2nd, Toth C. (2009) Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes 58:934–945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Freedman BI, Wuerth JP, Cartwright K, Bain RP, Dippe S, Hershon K, Mooradian AD, Spinowitz BS. (1999) Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control Clin Trials 20:493–510 [DOI] [PubMed] [Google Scholar]
- Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. (2006) Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ 174:1589–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes P, King GL. (2010) Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106:1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel JS, Richards P, Portenoy RK. (2003) Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology 60:927–934 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Clemente JM, Mauricio D, Richart C, Broch M, Caixas A, Megia A, Gimenez-Palop O, Simon I, Martinez-Riquelme A, Gimenez-Perez G, et al. (2005) Diabetic neuropathy is associated with activation of the TNF-alpha system in subjects with Type 1 diabetes mellitus. Clin Endocrinol (Oxf) 63:525–529 [DOI] [PubMed] [Google Scholar]
- Gooch C, Podwall D. (2004) The diabetic neuropathies. Neurologist 10:311–322 [DOI] [PubMed] [Google Scholar]
- Greene DA, Mackway AM. (1986) Decreased myo-inositol content and Na+-K+-ATPase activity in superior cervical ganglion of STZ-diabetic rat and prevention by aldose reductase inhibition. Diabetes 35:1106–1108 [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. (1996) Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci 16:6107–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DCCT Research Group (1988) Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). Diabetes 37:476–481 [PubMed] [Google Scholar]
- DCCT Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, Burt D, Pinach S, Schalkwijk C, Stehouwer CD, Witte DR, Fuller JH, Cavallo-Perin P, et al. (2009) ANTI-HSP60 and ANTI-HSP70 antibody levels and micro/macrovascular complications in type 1 diabetes: the EURODIAB Study. J Intern Med 266:527–536 [DOI] [PubMed] [Google Scholar]
- Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA. (2005) Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci 25:3478–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford BL, Ryals JM, Wright DE. (2011) Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57Bl/6 mice. Exp Diabetes Res http://dx.doi.org/10.1155/2011/848307 [DOI] [PMC free article] [PubMed]
- Gumy LF, Bampton ET, Tolkovsky AM. (2008) Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci 37:298–311 [DOI] [PubMed] [Google Scholar]
- Guo G, Kan M, Martinez JA, Zochodne DW. (2011) Local insulin and the rapid regrowth of diabetic epidermal axons. Neurobiol Dis 43:414–421 [DOI] [PubMed] [Google Scholar]
- Guo S, Wharton W, Moseley P, Shi H. (2007) Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 12:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harati Y. (2007) Diabetic neuropathies: unanswered questions. Neurol Clin 25:303–317 [DOI] [PubMed] [Google Scholar]
- Harati Y, Gooch C, Swenson M, Edelman S, Greene D, Raskin P, Donofrio P, Cornblath D, Sachdeo R, Siu CO, et al. (1998) Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 50:1842–1846 [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. (2004) The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J 23:3061–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. (2011) Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Ann Rev Biochem 80:825–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt E, Ledermann H, Köpcke W. (2005) Benfotiamine in the treatment of diabetic polyneuropathy–a three-week randomized, controlled pilot study (BEDIP study). Int J Clin Pharmacol Ther 43:71–77 [DOI] [PubMed] [Google Scholar]
- Hellweg R, Hartung HD. (1990) Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: a possible role for NGF in the pathogenesis of diabetic neuropathy. J Neurosci Res 26:258–267 [DOI] [PubMed] [Google Scholar]
- Hellweg R, Raivich G, Hartung HD, Hock C, Kreutzberg GW. (1994) Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp Neurol 130:24–30 [DOI] [PubMed] [Google Scholar]
- Ho EC, Lam KS, Chen YS, Yip JC, Arvindakshan M, Yamagishi S, Yagihashi S, Oates PJ, Ellery CA, Chung SS, et al. (2006) Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes 55:1946–1953 [DOI] [PubMed] [Google Scholar]
- Homs J, Ariza L, Pagès G, Verdú E, Casals L, Udina E, Chillón M, Bosch A, Navarro X. (2011) Comparative study of peripheral neuropathy and nerve regeneration in NOD and ICR diabetic mice. J Peripher Nerv Syst 16:213–227 [DOI] [PubMed] [Google Scholar]
- Hooper PL. (1999) Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 341:924–925 [DOI] [PubMed] [Google Scholar]
