Abstract
We studied the structural determinants of binding affinity and efficacy of adenosine receptor (AR) agonists. Substituents at the 2-position of adenosine were combined with N6-substitutions known to enhance human A3AR affinity. Selectivity of binding of the analogues and their functional effects on cAMP production were studied using recombinant human A1, A2A, A2B, and A3ARs. Mainly sterically small substituents at the 2-position modulated both the affinity and intrinsic efficacy at all subtypes. The 2-cyano group decreased hA3AR affinity and efficacy in the cases of N6-(3-iodobenzyl) and N6-(trans-2-phenyl-1-cyclopropyl), for which a full A3AR agonist was converted into a selective antagonist; the 2-cyano-N6-methyl analogue was a full A3AR agonist. The combination of N6-benzyl and various 2-substitutions (chloro, trifluoromethyl, and cyano) resulted in reduced efficacy at the A1AR. The environment surrounding the 2-position within the putative A3AR binding site was explored using rhodopsin-based homology modeling and ligand docking.
Keywords: Purines, Cyclic AMP, Binding, Antagonists, Agonists, GPCR, Molecular modeling
1. Introduction
Receptors for the extracellular local modulator adenosine consist of four subtypes: A1, A2A, A2B, and A3.1 The selective activation of the A3AR (adenosine receptor) is both cardioprotective and cerebroprotective in a variety of ischemic models.2,3 The activation of this receptor subtype has also been associated with a cytostatic, anticancer effect in several tumor models.4 Thus, selective A3AR agonists have therapeutic potential.
Previous medicinal chemical studies demonstrated that an adenosine derivative’s ability to activate the A3AR is more structure sensitive than at other AR subtypes.5–7 Changes in various regions of the adenosine molecule have been shown to reduce A3AR efficacy, which leads to nucleoside analogues that are partial agonists or antagonists. A structure–efficacy relationship at this subtype, separate from the structure–affinity relationship derived from receptor binding experiments, was analyzed.7 Substitution with N6-benzyl groups, 2-chloro substitution of the adenine moiety, and conformational constraint of the ribose moiety all contribute to a reduction of efficacy. In the present study, we evaluated the binding affinity and functional properties of adenosine derivatives modified at the N6-position to achieve high A3AR affinity and at the 2-position with simple substitutions, such as cyano, carbonyl, aminomethyl, and trifluoromethyl. Previously, the effects of such substitutions at AR subtypes had not been fully evaluated.8
2. Results and discussion
2.1. Chemistry
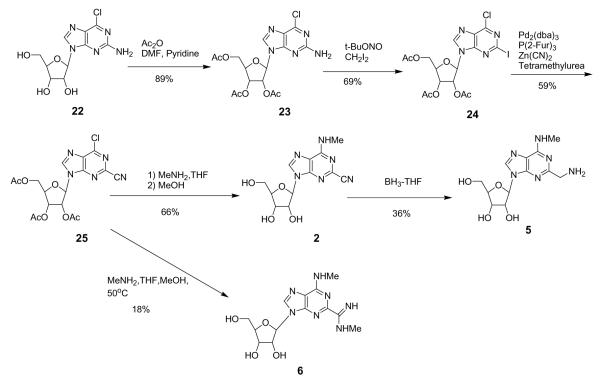
Adenosine agonists 2–17 and 19–21 were prepared (Schemes 1–3) to study the effects of 2-position substitution in interactions with ARs. Key synthetic intermediates 24 and 25 contained the 2-iodo and 2-cyano group, respectively, in combination with 6-chloro substitution. The introduction of the 2-cyano group was carried out by the reaction of zinc cyanide on the corresponding 2-iodo analogue with palladium chemistry (Scheme 1). We chose the protected 2-amino nucleoside 23 as the synthetic intermediate because of the simplicity of its synthesis from (−)-2-amino-6-chloropurine riboside 22. An attempted Sandmeyer-type cyanation (t-BuONO, CuCN, MeCN, 65 °C) on 23 resulted in decomposition without isolation of the desired 2-CN compound. We then applied Pd chemistry to this cyanation. The 2-amino group of 23 was converted to 2-iodo using a neutral purinyl radical, which was generated transiently from the thermal homolysis of the 2-diazonium intermediate.9 Stille-type conditions (PdCl2(PPh3)2, Bu3SnCN) or use of potassium cyanide (PdCl2(PPh3)2, KCN)10 on the iodo derivative 24 resulted in decomposition. Only use of zinc cyanide and a Pd(0) complex generated in situ from tris-2-furylphosphine and Pd2(dba)3 (tris(dibenzylideneacetone)dipalladium(0) chloroform adduct)11 effected cyanation of 24. Use of tetramethylurea as solvent gave a higher yield than N-methyl-2-pyrrolidinone, which was recommended in the literature.
Scheme 1.
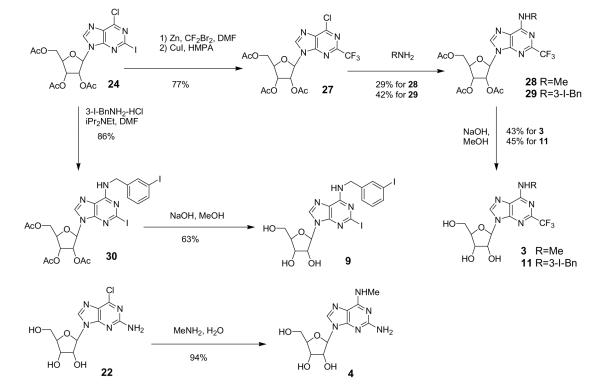
Scheme 3.
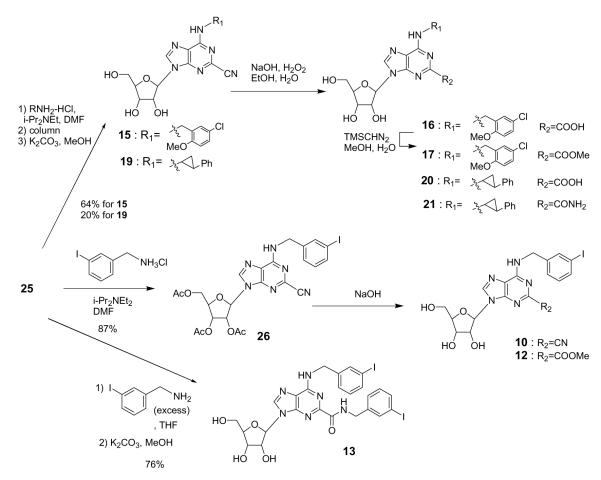
Intermediate 25 was coupled with the selected amines (methylamine, 5-chloro-2-methoxybenzylamine, or trans-2-phenyl-1-cyclopropylamine) at room temperature, and the acetyl groups were hydrolyzed to give the final target compounds 2, 15, and 19 (Schemes 1 and 2). The 2-cyano group could be reduced with BH3 to give the 2-aminomethyl derivative 5 (Scheme 1) and in other cases was hydrolyzed to give two 2-carboxylic acid derivatives 16 and 20 (Scheme 2). In the case of the N6-methyl derivative 2, the 2-cyano group also reacted with methylamine at elevated temperature and provided a N-methyl-carboxyamidine (C(=NH)NHMe), which was hydrolyzed to give 6. In the case of the 3-iodobenzylamine derivative 26, the methanol solvent reacted with the 2-cyano group and upon hydrolysis produced the 2-methoxycarbonyl derivative 12. In methanol-free hydrolysis conditions, the desired N6-(3-iodobenzyl) derivative 10 was obtained. Adding excess 3-iodobenzylamine to 25 and heating resulted in amine addition at the 2-cyano group to give the 2-(3-iodobenzylamide) derivative 13 upon hydrolysis.
Scheme 2.
Trifluoromethylation was performed on the intermediate 24 by treatment with ‘CF3Cu’ species, which was generated in situ from CF3ZnBr and CuI (Scheme 3).12,13 Intermediate 27 was coupled with methylamine or 3-iodobenzylamine, and the acetyl groups were hydrolyzed to give the final target compounds 3 and 11.
A 2-iodoadenosine derivative 9 was also synthesized from 24 by treatment with 3-iodobenzylamine followed by hydrolysis. A 2-amino-N6-methyl derivative 4 was synthesized by treatment of 22 with methylamine with-out the use of a protecting group. Each compound obtained was purified by column chromatography, preparative thin-layer chromatography, or HPLC.
2.2. Biological activity
We measured the binding affinities of the adenosine derivatives examined in this study (1–21) at hA1, hA2A, and hA3 (human) ARs and at the rA3AR (rat) and their degree of activation at the four hAR subtypes (Table 1). The efficacy of each of these adenosine derivatives in activation of the hARs was evaluated at a fixed concentration of 10 μM. Four different N6-substitutions were included: methyl, 3-iodobenzyl, 5-chloro-2-methyloxybenzyl, and trans-2-phenyl-1-cyclopropyl. The choice of these four N6-substituents was based on either their prior use as groups that enhance hA3AR affinity or selectivity6,7,14 or structural similarity to such a moiety.15 Stereoselectivity of the N6-functional group of adenosine derivatives in receptor binding has been established at A3ARs.7 Although the trans-2-phenyl-1-cyclopropyl analogues 18–21 shown in Table 1 are diastereomeric mixtures, it was previously established that the principal biological activity at the A3AR for this series is associated with the (1S,2R) isomer.7 These N6-substitutions were combined with various 2-modifications, including chloro, iodo, cyano, trifluoromethyl, carboxylic acid, amide, ester, or aminomethyl groups.
Table 1.
