Abstract
Background
Due to the lack of specific clinical manifestations and imaging features, the diagnosis of pulmonary mycosis is difficult. This study aimed to investigate the pathogens, clinical manifestations, imaging features, diagnosis and management of pulmonary mycosis.
Methods
Data on 68 patients diagnosed as pulmonary mycosis in Xiang Ya hospital from January 2001 to December 2010 were collected and their clinical manifestations, radiographic characterization, diagnostic methods and management were analyzed.
Results
All patients were diagnosed by pathological examination. Of the 68 cases, 38 (55.9%) had pulmonary aspergillosis and 19 (27.9%) pulmonary cryptococcosis. Open-lung surgery was performed in 38 patients (55.9%), transbronchial biopsy in 15 (22.0%), and computerized tomography (CT) guided percutaneous needle biopsy in 11 (16.2%). Main symptoms were as follows: cough in 51 cases (75.0%), expectoration in 38 (55.9%), hemoptysis in 25 (37.8%), fever in 20 (29.4%), while 6 cases (11.1%) were asymptomatic. X-ray and chest CT showed masses or nodular lesions in 52 cases (76.5%), patchy lesions in 10 (14.7%), cavity formation in 15 (22.0%), and diffuse miliary nodules in 1 case. In 51 cases (75.0%) misdiagnosis before pathological examination occurred. Surgical resection was performed in 38 patients (55.9%). In 25 patients (36.7%) systemic antifungal therapy was administered, and 20 patients (29.4%) experienced complete responses or partial responses.
Conclusion
The main pathogens of pulmonary mycosis are Aspergillus, followed by cryptococcosis. Final diagnosis of pulmonary mycosis mainly depends on pathological examination. The clinical manifestations, imaging features, diagnostic methods and management differ depending on the pathogens. Satisfactory therapy can be obtained by both antifungal and surgical treatment.
Keywords: Clinical manifestations, imaging features, pulmonary mycosis.
The incidence of pulmonary mycosis has increased over the past few decades due to the wide use of broad-spectrum antibiotics, immunosuppressive and chemotherapy agents as well as the increased incidence of respiratory diseases, including chronic obstructive pulmonary disease (COPD), lung cancer and tuberculosis [1]. It has been reported that the overall incidence of systemic fungal infection is up to 11.3% and 60% of them involve the bronchi and lungs at autopsy [2]. However, due to the lack of specific clinical manifestations and imaging features, the diagnosis of pulmonary mycosis is difficult. In order to further investigate the pathogenesis, clinical manifestations, prognosis and the risk factors of pulmonary mycosis, we performed a retrospective analysis of patients with pathologically confirmed pulmonary mycosis from a single institution during the period January 2001 to December 2010.
Methods
Patient selection
From January 2001 to December 2010, 68 patients were diagnosed as pulmonary mycosis by pathological examination at the Xiang Ya Hospital (Hunan, China). All cases met the diagnostic criteria involving clinical, microbiological and radiological analyses [3,4]. The study was approved by the Ethics Committee of Xiang Ya Hospital and all patients gave their informed consent.
Treatment efficacy evaluation
The overall response to treatment was defined according to specific criteria [3,4]. 'Complete response' was defined as the disappearance of all symptoms, signs and radiographic or bronchoscopic abnormalities present at the enrollment. 'Partial response' was defined as a major improvement. 'Stable disease' was considered to be minor or no improvement, but without deterioration. 'Progressing disease' was defined as new disease sites or original disease worsened. 'Failure' was defined as a deterioration necessitating alternative antifungal therapy or resulting in death.
Drug safety assessments
Each patient was evaluated for adverse events. Adverse events and laboratory abnormalities were recorded, which included hematology, blood biochemistry, and urine analysis.
Statistical analysis
Data among different groups were analyzed by χ2 test using the SPSS 16.0 statistical package. A p value < 0.05 was considered statistically significant.
Results
Etiology
Among the 68 patients, 38 cases (55.9%) were identified as pulmonary aspergillosis, 19 (27.9%) as pulmonary cryptococcosis, 5 (7.4%) as pulmonary candidiasis, 4 (5.8%) as pulmonary histoplasmosis, 1 (1.5%) as pulmonary sporotrichosis and 1 (1.5%) as actinomyces pneumonia.