- Hooper PL. (2003) Diabetes, nitric oxide, and heat shock proteins. Diabetes Care 26:951–952 [DOI] [PubMed] [Google Scholar]
- Huang TJ, Price SA, Chilton L, Calcutt NA, Tomlinson DR, Verkhratsky A, Fernyhough P. (2003) Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes 52:2129–2136 [DOI] [PubMed] [Google Scholar]
- Huang TJ, Verkhratsky A, Fernyhough P. (2005) Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons. Mol Cell Neurosci 28:42–54 [DOI] [PubMed] [Google Scholar]
- Hung JW, Liou CW, Wang PW, Yeh SH, Lin LW, Lo SK, Tsai FM. (2009) Effect of 12-week tai chi chuan exercise on peripheral nerve modulation in patients with type 2 diabetes mellitus. J Rehabil Med 41:924–929 [DOI] [PubMed] [Google Scholar]
- Hur J, Sullivan KA, Pande M, Hong Y, Sima AA, Jagadish HV, Kretzler M, Feldman EL. (2011) The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain 134:3222–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. (2003) Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest 111:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack MM, Ryals JM, Wright DE. (2012) Protection from diabetes-induced peripheral sensory neuropathy–a role for elevated glyoxalase I? Exp Neurol 234:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Calcutt NA, Ramos KM, Rames KM, Mizisin AP. (2006) Novel sites of aldose reductase immunolocalization in normal and streptozotocin-diabetic rats. J Peripher Nerv Syst 11:274–285 [DOI] [PubMed] [Google Scholar]
- Jinwal UK, Miyata Y, Koren J, 3rd, Jones JR, Trotter JH, Chang L, O'Leary J, Morgan D, Lee DC, Shults CL, et al. (2009) Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci 29:12079–12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivalt CG, Fineman M, Deacon CF, Carr RD, Calcutt NA. (2011) GLP-1 signals via ERK in peripheral nerve and prevents nerve dysfunction in diabetic mice. Diabetes Obes Metab 13:990–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivalt CG, Mizisin LM, Nelson A, Cunha JM, Ramos KM, Bonke D, Calcutt NA. (2009) B vitamins alleviate indices of neuropathic pain in diabetic rats. Eur J Pharmacol 612:41–47 [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. (2001) Molecular mechanisms of nociception. Nature 413:203–210 [DOI] [PubMed] [Google Scholar]
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407–410 [DOI] [PubMed] [Google Scholar]
- Kamiya H, Zhang W, Sima AA. (2006) Degeneration of the Golgi and neuronal loss in dorsal root ganglia in diabetic BioBreeding/Worcester rats. Diabetologia 49:2763–2774 [DOI] [PubMed] [Google Scholar]
- Kamiya H, Zhangm W, Sima AA. (2005) Apoptotic stress is counterbalanced by survival elements preventing programmed cell death of dorsal root ganglions in subacute type 1 diabetic BB/Wor rats. Diabetes 54:3288–3295 [DOI] [PubMed] [Google Scholar]
- Karachalias N, Babaei-Jadidi R, Rabbani N, Thornalley PJ. (2010) Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes. Diabetologia 53:1506–1516 [DOI] [PubMed] [Google Scholar]
- Kato K, Thomas TP, Stevens MJ, Greene DA, Nakamura J. (1999) 2-Chloroadenosine reverses hyperglycemia-induced inhibition of phosphoinositide synthesis in cultured human retinal pigment epithelial cells and prevents reduced nerve conduction velocity in diabetic rats. Metabolism 48:827–833 [DOI] [PubMed] [Google Scholar]
- Kellogg AP, Pop-Busui R. (2005) Peripheral nerve dysfunction in experimental diabetes is mediated by cyclooxygenase-2 and oxidative stress. Antioxid Redox Signal 7:1521–1529 [DOI] [PubMed] [Google Scholar]
- Kellogg AP, Wiggin TD, Larkin DD, Hayes JM, Stevens MJ, Pop-Busui R. (2007) Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes 56:2997–3005 [DOI] [PubMed] [Google Scholar]
- Kim B, Feldman EL. (2012) Insulin resistance in the nervous system. Trends Endocrinol Metab 23:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluding P, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, Sharma N, Wright DE. (2012) The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications http://dx.doi.org/10.1016/j.jdiacomp.2012.05.007 [DOI] [PMC free article] [PubMed]
- Koren J, 3rd, Jinwal UK, Jin Y, O'Leary J, Jones JR, Johnson AG, Blair LJ, Abisambra JF, Chang L, Miyata Y, et al. (2010) Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J Biol Chem 285:2498–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kürthy M, Mogyorósi T, Nagy K, Kukorelli T, Jednákovits A, Tálosi L, Bíró K. (2002) Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. Ann NY Acad Sci 967:482–489 [DOI] [PubMed] [Google Scholar]
- Lehning EJ, LoPachin RM, Mathew J, Eichberg J. (1994) Changes in Na-K ATPase and protein kinase C activities in peripheral nerve of acrylamide-treated rats. J Toxicol Environ Health 42:331–342 [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Vincent AM, Feldman EL. (2004) The role of growth factors in diabetic peripheral neuropathy. J Peripher Nerv Syst 9:26–53 [DOI] [PubMed] [Google Scholar]
