Abstract
The efficacy of T-cell–based immunotherapy to treat cancer patients remains a challenge partly because of the weak activity toward subdominant tumor-antigens (TAg) and to tumors expressing suboptimal TAg levels. Recent reports indicate that Toll-like receptor (TLR) stimulation on T-cells can lower the activation threshold. In this study, we examined the anti-tumor activity and survival of TLR2-MyD88–stimulated CD8 T-cells derived from melanoma patients and T-cell receptor transgenic pmel mice. TLR2-stimulated pmel CD8 T-cells but not TLR2−/−pmel or MyD88−/−pmel T-cells responded to significantly lower TAg levels and resulted in increased production of effector molecules and cytotoxicity. Wild-type or MyD88−/− mice treated with TLR2 ligand and pmel T-cells, but not TLR2−/−pmel or MyD88−/−pmel T-cells, showed tumor regression of an established melanoma tumor. Over-expressing TLR2 in TA-specific T-cells eradicated tumors; four-times fewer cells were needed to generate anti-tumor responses. The enhanced anti-tumor activity of TLR2-MyD88–stimulated T-cells was associated with increased effector function but perhaps more importantly with improved survival of T-cells. Activating TLR-MyD88 signals in patient-derived T-cells also reduced the activation threshold to several weakly immunogenic TAgs, resulting in increased cytokine production, expansion and cytotoxicity. These data highlight a previously unappreciated role for activating TLR-MyD88 signals in tumor-reactive T-lymphocytes.
Keywords: Toll-like receptors, T-cell activation, co-stimulation, tumor immunotherapy, subdominant antigens
Introduction
Recent advances highlight the potential for using T-cell–based immunotherapies to treat patients with metastatic cancers. However, several challenge in achieving effective anti-tumor responses stems from the fact that tumor-reactive T-cells can display: reduced avidity for TAg, are generally present at low frequencies, and can exhibit diminished cytolytic function (1–6). In addition, some tumors can down-regulate major histocompatibility complex (MHC) class I-TAg expression and consequently evade detection by T-cells. Therefore, strategies aimed at amplifying T-cell responses to weakly-expressed or subdominant TAgs is a major goal in designing effective cancer immunotherapies.
Emerging studies from several groups, including ours, indicate that activating TLR-MyD88 signals within CD4 or CD8 T-cells can lower the activation threshold. TLRs recognize pathogen-associated molecular patterns derived from all known microorganisms. Each TLR can recognize and form homo- or heterodimers that presumably aid in the detection of a broader array of microbial products (7). In CD4 T-helper cells, TLR1/2, TLR5, TLR7/8, and TLR9 engagement has been shown to enhance IL-2 production (8–10). TLR2 or TLR9 ligation on CD4 and CD8 T-cells also enhances survival by modulating the expression levels of the anti-apoptotic protein including A1, bcl2 and bcl-xl (11–14). TLR1/2 stimulation on CD8 T-cells has also been shown to enhance IFN-γ production and increase cytotoxicity in vitro (11;12). We recently demonstrated that TLR1/2 engagement on OT-1 T-cell receptor (TCR) transgenic CD8 T-lymphocytes enhanced the anti-tumor activity against an established tumor expressing the ovalbumin protein (11). Admittedly, one of the major limitations to those studies was that the ovalbumin model does not represent an authentic TAg, as it is an immunodominant xenoantigen and therefore does not deal with the parameters of tolerance or low-avidity.
We report that activating TLR2-MyD88 signals within a bona fide population of human and murine tumor-specific CD8 T-cells enhances responses against suboptimal concentrations of weakly-immunogenic TAgs. We used the synthetic ligand [tripalmitoyl-S-(bis(palmitoyloxy)propyl)-Cys-Ser-(Lys)3-Lys] as a TLR1/2 agonsist because this TLR agonist has been shown to enhance CD4 and CD8 T cell response in vitro and in vivo (10–12;15;16). TLR2 stimulation on patient CD8 T-cells or T-cells from TCR transgenic ‘pmel’ mice–which recognize the weakly immunogenic melanoma TAg gp10010–25–lowered the activation threshold consequently, increasing T-cell expansion, cytokine production and cytolytic activity in vivo and in vitro (17). Adoptive cell transfer (ACT) of pmel T-cells in combination with TLR1/2 ligand, but not TLR1/2 ligand and TLR2−/−pmel or MyD88−/−pmel cells, into WT or MyD88−/− reduced tumor growth kinetics. Furthermore, over-expressing TLR2 on tumor-reactive T-cells cured mice bearing an established melanoma tumor and four-times fewer pmel T-cells were required to generate anti-tumor responses. The enhanced anti-tumor effects appeared to be due in part to enhanced T-cell survival. These results reveal that the activating TLR2-MyD88 signals within tumor-specific T-cells lowers the activation threshold to weakly immunogenic TAgs and increases their efficiency by enhancing the duration and magnitude of T-cell responses.
Methods
Mice
Studies were approved by the LSUHSC Institutional Animal Care and Use Committee. C57BL6 mice were obtained from Charles River Laboratories (Wilmington, MA), MyD88−/− mice were a kind gift from Dr. Douglas Golenbock (Boston University, Boston, MA), B6.129-TLR2tm1kir/J (TLR2−/−) mice, and pmel (B6.Cg-Thy1/Cy Tg(TcraTcrb)8Rest/J) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). MyD88−/−pmel and TLR2−/−pmel mice were generated by crossing with pmel for over nine generations.
T-cell sorting and functional studies
CD8 T-cells were purified by negative selection (Stemcell Technology, Vancouver, BC, Canada) followed by positive selection (Miltenyi Biotec, Auburn, CA) and activated with MyD88−/− splenocytes pulsed with mouse gp10025–33 peptide (EGSRNQDWL; GenScript Corp; Piscataway, NJ.) with or without TLR1/2 agonist Pam3Cysk4 (10 µg/ml; Invitrogen, Carlsbad, CA) or plate-bound anti-CD3 antibodies. T-cell proliferation was determined by measuring [3H] thymidine (0.5 µCi/well) uptake. Cytokine production after 96 hrs was determined by ELISA using BD Pharmingen’s OptEIA ELISA kit (BD Pharmingen, San Jose, CA). T-cell proliferation and apoptosis was measured by labeling cells with 5 µM 5-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) followed by staining with PE-labeled Annexin V (BD Pharmingen) and analyzed by flow cytometry. For in vivo T-cell survival/proliferation studies, T-cells were labeled with 10µM CFSE and injected (i.v.) into WT mice followed by injection with mgp100 peptide and TLR2 ligand (i.v). In other experiments, CD90.1+CD45.2+ pmel and CD90.2+CD45.2+ MyD88−/−pmel T-cells were activated in vitro with mgp100 peptide-pulsed WT splenocytes and one day later, were enriched by negative selection, mixed at a 1:1 ratio and intravenously injected into CD45.1+ mice.
