Abstract
Background: Muscular dystrophies (MDs), characterized by progressive muscle wasting, are associated with 1 in 2,500 deaths in the United States. Although treatments slow the progression, these disorders lead to early death, usually due to cardiac or respiratory failure.
Methods: We analyzed death record data from 18,315 MD-associated deaths that occurred in the United States in 1986 through 2005 to assess trends in the age at death of people with MDs.
Results: From 1986 through 2005, the MD-associated mortality rate did not change among blacks, whites, males, or females. The median age at death among white females with MDs was 12 years higher than among black females. The frequency of reported cardiomyopathy increased among white but not black male decedents with MDs, although cardiomyopathy remained more commonly reported among black males. Among white males, the median age at death increased by 0.2 annually for those with and 1.3 for those without indications of cardiomyopathy. Among black males, the median age at death increased 0.3 years annually among those without reported cardiomyopathy. Among white males, the frequencies of pulmonary failure and pulmonary infection decreased significantly over time.
Conclusions: Changes in age at death and reported clinical comorbidities reflect improvements in the treatment of MDs. White males with MDs have shown a greater increase in age at death over time than black males. Contributing factors to this difference might include differences in types of MDs, rates of genetic and environmental modifiers, natural history, socioeconomic factors, and access to and use of treatment options.
Keywords: BMD = Becker muscular dystrophy; DMD = Duchenne muscular dystrophy; EDMD = Emery-Dreifuss muscular dystrophy; FSHD = facioscapulohumeral muscular dystrophy; ICD = International Classification of Diseases; LGMD = limb-girdle muscular dystrophy; MD = muscular dystrophy; NIV = noninvasive ventilation.
Muscular dystrophies (MDs) are genetic disorders associated with progressive muscle wasting and loss of motor function. Individual MDs are characterized by varying age at onset, rate of progression, distribution of muscle weakness, genetics, and presence of respiratory and cardiac involvement.1
Noninvasive ventilation (NIV) and corticosteroids are common interventions, particularly for the most common MD, Duchenne MD (DMD). NIV decreases the frequency of early death from respiratory failure,2–6 and might slow deterioration of cardiac muscle,7,8 although the frequency of cardiomyopathy increases with lifespan.9–11 Corticosteroids slow the progression of DMD7,12 and decrease or delay the risk of cardiomyopathy.13,14
In the United States, MDs account for about 1 in 2,500 deaths, and whites with MDs have a higher age at death than blacks,15 possibly due to differences in prevalences of individual MDs, although prevalence data for minority groups in the United States are lacking. Sociocultural barriers to good health that disproportionally affect blacks or African Americans16–18 and people with disabilities19 in the United States also might contribute to differences in age at death; health care preferences and treatment choices are additional factors. In addition, cardiomyopathy, common in some MDs,20–23 was more often reported among black decedents with MDs15 and among black decedents in the general US population (2.1% white and 3.0% black decedents).24 Further research is required to sufficiently describe the racial differences in natural history among people with MDs in the United States. We report the analysis of trends related to MD-associated deaths in the United States from 1986 to 2005.
METHODS
We analyzed 1986–2005 records from the National Center for Health Statistics' Multiple Cause Mortality Files, which contain data from all death certificates in the United States.25 Data include decedent's demographic information and immediate and underlying causes of death coded with the International Classification of Diseases (ICD). Prior to 1999, version ICD-926 was used; version ICD-1027 has been in use since. Changes in coding did not appear to introduce bias (appendix e-1 on the Neurology® Web site at www.neurology.org).
MDs are coded in ICD-9 by code 359.1, and in ICD-10 by code G71.0 (table e-1). These codes include DMD, Becker MD (BMD), Emery-Dreifuss MD (EDMD), limb-girdle MD (LGMD), facioscapulohumeral MD (FSHD), and other less-common MDs. MD-associated deaths were defined as those containing an ICD code for MD as an immediate or underlying cause of death. The definition of MD-associated death did not include those with a code for congenital MD (359.0/G71.2), because the distribution of age at death indicated that they were clinically distinct from those with code 359.1/G71.0, as expected (figure e-1).
Conditions in the analysis included those known to be associated with MDs (cardiomyopathy, heart failure, and respiratory failure) or found on at least 1% of MD-associated death records (table e-2).
