Abstract
Background and aims
Recently a multidimensional grading system based on the body mass index (B), degree of airflow obstruction (O), dyspnea (D) and exercise capacity (E) - the BODE index - has begun to be used increasingly for the evaluation of chronic obstructive pulmonary disease (COPD) patients. The aim of our study was to investigate the relationship between the BODE index and disease duration, annual exacerbation and hospitalization rates, health related quality of life and systemic inflammatory markers like C-reactive protein (CRP), tumor necrosis factor (TNF)-α and interleukin (IL)-8.
Materials and methods
In 88 stable COPD patients we evaluated the body-mass index, pulmonary function tests, Modified Medical Research Council dyspnea scale and six-minute walk test (6 MWT). BODE scores were determined. Disease duration, number of exacerbations and hospitalization in the previous year were recorded. We also performed arterial blood gases analysis, administered the St. George's Respiratory Questionnaire (SGRQ) and measured serum levels of CRP, TNF-α, IL-8.
Results
According to BODE score 52% of patients were BODE 1, 21% BODE 2, 15% BODE 3 and 12% were BODE 4. There was a significant relationship between BODE index and COPD stage as classified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (p < 0.001). Correlations between BODE score and disease duration (p = 0.011), number of exacerbations (p < 0.001) and hospitalizations (p < 0.001) in the last year were also observed. SGRQ symptom, activity, emotion scores and total scores were found to be significantly correlated to BODE (p < 0.001). Serum CRP levels and BODE were also correlated (p = 0.014); however, no correlation was found between serum levels of TNF-α and IL-8 and BODE.
Conclusions
As the BODE index shows a strong correlation with various prognostic and follow up parameters of COPD and systemic inflammation, its use should be considered for the evaluation of COPD patients.
Keywords: Biomarkers, BODE index, COPD, quality of life
Introduction
Currently, chronic obstructive pulmonary disease (COPD) is regarded as a systemic disease causing structural and functional changes in many organs as well as in the lung. Malnutrition, weight loss, and peripheral muscle weakness are some of the systemic manifestations of COPD that seriously affect the health related quality of life and exercise capacity of patients [1,2]. Advances in understanding the systemic nature of COPD have given rise to the development of a combined index of multiple mortality predictors for this disease known as the "BODE index". The components of the index are: body mass index (BMI), airway obstruction (O), dyspnea (D) and exercise capacity (E). The BODE index includes both symptoms and physiological measurements and it has been reported as a better mortality predictor than forced expiratory volume in 1 second (FEV1) [3]. It predicts mortality from any cause as well as respiratory causes and gives more comprehensive information than the FEV1-based staging system described in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [3-5].
In view of the systemic nature of COPD, other tools such as quality of life questionnaires (QoLQ) have been developed to establish the systemic impacts of the disease. It has been recommended to administer QoLQs to determine disease severity and treatment responses in collaboration with physiological measurements [6]. St. George's Respiratory Diseases Questionnaire (SGRQ) was designed specifically for COPD patients and demonstrates the impact of the disease on daily life [6,7].
Since systemic inflammation has been recognized as an indisputable component of COPD, the role of inflammatory cytokines has also been widely investigated in the natural history of COPD [8]. It has been shown that C-reactive protein (CRP) levels are elevated in the serum of COPD patients even in stable disease [9]. In a follow up study of 8 years, basal CRP levels significantly predicted overall mortality, cardiovascular disease-related mortality and cancer-related mortality in patients with mild and moderate COPD. FEV1 loss was shown to be correlated to CRP levels [1] and CRP was found to be a specific marker of COPD exacerbations [10]. Increased levels of tumor necrosis factor (TNF)-α have been found in serum, induced sputum and bronchial biopsies of COPD patients, especially those in whom COPD was associated with weight loss [11-13]. Also, interleukin (IL)-8, a strong selective neutrophil chemo-attractant, showed increased levels in serum of COPD patients with respect to healthy controls [8].