In vitro and in vivo cytotoxicity assays
For in vitro cytotoxicity assays purified pmel T-cells were activated using antigen-pulsed MyD88−/− splenocytes with or without TLR2 ligand. After 5 days, cytolytic activity against B16 melanoma cells or mg100-pulsed EL4 thymoma cells was measured at different effectors to target ratios in a 4-hour 51Cr release assay. Target cells (3 × 106/ml) were labeled with 200 µCi of Na51Cr (GE Healthcare, Piscataway, NJ) for 90 min at 37°C. Cells lines, purchased within the last two years, were authenticated by ATCC by use of isoenzymology and/or the Cytochrome C subunit I (COI) PCR assay and by observations of recovery and growth along with morphological appearance. B16 cells were further authenticated to express the gp100 antigen by our group based on the ability of these cells to serve as targets in cytotoxicty assays and to induce cytokine production by the gp100-specific TCR transgenic T cells. For in vivo cytotoxicity assays, pmel T-cells were activated in vitro using antigen-pulsed splenocytes and four days later, 1×106 purified pmel or TLR2−/−pmel T-cells were injected (i.v.) into MyD88−/− mice. One day after T-cell transfer, mice were injected i.v. with target cells (2×107 MyD88−/− splenocytes) labeled with 0.1 µM CFSE dye and pulsed with the H-2Db restricted mouse gp100 peptide (100ng/ml106 cells) or labeled with 5 µM CFSEhigh and pulsed with an irrelevant H-2Db-restricted CTL epitope (NP396–404; FQPQNGQFI) with or without the TLR1/2 agonist (10µg). The percentage of targets recovered 24 hours after cell transfer (ACT) was evaluated by FACS analysis.
B16 melanoma tumor challenge
MyD88−/− or WT mice were injected (s.c.) with 5×104 B16 tumor cells in the rear, leg flank and were allowed to grow to 50 mm2, followed by irradiation (400 cGy) and intravenous (i.v.) injection with 106 T-cells; four days earlier, T-cells were activated in vitro with gp100-pulsed splenocytes which served as antigen-presenting cells (APCs) . B16 tumor cells lines were purchased and used within 6 months of use from the ATCC (Manassas, VA, USA). One day later, mice were injected intraperitoneally (i.p.) with mgp100 (5µg) anti-CD40 Ab (50µg) and TLR1/2 ligand (5µg) and peritumorally injected with TLR1/2 ligand (5µg) or control PBS, weekly. The anti-CD40 Ab was included in vaccine formulations as the combination of anti-CD40 Ab and TLR agonists synergistically enhances T cell responses (18). Tumor sizes (mm2) were analyzed using a mixed model approach for repeated measurements; mean comparisons at each time interval were performed using the SAS TUKEY option to adjust the p-values for multiple comparisons; mouse survival data were analyzed with the exact log-rank test. MyD88−/− or WT mice were injected (s.c.) with 5×104 B16 tumor cells in the rear leg flank and allowed to grow to 50 mm2 followed by irradiation (400 cGy) and intravenous (i.v.) injection with 106 T-cells, activated with gp100-pulsed splenoytes, which served as antigen-presenting cells (APCs), in vitro four days earlier. One day later, mice were injected intraperitoneally (i.p.) with mgp100 (5µg) anti-CD40 Ab (50µg) and TLR1/2 ligand (5µg) and injected peritumorally with TLR1/2 ligand (5µg) or control PBS weekly. Tumor sizes (mm2) were analyzed using a mixed model approach for repeated measurements; mean comparisons at each time were performed using the TUKEY option of SAS to adjust the p-values for multiple comparisons, and mouse survival data were analyzed with the exact log-rank test.
Retroviral vectors and transduction
The gene encoding murine TLR2 was excised from the pDUO-mTLR1/TLR2 vector (InvivoGen, San Diego, CA) and ligated into the multiple cloning site, upstream of the internal ribosomal entry site (IRES) of the moloney murine leukemia virus based pBMN-GFP vector (Orbigen, San Diego, CA) whereas, GFP was constructed downstream . Retroviruses were produced by calcium phosphate-mediated transient transfection of Phoenix eco packaging cells (Orbigen, San Diego, CA). Phoenix eco packaging cells, purchased within the last two years, were expanded and frozen stocks cell lines were generated upon receipt. Cells were characterized by our group based on their ability to produce retroviral particles. For transduction, pmel T-cells were activated for 48 hours prior to infection using human gp100 (KVPRNQDWL)–pulsed WT splenocytes and transduced by adding virus, concentrated via ultracentrifugation, in the presence of 8µg/ml polybrene, centrifuged for 4 hours at 2000 rpm at room temperature followed by incubation at 37C°, 7% CO2 for 48 hours in the presence of IL-2 (100U/ml) and IL-7 (50ng/ml).
Isolation, expansion and activation of patient T-cells
Peripheral blood mononuclear cells (PBMC) were collected from consented melanoma patients (HLA-A2+). T-cells were expanded using CD3/CD28-coated beads (Invitrogen) in the presence of IL-2 (250U/m), IL-15 (50ng/ml) and irradiated PBMCs in RPMI 1640 supplemented with 2 mM glutamine, 25 mM HEPES buffer, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% heat-inactivated human AB sera (Sigma-Aldrich, St. Louis, MO). Mart126–35 (ELAGIGILTV)–, MPS160 glycoprotein 100-derived melanoma antigen (MLGTHTMEV), and tyrosinase 188–196 (AFLPWHRLF)–specific T-cells were isolated using biotinylated-pentamers (ProImmune, Bradenton, FL) and strepevidin-coated magnetic beads (Invitrogen) and expanded. TAg-specific T-cells were co-cultured with the HLA-A2+ MART-1-, tyrosinase-, gp100-defective A375 melanoma cell line pulsed with varying concentrations of peptides (starting at 1µg/ml and diluted 10-fold) or pulsed with 0.001µg/ml peptide for in vitro cytotoxicity assays (6-hr 51Cr release assay (19)). Alternatively, T-cell cytotoxicity was measured against the HLA-A2+ gp100-expressing cell line SK-MEL-23 at varying effector to target ratios.