Decedents were categorized by age at death as 10–34 years and 35 or older. Ten years was selected because it marks the beginning of the large peak in age at death among males that is presumably the result of DMD. Age 34 was selected because it is near the beginning of the peak among older males (figure 1), and the frequencies of age-related conditions began to increase after 34 years in the study population. Females were also categorized as less than 45 years and 45 or older.
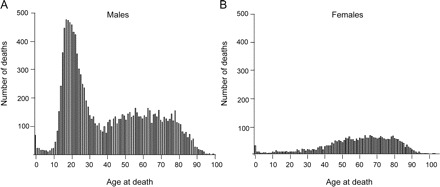
Figure 1 Distribution of age at muscular dystrophy–associated death
Age at muscular dystrophy–associated death in the United States, 1986–2006, among (A) males and (B) females.
Race was categorized as white, black, and other, as provided in the Multiple Cause Mortality Files (appendix e-1). We focused on blacks and whites because the number of MD-associated deaths among other decedents was too small to produce reliable estimates, and race data on death records were less accurate for this group.28
Age-adjusted mortality rates were calculated as previously described.2 Linear regression (SAS version 9.1) was used to assess trends in mortality rates and median age at death by year. Median age at death and frequencies of conditions were compared between groups using the Mann-Whitney U test and χ2 analysis (SPSS software). Records with missing values were excluded.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Centers for Disease Control and Prevention Institutional Review Board. Consent was not required because the study data were obtained from a public-access, de-identified death record database.
RESULTS
Refer to tables 1 and 2 (and appendix e-1) for complete results. In this section, we provide the significant results and pertinent negative results. There were 18,315 MD-associated deaths in the United States from 1986 through 2005. Of these, 71.7% were male and 28.3% were female; 90.6% were white, 7.7% were black, and 1.7% were of other races. MDs were associated with 1 in 2,502 deaths overall, and with 1 in 1,759 male, 1 in 4,388 female, 1 in 3,944 black, and 1 in 2,380 white decedents. The overall age-adjusted MD-associated mortality rate was 0.347 per 100,000 persons per year. The mortality rate was 0.523 for males, 0.182 for females, 0.220 for blacks, and 0.374 for whites. The mortality rate did not change over time for any group (figure e-2). About half of the MD-associated deaths occurred in hospitals, clinics, or medical centers, although there were significant differences in location of death between blacks and whites (table e-4).
Table 1 Race- and sex-specific median age at death by cardiomyopathy status, size of town, and geographic region of residence
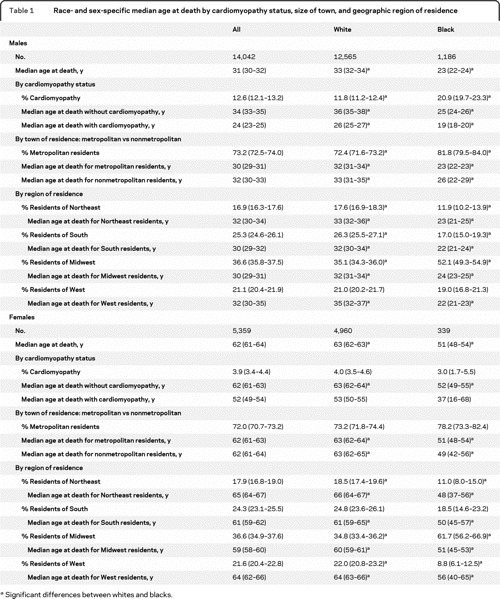
Age at death.
Distributions of age at death by sex are shown in figure 1. The median age at death was lower among black females (51 years, n = 329) than among white females (63 years, n = 4,785) (p ≤ 0.001). The median age at death among white males increased by 1.09 per year (n = 11,806, p ≤ 0.001), and 0.25 per year among black males (n = 1,078, p = 0.004) (figure 2). The proportion of white female decedents who were 45 years or older at death increased from 78.7% in the first decade of the study period to 83.8% in the second decade (p ≤ 0.001). In comparison, only about 63% of black females were 45 years or older at death, and this proportion did not change over time (table e-3). White female decedents in the South died at a younger age than those in the Northeast (p ≤ 0.001) or in the West (p ≤ 0.001). Otherwise there were no racial or sex differences by region or size of town of residence (table 1).