- Lennertz RC, Medler KA, Bain JL, Wright DE, Stucky CL. (2011) Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. J Neurophysiol 106:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GS, Shi JY, Lai CL, Hong YR, Shin SJ, Huang HT, Lam HC, Wen ZH, Hsu KS, Chen CH, et al. (2009) Peripheral gene transfer of glial cell-derived neurotrophic factor ameliorates neuropathic deficits in diabetic rats. Hum Gene Ther 20:715–727 [DOI] [PubMed] [Google Scholar]
- Love S, Cruz-Höfling MA, Duchen LW. (1986) Morphological abnormalities in myelinated nerve fibres caused by Leiurus, Centruroides and Phoneutria venoms and their prevention by tetrodotoxin. Q J Exp Physiol 71:115–122 [DOI] [PubMed] [Google Scholar]
- Lu Y, Ansar S, Michaelis ML, Blagg BS. (2009) Neuroprotective activity and evaluation of Hsp90 inhibitors in an immortalized neuronal cell line. Bioorg Med Chem 17:1709–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Dou F, Rodina A, Chip S, Kim J, Zhao Q, Moulick K, Aguirre J, Wu N, Greengard P, et al. (2007) Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci 104:9511–9516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Rodina A, Chiosis G. (2008) Heat shock protein 90: Translation from cancer to Alzheimer's disease treatment? BMC Neurosci 9 (Suppl 2):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari R, Del Corso A, Giglio M, Moschini R, Mura U, Ottanà R. (2011) In vitro evaluation of 5-arylidene-2-thioxo-4-thiazolidinones active as aldose reductase inhibitors. Bioorg Med Chem Lett 21:200–203 [DOI] [PubMed] [Google Scholar]
- Madonna R, De Caterina R. (2011) Cellular and molecular mechanisms of vascular injury in diabetes–part I: pathways of vascular disease in diabetes. Vascul Pharmacol 54:68–74 [DOI] [PubMed] [Google Scholar]
- Madrigal-Matute J, Fernandez-Garcia CE, Gomez-Guerrero C, Lopez-Franco O, Muñoz-Garcia B, Egido J, Blanco-Colio LM, Martin-Ventura JL. (2012) Hsp90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovasc Res 95:116–123 [DOI] [PubMed] [Google Scholar]
- Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I, Sharma AK, Boulton AJ, King RH, Thomas PK, et al. (2005) Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 48:578–585 [DOI] [PubMed] [Google Scholar]
- Malik RA, Veves A, Tesfaye S, Smith G, Cameron N, Zochodne D, Lauria G, and the Toronto Consensus Panel on Diabetic Neuropathy (2011) Small fibre neuropathy: Role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev 27:678–684 [DOI] [PubMed] [Google Scholar]
- Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. (2000a) The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem 275:37181–37186 [DOI] [PubMed] [Google Scholar]
- Marcu MG, Schulte TW, Neckers L. (2000b) Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst 92:242–248 [DOI] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR. (1991) Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266:4706–4712 [PubMed] [Google Scholar]
- Mayer MP, Bukau B. (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Rouen S, Siegfreid E, Wright DE, Dobrowsky RT. (2009) Caveolin-1 and altered neuregulin signaling contribute to the pathophysiological progression of diabetic peripheral neuropathy. Diabetes 58:2677–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Sahenk Z. (2003) Clinical practice. Painful sensory neuropathy. New Engl J Med 348:1243–1255 [DOI] [PubMed] [Google Scholar]
- Mijnhout GS, Alkhalaf A, Kleefstra N, Bilo HJ. (2010) Alpha lipoic acid: a new treatment for neuropathic pain in patients with diabetes? Neth J Med 68:158–162 [PubMed] [Google Scholar]
- Mizisin AP, Calcutt NA, Tomlinson DR, Gallagher A, Fernyhough P. (1999) Neurotrophin-3 reverses nerve conduction velocity deficits in streptozotocin-diabetic rats. J Peripher Nerv Syst 4:211–221 [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. (2005) Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6:11–22 [DOI] [PubMed] [Google Scholar]
- Myers RR, Powell HC. (1984) Galactose neuropathy: impact of chronic endoneurial edema on nerve blood flow. Ann Neurol 16:587–594 [DOI] [PubMed] [Google Scholar]
- Najemnikova E, Rodgers CD, Locke M. (2007) Altered heat stress response following streptozotocin-induced diabetes. Cell Stress Chaperones 12:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. (2010) Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer's and Parkinson's diseases. Apoptosis 15:1354–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, et al. (2012) A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73:729–742 [DOI] [PubMed] [Google Scholar]
- Nave KA, Salzer JL. (2006) Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol 16:492–500 [DOI] [PubMed] [Google Scholar]