Results
Stimulating TLR2-MyD88 signals in pmel CD8 T-cells lowers the activation threshold to a weakly-immunogenic tumor-antigen
We sought to determine if TLR2 engagement on a bona fide population of TAg-specific CD8 T-cells enhanced responses to the weakly-immunogenic mouse TAg gp10025–33. Purified pmel T-cells were activated with MyD88−/− splenocytes pulsed with varying concentrations of gp10025–33. The use of MyD88−/−APCs ensures that the co-stimulatory effects of TLR1/2 ligand occur through TLR stimulation on CD8 T-cells but not APCs (15;20)
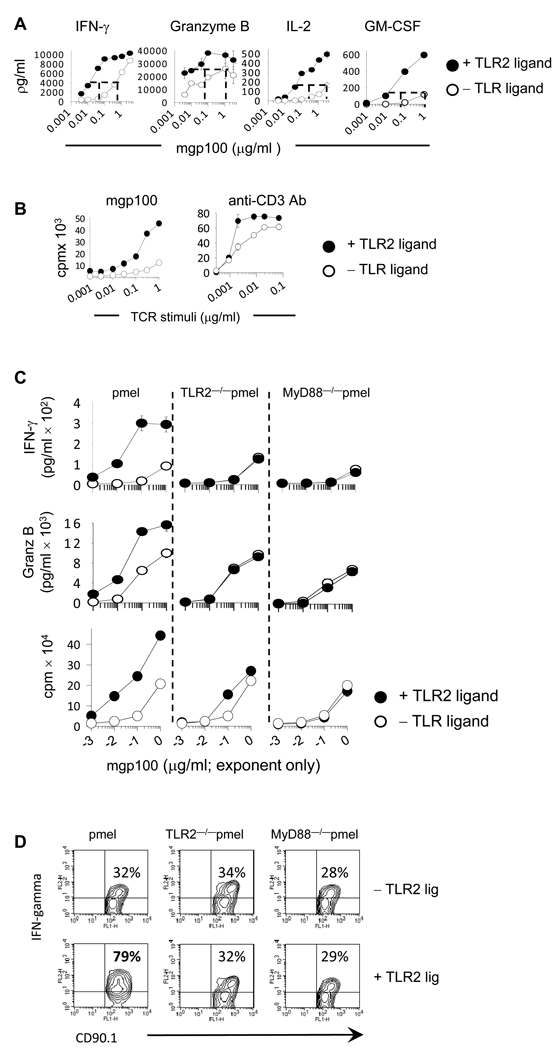
Significantly higher levels of IFN-γ, granzyme B, IL-2, and GM-CSF were detected in supernatants from TLR2-ligated CD8 T-cells (Fig 1A, p<0.005). Furthermore, TLR2-ligated pmel T-cells responded to 30 to 70-times lower levels of antigen (Fig 1A; p<0.005). TLR2 engagement also increased T-cell proliferation in response to sub-optimal concentrations of antigen (Fig 1B; left panel). To confirm the effects of TLR2 ligand occurred in the absence of APCs, purified pmel T-cells were stimulated with varying concentrations of plate-bound anti-CD3 antibodies in each the absence or presence of TLR2 ligand. TLR2-activated T-cells demonstrated enhanced expansion (Fig 1B; right panel) and cytokine production (data not shown).
Figure 1. Activating TLR2-MyD88 signals in tumor-reactive CD8 T-cells lowers the activation threshold to a weakly immunogenic tumor-antigen.
Purified pmel, TLR2−/−pmel and MyD88−/− pmel CD8 T-cells were activated with MyD88−/− splenocytes pulsed with varying concentrations of the mgp100 peptide or plate-bound anti-CD3 antibody with or without TLR2 agonist. Four days later cytokine levels determined were by ELISA whereas proliferation was determined by 3H-thymidine uptake. (D) The intracellular level of IFN-γ and granzyme B were determined by flow cytometry 4 days following activation mgp100-pulsed APCs. Shown on the upper right-hand of each plot are the percent of cytokine-positive cells. All data are representative of three or more independent experiments each yielding identical trends.
To validate the effects of TLR2 ligand occurred via a TLR2– and MyD88–dependent manner in CD8 T-cells, we generated TLR2−/−pmel and MyD88−/−pmel mice and examined the production levels of effector molecules and proliferation. TLR2–stimulated pmel T-cells, but neither TLR2−/− pmel nor MyD88−/−pmel cells, showed substantial expansion and increased production of IFN-γ and granzyme B (Fig 1D and Supplemental-1). The increased levels of effector molecules were due in part to the increased percentage of cells producing cytokines.
TLR2-ligated CD8 T-cells show enhanced cytotoxicity against suboptimal tumor-antigen concentrations
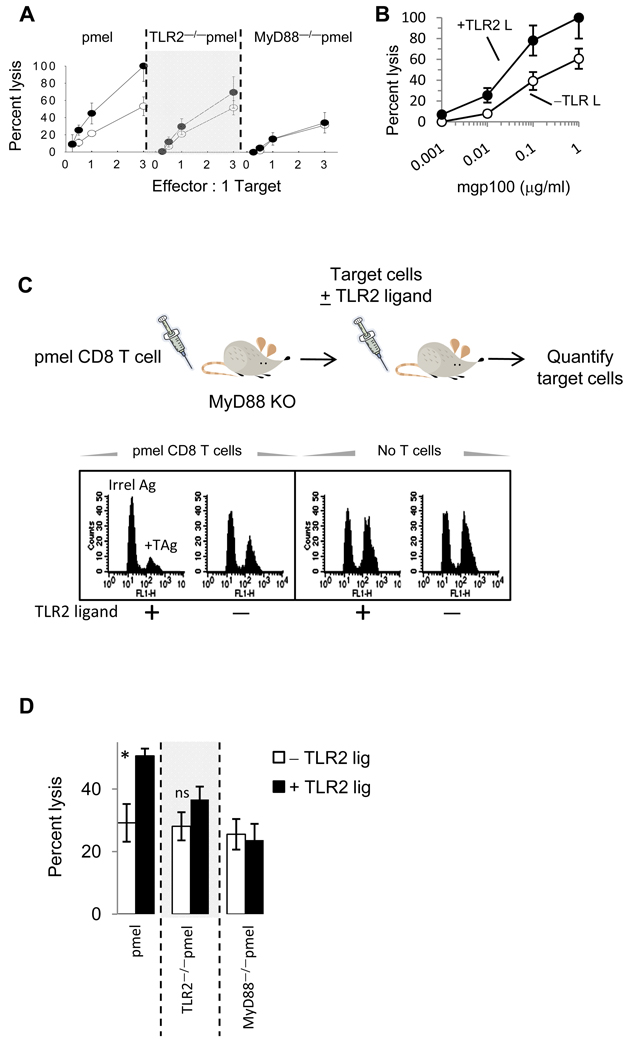
We examined the cytolytic activity of TLR2–stimulated pmel T-cells in vitro and in vivo. TLR2-stimulated T-cells demonstrated significantly higher cytotoxicity against B16 melanoma tumor cells at various effector to target ratios (Fig 2A). In contrast, TLR2 ligand did not increase TLR2−/−pmel or MyD88−/−pmel T-cells cytotoxicity. Moreover, TLR2 stimulation increased pmel cytotoxicity against EL4 tumor cells expressing suboptimal concentrations of TAg (Fig 2B). However, at levels below 1 ng/ml, TLR2-stimulated T-cells did not lyse target cells, indicating that TLR2 stimulation alone did not enhance non-specific killing (Fig 2B).
Figure 2. TLR2 engagement on pmel CD8 T-cells enhances cytolytic function against a weakly immunogenic melanoma tumor.