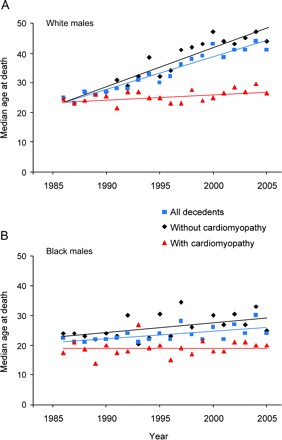
Figure 2 Age at muscular dystrophy–associated death among males by year
Median age at muscular dystrophy–associated death among all male decedents, males with cardiomyopathy, and males without cardiomyopathy on death certificate, United States, 1986–2005, for (A) white males and (B) black males.
Cardiomyopathy.
There was a linear increase in the frequency of cardiomyopathy reported among white males (0.2% per year, p = 0.0138), but not among black males (figure 3). In the 5 most recent years, cardiomyopathy was reported for 24.5% of black males, double that reported for white males (12.8%, p ≤ 0.001). Among females, the frequency of cardiomyopathy did not differ between blacks and whites, and did not change by decade (table 2).
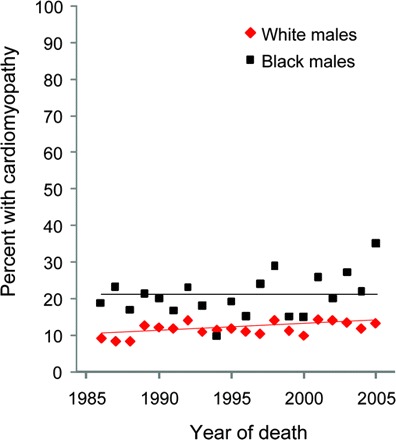
Figure 3 Race-specific percent of cardiomyopathy among muscular dystrophy–associated male decedents over time
Race-specific percent of cardiomyopathy among male decedents with muscular dystrophy–associated deaths by year, with significant linear trend among white but not black males.
Table 2 Secondary and age-related conditions among decedents with muscular dystrophy
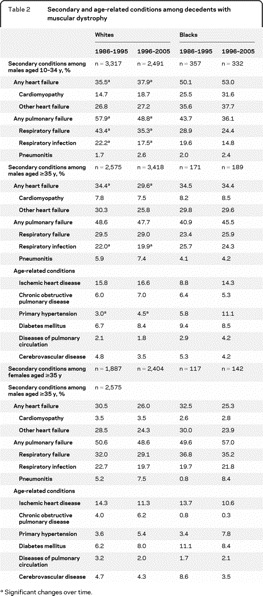
Overall, the median age at death for males with cardiomyopathy was lower than among those without, among both blacks and whites (table 1). Regardless of cardiomyopathy status, the median age at death was lower among black than white males. Among white males, the median age at death increased at 1.3 years annually (p ≤ 0.001) among those without cardiomyopathy and 0.2 years annually (p = 0.017) among those with cardiomyopathy (figure 2). Among black males, the median age at death increased for those without (0.33 years annually, p = 0.031), but not those with cardiomyopathy.
The median age at death among white females was lower among those with cardiomyopathy (53 years) than without (63 years, p < 0.001). Among females without cardiomyopathy, the median age at death was higher among whites (63 years) than among blacks (52 years, p < 0.001). The median age at death among black females with cardiomyopathy was 37 years, but did not differ from other female groups.
Secondary and age-related conditions by age group.
Among white males who died at age 10–34 years, reports of cardiomyopathy increased over time, while the frequencies of pulmonary infection and pulmonary failure decreased, and the frequency of other heart failure remained the same (table 2). Among white males in this age group without cardiomyopathy or other heart failure, the frequency of pulmonary complications decreased over time. Among black decedents aged 10–34 years, cardiomyopathy was more common and respiratory failure was less common (p < 0.001) than among white males.
Among white males who died at 35 years or older, the frequencies of pulmonary infection, pulmonary failure, and heart failure decreased over time, and the frequency of cardiomyopathy remained the same (table 2). The frequency of pulmonary complications decreased significantly over time among white males without cardiomyopathy, but not among those with cardiac indications. Among black males, the frequencies of these conditions did not change significantly over time. The frequencies of cardiomyopathy, cardiac failure, respiratory failure, and respiratory infection did not differ between white and black males. In addition to these common secondary conditions, several age-related conditions appeared on 1% or more of death records for this age group (table 2). Primary hypertension was more commonly reported among black than white males (p < 0.001); otherwise, males in this age group did not differ by race in frequencies of age-related conditions.
