Abstract
We present a case of severe interstitial pneumonitis, mild polyarthritis and polymyositis, and Raynaud's syndrome with the presence of anti-Jo-1 antibodies, which had been diagnosed as anti-synthetase syndrome. The presence, however, of anti-Ro/SSA antibodies led us to understand that we were dealing here with a more severe form of interstitial lung disease. The patient was treated for acute respiratory failure but he showed resistance to glucocorticoids and cyclosporine. Thus, he was treated with infusions of anti-CD20 therapy (rituximab): his clinical conditions improved very rapidly and a significant decrease in the activity of pulmonary disease was detected using high-resolution computerized tomography (HRCT) of the thorax and pulmonary function tests.
Keywords: Acute respiratory failure, immunology, interstitial lung disease, rituximab
Introduction
The anti-synthetase syndrome (ASS) is a subgroup of idiopathic inflammatory muscle disease. Its characteristics are interstitial lung disease, myositis, poly-arthritis, mechanic's hand, Raynaud's syndrome and the presence of antisynthetase antibodies (anti-Jo-1). Interstitial lung disease (ILD) is the major determinant of morbidity and mortality in the anti-synthetase syndrome (ASS).
Here we report a case of ASS with pulmonary involvement, successfully treated with rituximab.
Case report
The case involves a 58-year old Caucasian male, a farmer up until the age of 45 and thereafter employed as a clerk. At the age of 40 the patient began experiencing fever and asthenia following prolonged, strenuous activity. The patient reported suffering moderate muscle weakness and pain in the previous 4 years, with painful synovitis of both hands, Raynaud's phenomenon and episodic fever. In the last year a dry cough and weight loss were also experienced. The patient had been a heavy smoker (10-15 cigarettes/day) but had quit smoking completely one year prior to his admission to our hospital. His medical history was unremarkable and he was not taking any kind of pharmaceutical treatment.
The patient was referred to our hospital (time 0) due to a 2 month history of rapidly worsening dyspnea, experienced after only mild exertion, and to the persistence and worsening of his dry cough. Two months prior, he had been diagnosed with inter stitial lung disease and was treated with low doses of corticosteroids (prednisone) and N-acetylcysteine for a short period (15 days), but with no improvement of symptoms. On admission to our hospital, the patient was also suffering asthenia of the upper and lower limbs, persistent fever and muscle pain, which he had been experiencing for the past 4 years.
Over both lungs, diffused fine crackles were heard. Blood gas analysis showed desaturation with low PaO2 and PaCO2, while high resolution computerized tomography (HRCT) scan showed a significant worsening of the pulmonary fibrosis with areas of honeycomb and ground glass opacities (Figure 1).
Figure 1.
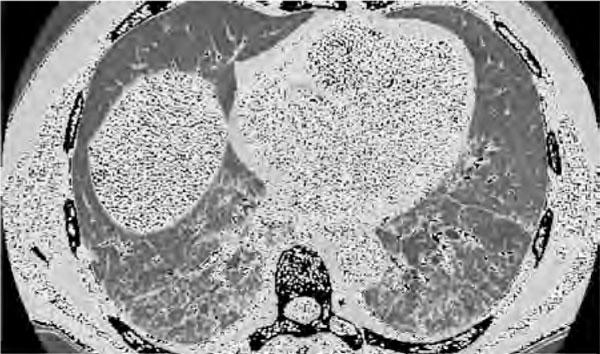
High-resolution chest ct scan showing pulmonary fibrosis with areas of honeycomb and ground glass opacities particularly in the dorsobasal areas. Definition of abbreviation: CT, computerized tomography.
The phenomenon of Reynaud was also present. Serology revealed positive anti-Jo-1 antibodies with increased inflammatory markers. The pulmonary function test showed marked restrictive syndrome (FEV1 = 50% pred.; FVC = 45% pred.; TLC = 65% pred.) with severe reduction of DLCO (32% pred.). Capillaroscopy pointed out nonspecific capillaroscopic abnormalities: non-homogeneous morphology and distribution of capillaries which were coiled; large and irregular hemosiderin deposition due to hemorrhage; fragmentation of the blood column with formation of plasma gaps and a granular aspect of the cells (Figure 2).