Demographics
Of the 68 patients, 41 (63.0%) were males and 27 (37.0%) females (male to female ratio = 1.5:1). Mean age was 45 years (range, 6 to 71). A total of 53 patients (77.9%) had underlying diseases, including 16 cases of tuberculosis (23.5%), 13 of COPD (19.1%), 6 cases of bronchiectasis, 6 of lung cancer (8.2%), 4 cases of inflammatory pseudo tumor, 3 of pulmonary cysts, 2 cases each of lung abscess, gout and diabetes, and 1 case each of severe pneumonia, empyema, bronchopleural fistula, idiopathic thrombocytopenic purpura, systemic lupus erythematosus, drug-induced neutropenia, pemphigus, acute immunodeficiency syndrome (AIDS), cytomegalovirus infection, and asthma. Four cases had used corticosteroids for more than 6 months. Three cases had received lung surgery previously, and 2 cases chemotherapy previously. The incidence of other diseases was 89.5% in pulmonary aspergillosis patients and 52.6% in pulmonary cryptococcosis patients, the difference between them being significant (p < 0.05). Details are given in Table 1.
Table 1.
Demographic Data For 68 Patients With Pulmonary Mycosis
| Pulmonary aspergillosis n = 38 |
Pulmonary cryptococcosis n = 19 |
Pulmonary histoplasmosis n = 4 |
Pulmonary sporotrichosis n = 1 |
Pulmonary actinomycosis n = 1 |
Pulmonary candidiasis n = 5 |
Total | |
|---|---|---|---|---|---|---|---|
| Male/female | 1/1 | 3/1 | 1/4 | -- | -- | 4/1 | 1.5/1 |
| Mean age | 47.7 | 39.2 | 32.7 | 49 | 58 | 51 | 45.0 |
| Underlying diseases | 34 | 10 | 3 | 1 | 0 | 5 | 53 |
Diagnostic methods
Of the 68 patients, 38 (55.9%) were diagnosed by open-lung biopsy; 15 cases (22.1%) were confirmed by transbronchial biopsy; 11 cases (16.2%) were diagnosed by computerized tomography (CT) guided percutaneous needle biopsy. Among the 14 cases of non-surgical pulmonary aspergillosis, 11 patients (78.6%) were confirmed by transbronchial biopsy. Among the 10 cases of non-surgical pulmonary cryptococcosis, 8 patients (80.0%) were confirmed by CT guided percutaneous needle biopsy. Details are given in Table 2.
Table 2.
Diagnostic Methods used in the 68 Patients with Pulmonary Mycosis
| Pulmonary aspergillosis n = 38 |
Pulmonary cryptococcosis n = 19 |
Pulmonary histoplasmosis n = 4 |
Pulmonary sporotrichosis n = 1 |
Pulmonary actinomycosis n = 1 |
Pulmonary candidiasis n = 5 |
Total | |
|---|---|---|---|---|---|---|---|
| Open-lung biopsy | 24 | 9 | 3 | 0 | 0 | 2 | 38 |
| Transbronchial biopsy | 11 | 1 | 0 | 1 | 1 | 1 | 15 |
| CT guided percutaneous needle biopsy | 2 | 8 | 0 | 0 | 0 | 1 | 11 |
Clinical manifestations
The main symptoms of the patients were as follows: cough in 51 cases (75.0%); expectoration in 38 cases (55.9%), with blood in sputum in 18 cases, white phlegm in 12 cases, and purulent sputum in 8 cases; hemoptysis in 25 cases (36.8%); fever in 20 cases (29.4%); chest pain and shortness of breath in 5 cases; headache, nausea and vomiting in 3 cases. Only 6 patients (11.1%) were asymptomatic. Notably, 20 out of the 38 apergillosis patients (52.6%) had hemoptysis while only 3 out of the 19 pulmonary cryptococcosis cases (15.8%) had hemoptysis. The incidence of hemoptysis in pulmonary aspergillosis was significantly higher than in the other groups of pulmonary mycosis (p < 0.05). The main signs included: ipsilateral decreased breath sounds, percussion dullness in 30 cases (44.1%); focal wet rales in 13 cases (19.1%); superficial lymphadenectasis in 8 cases. Details are given in Table 3.
Table 3.