Currently, clinical outcomes and biomarkers are two parameters being used to determine the pulmonary function and systemic effects of COPD. We think that evaluating COPD, a multicomponent disease, functionally and systemically will improve understanding of this disease. In our study we hypothesized that a composite index of clinical outcomes, the BODE index, was a better predictor of health status and systemic inflammation in COPD than FEV1 alone and aimed to investigate the relationship of the components of BODE and the BODE index itself with systemic inflammatory biomarkers and quality of life as well as with prognostic factors like disease duration and annual exacerbation and hospitalization rates.
Materials and methods
Eighty-eight stable COPD patients diagnosed according to GOLD guidelines in our pulmonary diseases outpatient clinic were included in the study consecutively between November 2006 and May 2007. The study was approved by the human-research Ethical Review board and all patients provided written informed consent. Inclusion criteria were: COPD patients in stable conditions (no exacerbations due to any reason in the last 6 weeks). COPD was defined as a history of smoking of more than 20 pack-years and a FEV1/forced vital capacity (FVC) ratio of less than 70% after 20 minutes after salbutamol administration [14]. Exclusion criteria were: patients with other inflammatory diseases (inflammatory bowel disease, rheumatologic diseases, vasculitis), interstitial lung diseases, active pulmonary tuberculosis, presence of atopy, history of myocardial infarction in the last 6 months, decompensated cardiovascular disease and walking disability.
Demographic features and medical history of the patients were recorded. Emergency service visits due to acute exacerbations and hospitalization to general ward or intensive care unit in the last year were investigated. Weight, height, dyspnea severity were measured and the six-minute walking test (6 MWT), pulmonary function tests (PFT) were performed. SGRQ, arterial blood gases (ABG) analysis and measurement of serum levels of inflammatory cytokines (CRP, TNF-α, IL-8) were also performed.
Pulmonary Function Tests: PFT were performed with the Jaeger Master Screen Pneumo device. The best test from three consecutive tests was accepted. FEV1, FVC, FEV1/FVC were measured according to ATS criteria. COPD staging was done according to GOLD 2006 [14].
Body Mass Index: BMI was calculated according to the formula weight (kg)/height (m)2 [15].
Dyspnea Severity: The Modified Medical Research Council (MMRC) scale was used for the evaluation of dyspnea [16].
Six-minute walking test: 6 MWT was performed in a 35 m long corridor. Patients were motivated to walk at the fastest speed they could. Oxygen saturation was measured before and after the test and the distance walked was recorded [17].
BODE index: The overall index was calculated according to BMI, FEV1, 6 MWT, MMRC by summing the points as shown in Table 1. Further subgroupings like BODE 0, 1, 2, 3 were made, as defined in the formula [3].
Table 1.
Scoring for the components of the bode index: body-mass index, degree of airflow obstruction, dyspnea, and exercise capacity
| Points on BODE Index | ||||
|---|---|---|---|---|
| Parameter | 0 | 1 | 2 | 3 |
| Body mass index (kg/m2) | > 21 | ≤ 21 | - | - |
| FEV1 (% predicted) | ≥ 65 | 50-64 | 36-49 | ≤ 35 |
| 6 minute walking distance (m) | ≥ 350 | 250-349 | 150-249 | ≤ 149 |
| MMRC dyspnea scale (score) | 0-1 | 2 | 3 | 4 |
Definition of abbreviation: FEV1, forced expiratory volume in 1st second; MMRC, Modified Medical Research Council.
BODE groups are classified as: 1-2 points: BODE 0; 2-4 points: BODE 1; 4-7 points: BODE 2; 7-10 points: BODE 3.
Quality of Life Questionnaire: The Turkish translation of the St. George's Respiratory Diseases Questionnaire (SGRQ) was used to determine the quality of life [18].
Arterial Blood Gases Analysis: ABG samples were obtained from the radial artery with heparinized injectors and studied with a Roche Diagnostics GmbH OMNI C, Mannheim (Germany) device with original reactive analyzers.