- Nayak B, Kondeti VK, Xie P, Lin S, Viswakarma N, Raparia K, Kanwar YS. (2011) Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J Biol Chem 286:27594–27611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef DW, Jaeger AM, Thiele DJ. (2011) Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov 10:930–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi G, Kumar A, Kaundal RK, Gulati A, Sharma SS. (2010a) Functional and biochemical evidence indicating beneficial effect of melatonin and nicotinamide alone and in combination in experimental diabetic neuropathy. Neuropharmacology 58:585–592 [DOI] [PubMed] [Google Scholar]
- Negi G, Kumar A, Sharma SS. (2010b) Concurrent targeting of nitrosative stress–PARP pathway corrects functional, behavioral and biochemical deficits in experimental diabetic neuropathy. Biochem Biophys Res Commun 391:102–106 [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. (2009) Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal 11:3071–3109 [DOI] [PubMed] [Google Scholar]
- Nelson KM, Reiber G, Boyko EJ. NHANES III (2002) Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care 25:1722–1728 [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Johnson-Cadwell L, Vesce S, Jekabsons M, Yadava N. (2007) Bioenergetics of mitochondria in cultured neurons and their role in glutamate excitotoxicity. J Neurosci Res 85:3206–3212 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790 [DOI] [PubMed] [Google Scholar]
- Noble EG, Milne KJ, Melling CW. (2008) Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab 33:1050–1065 [DOI] [PubMed] [Google Scholar]
- Oates PJ. (2008) Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets 9:14–36 [DOI] [PubMed] [Google Scholar]
- Obrosova IG. (2009a) Diabetes and the peripheral nerve. Biochim Biophys Acta 1792:931–940 [DOI] [PubMed] [Google Scholar]
- Obrosova IG. (2009b) Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics 6:638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, Yorek MA. (2007a) Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab 293:E1645–1655 [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. (2005) Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes 54:3435–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. (2007b) High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes 56:2598–2608 [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Li F, Abatan OI, Forsell MA, Komjáti K, Pacher P, Szabó C, Stevens MJ. (2004) Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes 53:711–720 [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Nadler JL, Schmidt RE. (2010) Different roles of 12/15-lipoxygenase in diabetic large and small fiber peripheral and autonomic neuropathies. Am J Path 177:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Xu W, Lyzogubov VV, Ilnytska O, Mashtalir N, Vareniuk I, Pavlov IA, Zhang J, Slusher B, Drel VR. (2008) PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropathy. Free Radic Biol Med 44:972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T, Oishi Y, Higashida K, Higuchi M, Muraoka I. (2009) Prolonged exercise training induces long-term enhancement of HSP70 expression in rat plantaris muscle. Am J Physiol Regul Integr Comp Physiol 296:R1557–1563 [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Xu LJ, Sun YJ, Giffard RG. (2006) Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones 11:180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Szabo C. (2008) Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol 173:2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande M, Hur J, Hong Y, Backus C, Hayes JM, Oh SS, Kretzler M, Feldman EL. (2011) Transcriptional profiling of diabetic neuropathy in the BKS db/db mouse: a model of type 2 diabetes. Diabetes 60:1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen G, Vissing K, Kalhovde JM, Ugelstad I, Bayer ML, Kadi F, Schjerling P, Hallén J, Raastad T. (2007) Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am J Physiol Regul Integr Comp Physiol 293:R844–853 [DOI] [PubMed] [Google Scholar]
- Pavlik A, Aneja IS. (2007) Cerebral neurons and glial cell types inducing heat shock protein Hsp70 following heat stress in the rat. Prog Brain Res 162:417–431 [DOI] [PubMed] [Google Scholar]
- Pavlik A, Aneja IS, Lexa J, Al-Zoabi BA. (2003) Identification of cerebral neurons and glial cell types inducing heat shock protein Hsp70 following heat stress in the rat. Brain Res 973:179–189 [DOI] [PubMed] [Google Scholar]
- Peppin JF, Majors K, Webster LR, Simpson DM, Tobias JK, Vanhove GF. (2011) Tolerability of NGX-4010, a capsaicin 8% patch for peripheral neuropathic pain. J Pain Res 4:385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH. (2002) A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther 300:958–966 [DOI] [PubMed] [Google Scholar]
