Abstract
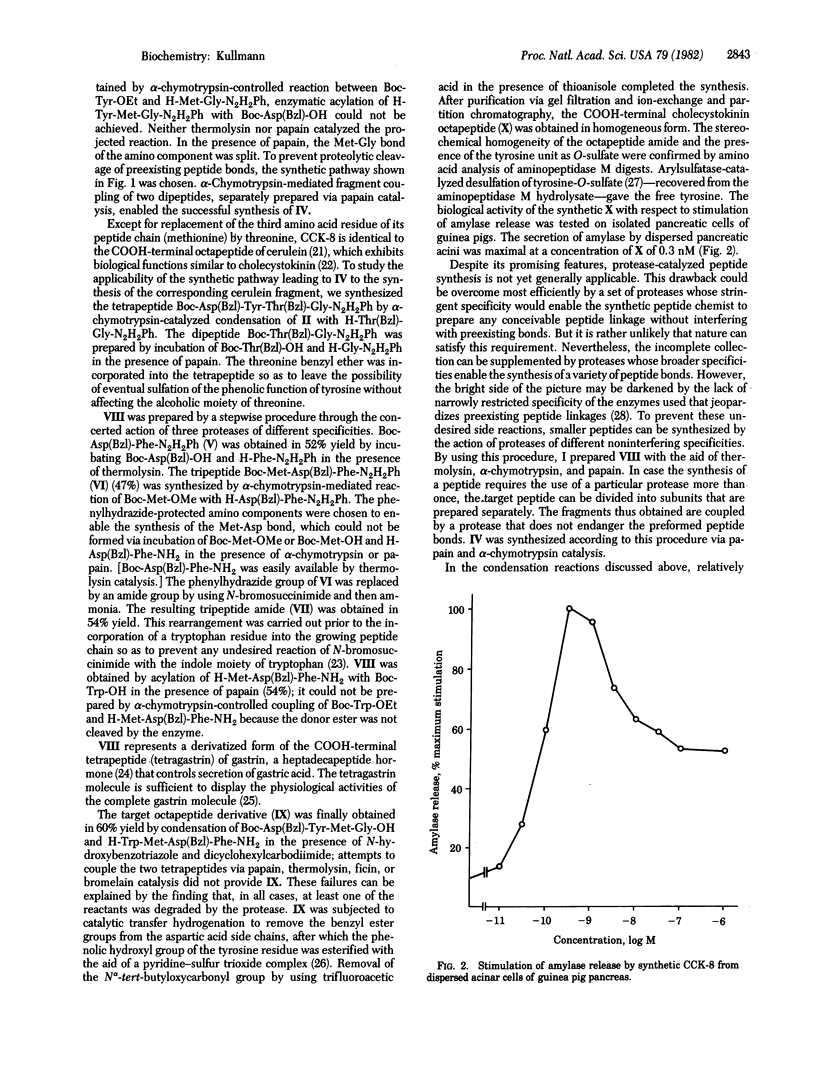
This study of protease-catalyzed peptide synthesis reports the preparation of the COOH-terminal octapeptide amide of cholecystokinin. The octapeptide was assembled by chemical condensation of two tetrapeptide segments that had been synthesized through the concerted catalytic reactions of several proteases of different specificities. The resulting octapeptide derivative was subjected to catalytic transfer hydrogenation, followed by sulfation of its tyrosine residue and removal of the N alpha-protecting group. The homogeneous target peptide was obtained after purification via partition chromatography, gel filtration, and ion-exchange chromatography. The synthetic octapeptide stimulated amylase release from pancreatic acinar cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Erspamer V., Endean R. Isolation and structure of caerulein, an active decapeptide from the skin of Hyla caerulea. Experientia. 1967 Sep 15;23(9):699–700. doi: 10.1007/BF02154119. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., ROSE F. A., TUDBALL N. Studies on sulphatases. 23. The enzymic desulphation of tyrosine O-sulphate. Biochem J. 1959 Jan;71(1):10–15. doi: 10.1042/bj0710010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V., Bertaccini G., De Caro G., Endean R., Impicciatore M. Pharmacological actions of caerulein. Experientia. 1967 Sep 15;23(9):702–703. doi: 10.1007/BF02154121. [DOI] [PubMed] [Google Scholar]
- Fruton J. S. Proteinase-catalyzed synthesis of peptide bonds. Adv Enzymol Relat Areas Mol Biol. 1982;53:239–306. doi: 10.1002/9780470122983.ch7. [DOI] [PubMed] [Google Scholar]
- GREGORY H., HARDY P. M., JONES D. S., KENNER G. W., SHEPPARD R. C. THE ANTRAL HORMONE GASTRIN. STRUCTURE OF GASTRIN. Nature. 1964 Dec 5;204:931–933. doi: 10.1038/204931a0. [DOI] [PubMed] [Google Scholar]
- Gillessen D., Trzeciak A., Müller R. K., Studer R. O. Syntheses and biological activities of methoxinine-analogues of the C-terminal octapeptide of cholecystokinin-pancreozymin. Int J Pept Protein Res. 1979 Feb;13(2):130–136. doi: 10.1111/j.1399-3011.1979.tb01860.x. [DOI] [PubMed] [Google Scholar]