Measurement of CRP, TNF-α, IL-8 levels: Venous blood samples were centrifuged and serums were separated and preserved at -20°C to be analyzed together. Serum CRP levels were studied with original reactive analyzers (Beckman Coulter Inc. Unicel DxC 800 Synchron Clinical Systems Galway, Ireland). Serum TNF-α and IL-8 levels were studied according to the manufacturer's recommendations (BioSource Europe S.A Nivelles, Belgium) with the Enzyme Linked-Immuno-Sorbent Assay (ELISA) method.
Intra-assay variation coefficient (%CV) values for IL-8 kit were 3.9% for 74.9 pg/ml, 2.6% for 186.2 pg/ml, 5.3% for 991.8 pg/ml while the inter-assay variation coefficients were 5% for 89.8 pg/ml, 5.5% for 223.1 pg/ml, 7.8% for 981.2 pg/ml. Lowest measurement level for IL-8 kit was < 5.0 pg/ml. Intra-assay variation coefficient values for TNF-α kit were 5.2% for 8 pg/ml, 4.1% for 167 pg/ml, 3.9% for 459 pg/ml, while the inter-assay variation coefficients were 8.5% for 47 pg/ml, 8.2% for 170 pg/ml, 5.9% for 438 pg/ml. Lowest measurement level for TNF-α kit was 1.7 pg/ml. IL-8 and TNF-α levels were measured in only 65 of the patients, and CRP levels in 86 patients according to a table of random numbers, due to an inadequate number of kits.
Statistical analysis
Data were analyzed by the SPSS 15.0 package programme. Spearman's rank correlations and Pearson correlations were used to analyze comparisons.
Results
Demographic characteristics of the 88 patients are shown in Table 2. Duration of the disease ranged widely, from 6 months to 40 years, in the study population. Sixteen of the patients (18%) were non smokers but had a history of passive exposure while 72 (82%) were current smokers. Most patients (53%) had comorbid diseases like hypertension, congestive heart failure and diabetes mellitus. Fifty-one (58%) patients had had no exacerbation and 65 (74%) had not been hospitalized in the previous 12 months. Functional parameters and serum cytokine levels of the study population are shown in Table 3. Of the 88 patients, 16% were stage I, 42% were stage II, 27% were stage III, and 15% were stage IV according to GOLD guidelines. When patients were classified with respect to BODE score, 52% were BODE 1, 21% were BODE 2, 15% were BODE 3 and 12% were BODE 4. BODE scores and disease stages of the patients according to GOLD were significantly correlated, as expected (p < 0.001) (Figure 1).
Table 2.
Demographic characteristics of study population (N = 88)
| Age (mean ± SD) | 63.6 ± 10.5 |
|---|---|
| Sex (female/male) | 12/76 |
| Disease duration, median (25th to 75th percentile) | 5 (2-10) years |
| Smoking history, median (25th to 75th percentile) | 40 (20-60) pack/year |
| Number of exacerbations, median (25th to 75th percentile) | 0 (0-1)/last year |
| Number of hospitalizations, median (25th to 75th percentile) | 0 (0-1)/last year |
| Comorbid diseases (present/absent) | 47/41 |
| Oxygen therapy (present/absent) | 8/80 |
| Maintenance therapy with inhaled steroid (present/absent) | 49/39 |
Table 3.
Summary of functional parameters and serum cytokine levels for the study population
| Number (n) | Mean ± SD | Minimum | Maximum | |
|---|---|---|---|---|
| BMI (kg/m2) | 88 | 25.7 ± 4.9 | 16.5 | 37.9 |
| FEV1 (L) | 88 | 1.51 ± 0.6 | 0.3 | 2.8 |
| FEV1% predicted | 88 | 53.3 ± 17.4 | 21 | 103 |
| FEV1/FVC | 88 | 55.9 ± 10.2 | 28 | 70 |
| MMRC | 88 | 1.9 ± 1.1 | 0 | 4 |
| 6 MWT (m) | 88 | 375.2 ± 137.0 | 65 | 640 |
| BODE index | 88 | 3.1 ± 2.6 | 0 | 10 |
| Number (n) | Median ± IQR (25th - 75th) | Minimum | Maximum | |
| SGRQ total score | 88 | 43 (31-62) | 7.4 | 95.8 |
| CRP (pg/ml) | 88 | 0.6 (0.25-1.20) | 0.05 | 14.4 |
| TNF-α (pg/ml) | 65 | 19 (15-21) | 12.4 | 57.7 |
| IL-8 (pg/ml) | 65 | 14 (8-23) | 2.5 | 391.1 |
Definition of abbreviations: BMI, body mass index; CRP, C-reactive protein; FEV1 (L), forced expiratory volume in 1 second, liter; FVC, forced vital capacity; MMRC, Modified Medical Research Council; 6 MWT, 6-minute walking test; SGRQ, St. George's Respiratory Disease Questionnaire; TNF-α, tumor necrosis factor-α; IL-8, interleukin 8.