- Peterson LB, Blagg BS. (2009) To fold or not to fold: modulation and consequences of Hsp90 inhibition. Future Med Chem 1:267–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piyapolrungroj N, Li C, Bockbrader H, Liu G, Fleisher D. (2001) Mucosal uptake of gabapentin (Neurontin) vs. pregabalin in the small intestine. Pharm Res 18:1126–1130 [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. (2004) The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 127:1606–1615 [DOI] [PubMed] [Google Scholar]
- Pop-Busui R, Sima A, Stevens M. (2006) Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev 22:257–273 [DOI] [PubMed] [Google Scholar]
- Powell HC, Myers RR. (1983) Schwann cell changes and demyelination in chronic galactose neuropathy. Muscle Nerve 6:218–227 [DOI] [PubMed] [Google Scholar]
- Powers MV, Workman P. (2007) Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett 581:3758–3769 [DOI] [PubMed] [Google Scholar]
- Purwata TE. (2011) High TNF-alpha plasma levels and macrophages iNOS and TNF-alpha expression as risk factors for painful diabetic neuropathy. J Pain Res 4:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani N, Thornalley PJ. (2011) Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol 22:309–317 [DOI] [PubMed] [Google Scholar]
- Ramos KM, Jiang Y, Svensson CI, Calcutt NA. (2007) Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes 56:1569–1576 [DOI] [PubMed] [Google Scholar]
- Rapposelli S, Da Settimo F, Digiacomo M, La Motta C, Lapucci A, Sartini S, Vanni M. (2011) Synthesis and biological evaluation of 2′-oxo-2,3-dihydro-3′h-spiro[chromene-4,5′-[1,3]oxazolidin]-3′yl]acetic acid derivatives as aldose reductase inhibitors. Arch Pharm (Weinheim) 344:372–385 [DOI] [PubMed] [Google Scholar]
- Raskin J, Pritchett YL, Wang F, D'Souza DN, Waninger AL, Iyengar S, Wernicke JF. (2005) A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Medicine 6:346–356 [DOI] [PubMed] [Google Scholar]
- Romanovsky D, Cruz NF, Dienel GA, Dobretsov M. (2006) Mechanical hyperalgesia correlates with insulin deficiency in normoglycemic streptozotocin-treated rats. Neurobiol Dis 24:384–394 [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. (2004) Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 110:628–638 [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, et al. (2003) Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest 111:959–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Smith DR, Balakrishnan S, Dunn L, Martens C, Tweed CW, Fernyhough P. (2011) Tumor necrosis factor-α elevates neurite outgrowth through an NF-κB-dependent pathway in cultured adult sensory neurons: diminished expression in diabetes may contribute to sensory neuropathy. Brain Res 1423:87–95 [DOI] [PubMed] [Google Scholar]
- Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. (2011) Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem Biol Interact 191:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeski PP, Kudlow JE. (1996) Glucose metabolism to glucosamine is necessary for glucose stimulation of transforming growth factor-α gene transcription. J Biol Chem 271:15237–15243 [DOI] [PubMed] [Google Scholar]
- Schemmel KE, Padiyara RS, D'Souza JJ. (2010) Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications 24:354–360 [DOI] [PubMed] [Google Scholar]
- Schmidt O, Pfanner N, Meisinger C. (2010) Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol 11:655–667 [DOI] [PubMed] [Google Scholar]
- Schwartz S, Etropolski M, Shapiro DY, Okamoto A, Lange R, Haeussler J, Rauschkolb C. (2011) Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin 27:151–162 [DOI] [PubMed] [Google Scholar]
- Sekido H, Suzuki T, Jomori T, Takeuchi M, Yabe-Nishimura C, Yagihashi S. (2004) Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem Biophys Res Commun 320:241–248 [DOI] [PubMed] [Google Scholar]
- Selagzi H, Buyukakilli B, Cimen B, Yilmaz N, Erdogan S. (2008) Protective and therapeutic effects of swimming exercise training on diabetic peripheral neuropathy of streptozotocin-induced diabetic rats. J Endocrinol Invest 31:971–978 [DOI] [PubMed] [Google Scholar]
- Shankarappa SA, Piedras-Rentería ES, Stubbs EB., Jr (2011) Forced-exercise delays neuropathic pain in experimental diabetes: effects on voltage-activated calcium channels. J Neurochem 118:224–236 [DOI] [PubMed] [Google Scholar]
- Shaw JE, Sicree RA, Zimmet PZ. (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14 [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Yorek MA. (2010) Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal 12:537–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, et al. (2006) Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 29:1294–1299 [DOI] [PubMed] [Google Scholar]