- Gutte B., Däumigen M., Wittschieber E. Design, synthesis and characterisation of a 34-residue polypeptide that interacts with nucleic acids. Nature. 1979 Oct 25;281(5733):650–655. doi: 10.1038/281650a0. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Finn F. M., Limetti M., Montibeller J., Zanetti G. Studies on polypeptides. XXXIV. Enzymic properties of partially synthetic De(16-20)- and De(15-20)-ribonucleases S'1-3. J Am Chem Soc. 1966 Aug 5;88(15):3633–3639. doi: 10.1021/ja00967a030. [DOI] [PubMed] [Google Scholar]
- Jorpes J. E. The isolation and chemistry of secretin and cholecystokinin. Gastroenterology. 1968 Aug;55(2):157–164. [PubMed] [Google Scholar]
- Kullmann W. Enzymatic synthesis of Leu- and Met-enkephalin. Biochem Biophys Res Commun. 1979 Nov 28;91(2):693–698. doi: 10.1016/0006-291x(79)91577-8. [DOI] [PubMed] [Google Scholar]
- Kullmann W. Protease-mediated peptide bond formation. On some unexpected outcomes during enzymatic synthesis of leu-enkephalin. J Biol Chem. 1981 Feb 10;256(3):1301–1304. [PubMed] [Google Scholar]
- Kullmann W. Proteases as catalysts for enzymic syntheses of opioid peptides. J Biol Chem. 1980 Sep 10;255(17):8234–8238. [PubMed] [Google Scholar]
- Milne H. B., Carpenter F. H. Peptide synthesis via oxidation of N-acyl-alpha-amino acid phenylhydrazides. 3. Dialanyl-insulin and diphenylalanyl-insulin. J Org Chem. 1968 Dec;33(12):4476–4479. doi: 10.1021/jo01276a038. [DOI] [PubMed] [Google Scholar]
- Moroder L., Hallett A., Wünsch E., Keller O., Wersin G. Di-tert.-butyldicarbonat--ein vorteilhaftes Reagenz zur Eingührung der tert.-Butyloxycarbonyl-Schutzgruppe. Hoppe Seylers Z Physiol Chem. 1976 Nov;357(11):1651–1653. [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem. 1968 Oct 17;6(1):156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Pluscec J., Sabo E. F., Sheehan J. T., Williams N. Synthesis of cholecystokinin-pancreozymin. I. The C-terminal dodecapeptide. J Am Chem Soc. 1970 Jan 14;92(1):195–199. doi: 10.1021/ja00704a033. [DOI] [PubMed] [Google Scholar]
- Peikin S. R., Rottman A. J., Batzri S., Gardner J. D. Kinetics of amylase release by dispersed acini prepared from guinea pig pancreas. Am J Physiol. 1978 Dec;235(6):E743–E749. doi: 10.1152/ajpendo.1978.235.6.E743. [DOI] [PubMed] [Google Scholar]
- TRACY H. J., GREGORY R. A. PHYSIOLOGICAL PROPERTIES OF A SERIES OF SYNTHETIC PEPTIDES STRUCTURALLY RELATED TO GASTRIN I. Nature. 1964 Dec 5;204:935–938. doi: 10.1038/204935a0. [DOI] [PubMed] [Google Scholar]




