Abstract
Lung resection is the mainstay of treatment in patients with early stage non-small cell lung cancer. However, lung cancer patients often suffer from comorbidities and the respiratory reserve should be carefully evaluated preoperatively in order to avoid postoperative complications. Forced expiratory volume in 1 second (FEV1) is considered to be an index that depicts the patient's respiratory efficacy and its prediction has a key role in the preoperative evaluation of lung cancer patients with impaired lung function. Prediction of postoperative FEV1 is currently possible with the use of perfusion radionuclide lung scanning.
Quantitative CT is the analysis of data acquired during normal chest CT scan using the system's software. By applying a dual threshold of -500 to -910 Hounsfield Units, functional lung volumes are estimated and postoperative FEV1 can be predicted by reducing the preoperative measurement by the fraction of the part to be resected.
Studies have shown that preoperative predictions correlate well with the actual postoperative measurements. Additionally, quantitative CT results are in good agreement with perfusion scintigraphy predictions. Newer radiological techniques such as perfusion MRI and co-registered SPECT/CT have also been used in the preoperative evaluation with similar results.
In conclusion, chest CT which is obligatory for staging, can be used for quantitative analysis of the already available data. It is technically simple, providing an accurate prediction of postoperative FEV1. Thus, quantitative CT appears to be a useful tool in the preoperative evaluation of lung cancer patients undergoing lung resection.
Keywords: Lung cancer, lung resection, predicted postoperative FEV1, quantitative CT
Introduction
Lung resection is the mainstay of treatment in patients with early stage non-small cell lung cancer. Operability is determined based on the stage, histology and the respiratory reserve which has to be carefully evaluated preoperatively, in order to avoid serious postoperative complications. According to current guidelines, this evaluation includes measurement of the forced expiratory volume in 1 second (FEV1), diffusing capacity for carbon monoxide (DLCO) and values < 80% predicted require further investigation with exercise testing and estimation of VO2 max [1]. If exercise testing is not available, it can be replaced by stair climbing. However, if altitude reaching is less than 22 meters, then VO2 max measurement is highly recommended. Values < 10 ml/kg/min indicate increased risk and other treatment modalities should be chosen. Values > 20 ml/kg/min indicate that the patient can undergo resection up to pneumonectomy. Values from 10 to 20 ml/kg/min require prediction of postoperative lung function. Patients with predicted postoperative (ppo) FEV1 and DLCO > 30% pred. are suitable for surgery. If either of them is < 30% pred., then ppo VO2 max should be estimated and if it is < 10 ml/kg/min or < 35% pred. other treatment options should be considered. Thus, preoperative testing regarding the respiratory reserve is complete and every patient is fully evaluated so as not to be excluded from the only, potentially curative treatment.
Prediction of postoperative lung function is currently possible using perfusion radionuclide lung scanning [2,3]. Postoperative FEV1 is predicted by reducing the preoperative value by the fraction of the regional radioactivity counts of the part to be resected to total radioactivity counts of both lungs. However, perfusion scintigraphy is a test that requires special equipment, leads to radiation expo-sure of the patients and their environment, is an additional economic burden and is not accurate in chronic obstructive pulmonary disease (COPD) patients. On the other hand, chest computer tomography (CT) scan is in any case available since it is necessary for staging. Data acquired during normal CT scan can be processed using the system's software and quantitative measurements can be performed. Lung volumes estimated by quantitative CT can be used to predict postoperative FEV1, by reducing the preoperative measurement by the fraction that the part to be resected contributes to the total volume of both lungs.
CT analysis
All lung cancer patients are submitted to chest CT scan for staging reasons. Images acquired during a normal CT scan before the contrast administration can be analyzed using the system's software. Lung parenchyma is isolated from the mediastinum and chest wall and then segmented in three areas according to the attenuation of each voxel, using the dual threshold of -500 to -910 Hounsfield Units (HU). Areas between these limits correspond to areas of functional lung parenchyma, whereas areas < -910 HU correspond to areas of emphysema and areas > -500 HU to areas of tumor, postobstructive atelectasis or pneumonitis. Using the software, the volume of the functional lung parenchyma of both lungs can be automatically calculated (Figure 1). In addition, guided by the fissures between the different lobes and by delineating the region of interest (i.e. the boundaries of the part to be resected) in every slice with the cursor, functional lung volume of the part to be resected can be estimated (Figures 2, 3). Postoperative FEV1 can be predicted by using the following formula:
Figure 1.