The same trends were seen among both black and white females in the 35-and-older group, but not significantly. The frequencies of secondary and age-related conditions did not differ between males and females, except for cardiomyopathy and ischemic heart disease, which were more common among white males than white females.
DISCUSSION
Increases in age at death were observed among decedents with MDs, as expected due to evolving health care treatments for MDs such as corticosteroids and NIV, with an older age at death among whites than blacks with MDs, among both males and females, and a greater increase in age at death among white males than black males with MDs. The distribution of age at death is consistent with the mix of MDs classified together in the ICD system. The small peak at zero years of age and the peak in the 60-year age range are present in both sexes. In the latter group, there were more male than female decedents, consistent with a mixture of autosomal MDs such as FSHD and LGMD, and X-linked MDs such as BMD and EDMD. Among male decedents, there is an additional peak around 20 years of age, which is likely to be composed predominantly of deaths from DMD. The overall MD-associated mortality rate was lower among blacks than among whites, which might be due to a lower prevalence of MDs, underdiagnosis of MDs, or underascertainment through death records for MDs among blacks.
Cardiomyopathy, a major contributor to morbidity and mortality for MDs, was more often reported among blacks than among whites, even early in the study period before the widespread use of corticosteroids and NIV were likely to have had an effect on patients with DMD. Cardiomyopathy is not associated with all types of MD, but is common in DMD,20 BMD,21 EDMD,22 and some of the LGMDs.23 Therefore, this finding may be indicative of racial differences in prevalences or natural histories of the different types of MDs. It might also be related to the higher frequency of cardiomyopathy among blacks in general.24
Although it might have been difficult to distinguish clinical heart failure with or without cardiomyopathy, the differences in age at death between decedents with and without reported cardiomyopathy suggested clinically relevant differences between the 2 groups. From 1986 through 2005, the frequency of cardiomyopathy increased steadily among white males, likely the result of increased age at death associated with NIV in patients with DMD and health care improvements for people with MDs in general. At the same time, the age at death of white males with cardiomyopathy gradually increased, consistent with the increased age at death among white males with MDs in general. Neither the frequency of cardiomyopathy nor age at death among those with cardiomyopathy increased among black males.
The racial differences in changes in age at death and frequency of cardiomyopathy might have been related to different prevalences of the various types of MD, different natural histories, or differences in environmental, genetic, or behavioral risk factors. However, it is possible that the use of corticosteroids and NIV was less common among black than white patients, or that these treatments were less effective in blacks because of differences in types of MD or other factors that affect natural histories. While differences in use of interventions might be due to racial or geographic preferences in balancing quality of life against potentially life-extending treatments, differences in access to health care may also be a factor, as suggested by racial differences in locations of MD-associated deaths (appendix e-1).
We looked at the frequencies of cardiac and pulmonary conditions among males who died at ages 10–34 years, which we presume to be mostly comprised of cases of DMD. The increasing frequency of cardiomyopathy and decreasing frequency of pulmonary complications among those without cardiac problems are consistent with the improvements in DMD treatment, which reduce pure pulmonary failure and therefore increase the age at death and the likelihood of cardiomyopathy.
Cardiomyopathy was less common among male decedents over the age of 35 years than among those aged 10–34 years. This finding was expected because males in the older age group are more likely to have one of the autosomal MDs that have lower frequencies of cardiomyopathy, such as FSHD.
As individuals with MDs lived longer, age-related conditions became apparent. The same trends were seen among both races and sexes, although the trends were not significant among blacks and females, likely due to smaller sample sizes. The frequencies of cardiomyopathy and ischemic heart disease were higher among white males than females with MDs, as they are among decedents in the general population.24 Along with the racial difference in cardiomyopathy among all decedents, this suggests that risk factors for heart disease in the general population might also affect the natural histories of MDs.
There were several limitations to this study. This dataset did not include information about quality of life, so increases in age at death do not necessarily equate with improvements in quality of life.29 Interpretation was complicated by the coding of MDs as a group in the ICD system, and by the lack of age-, race-, and sex-specific prevalence and age at death estimates for MDs. The sample sizes for female and black decedents limited the power of some race- or sex-specific analyses, especially when the need to dichotomize year of death could have masked small gradual changes. The dataset did not include age at onset or age at diagnosis; thus, we could not assess disease-related longevity relative to these events. While the database contains some possible social effect modifiers, such as education and marital status, it does not include information to assess sufficiently other sociocultural variables, such as health insurance and family structure.