Main Clinical Manifestations of 68 Patients with Pulmonary Mycosis
| Pulmonary aspergillosis n = 38 |
Pulmonary cryptococcosis n = 19 |
Pulmonary histoplasmosis n = 4 |
Pulmonary sporotrichosis n = 1 |
Pulmonary actinomycosis n = 1 |
Pulmonary candidiasis n = 5 |
Total | |
|---|---|---|---|---|---|---|---|
| Cough | 30 | 13 | 2 | 1 | 1 | 4 | 51 |
| Expectoration | 24 | 7 | 1 | 1 | 1 | 4 | 38 |
| Fever | 5 | 8 | 1 | 1 | 1 | 4 | 20 |
| Hemoptysis | 20 | 3 | 0 | 0 | 0 | 2 | 25 |
| Asymptomatic | 1 | 3 | 2 | 0 | 0 | 0 | 6 |
| Percussion dullness | 19 | 5 | 0 | 1 | 1 | 4 | 30 |
| Focal wet rales | 8 | 0 | 1 | 1 | 0 | 3 | 13 |
| Superficial lymphadenectasis | 2 | 3 | 1 | 1 | 0 | 1 | 8 |
Radiographic characteristics
Type: masses or nodule lesions in 52 cases (76.5%), patchy lesions in 10 cases (14.7%), cavity formation in 15 cases (22.0%), and diffuse miliary nodules in 1 case. In the 38 pulmonary aspergillosis cases, 13 patients (34.2%) showed the formation of cavities, while only 2 cases (6.7%) showed cavities in other pulmonary mycosis. Pulmonary aspergillosis patients had a significantly higher incidence of cavity formation than other pulmonary mycosis patients (p < 0.05). Details are given in Table 4.
Table 4.
Chest Imaging Features of 68 Patients with Pulmonary Mycosis
| Pulmonary aspergillosis n = 38 |
Pulmonary cryptococcosis n = 19 |
Pulmonary histoplasmosis n = 4 |
Pulmonary sporotrichosis n = 1 |
Pulmonary actinomycosis n = 1 |
Pulmonary candidiasis n = 5 |
Total | |
|---|---|---|---|---|---|---|---|
| Masses or nodule lesions | 28 | 16 | 3 | 1 | 1 | 3 | 52 |
| Patchy lesions | 6 | 2 | 0 | 0 | 0 | 2 | 10 |
| Cavity formation | 13 | 2 | 0 | 0 | 0 | 0 | 15 |
| Diffuse miliary nodules | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
Site: 9 cases (13.2%) involved bilateral lung; 33 cases (48.5%) involved the right lung, 26 cases (38.2%) the left lung. The upper lobes were mainly involved in pulmonary aspergillosis, while the right lower lobe was mainly involved in pulmonary cryptococcosis.
Specific signs: 'air-crescent sign' was observed in 6 cases of pulmonary aspergillosis (15.8%), whereas 'halo sign' was found in 9 (23.7%) pulmonary as pergillosis patients and 6 (31.5%) pulmonary cryptococcosis patients. The lesions of pulmonary cryptococcal patients were located in the peripheral lung fields close to the pleura. Other signs included: lung hilum or mediastinal lymphadenectasis in 25 cases (36.7%), pleural thickening and adhesion in 9 cases and pleural effusion in 8 cases (Figures 1, 2, 3, 4).
Figure 1.
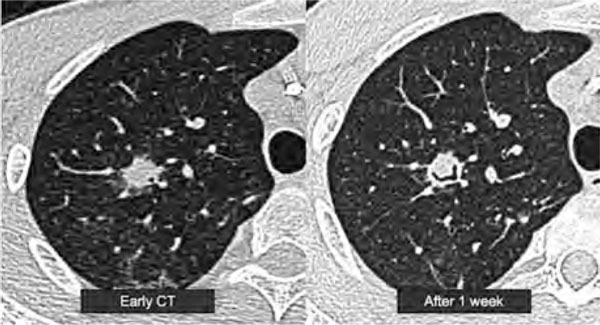
Representative Radiographic Pictures of Pulmonary Aspergillosis. CT examination had earlier shown the 'halo sign'; with the progression of disease, 1 week later, it showed 'air-crescent sign'. Definition of abbreviation: CT, computerized tomography.
Figure 2.