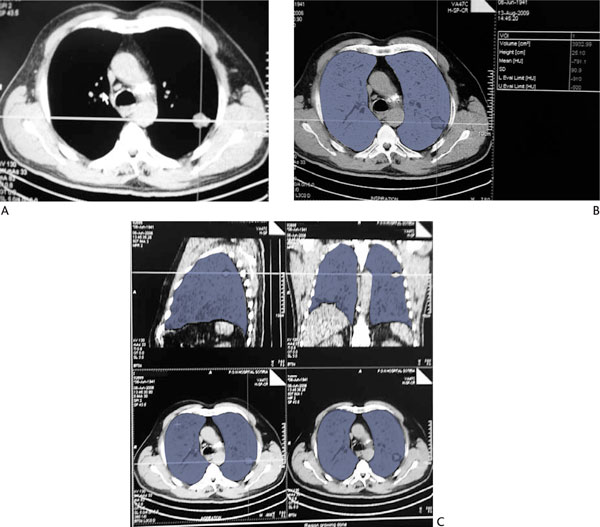
Quantitative Ct Volume Estimations. (A): Chest CT scan of a patient with a tumor in the left upper lobe. (B): and (C): Quantitative analysis of functional lung parenchyma of both lungs, using the dual threshold of -500 to -910 HU. Areas in blue correspond to voxels within these attenuation limits. Total functional lung volume of both lungs is estimated to be 3932.99 mL.
Figure 2.
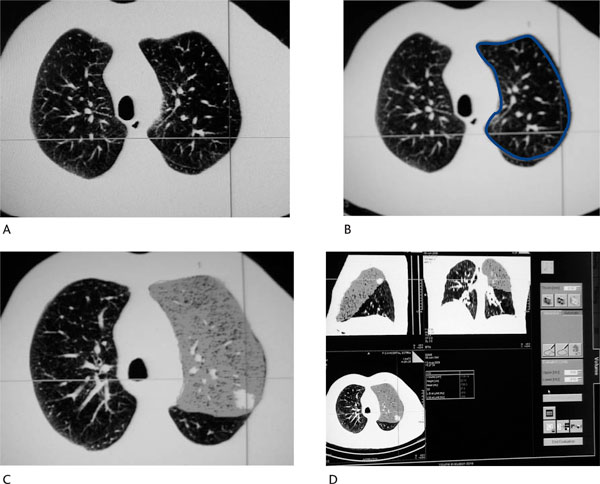
Volumetric Analysis of the Resected Lobe (Same Patient as in Figure 1). (A): Fissure identification between left upper and lower lobe. (B): Delineation of the region of interest (limits of the lobe to be resected) in all transaxial images. (C): and (D): Volumetric analysis of the left upper lobe. Regional functional lung volume is estimated to be 1173.14 mL.
Figure 3.
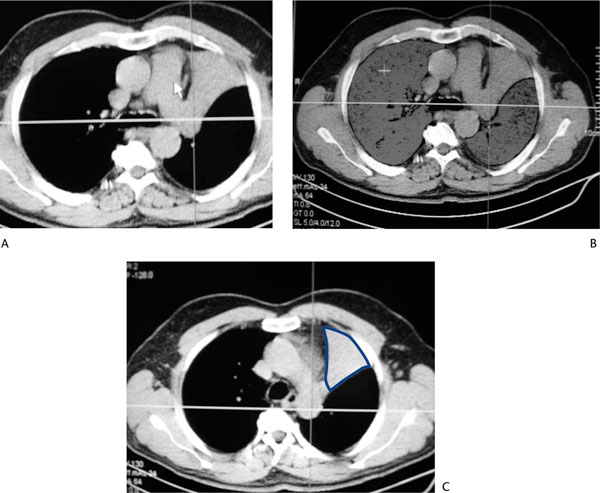
Quantitative Analysis of a Patient With a Tumor Causing Atelectasis of the Left Upper Lobe. (A): Chest CT scan. (B): Volumetric analysis of both lungs. Total functional lung volume is estimated to be 3972 mL. (C): Regional functional lung volume of the left upper lobe is only 9 mL due to the atelectasis. In this case, predicted postoperative FEV1 is very similar to the preoperative measurement.