Figure 2.
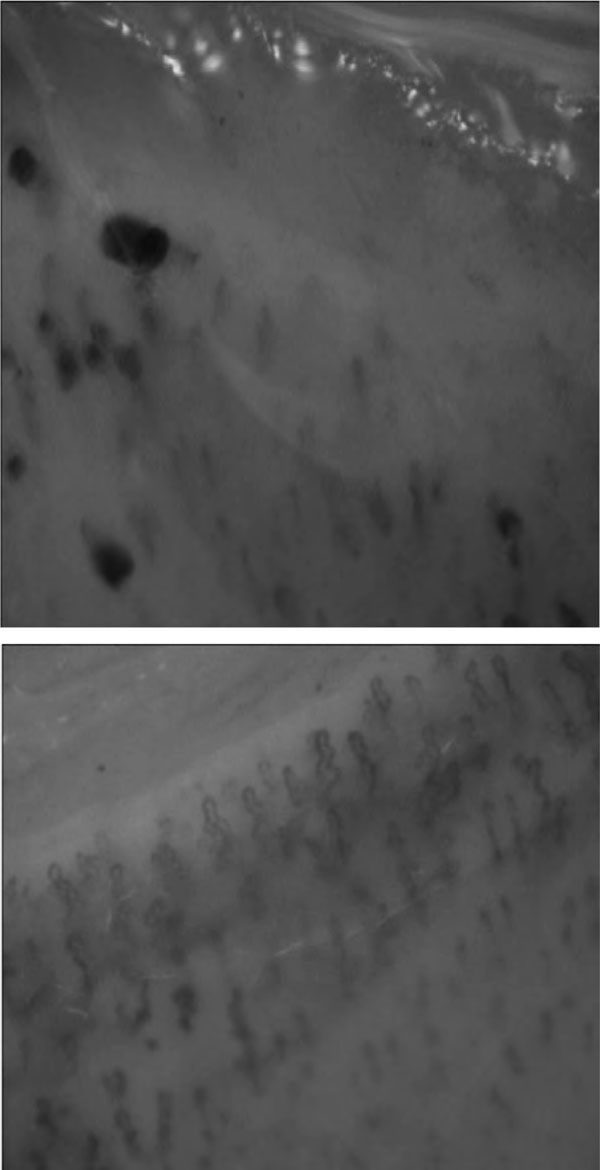
Capillaroscopy showing a nonspecific pattern of abnormalities and non-homogeneity in capillary length and loop size.
Electromyography showed myopathic injury associated with signs of low grade chronic nerve damage without denervation in the lumbar-sacral root distribution. The echocardiogram underlined mild pulmonary hypertension (35 mm Hg). The patient was unable to perform a 6-minute walking test (6MWT). Based on the data collected, the patient was diagnosed with anti-synthetase syndrome, classified as a rare inflammatory autoimmune disease.
A high dose of steroid and immune suppression therapy was quickly begun (methylprednisolone i.v. 2 mg/Kg/die and azathioprine 50 mg/die) which appeared to result in an initial decrease of the patient's symptoms, although not objectively verifiable, but with functional respiratory deterioration (reduction of DLCO) and apparent worsening of the radiological features.
Treatment with cyclosporin at 5 mg/Kg was then begun; however, a progressive functional and symptomatic worsening was observed (the patient was unable to walk a few meters). Therefore, based on data in the medical literature, it was decided to proceed with the rituximab (Mabthera®: monoclonal antibody anti-CD20) treatment. According to the protocol, the treatment would involve a dosage of 1,000 mg i.v. administered twice in the course of 15 days. A second cycle would be repeated 6 months later. Already the day following the first administration of the drug (time 1), the patient began showing mild but progressive reduction of the dyspnea and increased exercise tolerance. After 10 days, the patient was discharged from hospital based on the significant improvement of his respiratory functions. The second dose of rituximab was administered, as planned, after 2 weeks. Blood samples taken to evaluate the eventual reduction of the antibody anti-Jo-1 showed, however, no change in the results. A thorax HRCT was repeated resulting in a definite regression of ground glass attenuation and stabilization of fibrotic changes (Figure 3).