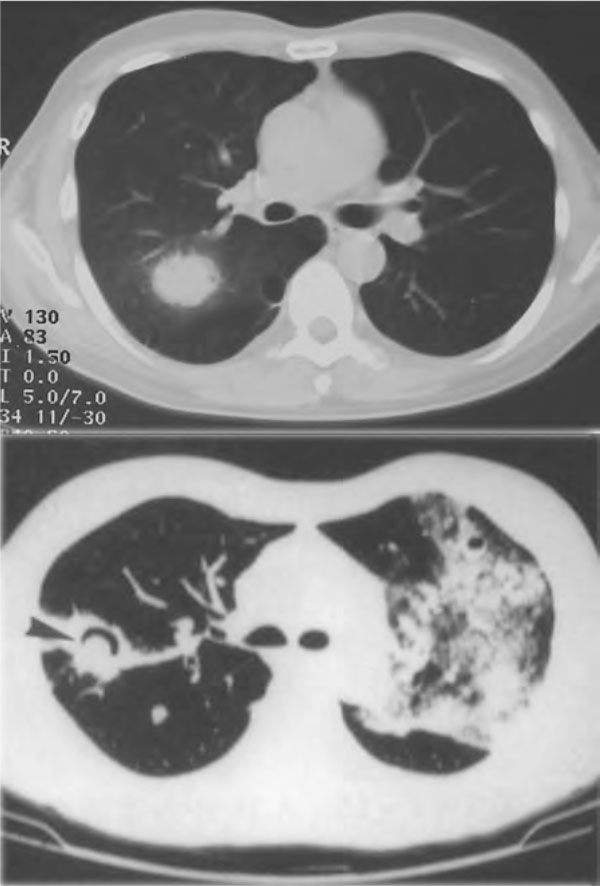
Representative radiographic pictures of pulmonary cryptococcosis. CT examination showed the "nodule lesion" (upper) and the "mass lesion" and the "cavity formation, air-crescent sign" (down).
Figure 3.
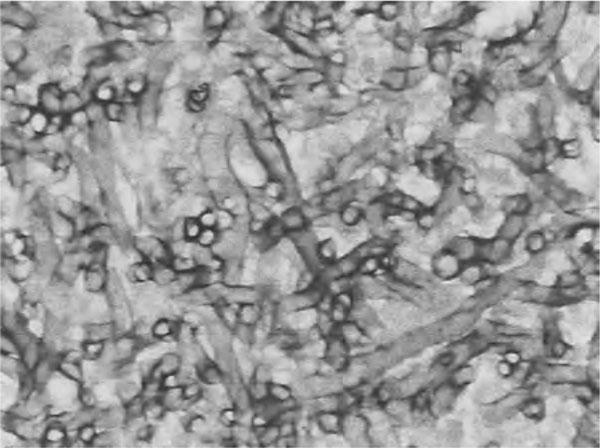
Representative pathological picture of pulmonary aspergillosis. Fungal mycelium was stained with light blue. The hyphae had similar thickness and were with branching, showing an acute angle of about 45°.
Figure 4.
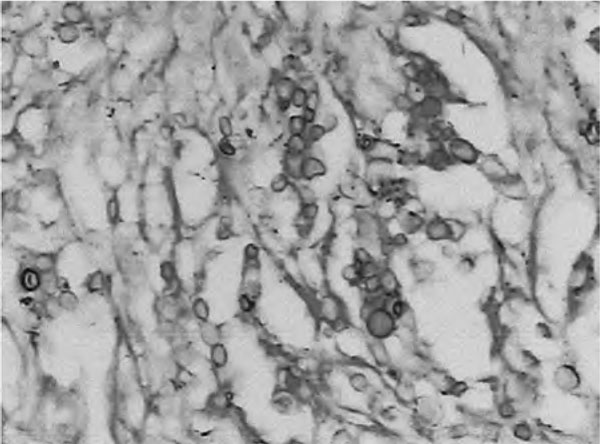
Representative pathological picture of pulmonary cryptococcosis. Cryptococcal granuloma and round spores were found on PAS staining (+).
Diagnosis of pulmonary mycosis by bronchoscopy
Bronchoscopy was performed in 38 cases (55.9%). The results showed endobronchial polypoid, cauliflower-like lumps in 10 cases, purulent inflammation in 3 cases, and bronchial stenosis in 3 cases.