Discussion
Non-small cell lung cancer is a disease usually detected in an advanced stage and despite the administration of chemotherapy and radiotherapy it has poor prognosis. Early detection gives the opportunity of surgical resection, which has better survival rates. However, lung cancer patients often suffer from comorbidities and careful preoperative evaluation should be performed to ensure postoperative respiratory efficacy. FEV1 is considered to be an index that depicts the patient's respiratory efficacy and its prediction has a key role in the preoperative evaluation of lung cancer patients with impaired lung function. Qualitative prediction of postoperative lung function is the simplest method frequently used by thoracic surgeons and is based on segment counting. Considering that right upper, middle and lower lobes have 3, 2 and 5 segments respectively and left upper and lower lobes 5 and 4 segments respectively, each segment is considered to represent 1/19 of lung function. Postoperative FEV1 is predicted based on the formula:
S = the number of segments that are going to be resected [4]. In the case of obstructed parts of the lung, qualitative prediction can be made using the Nakahara formula:
b = the number of total subsegments in the resected part, n = the number of obstructed subsegments based on findings of bronchoscopy and chest CT, assuming that b is 6, 4, 12 in the right upper, middle and lower lobes respectively and 10 each in the left upper and left lower lobes [5]. However, the functional contribution of each segment to the total pulmonary function can differ among patients, especially in the presence of underlying pulmonary disorders such as emphysema or interstitial lung disease.
Prediction of postoperative FEV1 using quantitative analysis of chest CT scan was first proposed by Wu et al. [6]. Based on the fact that the attenuation of lung parenchyma at chest CT is primarily determined by the relative proportions of blood, gas, extravascular fluid and pulmonary tissue, areas of emphysema or fibrosis can be detected, since their attenuation differs from functional lung parenchyma. Different attenuations also have areas of tumor, postobstructive atelectasis or pneumonitis. In order to quantify these findings, different dual thresholds of Hounsfield Units have been applied and, finally, it was observed that by using threshold limits of - 500 to -910 HU, areas of tumor, atelectasis, fibrosis and emphysema could be excluded and volumetric assessment of functional lung parenchyma could be performed, by measuring the volume of voxels within these attenuation limits. Prediction of postoperative FEV1 could be performed by reducing the preoperative value by the fraction of the regional functional lung volume of the part to be resected to the total functional lung volume of both lungs. Thirty-eight patients were evaluated and results were promising since CT-predicted values correlated well with the actual postoperative measurements (r = 0.93, p < 0.001).
Further studies evaluated the role of quantitative CT in predicting postoperative FEV1, by simultaneously comparing its results with the perfusion scintigraphy predictions [7,8]. Bolliger et al., based on correlation coefficients between predicted and measured postoperative values (44 patients evaluated with both methods, r = 0.91 for quantitative CT and 0.92 for perfusion scintigraphy), concluded that although both methods performed well in predicting postoperative lung function, perfusion lung scanning was slightly superior compared to quantitative CT. Wu et al. also evaluated 44 patients and results showed that both methods predicted postoperative FEV1 well in patients who underwent pneumonectomy (n = 28, r = 0.88 for quantitative CT vs. r = 0.86 for perfusion scintigraphy) and lobectomy (n = 16, r = 0.90 vs. r = 0.80). As a result, the use of quantitative CT was favored, based on its technical simplicity and availability, its ability to define lobar anatomy more objectively and its performance to the sub-group of patients with a predicted postoperative FEV1 < 40% pred. In this subgroup of patients it was observed that quantitative CT had no false-negative findings, in contrast with perfusion scintigraphy which had false-negative results. Quantitative CT was therefore proposed for widespread use in predicting postoperative FEV1.