Figure 3.
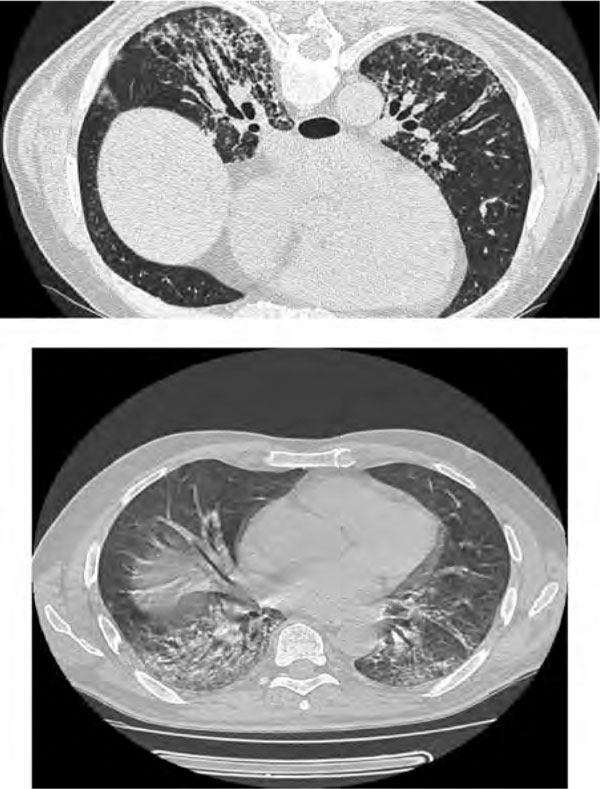
High resolution chest ct scan showing regression of ground glass attenuation and stabilization of fibrotic changes.
Figure 4.
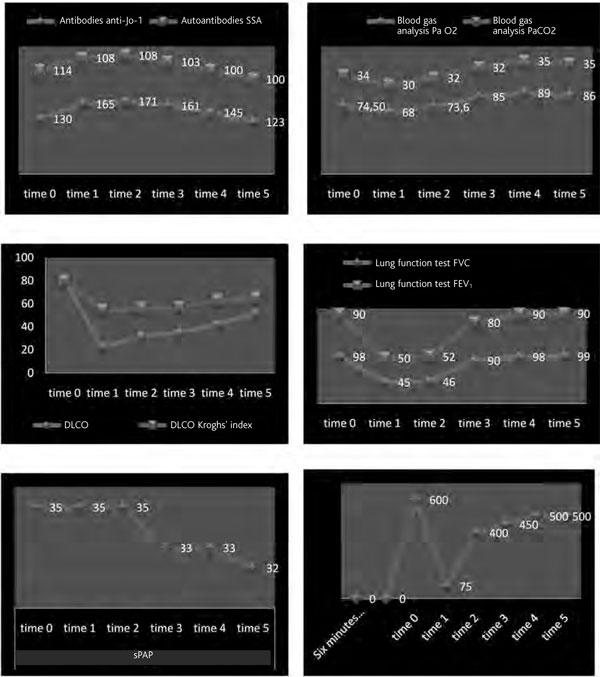
Graphs of parameters evaluated before, during and after treatment with rituximab.
The DLCO test increased its value to 52% pred.; blood gas analysis, performed without oxygen support, showed improvement of blood gases (PaO2 78.5 mm Hg and PaCO2 30 mm Hg) (time 2).
A second treatment course with rituximab (1,000 mg infusion with an interval of 14 days) was administered without side effects six months after the first line therapy (time 3).