(A and B) Purified pmel CD8 T-cells were activated with mgp100-pulsed MyD88−/− splenocytes with or without TLR2 agonist and five days later, cytolytic activity against B16 melanoma cells was determined at different effectors to target ratios (B) or against EL4 cells pulsed with varying concentrations of mgp100 peptide at a 3:1 E:T ratio. (C) In vivo cytotoxicity assays were conducted as described in the Materials and Methods section. Briefly, resting pmel, TLR2−/−pmel, or MyD88−/−pmel T-cells were were injected (i.v.) into MyD88−/− mice. One day later mice received CFSEhigh mgp100-peptide–pulsed MyD88−/− target cells and CFSElow cells pulsed with an irrelevant H-2Db–restricted peptide with or without the TLR1/2 agonist. D) The percentage of targets recovered from spleens 24 hours after adoptive transfer was evaluated by FACS analysis. Data are representative of 2 experiments (5 mice per group). *P ≤0.02; ANOVA; n.s. not significant.
For in vivo studies, previously-activated (but resting) pmel T-cells were injected (i.v.) into MyD88−/− mice. One day later, mice received an equal number of CFSEhigh target cells (MyD88−/− splenocytes) pulsed with gp100 peptide and CFSElow cells pulsed with an irrelevant peptide and injected with PBS or TLR2 ligand (Fig 2C). The frequency of each target population was determined by FACS analysis 24 hours after transfer. The histogram shown in Figure 2C demonstrates that fewer mgp100-pulsed targets were recovered from mice injected with pmel T-cells and TLR2 ligand than mice injected with pmel T-cells alone (left panel). However, similar number of target cells was recovered from mice injected with PBS or TLR2 ligand alone (Fig 2C, right panel). The average percent lysis from 5 mice per group is shown in Fig 2D. In contrast, TLR2 ligand did not increase TLR2−/−pmel or MyD88−/−pmel cytotoxicity. It is worth noting that TLR2 stimulation did not increase the killing of target cells pulsed with ten-fold lower levels of antigen (data not shown).
Treatment with pmel T-cells and TLR2 ligand enhances anti-tumor responses compared to treatment with TLR2−/−pmel or MyD88−/−pmel T-cells
We examined the anti-tumor activity of TLR2-stimulated pmel T-cells in tumor-bearing mice. As depicted in Figure 3A, WT B6 harboring an established B16 tumor (~50 mm2) were irradiated (21–24), and injected (i.v.) with pmel, TLR2−/−pmel or MyD88−/−pmel T-cells followed by injectioin (i.p.) with mgp10025–33 and anti-CD40 antibody with or without TLR2 ligand. Mice received weekly injections (peritumoral) of PBS or TLR2 ligand.
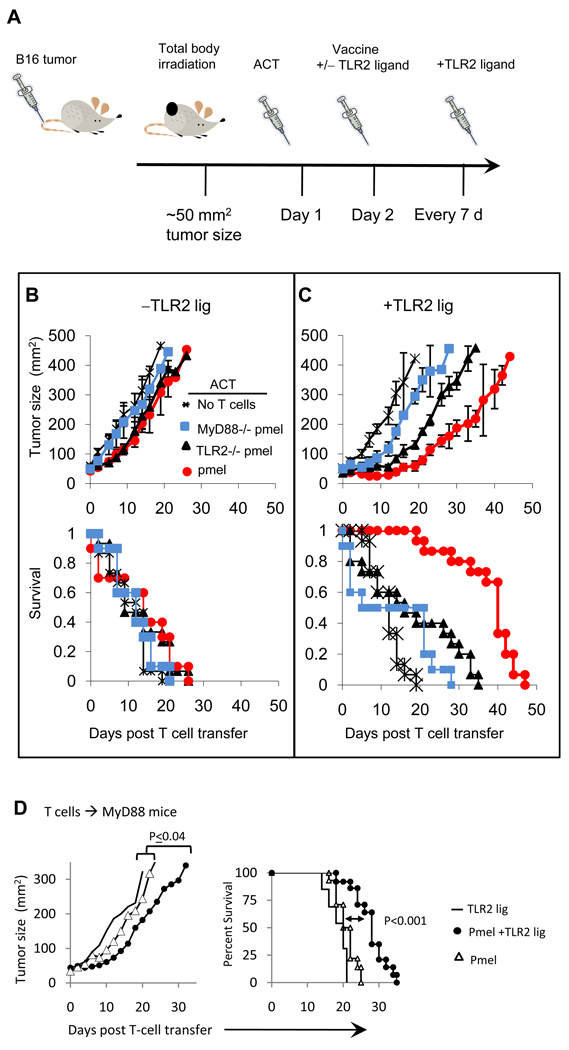
Figure 3. TLR2-MyD88 signals in pmel CD8 T-cells enhance antitumor activity against an established melanoma tumor.
A) Wild type BL6 or MyD88−/− mice (D) were injected (s.c.) with B16 melanoma cells and sublethally irradiated (400 cGy) when tumors reached a size of approximately of 50 mm2. One day later mice were injected (i.v.; 1×106) with previously-activated but resting pmel, TLR2−/−pmel or MyD88−/−pmel CD8 T-cells and injected i.p. with mgp10025–33 antigen and anti-CD40 antibody in each the absence or presence of TLR2 ligand. Mice received weekly peritumioral s.c. injections of TLR2 ligand or control saline. Tumor sizes were calculated by measuring perpendicular by longitudinal diameter. Data are compiled from four (B and C) or three independent (D) experiments, each yielding identical trends; *P< 0.001.
Tumor growth and mouse survival were comparable in mice receiving pmel, TLR2−/−pmel, or MyD88−/−pmel T-cells in the absence of TLR2 ligand (Fig 3B). These observations are in agreement with reports indicating that transfer of TAg-specific T-cells alone is insufficient to mediate significant anti-tumor responses (25). The lack of antitumor responses is likely due to the initial size and aggressive nature of this established tumor especially, in the absence of TLR and CD40 signals which are required to generate potent T-cell responses (18;26).
Treatment with pmel CD8 T-cells with TLR2 ligand delayed tumor growth (Fig 3C; circles) as compared with vaccinated mice not receiving T-cells (Fig 3C; asterisk). Although we observed tumor regression lasting up to 20 days after ACT, tumor growth resumed after this time point. The median survival time in mice receiving pmel T-cells and vaccine/TLR2 ligand increased to 34 days as compared to 13 days in mice receiving T-cells without TLR2 ligand (Fig 3E). It is worth noting that the absence of anti-CD40 Ab significantly reduced pmel’s anti-tumor activity (data not shown) despite injection with TLR2 ligand. These observations emphasize a critical need for APC activation with TLR and CD40 signals, as suggested by others (18). We also examined whether a second injection of pmel T-cells delayed tumor growth and found that although tumor growth was delayed and mouse survival increased as compared with mice receiving a single injection of T-cells, mice ultimately succumbed to tumor within 60 days (Supplemental-2)
Mice receiving TLR2−/−pmel CD8 T-cells and TLR2 ligand also showed delayed tumor growth (Fig 3C; triangles) as compared with mice receiving TLR2 ligand alone (Fig 3C; asterisks). Still, this treatment regime was not as effective as pmel T-cells and TLR2 ligand (circles), Fig 3C; p<0.001. The median survival time in mice receiving TLR2−/−pmel T-cells plus TLR2 ligand was 27 days.