Furthermore, special interest has been shown in examining the efficacy of quantitative CT in predicting postoperative FEV1 in patients with severe air-flow obstruction, since many lung cancer patients also suffer from COPD [9,10]. Sverzellati et al. evaluated by means of quantitative CT COPD patients with lung cancer scheduled for lobectomy. Using the same procedure and the same dual threshold of Hounsfield Units, postoperative FEV1 was predicted and analysis showed significant correlation with the actual postoperative measurements (r = 0.97, p < 0.001). It can therefore be used as an alternative tool to perfusion scans even in lung cancer patients with borderline pulmonary function.
Newer radiological techniques have lately been evaluated for their use in predicting postoperative FEV1 in lung cancer patients. Ohno et al. used per-fusion MRI by assessing blood volumes within total and resected lungs, quantitative CT by estimating total and regional functional lung volumes, qualitative CT by segment counting and nuclear medicine studies such as the commonly used perfusion planar imaging and also SPECT and co-registered SPECT/CT by assessing uptakes within total and resected lungs [11,12]. Results from these studies showed significantly good correlations between predicted and measured FEV1 for all techniques. However, the predictive capability was better for the state of the art radiological techniques, i.e. dynamic perfusion MRI (r = 0.88), quantitative CT (r = 0.88), and co-registered SPECT/CT (r = 0.88), when compared with the traditional methods of segment counting (r = 0.85), perfusion scintigraphy planar imaging (r = 0.83) and SPECT (r = 0.85). Similar results were reported by Yoshimoto et al., where quantitative CT and SPECT/CT were almost equally accurate and superior to the segment counting method [13].
Based on these studies, quantitative CT appears to be a useful tool in the preoperative evaluation of lung cancer patients, as its predictions seem to correlate well with the actual postoperative measurements. It has been tested in patients with marginal pulmonary function with good results and appears to be superior to traditional methods of prediction, especially if we consider the fact that a chest CT scan is in any case needed for staging reasons and quantitative CT is the processing of the already available raw data. Thus, no additional cost, patient discomfort or further exposure to radiation are needed to predict postoperative lung function. Additionally, its capability to predict postoperative chronic dyspnea is currently being investigated, since prediction of FEV1 as a single value is not an accurate index to reflect the patient's degree of dyspnea. Dyspnea ratings influence and predict general health status to a greater extent than do physiologic measurements [14,15]. This study is ongoing in our institution and results so far show that predicted volume loss, estimated preoperatively using quantitative CT, correlates well with the degree of chronic dyspnea evaluated using the modified Medical Research Council (mMRC) dyspnea scale 3 months after surgery. In addition, prediction of postoperative FEV1 is performed using the same procedure of quantitative analysis as in the previous studies, although we use the low-dose technique in performing the chest CT scan. Despite the lower level of tube current compared to previous studies, volumetric analysis is equally feasible and the results similarly accurate [16].
In conclusion, it appears that quantitative CT might simultaneously be used for staging, prediction of postoperative FEV1 and prediction of postoperative degree of dyspnea, thus substituting the other traditional methods of preoperative evaluation.
Note
The study was partly supported by the National University of Athens and Greek NHS.
Conflict of Interest Statement
None of the authors has any conflict of interest to declare in relation to the subject matter of this manuscript.