Binding affinities and maximal agonist effects of adenosine derivatives (upon structural variation at N6 and C2 positions) at human A1, A2A, A2B, and A3ARs and at rat A3ARs expressed in CHO (Chinese hamster ovary) cellsa
| # | N6-R1 | C-2 |
Ki (hA1AR)a or % displ. |
% Act. (hA1AR)b |
Ki (hA2AAR)a or % displ. |
% Act. (hA2AAR)b |
% Act. (hA2BAR)b |
Ki (hA3AR)a |
% Act. (hA3AR)b |
Ki (rA3AR)a |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CH3 | H | 5970±2030 | 38±10 | 17% | 5±5 | 16±3 | 9.3±0.4 | 96±3 | 6390 |
| 2 | CH3 | CN | 69.8±4.4 | 60±7 | 23% | 0 | 0 | 3.4±0.8 | 101±7 | >10,000 |
| 3 | CH3 | CF3 | 4650±580 | 4±3 | 9% | 20±3 | 0 | 64.5±3.8 | 93±3 | >10,000 |
| 4 | CH3 | NH2 | 484±22 | 13±2 | 15% | 29±6 | 11±2 | 39.0±2.4 | 98±3 | >10,000 |
| 5 | CH3 | CH2NH2 | 27% | 32±4 | 25% | 30±3 | 4±2 | 719±37 | 57±4 | >10,000 |
| 6 | CH3 | CH(=NH)– NHCH3 |
10% | 8±5 | 9% | 12±3 | 13±8 | 2730±268 | 7±1 | ND |
| 7 | IB | H | 7.4±1.7 | 78±6 | 135±22 | 90±7 | 58±1 | 5.8±0.4 | 46±8 | 9.5±1.4c |
| 8 | IB | Cl | 16.8±2.2 | 8±3 | 197±34 | 99±2 | 16±3 | 1.8±0.1 | 0 | 2.7±1.2 |
| 9 | IB | I | 191±12 | 0 | 1910±320 | 92±8 | 9±6 | 24.3±3.1 | 0 | ND |
| 10 | IB | CN | 1750±290 | 1±1 | 25% | 32±5 | 0 | 119±19 | 0 | 256±30 |
| 11 | IB | CF3 | 612±131 | 0 | 35% | 61±3 | 15±2 | 138±7 | 0 | 257±19 |
| 12 | IB | CO2CH3 | 73.1±22.9 | 38±4 | 390±160 | 91±9 | 18±2 | 3.21±0.17 | 0 | 6.05±0.16 |
| 13 | IB | CONH(3-IB) | 500±160 | 66±16 | 3990±150 | 52±18 | 5±2 | 133±19 | 0 | ND |
| 14 | CMB | H | 9.2±0.5 | 98±8 | 399±6 | 93±6 | 56±4 | 1.31±0.16 | 53±3 | ND |
| 15 | CMB | CN | 63.2±16.9 | 36±13 | 1260±190 | 90±15 | 16±3 | 2.76±0.51 | 29±6 | 12.8±2.3 |
| 16 | CMB | COOH | 41% | 33±10 | 9% | 31±6 | 6±7 | 211±36 | 0 | 698±100 |
| 17 | CMB | CO2CH3 | 6200±790 | 30±4 | 35% | 74±3 | 6±4 | 8.04±0.4 | 35±4 | ND |
| 18 | PC | H | 124±30 | 101±13 | 2530±720 | 88±23 | 20±15 | 0.86±0.09 | 101±5 | 399±28 |
| 19 | PC | CN | 1750±280 | 28±14 | 5640±780 | 51±8 | 6±4 | 8.7±2.1 | 0 | ND |
| 20 | PC | COOH | 30% | 6±2 | 0% | 5±5 | 3±1 | 1730±540 | 28±1 | ND |
| 21 | PC | CONH2 | 295±25 | 98±7 | 41% | 94±4 | 16±3 | 19.7±0.4 | 74±6 | ND |
All AR binding experiments were performed using adherent CHO cells stably transfected with cDNA encoding the human or rat ARs (unit: nM). Radioligands: 2nM [3H]R-PIA (A1), 15nM [3H]CGS21680 (A2A), and 0.5nM [125I]I-AB-MECA (A3). % displacement at 10 μM.
Percent activity at 10 μM, relative to 10 μM CPA (A1), 10 μM NECA (A2A, A2B), or 10 μM Cl-IB-MECA (A3). Values are expressed as mean±sem, n = 3–5.
Data from Kim et al.25 ND, not determined. IB, 3-iodobenzyl; CMB, 5-chloro-2-methyloxybenzyl; PC, trans-2-phenyl-1-cyclopropyl.
The codependence of N6 and C-2 regions in AR binding and activation was evident. With a small group at the N6 position (methyl), the affinity at the hA3AR was reduced by some groups at C-2 (trifluoromethyl 3, amino 4, aminomethyl 5, and N-methyl-carboxamidine 6) and enhanced by another group, cyano 2. In the case of N6-methyl derivatives, the 2-cyano, 2-trifluoromethyl, and 2-amino analogues 2–4 were full agonists, and 2 also showed moderate hA3AR selectivity (A1AR/A3AR 20-fold, A2AAR/A3AR > 1000-fold). Aminomethyl 5 and N-methyl-carboxamidine 6 analogues, being positively charged at physiological pH, decreased the affinity at ARs significantly. Also, by virtue of a charged group, 5 and 6 may prove to be neoligands for engineered ARs.16
In contrast to N6-methyl, with a larger N6-substituent, the 2-cyano substitution did not enhance the affinity, but rather resulted in a reduction in the ability to activate the receptor. For example, for N6-(5-chloro-2-methoxybenzyl), the efficacy of the 2-cyano analogue 15 was some-what reduced compared to the 2-H analogue 14 of similar A3AR affinity. Nevertheless, both were partial agonists. Similarly, a loss of efficacy was seen for the corresponding N6-(3-iodobenzyl) analogues, that is 10 compared with 7. The 2-cyano analogue 15 showed improved hA3AR selectivity (A1AR/A3AR 23-fold, A2AAR/A3AR 457-fold); 14 behaved as an = ′ A1AR full agonist and an A3AR partial agonist. For a pair of trans-2-phenyl-1-cyclopropyl derivatives, at the hA3AR the addition of the 2-cyano group transformed a selective full agonist 18 into a selective full antagonist 19.
Other 2-substitutions had varied effects. Curiously, when substituted with 2-COOMe, the high affinity of these N6-substituted adenosine analogues at the hA3AR was maintained or even increased (12 compared with 7 with N6-(3-iodobenzyl)). Also, 12 showed favorable hA3AR selectivity (A1AR/A3AR = 23-fold, A2AAR/A3AR = 130-fold). Compound 12 appeared to be an A3AR antagonist and an A1AR weak, partial agonist. Another 2-COOMe derivative, 17, was a highly selective A3AR partial agonist.
At the hA1AR, our compounds showed variable efficacy. In a previous report, 2-halo analogues showed full agonist activity at the A1AR.17 In our results, 14, 18, and 21 showed full agonism, and 9–11 were antagonists. Compounds 2, 7, 8, 12, and 15 appeared to be partial agonists at the hA1AR. hA2AAR affinities were moderate to weak, and there was no indication of partial agonism. In a previous report, 2-halo substituents reduced the efficacy at the A2AAR.17 At the hA2AAR, 2-Cl (8) and 2-I (9) analogues showed full agonism. Compounds 7, 12, 14, 15, and 21 were also full A2AAR agonists. At the hA2BAR, only 7 and 14 substantially activated at 10 μM.
The loss of A3AR affinity of 13, which was substituted with a 2-CONHBn group, could be explained by the bulkiness of this group. The less bulky 2-iodo analogue 9 was four-fold less potent in binding to the hA3AR than the 2-H, while the corresponding methyl ester 12 was twice as potent. Another electron withdrawing group, 2-CF3, was similar to 2-CN in its effect on A3AR affinity (11 compared with 10) when N6-(3-iodobenzyl) was present. However, with N6-methyl there was a 20-fold reduction (3 compared with 2). 2-COOH, which is also charged at physiological pH, decreased the affinity at several ARs significantly (16 compared with 14 and 20 compared with 18). However, 2-CONH2 21 retained A3AR affinity. Although compound 21 fully activated the A1AR, it was only a partial agonist at the A3AR.
The usefulness of the compounds may also be limited by species differences. For example, with the N6-methyl group, the affinity at the rA3AR was dramatically reduced (e.g., 1 and 2); this is similar to previous observations.7,18 Species differences at the A3ARs were also observed for larger N6-substituent derivatives, for example, 18. However, for substituted N6-benzyl derivatives, binding affinity at the rA3AR tended to be preserved. For example, 12 displayed a Ki value of 6 nM at the rA3AR, which was almost the same as its hA3AR affinity. Compound 12 also displayed substantial A3AR selectivity. Thus, 12 may prove to be an A3AR-selective antagonist in the study of rat models. In general, for N6-(3-iodobenzyl)–derivatized compounds, the affinity at the rA3AR was no more than three-fold weaker than at hA3AR, making these compounds attractive for testing in nonprimate animals.
2.3. Molecular modeling
To locate energetically favorable binding orientations of 2-H and 2-CN derivatives 1, 2, 7, 10, 14, and 15, the previously reported Cl-IB-MECA hA3AR complex was used as the starting geometry for the ribose-binding position.6 For the optimal interaction, various t0 angles for the N6-substituent and χ1 angles for the adenine ring were generated, and the resulting conformations were compared energetically in the putative binding site.