Following the first cycle of rituximab therapy, the patient showed clinical improvement with an increase in muscle strength and a decreased need of oxygen. After the second infusion of rituximab, six months later, the patient's condition was markedly improved and he was now able to resume normal daily activities without difficulty (time 4).
The last medical examination was carried out in December 2010, some fourteen months after the first cycle of treatment with rituximab and seven months after the second (time 5). The patient appeared in good condition, asymptomatic for dys pnea, cough, fever and muscle fatigue, as in previous visits. He underwent the anti-Jo-1 test, the value of which was reduced compared to the previous (123); the CO diffusion test (DLCO) also improved, with a rate of 52%, and CT of the chest, initially in the supine position, showed the presence of ground-glass opacity in basal areas of the lung and then in the prone position where ground-glass areas were significantly reduced and the lung was almost completely ventilated (Figure 6). The results of other tests, already normal in the previous controls, were virtually unchanged. Given the continuing good condition of the patient and continuing stability of the improvements achieved, it was not considered necessary to carry out a further administration of rituximab.
Figure 6.
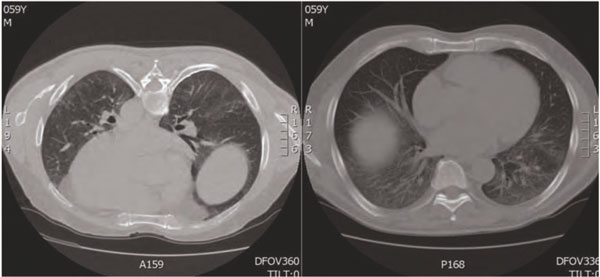
High resolution chest ct scan during last clinical examination, showing stabilization of radiographic changes.
During the follow up time (Table 1, Figure 5), the clinical evaluation of the pulmonary functional test (PFR) with DLCO and 6MWT and HRCT of the thorax showed a remarkable improvement of all functional parameters and radiographic imaging. Nevertheless anti Jo-1 antibodies remained elevated even in the absence of any disease activity.
Table 1.
Serial measurements before, during and after treatment with rituximab
| time 0* | time 1** | time 2*** | time 3**** | time 4***** | time 5****** | |
|---|---|---|---|---|---|---|
| Antibodies | Jo-1 | 130 | 165 | 160 | 161 | 145 |
| SSA | 114 | 110 | 105 | 103 | 100 | 100 |
| PaO2, mmHg | 74 | 68 | 73 | 85 | 89 | 86 |
| PaCO2, mmHg | 34 | 30 | 32 | 32 | 35 | 35 |
| DLCO, % pred. | 80 | 22 | 31 | 35 | 42 | 52 |
| Krogh index | 80 | 54,1 | 67 | 57 | 63 | 65 |
| FVC, % pred. | 98 | 45 | 46 | 90 | 98 | 99 |
| FEV1, % pred. | 90 | 50 | 52 | 80 | 90 | 90 |
| 6MWT, m | 600 | 75 | 150 | 450 | 500 | 500 |
| sPAP, mm Hg | 35 | 35 | 35 | 33 | 33 | 32 |
* Before treatment with rituximab. ** 1st course of rituximab administration. *** Interval between 1st and 2nd course of therapy with rituximab. **** 2nd course of rituximab administration. ***** After 2nd course of therapy with rituximab. ****** Final medical examination.
Definitions of abbreviations: 6MWT, 6-minute walking test; DLCO, diffusing lung capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; sPAP, systolic pulmonary artery pressure.
Figure 5.