Mice receiving MyD88−/−pmel CD8 T-cells and TLR2 ligand (squares) showed moderate but statistically insignificant anti-tumor responses than mice treated with TLR2 ligand alone, Figure 3C. Mice treated with TLR2 ligand or PBS alone demonstrated similar tumor growth kinetics, indicating that stimulating TLR2 on WT APCs was insufficient to suppress tumor development (Fig 3B and 3C).
We next examined the contribution of activating TLR2-MyD88 signals in CD8 T-cells in MyD88−/− mice. Administration of pmel T-cells plus TLR2 ligand delayed tumor growth as compared with mice treated with TLR2 ligand or pmel T-cells (Fig 3D; P≤0.04). Treatment with TLR2 ligand plus pmel T-cells moderately enhanced mouse survival (26 d) over mice receiving pmel T-cells (21 d; P<0.001) or TLR2 ligand (20d; P<0.0001).
Collectively, these data demonstrate that activating TLR2-MyD88 signals in T-cells enhances anti-tumor activity and that eliminating MyD88 signals in T-cells severely reduces anti-tumor responses. These results also emphasize the importance for activating TLR-MyD88 signals in APCs for generating anti-tumor T-cell responses.
Antitumor activity of TLR-MyD88–activated T-cells is associated with enhanced T-cell survival
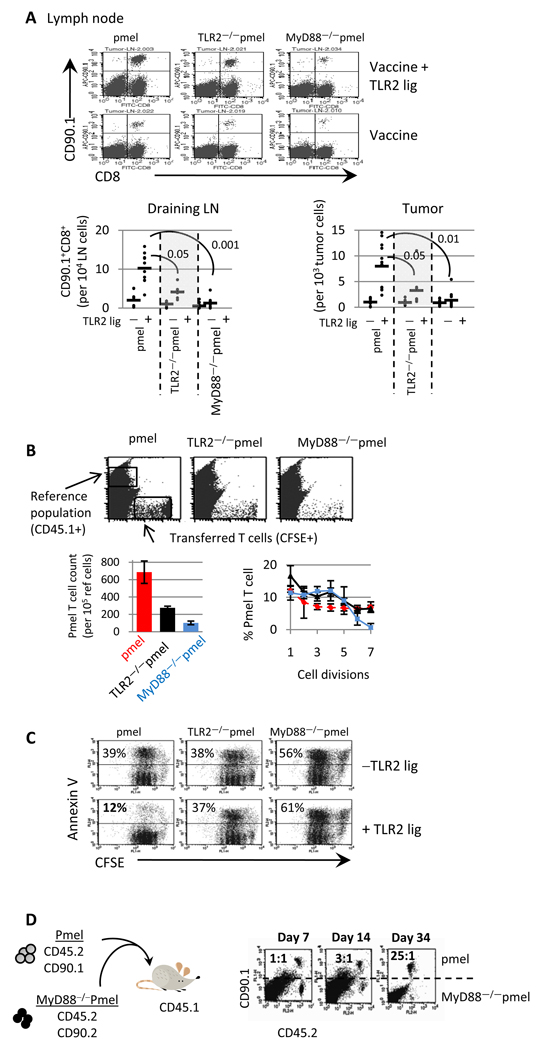
We examined if the improved anti-tumor activity in TLR ligand/T-cell–treated mice was associated with increased pmel T-cell numbers in tumor-bearing mice. As shown in Figure 4A, more pmel T-cells (CD90.1+) were detected in the draining lymph nodes and tumors following treatment with TLR2 ligand. The increase in T-cell numbers correlated with reduced tumor size at this time point (day 12) as compared with mice not receiving TLR2 ligand (Figure 3C). However, the tumor size began to increase 19 days after T-cell transfer (Figure 3). In accordance with increased tumor size, we found that the number of pmel T cells in the draining lymph nodes and tumor were reduced to an average of 937 ± 26 pmel T-cells per 105 tumor cells and 12 ± 3 per 105 LN cells 30 days after T cell transfer. The number of TLR2−/−pmel T-cells was also higher in TLR2 ligand-treated mice than TLR2−/−pmel T-cells in mice not receiving TLR2 ligand (Fig 4A; p<0.01, left panel). However, TLR2−/−pmel T-cell numbers were less than pmel T-cells, suggesting that TLR2 engagement on T-cells contributed to increased numbers (Fig 4A; p<0.05, left panel). In sharp contrast, MyD88−/−pmel T-cell numbers remained similar in TLR2 ligand-treated and untreated mice (Fig 4A; p<0.05, left panel). Furthermore, MyD88−/−pmel T-cell numbers were significantly lower than pmel and TLR2−/−pmel T-cell numbers.
Figure 4. TLR2-MyD88 signals within tumor-specific T-cells enhance antitumor activity by promoting T-cell longevity.
(A) Mice were challenged with tumor and treated with ACT as described in Figure 3. Twelve days after ACT CD90.1+CD8+ T-cells in the tumor and lymph nodes was determined by FACS. (B) Purified pmel, TLR2−/−pmel, and MyD88−/−pmel T-cells, were labeled with CFSE, and injected (i.v.) into WT mice followed by injection with mgp100 peptide and TLR2 ligand (i.v). Twelve days later, spleens were collected and the number of transferred cells was determined by setting the instrument gates to count a set number of the CD45.1 cells. Shown is a representative plot for two experiments; 5 mice per group. (C) Purified T-cells were labeled with CFSE and activated with mgp100–pulsed MyD88−/− splenocytes in the absence and presence of TLR2 ligand. Four days later cells were stained with Annexin-V. (D) Purified CD90.1+CD45.2+ pmel and CD90.2+CD45.2+ MyD88−/−pmel T-cells were activated in vitro with mgp100 peptide-pulsed WT splenocytes. One day later, pmel and MyD88−/−pmel T-cells were purified, mixed at a 1:1 ratio and intravenously injected into CD45.1+ mice. The ratio of pmel to MyD88−/−pmel T-cells was determined by staining cell suspensions with anti-CD90.1 and anti-CD45.2 antibodies. Shown is the data from one of two to three independent experiments; with 3–5 mice per group; *p<0.05; ANOVA.
We tested whether the elevated T-cell numbers, in response to the TLR2 stimulation, was due to enhanced cell division and/or survival. CFSE–labeled pmel, TLR2−/−pmel, and MyD88−/− pmel T-cells were injected (i.v.) into WT mice followed by injection with mgp10010–25 peptide and TLR2 ligand. To quantify the number of transferred T-cells we co-injected CD45.1+ splenocytes and set the instrument gates to count a fixed number of CD45.1 cells (Fig 4B). We detected 2.5-fold more pmel cells than TLR2−/−pmel and 7-fold more than MyD88−/−pmel T-cells (Figure 4B, bar graph). Although we detected more pmel T-cells, the number of pmel T-cells that underwent multiple divisions were only moderately increased as compared with TLR2−/− pmel or MyD88−/−pmel T-cells, suggesting that increased pmel T-cell numbers was primarily due to the enhanced survival.