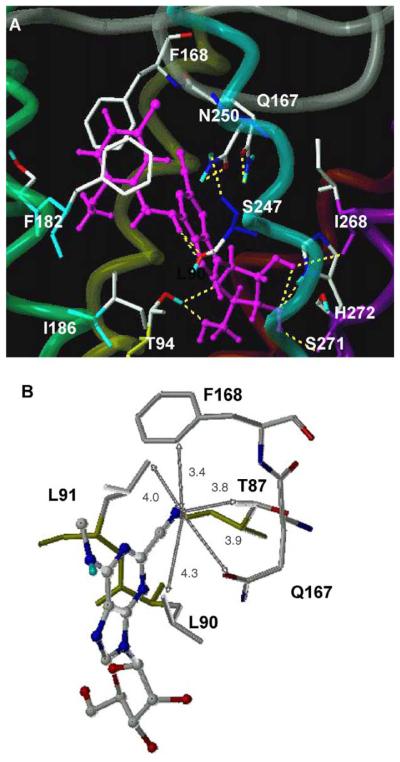
The hA3AR docking result of the high-affinity nucleoside 15 is shown in Figure 1. Residues in the putative binding site that were within 5-Å proximity to the ligand were L91 (3.33), T94 (3.36), H95 (3.37), Q167 (EL2), F168 (EL2), S181 (5.42), M177 (5.38), V178 (5.39), F182 (5.43), W243 (6.48), L246 (6.51), S247 (6.52), N250 (6.55), I268 (7.39), S271 (7.42), and H272 (7.43). Molecular modeling results were correlated with results of point mutation in TMs (transmembrane helical domains) 3, 6, 7, and EL2.6,19–21
Figure 1.
The complexes of the A3AR with 2-cyano ligands (A) the overall binding site of 15 and (B) the 2-cyano binding site of 2 with distances in Å. All ligands are displayed as ball-and-stick models in magenta color, and the side chains of the hA3AR are shown as stick models. The H bonding between the ligand and the hA3AR is displayed in yellow. The A3AR is represented by a tube model, with a different color for each TM (TM3 in yellow, TM5 in green, TM6 in cyano, TM7 in purple).
The purine ring was surrounded by a hydrophobic pocket, which was defined by L91 (3.33) and L246 (6.51). In addition, H-bonds formed between the exocyclic amine and the hydroxyl group of S247 (6.52), which was also in proximity to N250 (6.55), and between the purine N3 atom and the side chain of Q167 (EL2) (Fig. 1A). The 2′-OH group of the ribose moiety formed an H bond with the backbone carbonyl group of I268 (7.39), and the 3′-OH group H bonded with the backbone carbonyl group of S271 (7.42) and with the side chain of H272 (7.43), consistent with our A3 neoceptor model.19 The 5′-hydroxyl group also formed an H bond with T94 (3.36). The phenyl moiety showed an additional hydrophobic interaction with F168 (EL2), and the methyl of the methoxy group was stabilized through hydrophobic interaction with L91 (3.33) and I186 (5.47), consistent with its higher binding affinity.
In comparison to N6-methyl adenosine 1, the more potent 2-cyano-N6-methyl derivative 2 showed a more favorable nonbonding van der Waals interaction between the cyano group and the side chains of L90 (3.32) and L91 (3.33) and between the methyl group of the side chain of T87 (3.29) and the aromatic ring of F168 of EL2 (Fig. 1B). The distances between the N atom of the cyano group and the closest carbon atoms of the surrounding hydrophobic residues were 3.4–4.3 Å. The C-2 substituent was also proximal to the hydrophilic residue Q167 (EL2), with a distance of only 3.9 Å between the N atom of the cyano group and the side chain carbonyl O atom of Q167. There are some variable amino acids surrounding the binding site of the 2-cyano group. The amino acid corresponding to L90 (3.32) was Val in other ARs. T87 (3.29) was Ala in other human AR subtypes and Ser for the rA3AR. Q167 was a highly variable residue among the different subtypes and species, corresponding to Glu for A1AR, Leu for A2A and A2BAR, and His for rat A3AR. Thus, the hA3AR preference of 2-cyanoadenosine derivatives might be explained by an optimal van der Waals interaction. Another possibility is that a different binding preference for χ1 angle at each AR subtype would vary the residues of interaction with the C-2 substituents.
The preferred t0 and χ1 angles of the energetically favorable bound conformation, derived from hA3AR docking of various compounds, depended on the particular N6 and C-2 substituents. In the lowest-energy complexes of 1 and 2, the t0 and χ1 angles were −134.7° and −102.1° and −162.7° and −97.3°, respectively. For larger N6 groups, such as 3-I-benzyl, the preferred t0 and χ1 angles were −113.8° and −111.5° for 7 and −117.5° and −108.3° for 10. Compared to 2, the − binding of the 2-cyano group in 10 was directed more toward the methyl group of T87 (3.29), resulting in unfavorable van der Waals interactions, consistent with decreased binding affinity. The difference in 2-CN orientation between 2 and 10 was a consequence of additional hydrophobic interaction of the phenyl ring of the N6-substituent. Thus, the modeling has demonstrated how the interactions at the N6 and C-2 regions of the hA3AR might be interdependent.22
3. Conclusions
In previous studies, it was demonstrated that 2-chloro and other 2-substitutions of the adenine ring may increase the affinity and decrease the efficacy of the adenosine derivatives for the hA3AR.5–7 The 2-chloro modification has been incorporated into many of the more highly potent AR probes. At the A1ARs, 2-chloro has also been observed to enhance affinity of selective agents. Here we further demonstrated that the 2-substitution with the cyano group and other small groups has variable effects.
Although the SAR (structure–activity relationship) of the many 2-substitutions of adenosine at ARs has been thoroughly explored,23–26 the cyano, trifluoromethyl, simple ester, and other groups included here have not been among them. However, various 2-alkyne derivatives,18,27 such as 2-hexynyladenosine-5′-N-ethyluron-amide, have been found to bind potently to both A2A and A3ARs. The 2-iodo group was found previously to reduce efficacy at the A2AAR and retain efficacy at the A1AR.17 In our study, 2-iodo analogue 9 showed full agonism at the hA2AAR and antagonism at the hA1AR.
We have studied the effects of mainly sterically small groups substituted at the 2-position on the affinity and intrinsic efficacy at A1, A2A, A2B, and A3 receptors. The previously reported compound 8 appears to be a mixed A1/A3AR antagonist. Compound 12 appears to be an A3AR antagonist and an A1AR weak, partial agonist. Compound 14 behaves as an A1AR full agonist and an A3AR partial agonist, and compound 17 is a highly selective A3AR partial agonist. Compound 19 is a potent A3AR antagonist, and the related compound 21 is an A1AR full agonist and an A3AR partial agonist. Thus, we have modulated both the AR affinity and efficacy upon substitution at the 2-position in combination with various N6-substituents. This has resulted in a collection of adenosine analogues having widely differing pharmacological activities, both quantitatively and qualitatively. The dependence of intrinsic efficacy at the A3AR has recently been emphasized,5–7 and with this study we extend the analysis to other AR subtypes.
In conclusion, we have expanded the range of structures suitable as nucleoside antagonists of the A3AR. Such compounds include 12 and 16. A3AR antagonists are of interest for treatment of glaucoma and possibly asthma.1 Partial A1AR agonists, such as 2, 12, and 15, may be of interest for treatment of cardiac arrhythmias.26 Whether the presence of A3AR agonism or antagonism in these compounds might provide a therapeutic advantage is unknown. Compound 2 as a selective hA3AR agonist could be examined for activity as a cardioprotective and cerebroprotective agent.2,3,28 These compounds may also be useful as receptor probes for mutagenesis and for the design of neoceptors (e.g., moderately potent ligands that have either negative, for example, 16, or positive, for example, 5, charges near the 2-position).16,19–21 The expanded analysis of the SAR of nucleoside binding at the A3AR may now be applied to other agonists series, to probe the additivity with ribose ring modifications.6,7
4. Experimental materials
[125I]N6-(4-Amino-3-iodobenzyl)adenosine-5′-N-methyl-uronamide ([125I]I-AB-MECA; 2000 Ci/mmol), N6-[(R)-phenylisopropyl]adenosine ([3H]R-PIA; 34 Ci/mmol), and [3H]cyclic AMP (40 Ci/mmol) were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). 2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarbox-amido-adenosine ([3H]CGS21680; 47 Ci/mmol) was supplied by Perkin–Elmer Life Sciences (Boston, MA). Several N6-substituted adenosine derivatives 1, 7, 8, and 18 were prepared as reported (Ref. 7 and references therein). All other chemicals were of analytical grade from standard commercial sources.
4.1. Chemistry
Nucleosides and synthetic reagents were purchased from Sigma Chemical Co. (St. Louis, MO) and Aldrich (Milwaukee, WI). 1H NMR spectra were obtained with a Varian Gemini-300 spectrometer (300 MHz) with D2O, CDCl3, CD3OD, and DMSO-d6 as a solvent. Purity of the nucleosides was checked using a Hewlett–Packard 1100 HPLC equipped with a Luna 5 μ RP-C18(2) analytical column (250 × 4.6 mm; Phenomenex, Torrance, CA). System A: linear gradient solvent system: H2O/CH3CN/AcOH from 90/10/0.05 to 50/50/0.05 in 20 min and 10/90/0.05 in 50 min, flow rate 0.5 mL/min. System B: linear gradient solvent system: H2O/CH3CN/AcOH from 90/10/0.05 to 50/50/0.05 in 40 min and 0/100/0.05 in 60 min, flow rate 0.5 mL/min. System C: linear gradient solvent system: CH3CN/TBAP from 5/95 to 80/20 in 20 min, flow rate 1.0 mL/min. System D: linear gradient solvent system: H2O/CH3CN/AcOH from 80/20/0.05 to 60/40/0.05 in 5 min and 30/70/0.05 in 20 min, flow rate 1.0 mL/min. System E: linear gradient solvent system: H2O/CH3CN from 95/5 to 0/100 in 30 min, flow rate 1.0 mL/min. Peaks were detected by UV absorption with a diode array detector. All derivatives tested for biological activity showed ≥97% purity in the HPLC systems. Low-resolution mass spectra were measured with a Finnigan–Thermoquest LCQ with APCI (Atmospheric Pressure Chemical Ionization) interface. Vaporizer heater was 480 °C interfaced with HP1100 LC apparatus (System A). Low-resolution and high-resolution FAB (fast atom bombardment) mass spectrometry was performed with a JEOL SX102 spectrometer with 6-kV Xe atoms following desorption from a glycerol matrix.