Misdiagnosis
Of the 68 patients, 51 (75.0%) were misdiagnosed before pathological examination. Specifically, 21 cases (30.9%) were misdiagnosed as pulmonary tuberculosis, 19 (27.9%) as lung cancer, and 11 cases (16.2%) as bacterial infection and bronchiectasis.
Treatment and prognosis
Of the 68 patients, 38 cases (55.9%) underwent surgical resection of pulmonary lesions. One case suffered from cryptococcal meningitis after the operation. Twenty-five cases (36.8%) received systemic antifungal therapy, 20 cases (29.4%) experiencing complete or partial responses, and 5 cases showing no response or disease progression. Three cases were lost to follow up, and 2 cases died.
Adverse effects of antifungal drugs
After antifungal drug therapy, adverse effects occurred in 12 cases, among which 10 had received monotherapy and 2 combined treatment. After fluconazole therapy, 2 cases demonstrated an elevated serum aminotransferase level and 1 case developed hypokalemia. After amphotericin B therapy, fever and chills occurred in 3 cases, and phlebitis occurred in 1 case. After itraconazole therapy, 2 cases developed hypokalemia and 1 case demonstrated elevated serum bilirubin level. For the 2 cases of combined treatment with fluconazole and amphotericin B, 1 case showed renal dysfunction while another case demonstrated chills, fever, and elevated serum aminotransferase level.
Discussion
Fungi are widespread in nature and the majority of them are opportunistic pathogens which may be present in healthy people and cause disease when the host immunity is lower or defective. Aspergillus has been reported as the main pathogen accounting for 54.8% of deep fungal diseases, the lung being involved in all Aspergillus infections, while cryptococcosis accounted for 19.4% of fungal infections and mainly involved the central nervous system and lung [5]. Chen et al. reported that the most prevalent forms of pulmonary mycosis were pulmonary aspergillosis (57%), pulmonary cryptococcosis (21%), and pulmonary candidiasis (14%) [6]. Consistent with this study, here we have shown that the most prevalent organisms isolated from pulmonary mycosis were pulmonary aspergillosis (55.9%), pulmonary cryptococcosis (27.9%) and pulmonary candidiasis (7.4%). The incidence of pulmonary candidiasis is also very high [7]. However, these lesions are mainly diffuse and the pathological results were not readily available in our pathologic examination. Only when the diffuse lesions merge into masses or nodule lesions can we obtain the pathological results by surgical operations, transbronchial biopsy or CT guided percutaneous needle biopsy [7,8].
Final diagnosis of pulmonary mycosis is well known to depend on pathological examination. Given that thoracotomy may cause damage to the patients and increase the risk of complications, it is important to develop less invasive methods for pathological examination. The non-operative methods of diagnosis vary depending on the pathogens. Pulmonary aspergillosis is mainly confirmed by transbronchial biopsy while pulmonary cryptococcosis is mainly confirmed by CT guided percutaneous lung puncture. Pulmonary aspergillosis usually involves the bronchi, which makes trans-bronchial biopsy the best mode for the diagnosis. The main manifestation of pulmonary crypto coccosis is subpleural nodules. CT guided percutaneous needle biopsy of peripheral lung lesions is important to confirm the diagnosis of pulmonary cryptococcosis [9].
Depending on the pathogens, the clinical manifestations, imaging features and management of pulmonary mycosis differ. First, there are different predilection sites for different forms of pulmonary mycosis. Pulmonary aspergillosis occurs in the upper lobes similarly as for tuberculosis, while pulmonary cryptococcosis occurs in the right lower lobe with lesions located in peripheral lung fields close to the pleura. Second, cavity formation and hemoptysis are more likely to develop in pulmonary aspergillosis than in other forms of pulmonary mycosis. In our 38 pulmonary aspergillosis cases, 13 patients (34.2%) showed the formation of cavities, while 20 patients (52.6%) showed hemoptysis. Third, in this study 9 (23.7%) pulmonary as pergillosis patients and 6 (31.5%) pulmonary cryptococcosis patients showed the 'halo sign'. It is important to note that 'halo sign' is not specific for pulmonary aspergillosis. In fact, non-infectious diseases such as tumor invasion or organized pneumonia could lead to hemorrhagic pulmonary nodules, therefore also causing the imaging change. Other infectious diseases which cause hemorrhagic pulmonary nodules may also show 'halo sign'. Therefore, we could not exclude other diseases when the 'halo sign' was shown in chest imaging [10].
