Abstract
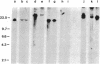
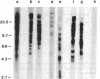
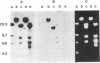
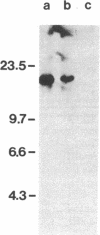
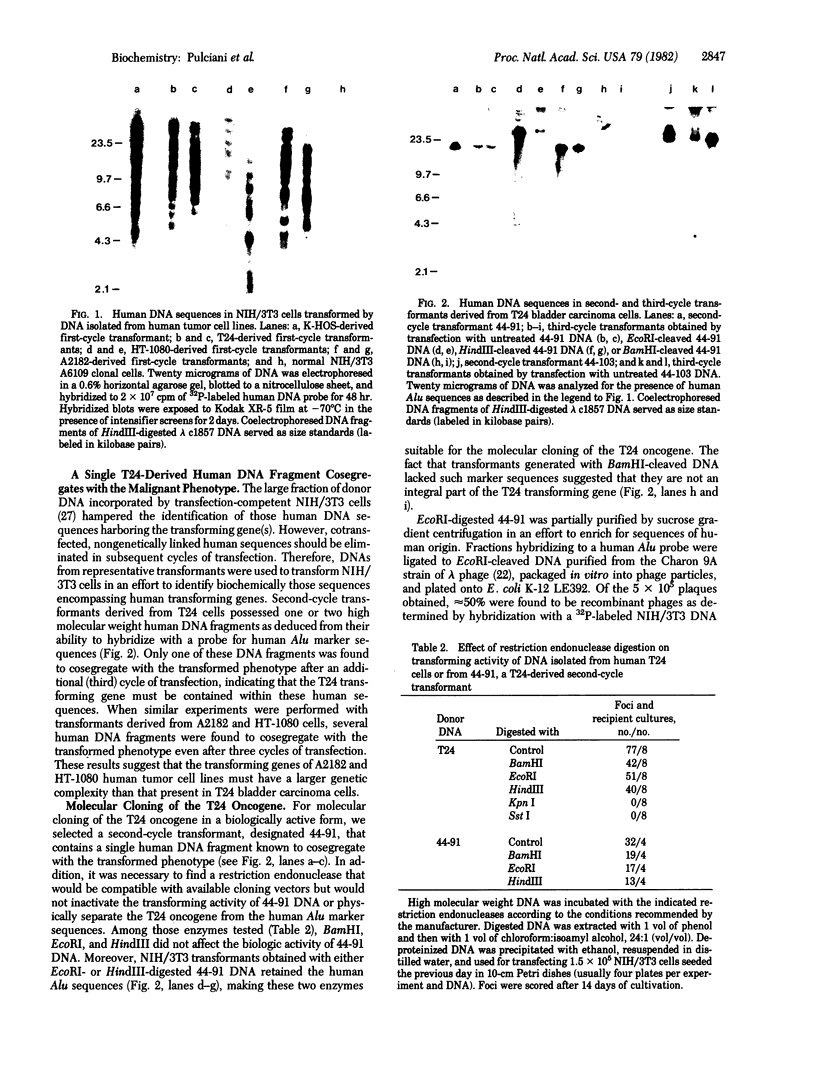
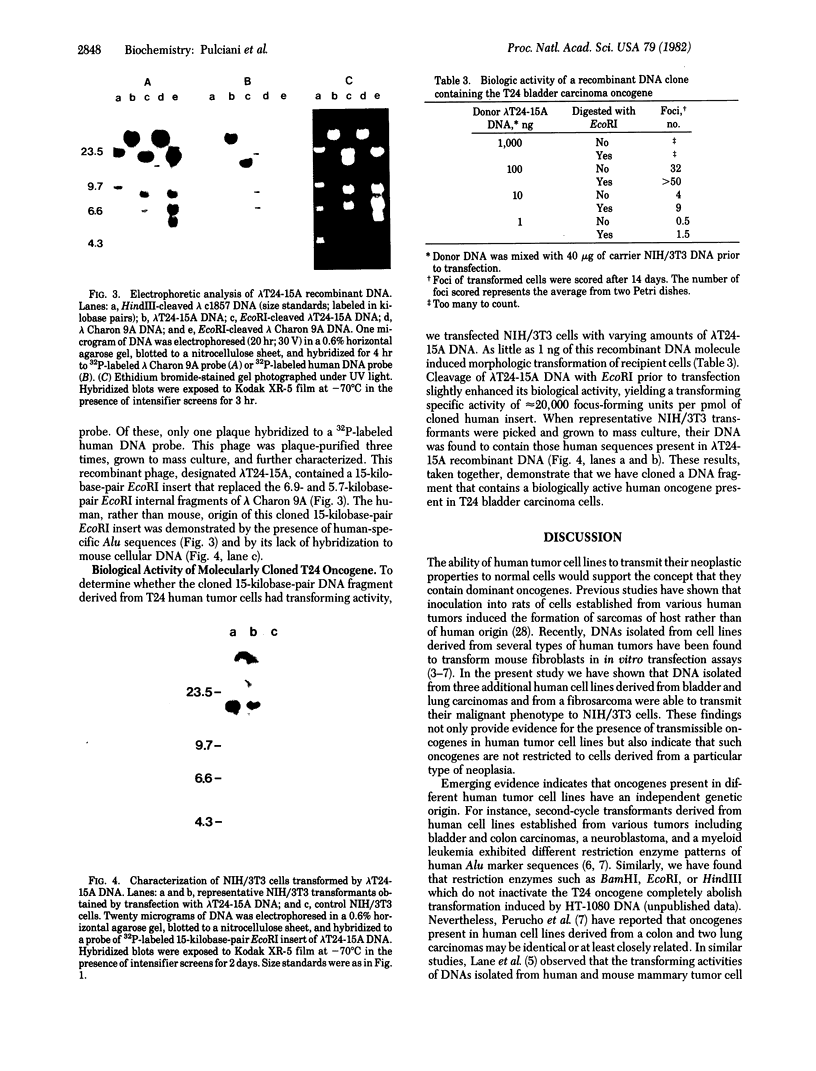
The presence of dominant transforming genes in human tumor cell lines has been investigated. High molecular weight DNAs isolated from cell lines established from carcinomas and sarcomas of various organs as well as from a glioblastoma and two melanomas were utilized to transfect NIH/3T3 mouse fibroblasts. The DNAs of T24 and A2182, two cell lines derived from a bladder and a lung carcinoma, respectively, and of HT-1080, a cell line established from a fibrosarcoma, were able to transform recipient NIH/3T3 cells. First-cycle transformants exhibited anchorage-independent growth and were tumorigenic in athymic and immunocompetent mice. Moreover, they contained human DNA sequences and were able to transmit their malignant phenotype in additional cycles of transfection. Southern blot analysis of T24-derived transformants showed that a single fragment of human DNA specifically cosegregated with the malignant phenotype, suggesting that it contained the T24 oncogene. Therefore, these human sequences were molecularly cloned with lambda Charon 9A as the cloning vector. The resulting recombinant DNA molecule, designated lambda T24-15A, was shown to contain a 15-kilobase-pair EcoRI insert of human cellular DNA. lambda T24-15A DNA (either intact or EcoRI digested) transformed NIH/3T3 fibroblasts with a specific activity of 20,000 focus-forming units per pmol of cloned DNA. Our results indicate that we have molecularly cloned a biologically active oncogene present in T24 human bladder carcinoma cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. SV40 T antigen induction and transformation in human fibroblast cell strains. Virology. 1968 Oct;36(2):254–261. doi: 10.1016/0042-6822(68)90142-6. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Cellular transformation by subgenomic feline sarcoma virus DNA. J Virol. 1981 Jan;37(1):518–523. doi: 10.1128/jvi.37.1.518-523.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Baresová M., Viklický V., Jakoubková J., Sainerová H., Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973 May;11(3):765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Fish D. C., Djurickovic D., Trimmer R. W., Bare A. L., Bare R. M., Smith G. T. Induction of rat sarcomas in rats treated with antithymocyte sera after transplantation of human cancer cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1793–1794. doi: 10.1073/pnas.76.