4.2. (2R, 3S, 4S, 5R)-2-(2-Cyano-6-methylamino-purin-9-yl)-5-hydroxy-methyl-tetrahydrofuran-3,4-diol (2)
A solution of 2.0 M MeNH2 in THF (2.0 mL) was added to a solution of 25 (42 mg, 0.096 mmol) in THF (0.80 mL), and the mixture was stirred at rt (room temperature) for 26 h. MeOH (2.0 mL) was added, and the reaction mixture was stirred at rt for 3 h. The solvent was removed under reduced pressure and the residue was purified by preparative TLC (silica gel, thickness 0.25 mm, solvent: CHCl3/MeOH = 10/1) to give 2 (19 mg, 66%). 1H NMR (DMSO-d6) δ 8.62 (s, 1H), 8.48 (br, 1H), 5.90 (d, 1H, J = 5.7 Hz), 5.52 (d, 1H, J = 6.1 Hz), 5.23 (d, 1H, J = 5.1 Hz), 5.05 (t, 1H, J = 5.7 Hz), 4.52 (m, 1H), 4.15 (m, 1H), 3.95 (m, 1H), 3.75–3.60 (m, 1H), 3.60–3.50 (m, 1H), 2.97 (d, 3H, J = 4.2 Hz); MS (m/e) (CI) 307 (M + H)+, HRMS (positive-FAB) calcd for C12H15N6O4 (M + H)+ 307.1155, found 307.1158; HPLC (System B) 24.6 min (99%) (System C), 9.9 min (99%).
4.3. (2R, 3S, 4S, 5R)-2-(6-Methylamino-2-trifluoromethyl-purin-9-yl)-5-hydroxymethyl-tetrahydrofuran-3,4-diol (3)
To a solution of 28 (2.6 mg, 0.0055 mmol) in MeOH (2.0 mL) was added 3 N NaOH (0.02 mL) and the mixture was stirred at rt for 4 h. The solvent was removed under reduced pressure and the residue was purified by preparative TLC (silica gel, thickness 0.25 mm, solvent AcOEt), to give 3 (0.8 mg, 43%). 1H NMR (CD3OD) δ 8.38 (s, 1H), 6.00 (d, 1H, J = 6.0 Hz), 4,72 (t, 1H, J = 5.4 Hz), 4.34 (dd, 1H, J = 3.3, 5.1 Hz), 4.14 (dd, 1H, J = 3.3, 6.3 Hz), 3.88 (dd, 1H, J = 3.0, 12.3 Hz), 3.75 (dd, 1H, J = 3.6, 12.3 Hz), 3.13 (br = s, 3H); MS (m/e) (positive-FAB) 350 (M + H)+, 372 (M + Na)+. HRMS (positive-FAB) calcd for C12H15N5O4F3 (M H)+ 350.1076, found 350.1069; HPLC (System C) 11.8 + min (99%) (System E), 4.3 min (99%).
4.4. (2R, 3S, 4S, 5R)-2-(2-Amino-6-methylamino-purin-9-yl)-5-hydroxy-methyl-tetrahydrofuran-3,4-diol (4)
A solution of 22 (19 mg, 0.063 mmol) in 30% methylamine in H2O (2.0 mL) was stirred at 60 °C for 7 h. The solvent was removed under reduced pressure and the residue was purified by C18 reverse-phase silica gel chromatography (eluted by a linear gradient of H2O/MeCN = 100/0 to 0/100), to give 4 (18 mg, 94%). 1H NMR (DMSO-d6) δ 7.94 (s, 1H), 5.90 (d, 1H, J = 6.6 Hz), 4.77–4.72 (m, 1H), 4.41 (dd, 1H, J = 3.0, 5.4 Hz), 4.27 (dd, 1H, J = 3.0, 6.0 Hz), 3.91 (dd, = 1H, J = 2.7, 12.9 Hz), 3.82 (dd, 1H, J = 3.3, 12.9 Hz), 3.04 (s, 3H); MS (m/e) (positive-FAB) 297 = (M + H)+. HRMS (positive-FAB) calcd for C11H17N6O4 (M H)+ 297.1311, found 297.1317; HPLC (System C) 5.5 + min (99%) (System E), 3.3 min (99%).
4.5. (2R, 3S, 4S, 5R)-2-(2-Aminomethyl-6-methylaminopurin-9-yl)-5-hydroxymethyl-tetrahydrofuran-3,4-diol (5)
To a solution of 2 (2.4 mg, 0.0079 mmol) in THF (0.50 mL) was added 1.0 M BH3–THF complex THF solution (1.5 mL), and the mixture was stirred at 60 °C for 20 h. MeOH (2.0 mL) was added to the reaction mixture, and the solvent was removed under reduced pressure. The residue was purified on an Amberlite CG-50 column (eluted by a linear gradient of ammonium bicarbonate 0–1.0 M) and HPLC (Luna 5 μ RP-C18(2) column, eluted by a linear gradient of H2O/MeCN/AcOH = 100/0/0.05 to 80/20/0.05 in 15 min), to give 5 as acetic acid salt (1.1 mg, 36%). 1H NMR (D2O) δ 8.23 (s, 1H), 6.10 (d, 1H, J = 3.9 Hz), 4.56 (m, 1H), 4.44 (m, 1H), 4.26 (m, 1H), 4.20–4.12 (br, 2H), 3.92–3.73 (m, 2H), 3.12 (br s, 3H) 1.90 (s, 3H); MS (m/e) (CI) 311 (M + H)+ (positive-FAB), 311 (M + H)+. HRMS (positive-FAB) calcd for C12H19N6O4 (M + H)+ 311.1468, found 311.1464; HPLC (System B) 5.1 min (99%) (System C), 2.4 min (99%).
4.6. (2R, 3S, 4S, 5R)-9-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-methylamino-9H-purine-2-carboxamidine (6)
To a solution of 25 (21 mg, 0.047 mmol) was added to a 2.0 M MeNH2 THF solution (2.0 mL), and the mixture was stirred at rt for 2 h. MeOH (0.5 mL) was added, and the reaction mixture was stirred at 50 °C for 2 h. The solvent was removed under reduced pressure and the residue was purified on a short column (silica gel 2.0 mL, eluent: CHCl3/MeOH = 10/1) and an Amberlite CG-50 column (eluted by linear gradient of ammonium bicarbonate 0–1.0 M), to give 6 (2.8 mg, 18%). 1H NMR (D2O) δ 8.61 (s, 1H), 6.20 (d, 1H, J = 6.9 Hz), 4.99 (t, 1H, J = 6.5 Hz), 4.49 (m, 1H), 4.21 (m, 1H) 3.82 (d, 2H, J = 3.6 Hz), 3.17 (s, 3H), 2.87 (s, 3H); MS (m/e) (CI) 338 (M + H)+ (positive-FAB), 338 (M + H)+. HRMS (positive-FAB) calcd for C13H20N7O4 (M + H)+ 338.1577, found 338.1571; HPLC (System B) 12.1 + min (99%) (System C), 2.1 min (99%).
4.7. (2R, 3S, 4S, 5R)-2-(2-Iodo-6-(3-iodo-benzylamino)-purin-9-yl)-5-hydroxymethyl-tetrahydrofuran-3,4-diol (9)
To a solution of 24 (18.5 mg, 0.034 mmol) in DMF (0.10 mL) was added 3-iodobenzylamine (25 mg) and i-Pr2NEt (0.10 mL), and the mixture was stirred at rt for 20 h. The solvent was removed under reduced pressure and the residue was purified by column chromatography (silica gel, eluent: AcOEt/petroleum ether = 2/1), to give triacetate (21 mg). To a solution of this = triacetate (21 mg) in MeOH (1.0 mL) was added 3 N NaOH (0.10 mL), and the mixture was stirred at rt for 3 h. The solvent was removed under reduced pressure, and the residue was purified by short-column chromatography (silica gel, eluent: AcOEt) and preparative TLC (silica gel, thickness 0.25 mm, solvent AcOEt), to give 9 (11 mg, 53%). 1H NMR (CD3OD) δ 8.15 (s, 1H), 7.79 (s, 1H), 7.60 (d, 1H, J = 7.8 Hz), 7.39 (d, 1H, J = 7.8 Hz), 7.09 (t, 1H, J = 7.8 Hz), 5.89 (d, 1H, J = 6.3 Hz), 4.65 (m, 1H), 4.30 (dd, 1H, J 3.3, 5.1 Hz), 4.13 (m, 1H), 3.88 (dd, 1H, J = 2.7, 12.6 ′Hz), 3.74 (dd, 1H, J = 3.3, 12.6 Hz); MS (m/e) (positive-FAB) 610 (M + H)+. HRMS (positive-FAB) calcd for C17H18N5O4I2 (M + H)+ 609.9448, found 609.9424; HPLC (System C) 16.0 min (99%) (System E), 6.9 min (99%).