- Smith AG, Singleton JR. (2008) Impaired glucose tolerance and neuropathy. Neurologist 14:23–29 [DOI] [PubMed] [Google Scholar]
- Smith T, Nicholson RA. (2007) Review of duloxetine in the management of diabetic peripheral neuropathic pain. Vasc Health Risk Manag 3:833–844 [PMC free article] [PubMed] [Google Scholar]
- Spruce MC, Potter J, Coppini DV. (2003) The pathogenesis and management of painful diabetic neuropathy: a review. Diabet Med 20:88–98 [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Stevens M, Wiley JW. (2000) Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes 49:1932–1938 [DOI] [PubMed] [Google Scholar]
- Stavniichuk R, Drel VR, Shevalye H, Maksimchyk Y, Kuchmerovska TM, Nadler JL, Obrosova IG. (2011) Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative–nitrosative stress and p38 MAPK activation. Exp Neurol 230:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Stevens MJ, Nadler JL, Obrosova IG. (2010) Role of 12/15-lipoxygenase in nitrosative stress and peripheral prediabetic and diabetic neuropathies. Free Radic Biol Med 49:1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavniichuk R, Shevalye H, Hirooka H, Nadler JL, Obrosova IG. (2012) Interplay of sorbitol pathway of glucose metabolism, 12/15-lipoxygenase, and mitogen-activated protein kinases in the pathogenesis of diabetic peripheral neuropathy. Biochem Pharm 83:932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storozhevykh TP, Senilova YE, Persiyantseva NA, Pinelis VG, Pomytkin IA. (2007) Mitochondrial respiratory chain is involved in insulin-stimulated hydrogen peroxide production and plays an integral role in insulin receptor autophosphorylation in neurons. BMC Neuroscience 8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG. (2008) Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes 116:600–605 [DOI] [PubMed] [Google Scholar]
- Strokov IA, Manukhina EB, Bakhtina LY, Malyshev IY, Zoloev GK, Kazikhanova SI, Ametov AS. (2000) The function of endogenous protective systems in patients with insulin-dependent diabetes mellitus and polyneuropathy: effect of antioxidant therapy. Bull Exp Biol Med 130:986–990 [PubMed] [Google Scholar]
- Sugimoto K, Murakawa Y, Sima AA. (2002) Expression and localization of insulin receptor in rat dorsal root ganglion and spinal cord. J Peripher Nerv Syst 7:44–53 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Murakawa Y, Zhang W, Xu G, Sima AA. (2000) Insulin receptor in rat peripheral nerve: its localization and alternatively spliced isoforms. Diabetes Metab Res Rev 16:354–363 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Yasujima M, Yagihashi S. (2008) Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des 14:953–961 [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Robinson JP. (2006) Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am 17:381–400 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenski RT, Kaneda Y, Sawa Y, Matsuda H, Yacoub MH. (2002) Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation 106 (12 Suppl 1):I270–I276 [PubMed] [Google Scholar]
- Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. (2010) Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci 30:6122–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syroid DE, Maycox PR, Burrola PG, Liu N, Wen D, Lee KF, Lemke G, Kilpatrick TJ. (1996) Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc Natl Acad Sci 93:9229–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvássy Z, Németh J, Kovács P, Paragh G, Sári R, Vígh L, Peitl B. (2013. Insulin resistance occurs in parallel with sensory neuropathy in streptozotocin-induced diabetes in rats: differential response to early vs late insulin supplementation. Metabolism 61:776–786 [DOI] [PubMed] [Google Scholar]
- Taldone T, Sun W, Chiosis G. (2009. Discovery and development of heat shock protein 90 inhibitors. Bioorg Med Chem 17:2225–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Rouen S, Barkus KM, Dremina YS, Hui D, Christianson JA, Wright DE, Yoon SO, Dobrowsky RT. (2003) Nerve growth factor blocks the glucose-induced down-regulation of caveolin-1 expression in Schwann cells via p75 neurotrophin receptor signaling. J Biol Chem 278:23151–23162 [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Malik RA. (2008) Management of painful diabetic neuropathy. Expert Opin Pharmacother 9:2969–2978 [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, et al. (2005) Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47:681–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH, and EURODIAB Prospective Complications Study Group (2005) Vascular risk factors and diabetic neuropathy. New Engl J Med 352:341–350 [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Vileikyte L, Rayman G, Sindrup S, Perkins B, Baconja M, Vinik A, Boulton A. (2011) Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev 27:629–638 [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW. (2007) High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 50:2164–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP., Jr (2008) Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain 12:203–213 [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Gardiner NJ. (2008) Glucose neurotoxicity. Nat Rev Neurosci 9:36–45 [DOI] [PubMed] [Google Scholar]