Surgical resection and antifungal drugs are the main treatment methods for pulmonary mycosis. In our report, 38 patients (55.9%) received surgical resection, and most of them had no recurrence with only 1 patient suffering from cryptococcal meningitis 3 months after the operation. Twenty-five cases (36.8%) received drug treatment and 20 of them experienced a complete or partial response. Satisfactory therapy can be obtained by both antifungal and surgical treatment. Pulmonary aspergillosis patients in the following situations should be considered for surgical resection: focal lesions associated with hemoptysis; failure of antifungal treatment; life-threatening hemoptysis or lesions close to large vessels. It has been proposed that antifungal therapy is not required after surgical resection [11,12]. In this study, 24 patients with pulmonary aspergillosis received only surgical resection and none of them showed complications or recurrence. Fourteen patients received antifungal therapy and 10 of them used itraconazole. Nine patients experienced complete or partial responses. It has been confirmed that sequential therapy with itraconazole is well tolerated, safe and effective in invasive pulmonary aspergillosis [13]. According to the guidelines of the Infectious Diseases Society of America [9], pulmonary cryptococcosis patients with mild or moderate clinical symptoms should be treated with fluconazole, while patients with severe clinical symptoms should be treated with amphotericin B. If antifungal drugs fail or the lesions cannot be distinguished from the tumor, surgical resection needs to be considered. In view of the recurrence of cryptococcal meningitis in some patients, systemic antifungal therapy is recommended after surgical resection [14].
Our results confirm the importance of systemic antifungal therapy following surgery.
Conflict of Interest Statement
None of the authors has any conflict of interest to declare in relation to the subject matter of this manuscript.
References
- Hsu LY, Ng ES, Kop LP. Common and emerging fungal pulmonary infections. Infect Dis Clin North Am. 2010;24:557–577. doi: 10.1016/j.idc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Chen WB. Etiological analysis of deep fungal infection. Chinese Journal of Practical Internal Medicine. 2002;22:5–6. [Google Scholar]
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Medical Association, Chinese Society of Respiratory Disease, Group of Infectious Diseases. Editorial Committee of Chinese Journal of Tuberculosis and Respiratory Diseases. Diagnosis and treatment of pulmonary mycosis. Chinese Journal of Tuberculosis and Respiratory Diseases. 2007;30:821–834. [Google Scholar]
- Hao F, Yan H, Ye Q. Clinical analysis of 31 cases with deep fungal infection. Chinese Journal of Dermatology. 2003;36:441–443. [Google Scholar]
- Chen KY, Ko SC, Hsueh PR, Luh KT, Yang PC. Pulmonary fungal infection: emphasis on microbiological spectra, patient outcome, and prognostic factors. Chest. 2001;120:177–184. doi: 10.1378/chest.120.1.177. [DOI] [PubMed] [Google Scholar]
- Liu YN, She DY, Sun TY, Tong ZH, He B, Xiao Y, He LX, Qu JM, Liu XQ, Li ER, Chen P, Ma ZS, Shi Y, Feng YL, Jiang SJ, Xiong SD, Hu CP. A multicentre retrospective study of pulmonarypulmonary mycosis clinically proven from 1998 to 2007. Zhonghua Jie He He Hu Xi Za Zhi. 2011;34:86–90. [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, Powderly WG, Sobel JD, Dismukes WE. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710–718. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- Franquet T, Müller NL, Oikonomou A, Flint JD. Aspergillus infection of the airways: computed tomography and pathologic findings. J Comput Assist Tomogr. 2004;28:10–16. doi: 10.1097/00004728-200401000-00002. [DOI] [PubMed] [Google Scholar]
- Zhu XM, Zhou X. Diagnosis and treatment of invasive pulmonary aspergillosis. Chinese Journal of Respiratory and Critical Care Medicine. 2005;4:316–320. [Google Scholar]
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- Caillot D, Bassaris H, McGeer A, Arthur C, Prentice HG, Seifert W, De Beule K. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin Infect Dis. 2001;33:e83–e90. doi: 10.1086/323020. [DOI] [PubMed] [Google Scholar]
- Fan YH, Chen YW. Analysis of domestic pulmonary aspergilloma 230 cases. Journal of Gannan Medical College. 2000;20:81–84. [Google Scholar]