4.8. (2R, 3S, 4S, 5R)-2-(2-Cyano-6-(3-iodo-benzylamino)-purin-9-yl)-5-hydroxymethyl-tetrahydrofuran-3,4-diol (10)
To a solution of 26 (7.4 mg, 0.012 mmol) in MeCN (1.00 mL) and H2O (0.030 mL) was added 3 N aqueous NaOH (0.030 mL), and the mixture was stirred at rt for 6 h. Aqueous citric acid (5%, 0.050 mL) was added to neutralize the reaction and the solvent was removed under reduced pressure. The residue was purified by column chromatography (silica gel, eluent: AcOEt then CHCl3/MeOH = 5/1) first and then purified again by HPLC (Luna 5 μ RP-C18(2) column, eluted by a linear gradient of H2O/MeCN/AcOH = 70/30/0.05), to give 10 (1.2 mg, 20%). 1H NMR (DMSO-d6) δ 8.51 (s, 1H), 7.34 (br s, 1H), 7.68 (m, 1H), 7.60 (m, 1H), 7.35 (m, 1H), 7.12 (m, 1H), 5.95 (m, 1H), 4.65 (br, 2H), 4.51 (m, 1H), 4.13–4.10 (m, 2H), 3.92 (m, 2H); MS (m/e) (CI) 509 (M + H)+, HRMS (positive-FAB) calcd for C18H18N6O4I (M + H)+ 509.0434, found 509.0429; HPLC (System B) 49.2 min (99%) (System C), 16.1 min (99%) (System D), 11.6 min (99%).
4.9. (2R, 3S, 4S, 5R)-2-(6-(3-Iodo-benzylamino)-2-trifluoromethyl-purin-9-yl)-5-hydroxymethyl-tetrahydrofuran-3,4-diol (11)
To a solution of 29 (9.2 mg, 0.014 mmol) in MeOH (1.0 mL) was added potassium carbonate (18.5 mg, 0.13 mmol), and the mixture was stirred at rt for 4 h. The solvent was removed under reduced pressure, and the residue was purified by column chromatography (silica gel, eluent: CHCl3/MeOH = 10/1) and preparative TLC (silica gel, thickness 0.25 mm, solvent AcOEt), to give 11 (3.4 mg, 45%). 1H NMR (CDCl3) δ 7.98 (s, 1H), 7.76 (s, 1H), 7.63 (d, 1H, J = 8.1 Hz), 7.37 (d, 1H, J = 7.2 Hz), 7.08 (t, 1H, J = 7.8 Hz), 6.65 (br s, 1H), 5.84 (d, = 1H, J = 6.9 Hz), 4.99 (m, 1H), 4.80 (m, 2H), 4.49 (d, 1H, J = 4.5 Hz), 4.35 (s, 1H), 3.95 (dd, 1H, J = 1.8, 12.6 Hz), = 3.77 (d, 1H, J = 12.6 Hz); MS (m/e) (positive-FAB) 552 (M + H)+. HRMS (positive-FAB) calcd for C18H18N5O4F3I (M + H)+ 552.0356, found 552.0336; HPLC (System C) 17.8 min (99%) (System E), 8.2 min (99%).
4.10. (2R, 3S, 4S, 5R)-2-(2-Methoxycarbonyl-6-(3-iodo-benzylamino)-purin-9-yl)-5-hydroxymethyl-tetrahydrofuran-3,4-diol (12)
To a solution of 26 (10.5 mg, 0.017 mmol) in i-PrOH (0.60 mL), MeOH (0.05 mL) and THF (0.20 mL) was added 3 N aqueous NaOH (0.040 mL), and the mixture was stirred at rt for 5 h. The solvent was removed under reduced pressure, and the residue was purified by column chromatography (silica gel, eluent: CHCl3/MeOH = 10/1), to give 12 (1.3 mg, 15%). 1H NMR (DMSO-d6) δ 8.74 (br s, 1H), 8.56 (s, 1H), 7.82 (br s, 1H), 7.58 (m, 1H), 7.39 (m, 1H), 7.10 (m, 1H), 5.93 (d, 1H, J = 6.0 Hz), 5.46 (d, 1H, J = 6.3 Hz), 5.22 (d, 1H, J = 4.5 Hz), 5.03 (t, 1H, J = 4.5 Hz), 4.65 (m, 1H), 4.58 (br, 2H), 4.12 (m, 1H), 3.94 (m, 1H), 3.85 (s, 3H), 3.65 (m, 2H); MS (m/e) (CI) 542 (M + H)+, HRMS (positive-FAB) calcd for C19H21N5O6I (M + H)+ 542.0537, found 542.0540; HPLC (System A) 28.6 min (99%) (System C), 14.5 min (99%).
4.11. (2R, 3S, 4S, 5R)-9-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-(3-iodo-benzylamino)-9H-purine-2-carboxylic acid 3-iodo-benzylamide (13)
To a solution of 25 (9 mg, 0.020 mmol) in THF (1.50 mL) was added 3-iodobenzylamine (19 mg, 0.082 mmol), and the mixture was stirred at 50 °C for 21.5 h. MeOH (2.0 mL) and potassium carbonate (24 mg, 0.174 mmol) were added, and stirring continued at rt for 3 h. The solvent was removed under reduced pressure and the residue was purified by preparative TLC (silica gel, thickness 0.25 mm, solvent CHCl3/MeOH = 5/1), to give 13 (11 mg, 76%). 1H NMR (DMSO-d6) d 9.05 (br s, 1H), 8.84 (s, 1H), 7.80 (d, 2H, J = 5.4 Hz), 7.58 (d, 2H, J = 8.4 Hz), 7.39 (d, 2H, J = 8.7 Hz), 7.09 (t, 2H, J = 8.0 Hz), 6.10 (d, 1H, J = 5.4 Hz), 5.49 (d, 1H, J = 6.0 Hz), 5.24 (d, 1H, J = 4.2 Hz), 4.89 (s, 2H), 4.76 (s, 2H), 4.55 (m, 1H), 4.17 (m, 1H), 3.98 (m, 1H), 3.65–3.50 (m, 2H); MS (m/e) (positive-FAB) 743 (M + H)+. HRMS (positive-FAB) calcd for C25H25N6O5I2 742.9976, found 742.9972; HPLC (System A) 24.6 min (99%) (System B), 35.0 min (99%) (System C), 12.9 min (99%).
4.12. (2R, 3S, 4S, 5R)-9-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-(5-chloro-2-methoxy-benzylamino)-9H-purine (14)
Compound 14 (240 mg, 0.57 mmol) was prepared in one step19 from 6-chloropurine riboside (180 mg, 0.64 mmol), 5-chloro-2-methoxy-benzylamine hydrochloride29 (200 mg, 0.96 mmol) and triethylamine (120 μL, 0.83 mmol) in 88% yield. 1H NMR (DMSO-d6) δ 8.41 (s, 1H), 8.35 (br s, 1H), 8.18 (s, 1H), 7.24 (dd, 1H, J = 2.5, 8.7 Hz), 7.04 (s, 1H), 7.00 (d, 1H), 5.89 (d, 1H, J = 5.9 Hz), 5.37 (t, 1H, J = 5.6 Hz), 5.20 (d, 1H, J = 4.7 Hz), 4.63 (br, 3H), 4.14 (d, 1H, J = 2.9 Hz), 3.95 (d, 1H, J = 2.9 Hz), 3.83 (s, 3H), 3.66 (m, ′2H), 3.54 (m, 2H); HRMS (ESI) calcd for C18H21ClN5O5 (M + H)+: 422.1231, found 422.1223; Anal. (C18H20ClN5O5) C, H, N.
4.13. (2R, 3S, 4S, 5R)-9-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-(5-chloro-2-methoxy-benzylamino)-9H-purine-2-carbonitrile (15)
To a solution of triacetate 25 (6.2 mg, 0.014 mmol) in DMF (0.10 mL) was added 5-chloro-2-methoxybenzylamine hydrochloride (7.0 mg, 0.034 mmol) and i-Pr2NEt (0.10 mL), and the mixture was stirred at rt for 24 h. The solvent was removed under reduced pressure, and the residue was purified by short-column chromatography (silica gel, eluent: AcOEt). The obtained material was dissolved in MeOH (1.0 mL) and added potassium carbonate (15 mg, 0.108 mmol), and the mixture was stirred at rt for 6 h. The solvent was removed under reduced pressure, and the residue was purified by column chromatography (silica gel, eluent: CHCl3/MeOH = 10/1 then 5/1), to give 15 (5.2 mg, 82%). 1H NMR (DMSO-d6) δ 8.49 (s, 1H), 7.28 (d, 1H, J = 2.7 Hz), 7.23 (dd, 1H, J = 2.7, 8.5 Hz), 6.97 (d, 1H, J = 8.5 Hz), 6.01 (d, 1H), 4.65 (t, 1H, J = 5.4 Hz), 4.32 (t, 1H, J = 4.7 Hz), 4.13 (s, 2H), 3.88 (m, 3H), 3.63 (s, 3H); MS (m/e) (CI) 447, 449 (M + H)+ (relative peak height ratio was 3:1), HRMS (positive-FAB) calcd for C19H20N6O5Cl (M + H)+ 447.1184, found 447.1188; HPLC (System A) 31.2 min (99%) (System C), 15.6 min (99%).