There was the potential for bias resulting from inaccurate recording of information on death records. While the accuracy of physician-provided race data on death records of black and white decedents was about 98% when compared to information gathered from next of kin,30,31 the reported cause of death and underlying conditions might have been incomplete or inaccurate.32,33 It is not known if accuracy of death record data differed by race, so it is possible that race-specific biases existed because of uniform differences in the reporting of cause of death and underlying conditions for black and white decedents.
There was a greater increase in median age at death among whites than blacks with MDs in the United States, particularly among males without cardiomyopathy. The trends of increasing age at death and frequencies of secondary and age-related conditions were consistent with a slowing of the progression of MDs from corticosteroid use, respiratory support provided by NIV, and improvements in MD-related health care. More studies are needed, such as those ongoing in the Muscular Dystrophy Surveillance Tracking and Research Network,34 to understand the racial differences in age at MD-associated death in the United States.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Kenneson.
ACKNOWLEDGMENT
The authors thank Scott Grosse and Melissa Adams for their comments on the analysis and interpretation.
DISCLOSURE
Dr. Kenneson receives research support from the National Center on Birth Defects and Developmental Disabilities of the Centers for Disease Control and Prevention. Dr. Vatave reports no disclosures. Dr. Finkel serves on advisory boards for PTC Therapeutics, Inc., DuchenneConnect, Families of SMA, the National Fabry Disease Foundation, and TREAT-NMD; has received travel expenses for lectures not funded by industry; receives research support from PTC Therapeutics, Inc., Genzyme Corporation, Santhera Pharmaceuticals, the NIH (U54 AR0526446-03 [Co-I] and 1U54 NS0657-12-01 [Co-I]), the SMA Foundation, the Muscular Dystrophy Association, and the Foundation for the Eradication of Duchenne; and his spouse serves on the editorial board of Arthritis Research and Therapy, holds and has received license fees for numerous patents related to T cell activation and HIV, and receives research support from Merck Serono and the NIH in the field of T cell activation, HIV, and genomics of juvenile arthritis.
Supplementary Material
Footnotes
Editorial, page 948
Supplemental data at www.neurology.org
Study funding: Data analysis and manuscript preparation were supported by the National Center on Birth Defects and Developmental Disabilities of the Centers for Disease Control and Prevention (CDC), Atlanta, GA, contract number 200-2003-01396, to McKing Consulting Corporation.
Disclosure: Author disclosures are provided at the end of the article.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Received October 12, 2009. Accepted in final form May 11, 2010.
REFERENCES
- 1.Emery AE. The muscular dystrophies. Lancet 2002;359:687–695. [DOI] [PubMed] [Google Scholar]
- 2.Aberion G, Alba A, Lee MH, Solomon M. Pulmonary care of Duchenne type of muscular dystrophy. NY State J Med 1973;73:1206–1207. [PubMed]
- 3.Alexander MA, Johnson EW, Petty J, Stauch D. Mechanical ventilation of patients with late stage Duchenne muscular dystrophy: management in the home. Arch Phys Med Rehabil 1979;60:289–292. [PubMed] [Google Scholar]
- 4.Bach J, Alba A, Pilkington LA, Lee M. Long-term rehabilitation in advanced stage of childhood onset, rapidly progressive muscular dystrophy. Arch Phys Med Rehabil 1981;62:328–331. [PubMed] [Google Scholar]
- 5.Curran FJ, Colbert AP. Ventilator management in Duchenne muscular dystrophy and postpoliomyelitis syndrome: twelve years' experience. Arch Phys Med Rehabil 1989;70:180–185. [PubMed] [Google Scholar]
- 6.Eagle M, Bourke J, Bullock R, et al. Managing Duchenne muscular dystrophy: the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord 2007;17:470–475. [DOI] [PubMed] [Google Scholar]
- 7.Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord 2008;18:365–370. [DOI] [PubMed] [Google Scholar]
- 8.Simonds AK. Recent advances in respiratory care for neuromuscular disease. Chest 2006;130:1879–1886. [DOI] [PubMed] [Google Scholar]