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Cooper G. M. Transforming activity of human tumor DNAs. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1181–1184. doi: 10.1073/pnas.78.2.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASFARGUES E. Y., OZZELLO L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958 Dec;21(6):1131–1147. [PubMed] [Google Scholar]
- Lane M. A., Sainten A., Cooper G. M. Activation of related transforming genes in mouse and human mammary carcinomas. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5185–5189. doi: 10.1073/pnas.78.8.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Gardner M. B., Greene A. E., Bradt C., Nichols W. W., Landing B. H. Cultivation in vitro of cells derived from a human osteosarcoma. Cancer. 1971 Feb;27(2):397–402. doi: 10.1002/1097-0142(197102)27:2<397::aid-cncr2820270224>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Shilo B. Z., Shih C., Cowing D., Hsu H. W., Weinberg R. A. Three different human tumor cell lines contain different oncogenes. Cell. 1981 Aug;25(2):355–361. doi: 10.1016/0092-8674(81)90054-4. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Robins D., Wold B., Sweet R., Jackson J., Lowy I., Roberts J. M., Sim G. K., Silverstein S., Axel R. Altering genotype and phenotype by DNA-mediated gene transfer. Science. 1980 Sep 19;209(4463):1414–1422. doi: 10.1126/science.7414320. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Rigby C. C., Franks L. M. A human tissue culture cell line from a transitional cell tumour of the urinary bladder: growth, chromosone pattern and ultrastructure. Br J Cancer. 1970 Dec;24(4):746–754. doi: 10.1038/bjc.1970.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Padhy L. C., Murray M., Weinberg R. A. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981 Mar 19;290(5803):261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Use of transfection to analyze genetic information and malignant transformation. Biochim Biophys Acta. 1981 Aug 31;651(1):25–35. doi: 10.1016/0304-419x(81)90003-2. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Daniels D. L., Schroeder J. L., Williams B. G., Denniston-Thompson K., Moore D. D., Blattner F. R. Restriction maps for twenty-one Charon vector phages. J Virol. 1980 Jan;33(1):401–410. doi: 10.1128/jvi.33.1.401-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]