- Toth C, Brussee V, Martinez JA, McDonald D, Cunningham FA, Zochodne DW. (2006) Rescue and regeneration of injured peripheral nerve axons by intrathecal insulin. Neuroscience 139:429–449 [DOI] [PubMed] [Google Scholar]
- Toth C, Rong LL, Yang C, Martinez J, Song F, Ramji N, Brussee V, Liu W, Durand J, Nguyen MD, et al. (2008) Receptor for advanced glycation end products (RAGEs) and experimental diabetic neuropathy. Diabetes 57:1002–1017 [DOI] [PubMed] [Google Scholar]
- Treberg JR, Quinlan CL, Brand MD. (2011) Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). J Biol Chem 286:27103–27110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. (2005) The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 28:2686–2690 [DOI] [PubMed] [Google Scholar]
- Tzellos TG, Papazisis G, Toulis KA, Sardeli Ch, Kouvelas D. (2010) A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 14:71–75 [PMC free article] [PubMed] [Google Scholar]
- Um HS, Kang EB, Koo JH, Kim HT, Jin-Lee, Kim EJ, Yang CH, An GY, Cho IH, Cho JY. (2011) Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer's disease. Neurosci Res 69:161–173 [DOI] [PubMed] [Google Scholar]
- Urban MJ, Dobrowsky RT, Blagg BS. (2012a) Heat shock response and insulin-associated neurodegeneration. Trends Pharmacol Sci 33:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MJ, Li C, Yu C, Lu Y, Krise JM, McIntosh MP, Rajewski RA, Blagg BS, Dobrowsky RT. (2010) Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro 2:e00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MJ, Pan P, Farmer KL, Zhao H, Blagg BS, Dobrowsky RT. (2012b) Modulating molecular chaperones improves sensory fiber recovery and mitochondrial function in diabetic peripheral neuropathy. Exp Neurol 235:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareniuk I, Pacher P, Pavlov IA, Drel VR, Obrosova IG. (2009) Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency. Int J Mol Med 23:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareniuk I, Pavlov IA, Drel VR, Lyzogubov VV, Ilnytska O, Bell SR, Tibrewala J, Groves JT, Obrosova IG. (2007) Nitrosative stress and peripheral diabetic neuropathy in leptin-deficient (ob/ob) mice. Exp Neurol 205:425–436 [DOI] [PubMed] [Google Scholar]
- Vareniuk I, Pavlov IA, Obrosova IG. (2008) Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia 51:2126–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veves A, Backonja M, Malik RA. (2008) Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med 9:660–674 [DOI] [PubMed] [Google Scholar]
- Vígh L, Literáti PN, Horváth I, Török Z, Balogh G, Glatz A, Kovács E, Boros I, Ferdinándy P, Farkas B, et al. (1997) Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med 3:1150–1154 [DOI] [PubMed] [Google Scholar]
- Vincent AM, Edwards JL, McLean LL, Hong Y, Cerri F, Lopez I, Quattrini A, Feldman EL. (2010) Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropath 120:477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. (2009a) Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes 58:2376–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. (2009b) Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 14:257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Perrone L, Sullivan KA, Backus C, Sastry AM, Lastoskie C, Feldman EL. (2007) Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology 148:548–558 [DOI] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Low P, Feldman EL. (2004) Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25:612–628 [DOI] [PubMed] [Google Scholar]
- Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ, 3rd, and MBBQ Study Group (2005) Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther 27:1164–1180 [DOI] [PubMed] [Google Scholar]
- Voloboueva LA, Duan M, Ouyang Y, Emery JF, Stoy C, Giffard RG. (2008) Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J Cereb Blood Flow Metab 28:1009–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada R, Yagihashi S. (2005) Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann NY Acad Sci 1043:598–604 [DOI] [PubMed] [Google Scholar]
- Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR. (2012) Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15:186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watcho P, Stavniichuk R, Ribnicky DM, Raskin I, Obrosova IG. (2010) High-fat diet-induced neuropathy of prediabetes and obesity: effect of PMI-5011, an ethanolic extract of Artemisia dracunculus L. Mediators Inflamm 2010:268547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CP, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J. (2003) Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain 105:71–78 [DOI] [PubMed] [Google Scholar]
- Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G. (2005) 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med 11:1088–1095 [DOI] [PubMed] [Google Scholar]
- Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, et al. (2003) RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 162:1123–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL. (2008) Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology 149:4928–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. (2009) Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 58:1634–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson CL, Dabkowski ER, Dillmann WH, Hollander JM. (2008) Mitochondria protection from hypoxia/reoxygenation injury with mitochondria heat shock protein 70 overexpression. Am J Physiol Heart Circ Physiol 294:H249–256 [DOI] [PubMed] [Google Scholar]
- Wolff RF, Bala MM, Westwood M, Kessels AG, Kleijnen J. (2010) 5% lidocaine medicated plaster in painful diabetic peripheral neuropathy (DPN): a systematic review. Swiss Med Wkly 140:297–306 [DOI] [PubMed] [Google Scholar]
- Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. (2010) Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 58:1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. (2009) Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab 29:365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Rabbani N, Momiji H, Imbasi P, Anwar MM, Kitteringham N, Park BK, Souma T, Moriguchi T, Yamamoto M, et al. (2011) Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem J 443:213–222 [DOI] [PubMed] [Google Scholar]
- Yagihashi S, Yamagishi S, Wada R. (2007) Pathology and pathogenetic mechanisms of diabetic neuropathy: correlation with clinical signs and symptoms. Diabetes Res Clin Pract 77 (Suppl 1):S184–S189 [DOI] [PubMed] [Google Scholar]
- Yamakawa I, Kojima H, Terashima T, Katagi M, Oi J, Urabe H, Sanada M, Kawai H, Chan L, Yasuda H, et al. (2011) Inactivation of TNF-α ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab 301:E844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. (2001) O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci 98:6611–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang F, Kudlow JE. (2002) Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3a: coupling protein O-GlcNAcylation to transcriptional repression. Cell 110:69–80 [DOI] [PubMed] [Google Scholar]
- Yao P, Meng LX, Ma JM, Ding YY, Wang ZB, Zhao GL, Tao R, Wu YX, Wang QS, Zhang Z, et al. (2012) Sustained-release oxycodone tablets for moderate to severe painful diabetic peripheral neuropathy: a multicenter, open-labeled, postmarketing clinical observation. Pain Med 13:107–114 [DOI] [PubMed] [Google Scholar]
- Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R. (2003) Diabetic neuropathy and nerve regeneration. Prog Neurobiol 69:229–285 [DOI] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU. (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112:41–50 [DOI] [PubMed] [Google Scholar]
- Yu C, Rouen S, Dobrowsky RT. (2008) Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination. Glia 56:877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D, Marchionni MA, Salzer JL. (2001) Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol 152:1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhao H, Blagg BS, Dobrowsky RT. (2012) A C-terminal heat shock protein 90 inhibitor decreases hyperglycemia-induced oxidative stress and improves mitochondrial bioenergetics in sensory neurons. J Proteome Res 11:2581–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WY, Li Wan Po A. (1994) The effectiveness of topically applied capsaicin. A meta-analysis. Eur J Clin Pharmacol 46:517–522 [DOI] [PubMed] [Google Scholar]
- Zherebitskaya E, Akude E, Smith DR, Fernyhough P. (2009) Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes 58:1356–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, et al. (2006) Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care 29:2365–2370 [DOI] [PubMed] [Google Scholar]
- Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J, et al. (2011) Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 Trial. Diabetes Care 34:2054–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW. (2007) Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve 36:144–166 [DOI] [PubMed] [Google Scholar]
- Zochodne DW. (2008) Diabetic polyneuropathy: an update. Curr Opin Neurol 21:527–533 [DOI] [PubMed] [Google Scholar]