References
- Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, Licker M, Ferguson MK, Faivre-Finn C, Huber RM, Clini EM, Win T, De Ruysscher D, Goldman L. European Respiratory Society and European Society of Thoracic Surgeons joint task force on fitness for radical therapy. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- Markos J, Mullan BP, Hillman DR, Musk AW, Antico VF, Lovegrove FT, Carter MJ, Finucane KE. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139:902–910. doi: 10.1164/ajrccm/139.4.902. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Wyser C, Roser H, Solèr M, Perruchoud AP. Lung scanning and exercise testing for the prediction of postoperative performance in lung resection candidates at increased risk for complications. Chest. 1995;108:341–348. doi: 10.1378/chest.108.2.341. [DOI] [PubMed] [Google Scholar]
- Beckles MA, Spiro SG, Colice GL, Rudd RM. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest. 2003;123(1 Suppl):105S–114S. doi: 10.1378/chest.123.1_suppl.105s. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Monden Y, Ohno K, Miyoshi S, Maeda H, Kawashima Y. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg. 1985;39:260–265. doi: 10.1016/S0003-4975(10)62591-X. [DOI] [PubMed] [Google Scholar]
- Wu MT, Chang JM, Chiang A, Lu JY, Hsu HK, Hsu WH, Yang CF. Use of quantitative CT to predict postoperative lung function in patients with lung cancer. Radiology. 1994;191:257–262. doi: 10.1148/radiology.191.1.8134584. [DOI] [PubMed] [Google Scholar]
- Wu MT, Pan HB, Chiang A, Hsu HK, Chang HC, Peng NJ, Lai PH, Liang HL, Yang CF. Prediction of postoperative lung function in patients with lung cancer: comparison of quantitative CT with perfusion scintigraphy. AJR Am J Roentgenol. 2002;178:667–672. doi: 10.2214/ajr.178.3.1780667. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Gückel C, Engel H, Stöhr S, Wyser CP, Schoetzau A, Habicht J, Solèr M, Tamm M, Perruchoud AP. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration. 2002;69:482–489. doi: 10.1159/000066474. [DOI] [PubMed] [Google Scholar]
- Sverzellati N, Chetta A, Calabrò E, Carbognani P, Internullo E, Olivieri D, Zompatori M. Reliability of quantitative computed tomography to predict postoperative lung function in patients with chronic obstructive pulmonary disease having a lobectomy. J Comput Assist Tomogr. 2005;29:819–824. doi: 10.1097/01.rct.0000179595.09092.ee. [DOI] [PubMed] [Google Scholar]
- Ueda K, Tanaka T, Li TS, Tanaka N, Hamano K. Quantitative computed tomography for the prediction of pulmonary function after lung cancer surgery: a simple method using simulation software. Eur J Cardiothorac Surg. 2009;35:414–418. doi: 10.1016/j.ejcts.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Koyama H, Nogami M, Takenaka D, Matsumoto S, Yoshimura M, Kotani Y, Sugimura K. Postoperative lung function in lung cancer patients: comparative analysis of predictive capability of MRI, CT and SPECT. AJR Am J Roentgenol. 2007;189:400–408. doi: 10.2214/AJR.07.2084. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Koyama H, Nogami M, Takenaka D, Onishi Y, Matsumoto K, Matsumoto S, Maniwa Y, Yoshimura M, Nishimura Y, Sugimura K. State-of-the-art radiological techniques improve the assessment of postoperative lung function in patients with non-small cell lung cancer. Eur J Radiol. in press doi:10.1016/j.ejrad.2009.07.024. [DOI] [PubMed]
- Yoshimoto K, Nomori H, Mori T, Kobayashi H, Ohba Y, Shibata H, Shiraishi S, Kobayashi T. Prediction of pulmonary function after lung lobectomy by subsegments counting, computed tomography, single photon emission computed tomography and computed tomography: a comparative study. Eur J Cardiothorac Surg. 2009;35:408–413. doi: 10.1016/j.ejcts.2008.10.057. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Faryniarz K, Tomlinson D, Colice GL, Robins AG, Olmstead EM, O'Connor GT. Impact of dyspnea and physiologic function on general health status in patients with chronic obstructive pulmonary disease. Chest. 1992;102:395–401. doi: 10.1378/chest.102.2.395. [DOI] [PubMed] [Google Scholar]
- Stavem K, Boe J, Erikssen J. Health status, dyspnea, lung function and exercise capacity in patients with chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 1999;3:920–926. [PubMed] [Google Scholar]
- Papageorgiou C, Karakontaki F, Palamidas A, Kaltsakas G, Tsangaridou I, Papamichalis G, Antoniou D, Koulouris NG. The role of quantitative CT in predicting postoperative FEV1 and chronic dyspnoea in patients undergoing lung resection. Eur Respir J. 2009;34(Suppl 53):823s. doi: 10.1186/2049-6958-5-3-188. [DOI] [PMC free article] [PubMed] [Google Scholar]