Figure 1.
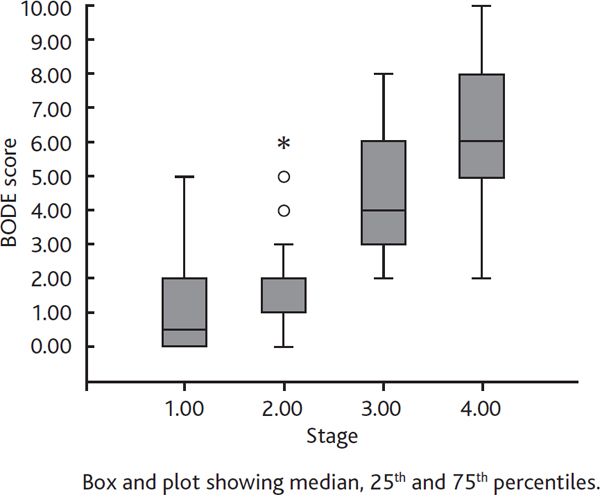
Relationship between bode index and copd stage according to gold. Definition of abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Each single BODE component and the BODE index itself were compared with disease duration, number of exacerbations and number of hospitalizations in the last year, separately. BMI was not found to be correlated with any of these parameters. A significant relationship was found between MMRC and disease duration (p = 0.003), number of exacerbations (p = 0.008) and number of hospitalizations (p = 0.001). 6 MWT was found to be correlated with disease duration (p = 0.041) and number of hospitalizations (p = 0.002). As for FEV1, a significant correlation was observed for disease duration (p = 0.001) and number of exacerbations (p < 0.001). The BODE index itself was found to be correlated with all these parameters, showing the strongest correlation for number of exacerbations and number of hospitalizations in the last year (p < 0.001 for both). Among the single components of the BODE index, BMI was the one that showed the least correlation (p = 0.020) with BODE (p < 0.001).
Components of BODE index including BMI, MMRC dyspnea scale, 6 MWT and pulmonary function parameters (FEV1, FVC and FEV1/FVC) were also compared. There was a significant relationship between BMI and FEV1/FVC (p = 0.011). MMRC and 6 MWT were also significantly correlated with FEV1 (p < 0.001 for both), FVC (p < 0.001 for both) and FEV1/FVC (p = 0.016, p = 0.006 respectively).
All components of BODE index and the BODE index itself were found to be significantly correlated with arterial PO2 (p < 0,05). The correlation was determined negative for BODE and PaO2, while it was positive for BODE and PaCO2 (p < 0.001).
A significant correlation was observed between MMRC, 6 MWT, FEV1 and SGRQ total score (p < 0.001 respectively). Also, BODE index and SGRQ symptom, emotion, activity and total scores were found to be significantly correlated (p < 0.001, respectively) (Figure 2).
Figure 2.