4.14. (2R, 3S, 4S, 5R)-9-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-(5-chloro-2-methoxy-benzylamino)-9H-purine-2-carboxylic acid (16)
To a solution of 15 (3.1 mg, 0.0069 mmol) in EtOH (0.50 mL) was added 3 N NaOH (0.100 mL) and 30% H2O2 (0.040 mL), and the mixture was stirred at rt for 4 h. The solvent was removed under reduced pressure, and the residue was purified by Sephadex column chromatography (eluted by a linear gradient of ammonium bicarbonate 0–0.5 M), to give 16 (1.1 mg, 33%). 1H NMR (D2O) δ 8.32 (s, 1H), 7.40 (d, 1H, J = 3.0 Hz), 7.33 (dd, 1H, J = 3.0, 9.0 Hz), 7.04 (d, 1H, J = 9.0 Hz), 6.12 (d, 1H, J = 5.7 Hz), 4.88 (m, 1H), 4.73 (s, 2H), 4.44 (m, 1H), 4.30 (m, 1H), 3.93 (dd, 1H, J = 2.7, 12.9 Hz), 3.88 (s, 3H), 3.83 (dd, 1H, J = 2.7, 12.9 ′Hz); MS (m/e) (CI) 466, 468 (M + H)+ (relative peak height ratio was 3:1) (negative-FAB), 464, 466 (M − H)+ (relative peak height ratio was 3:1). HRMS (negative-FAB) calcd for C19H19N5O7Cl (M − H)+ 464.0973, found 464.0969; HPLC (System B) 36.0 min (99%) (System C), 13.1 min (99%).
4.15. (2R, 3S, 4S, 5R)-9-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-(5-chloro-2-methoxy-benzylamino)-9H-purine-2-carboxylic acid methyl ester (17)
To a solution of 16 (1.0 mg, 0.0021 mmol) in MeOH (0.30 mL) and H2O (0.10 mL) was added (trimethylsilyl)diazomethane (0.100 mL, 2.0 M in ether), and the mixture was stirred at rt for 5 h. The solvent was removed under reduced pressure, and the residue was purified by preparative TLC (silica gel, thickness 0.25 mm, solvent CHCl3/MeOH 10/1), to give 17 (0.5 mg, 50%). 1H NMR (CD = 3OD) δ 8.41 (s, 1H), 7.54 (br s, 1H), 7.21 (dd, 1H, J = 3.0, 8.7 Hz), 6.95 (d, 1H, J = 8.7 Hz), 6.01 (d, 1H, J = 6.0 Hz), 4.70 (m, 1H), 4.58 (s, 2H), 4.33 (m, 1H), 4.16 (m, 1H), 3.98 (s, 3H), 3.88 (s, 3H), 3.75 (m, 1H), 3.55 (m, 1H); MS (m/e) (CI) 480, 482 (M + H)+ (relative peak height ratio was 3/1). HRMS (positive-FAB) calcd for C20H23N5O7Cl (M + H)+ 480.1286, found 480.1279; HPLC (System A) 25.5 min (99%) (System C), 14.1 min (99%).
4.16. (2R, 3S, 4S, 5R)-9-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-(2-phenyl-cyclopropyl-amino)-9H-purine-2-carbonitrile (19)
To a solution of 25 (8.1 mg, 0.019 mmol) in DMF (0.10 mL) was added trans-2-phenyl cyclopropylamine hydrochloride (10.0 mg, 0.059 mmol) and i-Pr2NEt (0.10 mL), and the mixture was stirred at rt for 26 h. The solvent was removed under reduced pressure, and the residue was purified by short-column chromatography (silica gel, eluent: AcOEt). The obtained material (5.7 mg) was dissolved in MeCN (1.00 mL) and H2O (0.030 mL), and the mixture was treated with 3 N NaOHaq (0.030 mL) and was stirred at rt for 4 h. 5% citric acid aq (0.050 mL) was added to neutralize the reaction, and the solvent was removed under reduced pressure. The residue was purified by column chromatography (silica gel, eluent: AcOEt), to give 19 (1.2 mg, 15%). 1H NMR (CDCl3) δ 8.02 (s, 1H), 7.34–7.02 (m, 5H), 6.44 (br s, 1H), 5.85 (d, 1H, J = 6.9 Hz), 4.95 (m, 1H), 4.70 (m, 1H), 4.52 (m, 1H), 4.35 (m, 1H), 3.99 (m, 1H), 3.83 (m, 1H), 3.44 (br, 1H), 3.15 (br, 1H), 2.87 (m, 1H), 2.22 (m, 1H), 0.88 (m, 2H); (DMSO-d6) δ 8.93 (br s, 1H), 8.64 (s, 1H), 7.32–7.10 (m, 5H), 5.90 (d, 1H, J = 5.7 Hz), 5.52 (d, 1H, J = 5.7 Hz), 5.23 (d, 1H, J = 5.4 Hz), 5.04 (t, 1H, J = 5.1 Hz), 4.50 (m, 1H), 4.14 (m, 1H), 3.95 (m, 1H), 3.65 (m, 1H), 3.57 (m, 1H), 3.10 (m, 1H), 2.16 (m, 1H), 1.45–1.30 (m, 2H); MS (m/e) (CI) 409 (M + H)+, HRMS (positive-FAB) calcd for C20H21N6O4 (M + H)+ 409.1624, found 409.1627; HPLC (System A) 30.2 min (99%) (System B), 46.7 min (99%) (System C), 15.3 min (99%).
4.17. (2R, 3S, 4S, 5R)-9-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-(2-phenyl-cyclopropyl-amino)-9H-purine-2-carboxylic acid (20) and 9-(3,4-dihydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-(2-phenyl-cyclopropyl-amino)-9H-purine-2-carboxamide (21)
To a solution of 19 (4.4 mg, 0.0108 mmol) in EtOH (0.50 mL) was added 3 N NaOH (0.100 mL) and 30% H2O2 (0.050 mL), and the mixture was stirred at rt for 28 h. The solvent was removed under reduced pressure, and the residue was purified by Sephadex column chromatography (eluted by a linear gradient of ammonium bicarbonate 0–0.5 M), to give 20 (1.0 mg, 21%) and 21 (1.2 mg, 26%). For 20: 1H NMR (D2O) δ 8.31 (s, 1H), 7.45–7.32 (m, 5H), 6.13 (d, 1H, J = 5.7 Hz), 5.05–5.00 (m, 1H), 4.43 (t, 1H, J = 4.8 Hz), 4.30 (m, 1H), 3.96–3.81 (m, 2H) 3.35 (m, 1H), 2.34 (m, 1H), 1.51–1.41 (m, 2H); MS (m/e) (CI) 428 (M + H)+. HRMS (positive-FAB) calcd for C20H22N5O6 (M + H)+ 428.1570, found 428.1571; HPLC (System B) 34.4 min (99%) (System C), 13.5 min (99%). For 21: 1H NMR (D2O) δ 8.36 (s, 1H), 7.43–7.23 (m, 5H), 6.14 (d, 1H, J = 5.7 Hz), 5.00–4.95 (m, 1H), 4.44 (t, 1H, J = 4.5 Hz), 4.29 (m, 1H), 3.96–3.80 (m, 2H) 3.07 (m, 1H), 2.15 (m, 1H), 1.47 (m, 2H); MS (m/e) (CI) 427 (M + H)+. HRMS (positive-FAB) calcd for C20H23N6O5 (M + H)+ 427.1730, found 427.1733; HPLC (System B) 35.0 min (99%) (System C), 12.1 min (99%).
4.18. (2R, 3S, 4S, 5R)-2-(2-Amino-6-chloro-purin-9-yl)-3,4-diacetoxy-5-acetoxymethyl-tetrahydrofuran (23)
To a solution of (−)-2-amino-6-chloropurine riboside 22 (995 mg, 3.30 mmol) in DMF (15 mL) was added pyridine (2.0 mL, 25 mmol) and acetic anhydride (2.0 mL, 21 mmol), and the mixture was stirred at 80 °C for 2 h. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (eluent: AcOEt/petroleum ether 1/1 then 1/0), to give 23 (1.26 g, 89%). 1H NMR (CDCl = 3) δ 7.87 (s, 1H), 6.01 (d, 1H, J = 4.8 Hz), 5.96 (t, 1H, J = 5.2 Hz), 5.75 (t, 1H, J = 5.0 Hz), 5.19 (br s, 2H), 4.49–4.36 (m, 3H), 2.15 (s, 3H), 2.10 (s, 3H), 2.09 (s, 3H); MS (m/e) (positive-FAB) 428, 430 (M + H)+ (relative peak height ratio was 3:1).
4.19. (2R, 3S, 4S, 5R)-2-(6-Chloro-2-iodo-purin-9-yl)-3,4-diacetoxy-5-acetoxymethyl-tetrahydrofuran (24)
To a solution of 23 (206 mg, 0.48 mmol) in MeCN (0.50 mL) and CH2I2 (2.0 mL) was added t-butyl nitrite (0.200 mL, 2.22 mmol), and the mixture was stirred at 80 °C for 3 h. The reaction mixture was directly purified by silica gel column chromatography (eluent: chloroform then AcOEt/petroleum ether 2/1), to give 2-iodo compound 24 (178 mg, 69%). 1H NMR = (CDCl3) δ 8.20 (s, 1H), 6.21 (d, 1H, J = 5.4 Hz), 5.78 (t, 1H, J = 5.6 Hz), 5.59 (t, 1H, J = 5.0 Hz), 4.48 (t, 1H, J = 3.9 Hz), 4.42 (m, 2H), 2.18 (s, 3H), 2.15 (s, 3H), 2.11 (s, 3H); MS (m/e) (positive-FAB) 539, 541 (M + H)+ (relative peak height ratio was 3:1).