We next examined cell division and apoptosis in vitro. CFSE-labeled pmel T-cells were activated with Ag-pulsed MyD88−/−splenocytes with or without TLR2 ligand. TLR2-ligated pmel T-cells showed significantly reduced apoptosis (12%) as compared with non-TLR2-stimulated T-cells (39%), Figure 4C. Identical trends were observed when staining cells with propidium iodide and Annexin-V (data not shown). In contrast, the addition of TLR2 ligand to TLR2−/−pmel or MyD88−/−pmel T-cells did not reduce cell death. Surprisingly, the absence of MyD88 in pmel T-cells rendered cells more susceptible to death than WT or TLR2−/− T-cells. Additionally, while pmel and TLR2−/−pmel T-cells underwent apoptosis after two cell divisions, MyD88−/−pmel T-cells began to die before dividing (Fig 4C, left panels). TLR2 engagement on pmel T-cells moderately increased cell division (Fig 4C). Noteworthy, the enhanced survival of TLR2-ligated T-cells correlated with increased levels of bcl-2 and bcl-xl at the protein and RNA level and a reduction in bim transcript levels (Supplemental 3).
To determine the requirement for intrinsic MyD88 signals within T-cells, we co-transferred MyD88−/−pmel (CD45.2+ CD90.2+) and pmel T-cells (CD45.2+ CD90.1+) into CD45.1+ mice and compared T-cell numbers at different time points, as depicted in Figure 4D. Pmel T-cells outnumbered MyD88−/−pmel T-cells as early as 14 days after ACT and became more procounced over time (Fig 4D). These results indicate that MyD88−/− T-cells could not be rescued despite conditions sufficient to promote the expansion/survival of pmel T-cells within the same environment.
All together, these data indicate that activating MyD88 via TLR2 on T-cells, in vivo, augments antitumor responses in part by rescuing T-cells from death and that MyD88 in T-cells is critical for their long-term survival.
Increased CTL effector function is achieved through over-expressing TLR2 on CD8 T-cells
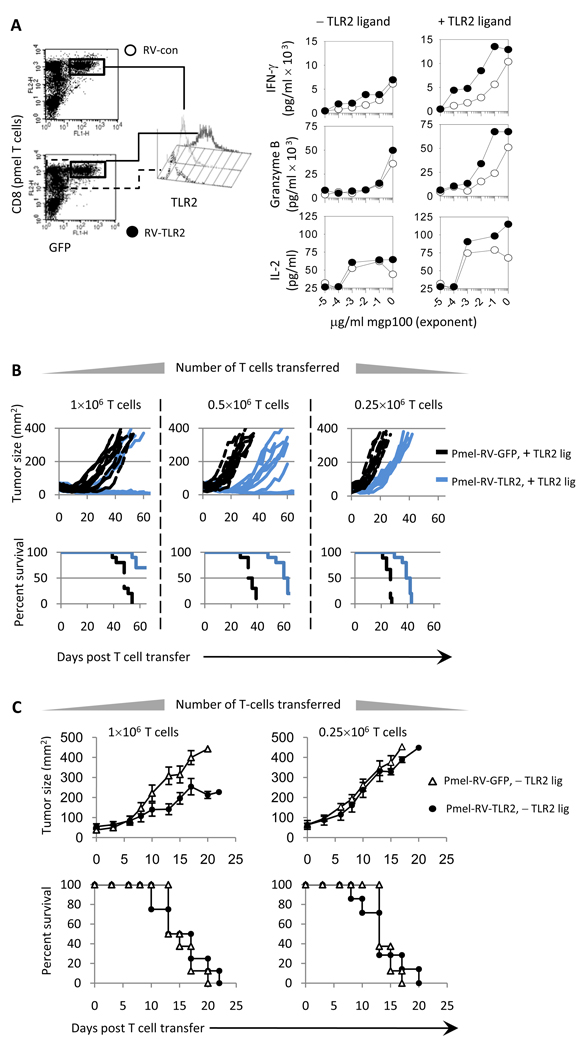
We sought to determine if over-expressing TLR2 on T-cells increased anti-tumor responses. Pmel T-cells infected with retroviruses, engineered to express TLR2-GFP (pmel-RV-TLR2) or GFP (pmel-RV-GFP), demonstrated higher TLR2 levels, Figure 5A. TLR2 stimulation on pmel-RV-TLR2 T-cells increased TCR sensitivity as demonstrated by significantly higher IFN-γ, granzyme B, and IL-2 levels (Fig 5A;P<0.0001; right panel). In the absence of TLR2 agonist, pmel-RV-TLR2 and pmel-RV-GFP T-cells produced similar cytokine levels.
Figure 5. Over-expressing TLR2 on tumor-reactive T-cells enhances the therapeutic efficacy of adoptively T-cell transfer.
A) pmel CD8 T-cells transduced with RV-expressing TLR2 and GFP (pmel-RV-TLR2) or GFP only (pmel-RV-con) were sorted by FACS. Cytokines production was determined by ELISA T-cells were activated with MyD88−/− APCs pulsed with varying concentrations of antigen in each the absence or presence of TLR2 ligand. B and C) BL6 mice bearing an established B16 tumor were treated with varying numbers of pmel-RV-TLR2 or pmel-RV-con with (B) or without (C) the TLR2 ligand. All mice were vaccinated with mgp100, anti-CD40 Ab and TLR2 ligand, as described in Figure 3. Data are compiled from 3 independent experiments with each experiment yielding similar results; *P< 0.001; MIXED procedure of SAS.
We examined the anti-tumor responses of pmel-RV-TLR2 and pmel-RV-GFP T-cells. Treatment with 106 RV-TLR2-pmel cells and TLR2 ligand successfully reduced tumor size in 7 of 10 mice bearing an established melanoma tumor (Fig 5B; solid lines). Mice treated with 106 pmel-TLR2-GFP cells and TLR2 ligand delayed tumor growth however, all mice succumbed to tumor by 45 days (Fig 5B; hashed lines). Treatment with 0.5×106 pmel-RV-TLR2 cells and TLR2 ligand reduced tumor growth and prolonged mouse survival (survival 59 days) as compared with mice treated with pmel-RV-GFP plus TLR2 ligand (median survival 32 days; p<0.0001). Mice receiving 0.25×106 pmel-RV-TLR2 and TLR2 ligand also showed tumor growth delay and prolonged survival (median survival 38 days) than the control group (survival 25 days). Interestingly, mice treated with pmel-RV-TLR2 in the absence of TLR2 ligand also demonstrated a statistically significant reduction in tumor growth kinetics although overall survival was not improved as compared with mice treated with pmel-RV-GFP (Figure 5C). Collectively, these data indicate that over-expressing TLR2 on tumor-specific T-cells could improve current approaches to treat cancer patients by augmenting sensitivity to subdominant TAgs and by requiring fewer T-cells.