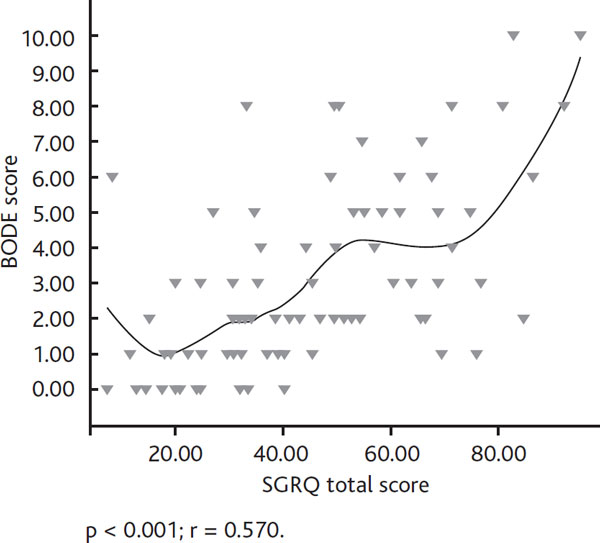
Relationship between bode index and sgrq total scores. Definition of abbreviation: SGRQ, St. George's Respiratory Diseases Questionnaire.
When inflammatory markers were compared with BODE index, CRP levels were shown to have a weak but statistically significant correlation (r = 0.2, p = 0.014) (Figure 3) while TNF-α and IL-8 did not show a correlation. CRP was not found to be correlated with FEV1, FVC and FEV1/FVC. However, a significant relationship was found between CRP and SGRQ total score (p = 0.015).
Figure 3.
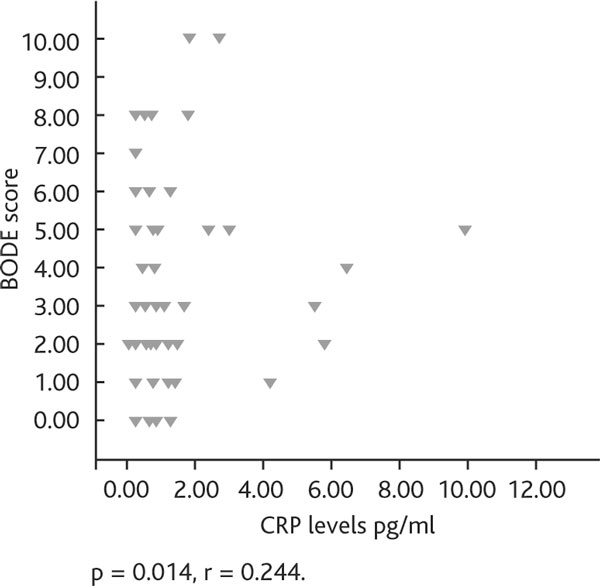
Relationship between bode index and serum crp (pg/ml) levels. Definition of abbreviation: CRP, C-reactive protein.
When the patients were divided into two groups according to the presence of comorbid diseases, there was no statistically significant difference with respect to dyspnea severity, 6 MWT and CRP levels between the two groups.
Discussion
The most recently discussed topic in COPD in the last few years is its inflammatory and systemic nature. Several clinical tests and biomarkers have been developed for the evaluation of systemic effects of the disease [1]. A weak correlation has been defined between pulmonary function tests, especially FEV1 and clinical outcomes including the severity of dyspnea and other symptoms, mortality, health status, quality of life and frequency of exacerbations [18,19]. The BODE index has been suggested as a new follow up tool for the evaluation of COPD patients [3]. CRP is another systemic biomarker that has been widely used for inflammatory diseases like COPD [8].
In our study, among the 88 COPD patients we found a correlation between BODE index and COPD stages according to GOLD; this was surely due to the impact of FEV1 in both GOLD staging and BODE index.
Ong et al. found the BODE index and number of emergency visits related in their study of 16 months follow up, and also showed a significant but lower grade relationship between number of emergency visits and FEV1 [20]. In another study, the BODE index was shown to be more significant for determining the severity of COPD exacerbations with respect to FEV1 [21]. In our study, we similarly found the BODE index to be related to annual rate of hospitalizations (p < 0.001), but this relationship was not observed for FEV1. However, both BODE and FEV1 had the same significant relationship for number of exacerbations (p < 0.001 for both). In the light of these results, we consider that FEV1 is an important marker in determining exacerbations within the other components of BODE, while for hospitalizations other components of BODE than FEV1 are more important.