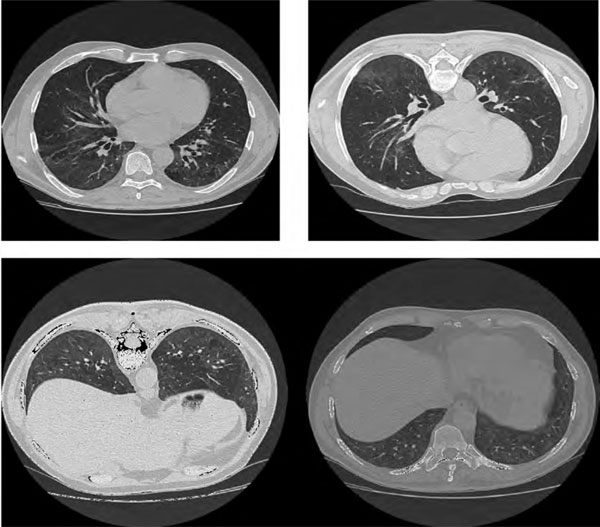
High resolution chest ct scan two months after the second treatment with rituximab, showing residual ground glass attenuation and almost complete resolution of fibrotic changes.
Conclusion
The advent of rituximab has undoubtedly generated considerable hope for the treatment of many diseases with immune pathogenesis. The treatment of interstitial pneumonia associated with anti-synthetase syndrome has not been standardized and, it should be noted, some forms of pulmonary diseases seem to be corticosteroid-resistant. In this case study of anti-synthetase syndrome in which the interstitial lung disease was persistent and acute and corticosteroid resistant, treatment with rituximab proved effective and successful, in line with published reports. These observations emphasize the need for additional studies to assess the optimal regimen of rituximab for the treatment of interstitial pneumonia associated with anti-synthetase syndrome, to determine the initial dose, the dosing interval, the number of cycles and the need for any additional associated therapy.
In fact, as was observed in the case described above, the highly positive results on HRCT (Figure 5) and the improvement in the patient's functional performances after the second infusion, suggest that a third cycle of rituximab repeated after another 6 months, could prove even more effective, even though the administration of a third cycle is not indicated in any research literature.
Unfortunately, studies conducted to date and described in the literature are too few and have too short a follow up to be able to predict the duration of treatment efficacy and the possible need for further doses of drug to standardize the treatment with rituximab. In our case report only two cycles of administration were required to block clinical and radiological manifestations of antibody-antisynthetase syndrome.
Further studies are also needed to identify possible side effects of rituximab and the possible effects of combination or interaction with other drugs. In our study no adverse symptoms were revealed that would have prevented continuing treatment.
Conflict of interest statement
None of the authors has any conflict of interest to declare in relation to the subject matter of this manuscript.
References
- Sem M, Molberg O, Lund MB, Gran JT. Rituximab treatment of the anti-synthetase syndrome: a retrospective case series. Rheumatology. 2009;48:968–971. doi: 10.1093/rheumatology/kep157. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke E, Grutters JC, Altenburg J, Boersma WG, ter Borg EJ, van den Bosch JM. Rituximab in life threatening antisynthetase syndrome. Rheumatol Int. 2009;29:1499–1502. doi: 10.1007/s00296-009-0859-x. [DOI] [PubMed] [Google Scholar]
- Fagedet D, Bernard S, Colombe B, Bosseray A, Baudet A, Bouillet L, Massot C. Acute respiratory distress syndrome as the presenting manifestation of an antisynthetase syndrome. Rev Med Interne. 2009;30:634–636. doi: 10.1016/j.revmed.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Mok CC, Ho LY, To CH. Rituximab for refractory polymyositis: an open-label prospective study. J Rheumatol. 2007;34:1864–1868. [PubMed] [Google Scholar]
- Brulhart L, Waldburger JM, Gabay C. Rituximab in the treatment of antisynthetase syndrome. Ann Rheum Dis. 2006;65:974–975. doi: 10.1136/ard.2005.045898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum. 2005;52:601–607. doi: 10.1002/art.20849. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- Imbert-Masseau A, Hamidou M, Agard C, Grolleau JY, Chérin P. Antisynthetase syndrome. Joint Bone Spine. 2003;70:161–168. doi: 10.1016/S1297-319X(03)00012-5. [DOI] [PubMed] [Google Scholar]