Human melanoma–specific T-cells respond to suboptimal concentrations of weakly-immunogenic tumor-antigens following TLR2 engagement
We examined the costimulatory effects of TLR2 engagement on TAg-specific CD8 T-cells derived from melanoma patients. Sorted T-cells were co-cultured with the A375 melanoma cell line pulsed with various concentrations of the following weakly-immunogenic TAgs: Mart126–35, tyrosine188–196 and melanoma gp100 antigen (MSP160), Fig 6A. In the presence of TLR2 ligand CD8 T-cells produced higher levels of IFN-γ, GM-CSF, and IL-2 and responded to significantly lower levels of TAg (Fig 6B). It is worth noting that although the increases in cytokine production were more compelling at the higher antigen concentrations, TLR2-stimulation significantly enhanced T-cell expansion (Fig 6B, lower panels) and augmented cytolytic activity (Fig 6C). To obtain a better understanding of the cytotoxic capacity of TLR2-stimulated T-cells, we examined the lytic activity of gp100-specific T-cells at varying effector to target ratios against an HLA-A2+ melanoma line which expresses the weakly immunogenic tumor antigen gp100. As shown in Figure 6D, TLR2 stimulation significantly augmented cytotoxicity at various effector to target ratios. These data highlight the potential for augmenting human T-cell responses against suboptimal expression levels of low-avidity TAgs by activating TLR-MyD88 signals within T-cells.
Figure 6. TLR2 stimulation on melanoma-specific patient T-cells lowers the activation threshold to subdominant tumor antigens.
Tumor antigen-specific CD8 T-cells from melanoma patients were expanded and sorted as described in the Materials and Methods section. Cytokine production, proliferation, and cytolytic activity of Mart1–, gp100–, and tyrosinase–specific CD8 T-cells were tested against peptide-pulsed HLA-A2+ A-375 melanoma target cells in each the presence or absence TLR2 ligand. The A375 melanoma cell line was pulsed with varying concentrations of peptides starting at 1µg/ml and diluted 10-fold or pulsed with 0.001µg/ml peptide for in vitro cytotoxicity assays. (B) Cytokine production was evaluated using a Milliplex cytokine array 48 hours after stimulation wheras, T-cell proliferation was measured at the end of 3 days via 3H-thymidine incorporation. (C) Cytotoxicity was determined in a 6-hour 51Cr-release assay at an effector to target ratio of 1:1. (D) Alternatively, gp100–specific CD8 T-cells were mixed with melanomas cells (SK-MEL-23) expressing endogenous mgp100, at varying effector to target ratios. Blood samples were collected from 10 melanoma patients at time of diagnosis. The data presented are from three (B and C) and two (D) different donors from which the highest number of tumor-specific T-cells could be generated. All experimental determinations were performed in triplicate; averages ±sd were consistently within 15% of the mean. Error bars represent mean ±sd of triplicate samples.*P<0.01; ANOVA.
Discussion
For several reasons, TAg-specific CD8 T-cells do not always persist in vivo or function effectively against established tumors. The present studies highlight that stimulating TLRs on TAg-specific T-cells: occurs in vivo, enhances longevity, lowers the activation threshold to suboptimal concentrations of TAg, and augments anti-tumor responses. Furthermore, over-expressing TLR2 enhances antitumor responses above wild type T-cells.
These results highlight several key points. First, although several studies have demonstrated the costimulatory effects of stimulating TLRs on T-cells in vitro the present studies emphasize the importance of activating TLR-MyD88 signals in T-cells in vivo. For example, WT mice receiving pmel CD8 T-cells plus TLR2 ligand but not TLR2−/−pmel or MyD88−/− pmel T-cells improved tumor regression and augmented the numbers of transferred T-cells (Fig 3). The contribution of ligating TLR2 on T-cells is further highlighted in experiments demonstrating that injection of TLR2 ligand and pmel T-cells into MyD88−/− mice augmented T-cell cytotoxicity (Fig 2) and delayed tumor growth (Fig 4). Studies by Tak and colleagues suggested that TLR9 and TLR2 agonists in the synovium of rheumatoid arthritis co-stimulated T-cells resulting in enhanced cytolytic function and IFN-γ production (27). Sobek et al. reported that TLR2 agonists sustained pathogen-induced chronic inflammatory joint disease perhaps by inducing T-cell proliferation and interferon IFN-γ secretion (28). Collectively, these findings stress a physiological role for activating TLR/MyD88 signals in T-cells and could make possible new approaches for the development of more effective immunotherapies by manipulating TLR signaling within T-cells.
Secondly, the studies presented here also show that that over-expressing TLR2 on pmel T cells (without provision of TLR2 ligand) significantly delayed tumor growth as compared with mice receiving control pmel T-cells transduced with GFP alone. Because TLR2 can also recognize endogenous TLR ligands released from dying cells such as heat shock proteins and high mobility group box-1 proteins (29–31), we speculate that overexpressing TLR2 on T-cells could have enhanced the detection of these endogenous danger-associated signals which may have served as a costimulatory signal to T-cells.
The studies presented here also show that the enhanced anti-tumor responses of TLR2-stimulated pmel T-cells correlated with improved persistence and accumulation at the tumor site (Fig 4). This appeared to be primarily a result of enhanced cell survival rather than increased proliferation. Recent studies by Bartholdy (32) and Rahman (33) show that MyD88 expression in lymphocytic choriomeningitis virus (LCMV)–specific CD8 T-cells plays a critical role in maintaining T-cell expansion. Furthermore, Zhoa et al (34) demonstrate that vaccinia virus-specific MyD88−/−CD8 T-cells failed to accumulate in vivo and underwent slow expansion in response to infection, highlighting a critical role for MyD88 signals in T-cells. Our present studies add to these findings by demonstrating that ligating TLR2 directly on CD8 T-cells occurs in vivo and enhances MyD88–mediated survival thus promoting anti-tumor immune responses. Zanin-Zhorov and colleagues (31) reported that TLR2-ligated T-cells change the chemokine receptor expression profile, which alters T-cell migration/retention patterns, suggesting that the accumulation of T-cells within the tumor may have also been due to enhanced recruitment and/or retention.
The mechanisms by which TLR2 stimulation on T-cells influences cell survival are unknown, but most likely involve the regulation of apoptosis-related molecules (12–14). Studies by several groups, including ours, demonstrated TLR3 or TLR9 stimulation on CD4 T-cells increased cell survival and was associated with the enhanced expression of Bcl-xL (13;14;35). In CD8 T-cells, TLR2 engagement increased the expression of bcl-xL and A1 (12). The importance of modulating apoptosis-related protein in TAg-specific T-cells is highlighted by Rosenberg et al. who reported that over-expression of Bcl-2 in tumor-specific T-cells augmented survival, consequently enhancing tumor regression (36). The data in supplemental data 3, showing an in an increase in bcl-2 and bcl-xl protein and mRNA expression levels and a reduction in bim are in agreement with these studies.