Weight loss is one of the common systemic effects of COPD. In a retrospective study of 400 patients Schols et al. reported increased mortality in severe COPD patients with chronic hypoxemia and a BMI < 25 kg/m2 [22]. Another study showed a significant correlation between fat free mass index (FFMI) and MRC, FEV1, FEV1/FVC. BMI and FFMI were found to be related to 6 MWT, but there was no correlation between BMI and disease severity. It was suggested that FFMI showed a better correlation with disease severity than BMI [23]. In our study, mean BMI of the patients was 25.7 kg/m2 and a significant positive correlation was found only with FEV1/FVC. No correlation was determined between BMI and disease severity according to GOLD. Also, the relationship between BMI and BODE index was the least with respect to the other components. This result leads us to consider that BMI has little contribution in determining disease severity
Dyspnea, one of the major symptoms of COPD, is a subjective symptom with perception differences depending on age or individual characteristics. Many dyspnea measurement scales have been developed and MMRC is one of the most widely accepted. Pulmonary function and dyspnea severity have been reported to be correlated in some studies; however in other studies dyspnea has been found uncorrelated with obstruction degree [24-27]. In our study, MMRC and FEV1, FVC, FEV1/FVC were found to be related. In addition, a difference of our study with respect to other studies was the relationship between MMRC and disease duration, annual exacerbation and hospitalization rates and disease severity as well as arterial blood gas parameters (PaO2, PaCO2).
6 MWT utilization has recently been increased in the evaluation of functional status and exercise performance of COPT patients. In our study, similarly to Marin's findings [28], 6 MWT was found correlated with MMRC, COPD stage according to GOLD and PFT parameters. There was also a significant relationship between 6 MWT and age, disease duration, and annual hospitalization rates. A distinctive finding of our study was the correlation between 6 MWT and arterial blood gas parameters. In addition, 6 MWT and SGRQ symptom, emotion, activity and total scores were found to be significantly related. In view of these data, 6 MWT is suggested as a first step test for demonstrating the unfavorable effects of COPD on quality of life and reflecting daily activities of the patients.
Recent studies have reported that functional parameters like FEV1 are insufficient to determine the health status in COPD, and quality of life measurements have gradually become more important for COPD [29]. In a study of COPD patients in which the relationship between BODE index and SGRQ was investigated, the BODE index was found to increase as SGRQ scores increased. There was a moderate and high relationship between BODE index and SGRQ; however COPD stage according to GOLD had a mild correlation with SGRQ [30]. In another study, a relationship between SGRQ total score and BODE severity scores was demonstrated; however this relationship was not observed between SGRQ total score and disease severity by GOLD stages [31]. In our study, we also found significant correlations between symptom, emotion, activity and total scores of SGRQ and BODE, and a weak correlation between all the SGRQ scores and COPD stage. These results suggest that the BODE index reflects the effects of disease on quality of life better than the GOLD stages do.
Several serum biomarkers have been defined in COPD. Among them, CRP, fibrinogen, interleukins (IL-6, IL-8), TNF-α and leucocytes are the ones most studied. Even in stable conditions, all these biomarkers have been shown to be elevated in COPD patients [8]. In our study, CRP, IL-8 and TNF-α levels were investigated. In a study by Wu et al., sputum/serum CRP levels and pulmonary function tests of 30 COPD patients were compared and an inverse relationship was observed between sputum/serum CRP levels and FEV1, and FEV1/FVC levels [32]. Broekhuizen et al. found lower post bronchodilator FEV1 levels in patients with high CRP levels in a group of 102 stable stage II and IV COPD patients. SGRQ scores were high and exercise capacity evaluated by 6 MWT was low in this group of patients also [33]. Garrod et al. demonstrated that COPD patients with high CRP levels had lower quality of life and exercise capacity and a greater decline in lung functions [34]. In our study, unlike other studies in similar case series, CRP and pulmonary function tests were not found to be correlated. However, SGRQ and CRP were found to be inversely correlated, while there was a positive relationship between BODE index, COPD stage and CRP.