4.20. (2R, 3S, 4S, 5R)-2-(6-Chloro-2-cyano-purin-9-yl)-3,4-diacetoxy-5-acetoxymethyl-tetrahydrofuran (25)
A solution of Pd2 (dba)3–CHCl3 (50 mg, 0.048 mmol) and tri-2-furylphosphine (150 mg, 0.646 mmol) in tetramethylurea (0.50 mL) was stirred at 80 °C for 30 min. The solution was cooled to rt and treated with 24 (110 mg, 0.204 mmol) and Zn(CN)2 (125 mg, 1.06 mmol) in tetramethyl urea (3.50 mL) and stirred at 80 °C for 22 h. The reaction mixture was passed through a short column (SiO2 3.0 mL and eluent AcOEt), and the eluted fraction was evaporated to dryness. The residue was purified twice by silica gel column chromatography (first column eluent: AcOEt/petroleum ether 2/1, second column eluent: AcOEt/petroleum ether 1/1), = to give 2-cyano compound 25 (52 mg, 59%). = 1H NMR (CDCl3) δ 8.51 (s, 1H), 6.28 (d, 1H, J = 5.7 Hz), 5.77 (t, 1H, J = 5.7 Hz), 5.54 (dd, 1H, J = 5.7, = 3.9 Hz), 4.53 (dd, 1H, J = 3.9, 7.5 Hz), 4.44 (m, 2H), 2.19 (s, 3H) 2.17 (s, 3H), 2.11 (s, 3H); MS (m/e) (CI) 438 (M + H)+.
4.21. (2R, 3S, 4S, 5R)-2-(2-Cyano-6-(3-iodo-benzyl-amino)-purin-9-yl)-3,4-diacetoxy-5-acetoxymethyl-tetrahydrofuran (26)
To a solution of 25 (8.3 mg, 0.019 mmol) in DMF (0.10 mL) was added 3-iodobenzylamine hydrochloride (9.0 mg, 0.033 mmol) and i-Pr2NEt (0.10 mL), and the mixture was stirred at rt for 25 h. The solvent was removed under reduced pressure, and the residue was purified by short-column chromatography (silica gel, eluent: AcOEt), to give triacetate 26 (10.5 mg, 87%). 1H NMR (CDCl3) δ 8.06 (s, 1H), 7.73 (s, 1H), 7.64 (d, 1H, J = 7.8 Hz), 7.36 (d, 1H, J = 7.8 Hz), 7.09 (t, 1H, J = 7.8 Hz), 6.36 (br s, 1H), 6.18 (d, 1H, J = 5.7 Hz), 5.78 (t, 1H, J = 5.6 Hz), 5.57 (t, 1H, J = 5.1 Hz), 4.80 (br, 2H), 4.45 (m, 1H), 4.40 (m, 2H), 2.17 (s, 3H) 2.16 (s, 3H), 2.10 (s, 3H); MS (m/e) (CI) 635 (M + H)+.
4.22. (2R, 3S, 4S, 5R)-Acetic acid 4-acetoxy-2-acetoxymethyl-5-(6-chloro-2-trifluoromethyl-purin-9-yl)-tetrahydrofuran-3-yl ester (27)
To a suspension of activated Zn powder (140 mg, 2.20 mmol) in DMF (0.50 mL) was added CF2Br2 (0.10 mL, 1.08 mmol), and the mixture was stirred at rt for 2.5 h. HMPA (0.40 mL) was added to the reaction mixture, and it was cooled to 0 °C. CuI (76 mg) was added to the reaction mixture. A solution of 24 (76 mg, 0.141 mmol) in DMF (0.50 mL) was added at 0 °C. The reaction mixture was stirred at 50 °C for 26.5 h and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (silica gel, solvent AcOEt/petroleum ether = 2/1), to give 27 (52 mg, 77%). 1H NMR (CDCl3) δ 9.09 (br s, 1H), 6.34 (d, 1H, J = 5.4 Hz), 5.84 (t, 1H, J = 5.4 Hz), 5.62 (t, 1H, J = 5.0 Hz), 4.55 (m, 1H), 4.45 (m, 2H), 2.20 (s, 3H), 2.15 (s, 3H), 2.09 (s, 3H); MS (m/e) (positive-FAB) 481 (M + H)+.
4.23. (2R, 3S, 4S, 5R)-Acetic acid 4-acetoxy-2-acetoxymethyl-5-(6-methylamino-2-trifluoromethyl-purin-9-yl)-tetrahydrofuran-3-yl ester (28)
A solution of 27 (8.8 mg, 0.019 mmol) in 2 N methylamine in THF (2.0 mL) was stirred at rt for 20 h and at 70 °C for 24 h. The solvent was removed under reduced pressure, and the residue was purified by column chromatography (silica gel, eluent: CHCl3/MeOH = 5/1) and preparative TLC (silica gel, thickness 0.25 mm, solvent AcOEt/petroleum ether = 1/1), to give 28 (2.6 mg, 29%). 1H NMR (CDCl3) δ 7.98 (s, 1H), 6.16 (d, 1H, J = 4.8 Hz), 6.04 (br s, 1H), 5.83 (t, 1H, J 5.3 Hz), 5.72 (t, 1H, J = 5.1 Hz), 4.50–4.35 (m, 3H), 3.22 = (s, 3H), 2.17 (s, 3H), 2.10(s, 3H), 2.09 (s, 3H); MS (m/e) (positive-FAB) 476 (M + H)+, 498 (M + Na)+.
4.24. (2R, 3S, 4S, 5R)-Acetic acid 4-acetoxy-2-acetoxymethyl-5-(6-(3-iodo-benzylamino)-2-trifluoromethyl-purin-9-yl)-tetrahydrofuran-3-yl ester (29)
To a solution of 27 (16.2 mg, 0.034 mmol) in DMF (0.40 mL) was added 3-iodobenzylamine hydrochloride (30 mg, 0.111 mmol) and i-Pr2NEt (0.10 mL), and the mixture was stirred at rt for 24 h. The solvent was removed under reduced pressure, and the residue was purified by column chromatography (silica gel, solvent AcOEt) and preparative TLC (silica gel, thickness 0.25 mm, solvent AcOEt/petroleum ether 1/1), to give 29 (9.5 mg, 42%). 1H NMR (CDCl3) δ 8.00′(s, 1H), 7.78 (s, 1H), 7.63 (d, 1H, J = 8.0 Hz), 7.38 (d, 1H, J = 8.0 Hz), 7.08 (t, 1H, J = 8.0 Hz), 6.31 (br s, 1H), 6.16 (d, 1H, J = 5.1 Hz), 5.84 (t, 1H, J = 5.3 Hz), 5.71 (t, 1H, J = 5.1 Hz), 4.80 (br, 2H), 4.50–4.30 = (m, 3H), 2.16 (s, 3H), 2.10 (s, 3H), 2.09 (s, 3H); MS (m/e) (positive-FAB) 678 (M + H)+, 700 (M + Na)+.
4.25. Biological assays
4.25.1. Cell culture and membrane preparation
CHO cells expressing recombinant human and rat A3ARs were cultured in DMEM (Dulbecco’s modified Eagle’s medium) and F12 (1:1) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, 2 μmol/mL glutamine, and 800 μg/mL geneticin. After harvest and homogenization, the cells were centrifuged at 500g for 10 min. The pellet was resuspended in 50 mM Tris–HCl buffer (pH 8.0) containing 10 mM MgCl2 and 1 mM EDTA. The suspension was homogenized with an electric homogenizer for 10 s, and was then recentrifuged at 20,000g for 20 min at 4 °C. The resulting pellets were resuspended in buffer containing 3 units/mL of adenosine deaminase, and the suspension was stored at −80 °C prior to the binding experiments. The rat A3AR was expressed recombinantly via transfection in CHO cells, and the procedure was the same as for the human subtype. The protein concentration was measured using the Bradford assay.30
4.25.2. Binding assay
For the A3AR binding experiments, the procedures used were similar to those previously described.6 Briefly, each tube contained 100 lL of membrane suspension, 50 μL of [125I]I-AB-MECA (final concentration 0.5 nM), and 50 lL of increasing concentrations of compounds in Tris–HCl buffer (50 mM, pH 7.4) containing 10 mM MgCl2, 1 mM EDTA. Non-specific binding was determined using 10 μM NECA (5′-N-ethyluronamidoadenosine). The mixtures were incubated at 25 °C for 60 min. Binding reactions were terminated by filtration through Whatman GF/B filters under reduced pressure using a MT-24 cell harvester (Brandel, Gaithersburg, MD). Filters were washed three times with ice-cold buffer. Radioactivity was determined in a Beckman 5500B γ-counter. The binding of [3H]R-PIA to the recombinant hA1AR and the binding of [3H]CGS21680 to the recombinant hA2AAR was performed as previously described.7,31
4.25.3. Cyclic AMP accumulation assay
Intracellular cyclic AMP levels were measured with a competitive protein binding method.32 CHO cells expressing recombinant human33 and rat34 ARs were harvested by trypsinization. After resuspension in the medium, cells were plated in 24-well plates in 0.5 mL medium/well. After 24 h, the medium was removed and cells were washed three times with 1 mL/well of DMEM, containing 50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4. Cells were then treated with agonists and/or test compounds in the presence of roli-pram (10 μM) and adenosine deaminase (3 units/mL) and incubated at 37 °C. For A2A and A2BARs, incubation was carried out for 1 h. For A1 and A3ARs, after 45 min forskolin (10 μM) was added to the medium, and incubation was continued for an additional 15 min. The reaction was terminated upon removal of the medium, and the cells were lysed with 200 μL/well of 0.1 M ice-cold HCl. The cell lysate was resuspended and stored at −20 °C. For determination of cyclic AMP production, protein kinase A (PKA) was incubated with [3H]cyclic AMP (2 nM) in K2HPO4/EDTA buffer (K2HPO4, 150 mM; EDTA, 10 mM), 20 μL of the cell lysate, and 30 μL 0.1 M HCl. Bound radioactivity was separated by rapid filtration through Whatman GF/C filters under reduced pressure and washed once with cold buffer. Bound radioactivity was subsequently measured by liquid scintillation spectrometry.