Understanding the integration of signaling cascades that can enhance CD8 T-cell cytotoxicity against weakly-immunogenic TAgs is a critical for developing effective cancer immunotherapies. TLR2 ligation on T-cells enhanced the production of various cytokines and increased cytotoxicity [Figures 1, 2, 6 and ref (11;12)]. Recent studies by our group suggest that TLR activation amplifies TCR signals in part by increasing the expression levels of several transcription factors including T-bet (Tbx21), known to transcriptionally regulate the expression of IFN-γ, perforin, and granzyme B (37) (manuscript submitted; Geng et al). However, it is worth noting that MyD88 activation in T-cells may have occurred via TLR-independent signals such as IL-1 or IL-18 or perhaps other endogenous danger-associated molecular patterns.
These findings reveal a novel role for TLR-MyD88 signals within tumor-specific T-cells which may inspire new approaches for enhancing immunotherapies by targeting or manipulating TLR-MyD88 signaling within T-cells.
Supplementary Material
Acknowledgements
National Cancer Institute 1R01CA140917-01, NIH Center for Biomedical Research Center Excellence grant (1P20 RR021970), the Louisiana Cancer Research Consortium and the University of Maryland Greenebaum Cancer Center.
Reference List
- 1.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005 Nov 1;175(9):6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 2.Coulie PG, Somville M, Lehmann F, Hainaut P, Brasseur F, Devos R, et al. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int J Cancer. 1992 Jan 21;50(2):289–297. doi: 10.1002/ijc.2910500220. [DOI] [PubMed] [Google Scholar]
- 3.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007 Feb 19;204(2):299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boon T, Cerottini JC, Van den EB, van der BP, Van PA. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 5.de Visser KE, Cordaro TA, Kioussis D, Haanen JB, Schumacher TN, Kruisbeek AM. Tracing and characterization of the low-avidity self-specific T cell repertoire. Eur J Immunol. 2000 May;30(5):1458–1468. doi: 10.1002/(SICI)1521-4141(200005)30:5<1458::AID-IMMU1458>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Morgan DJ, Kreuwel HT, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998 Jan 15;160(2):643–651. [PubMed] [Google Scholar]
- 7.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003 Jan 22;85(2):85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 8.Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999 Apr;29(4):1209–1218. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005 Aug 1;175(3):1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 10.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asprodites N, Zheng L, Geng D, Velasco-Gonzalez C, Sanchez-Perez L, Davila E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 2008 Jun 27; doi: 10.1096/fj.08-108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottalorda A, Verschelde C, Marcais A, Tomkowiak M, Musette P, Uematsu S, et al. TLR2 engagement on CD8 T cells lowers the thresholdfor optimal antigen-induced T cell activation. Eur J Immunol. 2006 Jul;36(7):1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 13.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004 May 15;172(10):6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng L, Asprodites N, Keene AH, Rodriguez P, Brown KD, Davila E. TLR9 engagement on CD4 T lymphocytes represses {gamma}-radiation-induced apoptosis through activation of checkpoint kinase response elements. Blood. 2008 Mar 1;111(5):2704–2713. doi: 10.1182/blood-2007-07-104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, et al. J Biol Chem. 2006 Apr 7;281(14):9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- 16.Cottalorda A, Mercier BC, Mbitikon-Kobo FM, Arpin C, Teoh DY, McMichael A, et al. TLR2 engagement on memory CD8(+) T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur J Immunol. 2009 Oct;39(10):2673–2681. doi: 10.1002/eji.200939627. [DOI] [PubMed] [Google Scholar]
- 17.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of "self"-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998 Jul 20;188(2):277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004 Mar 15;199(6):775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila E, Celis E. Repeated administration of cytosine-phosphorothiolated guanine-containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J Immunol. 2000 Jul 1;165(1):539–547. doi: 10.4049/jimmunol.165.1.539. [DOI] [PubMed] [Google Scholar]
- 20.Ingalls RR, Lien E, Golenbock DT. Differential roles of TLR2 and TLR4 in the host response to Gram-negative bacteria: lessons from a lipopolysaccharide-deficient mutant of Neisseria meningitidis. J Endotoxin Res. 2000;6(5):411–415. [PubMed] [Google Scholar]
- 21.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005 Oct 3;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellstrom KE, Hellstrom I. Evidence that tumor antigens enhance tumor growth in vivo by interacting with a radiosensitive (suppressor?) cell population. Proc Natl Acad Sci U S A. 1978 Jan;75(1):436–440. doi: 10.1073/pnas.75.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellstrom KE, Hellstrom I, Kant JA, Tamerius JD. Regression and inhibition of sarcoma growth by interference with a radiosensitive T-cell population. J Exp Med. 1978 Sep 1;148(3):799–804. doi: 10.1084/jem.148.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.North RJ. Radiation-induced, immunologically mediated regression of an established tumor as an example of successful therapeutic immunomanipulation. Preferential elimination of suppressor T cells allows sustained production of effector T cells. J Exp Med. 1986 Nov 1;164(5):1652–1666. doi: 10.1084/jem.164.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007 Aug;117(8):2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van dH I, Wilbrink B, Tchetverikov I, Schrijver IA, Schouls LM, Hazenberg MP, et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000 Mar;43(3):593–598. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Sobek V, Birkner N, Falk I, Wurch A, Kirschning CJ, Wagner H, et al. Direct Toll-like receptor 2 mediated co-stimulation of T cells in the mouse system as a basis for chronic inflammatory joint disease. Arthritis Res Ther. 2004;6(5):R433–R446. doi: 10.1186/ar1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002 Apr 26;277(17):15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 30.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006 Mar;290(3):C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 31.Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003 Aug;17(11):1567–1569. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- 32.Bartholdy C, Christensen JE, Grujic M, Christensen JP, Thomsen AR. T-cell intrinsic expression of MyD88 is required for sustained expansion of the virus-specific CD8+ T-cell population in LCMV-infected mice. J Gen Virol. 2009 Feb;90(Pt 2):423–431. doi: 10.1099/vir.0.004960-0. [DOI] [PubMed] [Google Scholar]
- 33.Rahman AH, Cui W, LaRosa DF, Taylor DK, Zhang J, Goldstein DR, et al. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J Immunol. 2008 Sep 15;181(6):3804–3810. doi: 10.4049/jimmunol.181.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, De TC, Flynn R, Ware CF, Croft M, Salek-Ardakani S. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol. 2009 May 15;182(10):6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelman AE, LaRosa DF, Zhang J, Walsh PT, Choi Y, Sunyer JO, et al. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006 Nov;25(5):783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charo J, Finkelstein SE, Grewal N, Restifo NP, Robbins PF, Rosenberg SA. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 2005 Mar 1;65(5):2001–2008. doi: 10.1158/0008-5472.CAN-04-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004 Nov;4(11):900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.