- 9.Bach JR, O'Brien J, Krotenberg R, Alba AS. Management of end stage respiratory failure in Duchenne muscular dystrophy. Muscle Nerve 1987;10:177–182. [DOI] [PubMed] [Google Scholar]
- 10.Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord 2002;12:926–929. [DOI] [PubMed] [Google Scholar]
- 11.Yasuma F, Konagaya M, Sakai M, Kuru S, Kawamura T. A new lease on life for patients with Duchenne muscular dystrophy in Japan. Am J Med 2004;117:363. [DOI] [PubMed] [Google Scholar]
- 12.Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev 2008;1:CD003725. [DOI] [PubMed] [Google Scholar]
- 13.Biggar WD, Harris VA, Eliasoph L, Alman B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord 2006;16:249–255. [DOI] [PubMed] [Google Scholar]
- 14.Silversides CK, Webb GD, Harris VA, Biggar DW. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. Am J Cardiol 2003;91:769–772. [DOI] [PubMed] [Google Scholar]
- 15.Kenneson A, Kolor K, Yang Q, Olney RS, Rasmussen SA, Friedman JM. Trends and racial disparities in muscular dystrophy deaths in the United States, 1983-1998: an analysis of multiple cause mortality data. Am J Med Genet A 2006;140:2289–2297. [DOI] [PubMed] [Google Scholar]
- 16.AHRQ. National Healthcare Disparities Report. Rockville, MD: Agency for Healthcare Research and Quality; 2005
- 17.Mukamel DB, Weimer DL, Buchmueller TC, Ladd H, Mushlin AI. Changes in racial disparities in access to coronary artery bypass grafting surgery between the late 1990s and early 2000s. Med Care 2007;45:664–671. [DOI] [PubMed] [Google Scholar]
- 18.Shen JJ, Washington EL, Chung K, Bell R. Factors underlying racial disparities in hospital care of congestive heart failure. Ethn Dis 2007;17:206–213. [PubMed] [Google Scholar]
- 19.CDC. 2006. Disability and Health State Chartbook: Profiles of Health for Adults With Disabilities. Atlanta: Centers for Disease Control and Prevention; 2006.
- 20.Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol 1990;26:271–277. [DOI] [PubMed] [Google Scholar]
- 21.Nigro G, Comi LI, Politano L, et al. Evaluation of the cardiomyopathy in Becker muscular dystrophy. Muscle Nerve 1995;18:283–291. [DOI] [PubMed] [Google Scholar]
- 22.Sanna T, Dello Russo A, Toniolo D, et al. Cardiac features of Emery-Dreifuss muscular dystrophy caused by lamin A/C gene mutations. Eur Heart J 2003;24:2227–2236. [DOI] [PubMed] [Google Scholar]
- 23.Finsterer J, Stollberger C. Cardiac involvement in Becker muscular dystrophy. Can J Cardiol 2008;24:786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC Wonder: Multiple Cause of Death, 1999–2004 Rockville, MD: Health and Human Services. Available at: http://wonder.cdc.gov/mcd.html Accessed July 14, 2009. [Google Scholar]
- 25.Israel RA, Rosenberg HM, Curtin LR. Analytical potential for multiple cause-of-death data. Am J Epidemiol 1986;124:161–179. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Based on the Recommendations of the Ninth Revision Conference, 1975. Geneva: World Health Organization; 1977
- 27.WHO. International Statistical Classification of Diseases and Health Related Problems, ICD-10. Geneva: World Health Organization; 2000
- 28.Rosenberg HM, Maurer JD, Sorlie PD, et al. Quality of death rates by race and Hispanic origin: a summary of current research, 1999. Vital Health Stat 2 1999;128:1–13. [PubMed] [Google Scholar]
- 29.Gibson B. Long-term ventilation for patients with Duchenne muscular dystrophy: physicians' beliefs and practices. Chest 2001;119:940–946. [DOI] [PubMed] [Google Scholar]
- 30.Caveney AF, Smith MA, Morgenstern LB, Lisabeth LD. Use of death certificates to study ethnic-specific mortality. Public Health Rep 2006;121:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poe GS, Powell-Griner E, McLaughlin JK, Placek PJ, Thompson GB, Robinson K. Comparability of the death certificate and the 1986 National Mortality Followback Survey. Vital Health Stat 2 1993;118: 1–53. [PubMed] [Google Scholar]
- 32.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol 1990;131:160–168. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med 1998;129:1020–1026. [DOI] [PubMed] [Google Scholar]
- 34.Miller LA, Romitti PA, Cunniff C, et al. The Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): surveillance methodology. Birth Defects Res A Clin Mol Teratol 2006;76:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