Nearly half of the patients in our study population had comorbid diseases like hypertension, congestive heart failure and diabetes mellitus related to systemic inflammation and this might have interfered with CRP levels, although these comorbidities had to be under control as an inclusion criterion. In an observational cohort study, in which moderate to severe COPD patients and two control groups without COPD (smokers and non-smokers) were tested using a high sensitivity CRP (hs-CRP) test, CRP levels were found to be elevated in patients with COPD independent of clinically significant ischemic heart disease and cigarette smoking [35]. Emerging laboratory and epidemiologic data have demonstrated that hs-CRP levels are associated with impaired insulin sensitivity and the development of type 2 diabetes [36]. Hence, diabetes mellitus might not be the cause of the high CRP levels in our study. However, we analyzed the CRP levels according to the presence or not of comorbidities and no statistically significant difference was observed between the two groups.
Many studies have shown a relationship between high TNF-α levels and weight loss in COPD [37,38]. In our study, we did not find a correlation between TNF-α levels and body mass index nor with the BODE index. IL-8 is another important biomarker in COPD and some studies have shown high levels of IL-8 in COPD patients [39], but also here we were not able to show any correlation between BODE index and IL-8.
In conclusion, in this study all components of BODE and the BODE index itself were compared with most of the clinical outcomes and biomarkers used in the evaluation of COPD. The most powerful correlation was observed between 6 MWT, MMRC dyspnea scale and FEV1. The BODE index is a comprehensive, feasible and simple clinical scoring system in the evaluation of COPD. It also reflects the quality of life and is correlated with CRP, one of the biological markers of systemic inflammation, while there is not a similar relationship with TNF-α and IL-8. Therefore, BODE is a clinical test which evaluates the pulmonary and extrapulmonary effects of the disease all together. In this study, we showed the feasibility of BODE and the efficacy of CRP. In the future, we suggest that BODE could replace FEV1 and could become the standard for the classification and clinical evaluation of COPD.
Conflict of interests statement
None of the authors has any conflict of interest to declare in relation to the subject matter of this manuscript.
References
- Agusti AG. COPD, a multicomponent disease: implications for management. Respir Med. 2005;99:670–682. doi: 10.1016/j.rmed.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Decramer M, De Benedetto F, Del Ponte A, Marinari S. Systemic effects of COPD. Respir Med. 2005;99(Suppl B):S3–10. doi: 10.1016/j.rmed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- Global Strategy of Diagnosis, Management and Prevention of COPD. 2006. http://www.goldcopd.com Date accessed: December 2006.
- Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56:880–887. doi: 10.1136/thorax.56.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Calverley PM, Sherwood Burge P, Jones PW. ISOLDE Study Group. Inhaled Steroids in Obstructive Lung Disease. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:122–128. doi: 10.1164/ajrccm.163.1.2005009. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres JP, Cordoba-Lanus E, López-Aguilar C, Muros de Fuentes M, Montejo de Garcini A, Aguirre-Jaime A, Celli BR, Casanova C. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J. 2006;27:902–907. doi: 10.1183/09031936.06.00109605. [DOI] [PubMed] [Google Scholar]
- Hurst JR, Donaldson GC, Perera WR, Wilkinson TM, Bilello JA, Hagan GW, Vessey RS, Wedzicha JA. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- de Godoy I, Donahoe M, Calhoun WJ, Mancino J, Rogers RM. Elevated TNF-alpha production by peripheral blood monocytes of weight-losing COPD patients. Am J Respir Crit Care Med. 1996;153:633–637. doi: 10.1164/ajrccm.153.2.8564110. [DOI] [PubMed] [Google Scholar]
- Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1453–1455. doi: 10.1164/ajrccm.150.5.7952575. [DOI] [PubMed] [Google Scholar]