4.26. Statistical analysis
Binding and functional parameters were estimated with GraphPAD Prism software (GraphPAD, San Diego, CA). IC50 values obtained from competition curves were converted to Ki values using the Cheng–Prusoff equation.35 Data were expressed as mean ± standard error.
4.27. Molecular modeling
All calculations were performed on a Silicon Graphics Octane workstation (300 MHz MIPS R12000 (IP30) processor). All ligand structures were modified from the lowest-energy conformation of Cl-IB-MECA6 with the Sketch Molecule of SYBYL 6.9.36 In all cases, MMFF force field37 and charge were applied using distance-dependent dielectric constants and conjugate gradient method until the gradient reached 0.05 kcal mol−1 Å−1.
A hA3AR model (PDB code: 1o74) constructed by homology to the X-ray structure of bovine rhodopsin with 2.8-Å resolution38 was used for the docking study. Compounds 1, 2, 7, 10, 14, and 15 in Table 1 were docked within the hA3AR model. The atom types of all ligands were manually assigned with Amber all-atom force field,39 and their charges were calculated before docking. The starting geometry of ligand conformation was chosen from the hA3AR complex model with Cl-IB-MECA, which was already validated by point mutation.6 The ribose-binding position of this series was fixed, using an atom-by-atom fitting method for the carbon atoms of the ribose ring. To determine the binding region of N6 derivatives and C-2 substituents, the flexible bonds, χ1 (O–C1′ –N9–C4) and t0 (C5–C6–N6–C) angles were variously searched in the putative binding cavity. χ1 was rotated by −60°, −110°, and −160° within the anti-conformational range and was rotated in 30° increments. Several conformations without any steric bump were selected for further optimization. The initial structures of all complexes were optimized using the Amber force field with fixed dielectric constant of 4.0 and terminating gradient of 0.1 kcal mol−1 Å−1.
Acknowledgements
M.O. thanks Toray Industries (Tokyo, Japan) for financial support. We thank Prof. Joel Linden (University of Virginia, Charlottesville) for the gift of CHO cells expressing the human A1AR and A2AAR. We thank Dr. Tom Spande and Dr. Victor Livengood (NIDDK) for mass spectral measurements.
References and notes
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. Pharmacol. Rev. 2001;53:527. [PMC free article] [PubMed] [Google Scholar]
- 2.Liang BT, Jacobson KA. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6995. doi: 10.1073/pnas.95.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Lubitz DK, Lin RC, Popik P, Carter MF, Jacobson KA. Eur. J. Pharmacol. 1994;263:59. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishman P, Bar-Yehuda S. Curr. Top. Med. Chem. 2003;3:463. doi: 10.2174/1568026033392147. [DOI] [PubMed] [Google Scholar]
- 5.van Tilburg EW, van der Klein PA, von Frijtag Drabbe Künzel J, de Groote M, Stannek C, Lorenzen A, IJzerman AP. J. Med. Chem. 2001;44:2966. doi: 10.1021/jm001114o. [DOI] [PubMed] [Google Scholar]
- 6.Gao ZG, Kim S-K, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. J. Med. Chem. 2002;45:4471. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao ZG, Blaustein J, Gross AS, Melman N, Jacobson KA. Biochem. Pharmacol. 2003;65:1675. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruns RF. Can. J. Physiol. Pharmacol. 1980;58:673. doi: 10.1139/y80-110. [DOI] [PubMed] [Google Scholar]
- 9.Nair V, Fasbender AJ. C-2 Tetrahedron. 1993;49:2169. [Google Scholar]
- 10.Tnaji K, Higashino T. Heterocycles. 1990;30:435. [Google Scholar]
- 11.Gundersen L-L. Acta Chemica Scand. 1996;50:58. [Google Scholar]
- 12.Burton DJ, Wiemer DM. J. Am. Chem. Soc. 1985;107:5014. [Google Scholar]
- 13.Wiemer DM, Burton DJ. J. Am. Chem. Soc. 1986;108:832. [Google Scholar]
- 14.Mogensen JP, Roberts SM, Bowler AN, Thomsen C, Knutsen LJ. Bioorg. Med. Chem. Lett. 1998;8:1767. doi: 10.1016/s0960-894x(98)00302-3. [DOI] [PubMed] [Google Scholar]
- 15.DeNinno MP, Masamune H, Chenard LK, DiRico KJ, Eller C, Etienne JB, Tickner JE, Kennedy SP, Knight DR, Kong J, Oleynek JJ, Tracey WR, Hill RJ. J. Med. Chem. 2003;46:353. doi: 10.1021/jm0255724. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson KA, Gao ZG, Chen A, Barak D, Kim SA, Lee K, Link A, Van Rompaey P, Van Calenbergh S, Liang BT. J. Med. Chem. 2001;44:4125. doi: 10.1021/jm010232o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchinson SA, Baker SP, Scammells PJ. Bioorg. Med. Chem. 2002;10:1115. doi: 10.1016/s0968-0896(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 18.Volpini R, Costanzi S, Lambertucci C, Taffi S, Vittori S, Klotz KN, Cristalli G. J. Med. Chem. 2002;45:3271. doi: 10.1021/jm0109762. [DOI] [PubMed] [Google Scholar]
- 19.Kim S-K, Gao Z-G, Van Rompaey P, Gross AS, Chen A, Van Calenbergh S, Jacobson KA. J. Med. Chem. 2003;46:4847. doi: 10.1021/jm0300431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Z-G, Kim S-K, Gross AS, Chen A, Blaustein JB, Jacobson KA. Mol. Pharmacol. 2003;63:1021. doi: 10.1124/mol.63.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Z-G, Chen A, Barak D, Kim S-K, Müller CE, Jacobson KA. J. Biol. Chem. 2002;277:19056. doi: 10.1074/jbc.M110960200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The relative energy differences for the 1/hA3AR complex according to various t0 and χ1 angles from the six different clusters was very small, that is within 2 kcal/mol energy, except for one cluster, which had 3.6 kcal/mol higher energy, whereas its 2-cyano analogue 2 displayed two clusters showing a wider distribution of 10 kcal/mol (data not shown). In addition, in the docking study of 2-cyano analogues having larger N6-(3-I)benzyl and (5-Cl-2-MeO)benzyl substituents, 10 and 15, most of the clusters displayed a higher relative energy for six different complexes. It was suggested the N6-methyladenosine introduced more conformational flexibility in the N6 binding site than the analogues having bulky N6-substituents, for which there was more conformational rigidity due to the additional binding of the phenyl ring and its substituent(s).
- 23.Thompson RD, Secunda S, Daly JW, Olsson RA. J. Med. Chem. 1991;34:3388. doi: 10.1021/jm00116a007. [DOI] [PubMed] [Google Scholar]
- 24.Daly JW, Padgett W. Biochem. Pharmacol. 1992;43:1089. doi: 10.1016/0006-2952(92)90616-q. [DOI] [PubMed] [Google Scholar]
- 25.Kim HO, Ji X-D, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. J. Med. Chem. 1994;37:3614. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zablocki J, Palle V, Blackburn B, Elzein E, Nudelman G, Gothe S, Gao Z, Li Z, Meyer S, Belardinelli L. Nucleos. Nucleot. Nucleic Acids. 2001;20:343. doi: 10.1081/NCN-100002306. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda A, Shinozaki M, Yamaguchi T, Homma H, Nomoto R, Miyasaka T, Watanabe Y, Abiru T. J. Med. Chem. 1992;35:241. doi: 10.1021/jm00080a007. [DOI] [PubMed] [Google Scholar]
- 28.Tracey WR, Magee WP, Oleynek JJ, Hill RJ, Smith AH, Flynn DM, Knight DR. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2780. doi: 10.1152/ajpheart.00411.2003. [DOI] [PubMed] [Google Scholar]
- 29.Yu G, Mason HJ, Wu X, Endo M, Douglas J, Macor JE. Tetrahedron Lett. 2001;42:3247. [Google Scholar]
- 30.Bradford MM. Anal. Biochem. 1976;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Hutchison AJ, Williams M, de-Jesus R, Yokoyama R, Oei HH, Ghai GR, Webb RL, Zoganas HC, Stone GA, Jarvis MF. J. Med. Chem. 1990;33:1919. doi: 10.1021/jm00169a015. [DOI] [PubMed] [Google Scholar]
- 32.Nordstedt C, Fredholm BB. Anal. Biochem. 1990;189:231. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 33.Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10365. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7432. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y-C, Prusoff WH. Biochem. Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 36.Sybyl Molecular Modeling System. version 6.9 Tripos Inc.; St. Louis, Missouri 63144, USA: [Google Scholar]
- 37.Halgren TA. J. Comput. Chem. 1999;20:730. doi: 10.1002/(SICI)1096-987X(199905)20:7<730::AID-JCC8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 38.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp TE, Yamamoto M, Miyano M. Science. 2000;289:739. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 39.Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Jr., Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. J. Am. Chem. Soc. 1995;117:5179. [Google Scholar]