- Mueller R, Chanez P, Campbell AM, Bousquet J, Heusser C, Bullock GR. Different cytokine patterns in bronchial biopsies in asthma and chronic bronchitis. Respir Med. 1996;90:79–85. doi: 10.1016/S0954-6111(96)90202-4. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A selfcomplete measure of health status for chronic airflow limitation. The St George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- Ong KC, Earnest A, Lu SJ. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest. 2005;128:3810–3816. doi: 10.1378/chest.128.6.3810. [DOI] [PubMed] [Google Scholar]
- Marin JM, Sanchez A, Alonso JE. et al. A multivariate grading system (BODE) as predictor of the severity of exacerbation in COPD (abstract) Am J Respir Crit Care Med. 2003;167:A23. [Google Scholar]
- Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest. 2007;132:164–169. doi: 10.1378/chest.06-2789. [DOI] [PubMed] [Google Scholar]
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs-Climent D, Le Gallais D, Varray A, Desplan J, Cadopi M, Préfaut CG. Factor analysis of quality of life, dyspnea, and physiologic variables in patients with chronic obstructive pulmonary disease before and after rehabilitation. Am J Phys Med Rehabil. 2001;80:113–120. doi: 10.1097/00002060-200102000-00007. [DOI] [PubMed] [Google Scholar]
- Sahebjami H, Sathianpitayakul E. Influence of body weight on the severity of dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:886–890. doi: 10.1164/ajrccm.161.3.9905023. [DOI] [PubMed] [Google Scholar]
- Paula S, Correia D, Morgado R, Fernandes D, Serrador A, Marques A, Valença J, Almeida A. Correlation between dyspnea and lung function evaluated by spirometry and body plethysmography in COPD patients. Rev Port Pneumol. 2005;11(Suppl 1):22–23. [Google Scholar]
- Marin JM, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1395–1399. doi: 10.1164/ajrccm.163.6.2003172. [DOI] [PubMed] [Google Scholar]
- Mahler DA. How should health-related quality of life be assessed in patients with COPD? Chest. 2000;117(2 Suppl):54S–57S. doi: 10.1378/chest.117.2_suppl.54s. [DOI] [PubMed] [Google Scholar]
- Ong KC, Lu SJ, Soh CS. Does the multidimensional grading system (BODE) correspond to differences in health status of patients with COPD? Int J Chron Obstruct Pulmon Dis. 2006;1:91–96. doi: 10.2147/copd.2006.1.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medinas Amorós M, Mas-Tous C, Renom-Sotorra F, Rubí-Ponseti M, Centeno-Flores M, Gorriz-Dolz M. Health-related quality of life is associated with COPD severity: a comparison between the GOLD staging and the BODE index. Chron Respir Dis. 2009;6:75–80. doi: 10.1177/1479972308101551. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Chen P, Jiang XN, Liu ZJ. C-reactive protein level and the correlation between lung function and CRP levels in patients with chronic obstructive pulmonary disease. Zong Nan Da Xue Xue Bao Yi Xue Ban. 2005;30:444–446. [PubMed] [Google Scholar]
- Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod R, Marshall J, Barley E, Fredericks S, Hagan G. The relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD) Prim Care Respir J. 2007;16:236–240. doi: 10.3132/pcrj.2007.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters EF, Creutzberg EC, Schols AM. Systemic effects in COPD. Chest. 2002;121(5 Suppl):127S–130S. doi: 10.1378/chest.121.5_suppl.127s. [DOI] [PubMed] [Google Scholar]
- Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, Celli BR. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61:23–28. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndumele CE, Pradhan AD, Ridker PM. Interrelationships between inflammation, C-reactive protein, and insulin resistance. J Cardiometab Synd. 2006;1:190–196. doi: 10.1111/j.1559-4564.2006.05538.x. [DOI] [PubMed] [Google Scholar]
- Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1453–1455. doi: 10.1164/ajrccm.150.5.7952575. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Kurihara N, Otsuka T, Fujii T, Tanaka S, Kudoh S, Hirata K, Takeda T. Clinical significance of serum concentration of interleukin 8 in patients with bronchial asthma or chronic pulmonary emphysema. Respiration. 1996;63:236–240. doi: 10.1159/000196552. [DOI] [PubMed] [Google Scholar]


