Abstract
A general theme in sensory perception is that exposure to a stimulus makes it seem more neutral such that perception of subsequent stimuli is shifted in the opposite direction. The visual motion aftereffect (MAE) is an extensively studied example of this. Although similar effects have been described in other sensory systems, it has not previously been described in the vestibular system. Velocity storage has been extensively studied in the vestibular system and suggests a persistence of perception in the direction of the initial movement. The current study sought to determine how motion perception is influenced by prior movement in darkness. Thirteen human subjects (mean age 41, range 21–68) underwent whole-body fore–aft translation. The threshold of vestibular motion discrimination perception was measured using a single interval (1I) of motion lasting 0.5 s in which subjects identified their direction of motion as forward or backward using an adaptive staircase. The translation aftereffect (TAE) was measured in 2-interval (2I) experiments: The adapting stimulus moved 15 cm in 1.5 s (peak velocity 20 cm/s, peak acceleration 42 cm/s2). After a fixed inter-stimulus interval (ISI) of 0.5, 1.0, 1.5, or 3 s, a second stimulus lasting 0.5 s was delivered and the subject identified the perceived direction of the second test stimulus. The test stimulus was determined using an adaptive staircase. The ISI was constant within the block, but adapting stimuli directions were randomly interleaved. During the 1I condition, the response bias was near zero in all subjects. With a 2I stimulus, 8 of 13 subjects demonstrated a significant bias. At an ISI of 0.5 s, a minority of subjects demonstrated a bias in the same direction as the adapter. When the ISI was 1, 1.5, or 3 s, all subjects who demonstrated a significant TAE had one in the opposite direction of the adapter, similar to that seen for MAE. When averaged across subjects, the TAE was significant with ISIs of 1.0 s and above. These findings demonstrate that perception of vestibular stimuli depends on prior motion. This has important implications for understanding and quantifying vestibular perception.
Keywords: Perception, Otolith, Human, Translation, Motion aftereffect (MAE), Vestibular
Introduction
Motion aftereffects (MAE) are an extensively studied phenomenon in which after viewing a moving image, a static pattern is perceived to move in the opposite direction. This is also known as the “waterfall illusion” and was first described by Robert Addams more than 175 years ago (Addams 1834), and has now been described for several types of visual motion in addition to perception of colors, line curvature, and facial identification (Thompson and Burr 2009). Although classically MAE is demonstrated after adaptation periods of several seconds, the effect has been demonstrated after an adaptation period as short as 320 ms (Kanai and Verstraten 2005). Analogous effects are not limited to vision but also have been observed in perception of sound intensity (Reinhardt-Rutland 1998), voice perception (Bestelmeyer et al. 2010), and proprioception (Seizova-Cajic et al. 2007). A general theme in sensory perception is that exposure to a stimulus makes it seem more neutral such that perception of subsequent stimuli is shifted in the opposite direction.
Motion through a rich environment produces an expanding visual pattern known as optic flow (Gibson 1950). Vection, an illusion of self-motion, can be caused by an expanding visual pattern (Johansson 1977; Andersen and Braunstein 1985; Ohmi and Howard 1988). Few studies on the MAE have tried to disambiguate the perception of environmental versus self-motion or vection, although a recent report demonstrated that the MAE may also cause an aftereffect in vection and may be separate from the visual MAE (Seno et al. 2010). There is some literature that demonstrates that MAE may be inhibited by concurrent vestibular stimulation (Wallach and Flaherty 1975; Harris et al. 1981). If the translation aftereffect (TAE) and MAE are additive, this finding suggests that a TAE may bias perception in the direction opposite the MAE. However, there are no studies to our knowledge of isolated TAE.
Vestibular-evoked eye movements such as the vestibulo-ocular reflex (VOR) can persist in the absence of vestibular stimulation such as that occurs with velocity storage during prolonged yaw rotation (Raphan et al. 1979; Hess and Angelaki 1997). Although velocity storage has been observed to have perceptual consequences (Bertolini et al. 2011), it is not likely to be responsible for sensory after-effects such as those seen in other sensory systems. Velocity storage is not present in roll (Bertolini et al. 2008) or with isolated linear (non-rotation) motion (Hess and Angelaki 1997). To our knowledge, the possibility of perceptual aftereffects has been not examined in fore–aft motion or other translation perception.
The current paper investigates the hypothesis that self-motion aftereffects may occur with vestibular stimulation alone independent of visual stimuli. This is a novel and potentially interesting sensory system to study aftereffects because both the vestibular interaction with the visual MAE (Wallach and Flaherty 1975; Harris et al. 1981) and velocity storage (Hess and Angelaki 1997) suggest that after vestibular stimulation, there may be a persistence of the sensory perception in the direction of the initial stimulation which if it occurs would be opposite the aftereffect seen with other sensory systems. The only effect in the vestibular system similar to other sensory aftereffects is the “Gillingham illusion,” which has been described in the aviation literature (Lyons et al. 1994). In a flight simulator-(Nooij and Groen 2011) and aircraft-based (Ercoline et al. 2000) experiments, pilots tend to inappropriately counter a previous roll with a motion in the same direction in more than two-thirds of trials suggesting a false perception of roll in the opposite direction.
In this paper, fore–aft motion was used because it is easy to maintain a visual fixation during this type of motion, and potentially confounding effects such as visual pursuit and the vestibulo-ocular reflex are minimized. Furthermore, fore–aft motion has previously been demonstrated to modulate visual MAE (Wallach and Flaherty 1975; Harris et al. 1981), suggesting it may be an appropriate model to study TAE in the absence of vision.
Materials and methods
Equipment
Motion stimuli were delivered using a 6-degree-of-freedom motion platform (Moog, East Aurora, NY, model 6DOF2000E) similar to that used in other laboratories for human motion perception studies (Grabherr et al. 2008; Fetsch et al. 2009; MacNeilage et al. 2010). Subjects were seated in a padded racing seat (Corbeau, Sandy UT, model FX-1) mounted on the platform. A four-point racing style harness held the body in place. The head was held in place using an American football style helmet. Helmets were available in 6 sizes to allow each subject to be fit appropriately. The helmets also included an inflatable liner to insure a sung fit. Once the subject was seated, the helmet was firmly pushed back against hard rubber pads and a strap was used to hold the helmet against the pad and to prevent any decoupling of the head within the helmet. A second rigid point of attachment on the side of the helmet further prevented any decoupling. The head was held in position so that the body midline and external auditory canals were directly over the center of the platform. The helmet covered the ears, thus reducing the sound made by the platform.
During the test stimulus, sound from the platform was masked using a white noise stimulus reproduced from two platform-mounted speakers on either side of the subject. Because the speakers moved with the subject, they could not give a cue to position in the room. The intensity of the masking noise varied with time as a half-sine wave so that the peak masking noise occurred at the same time the peak velocity was reached. This created a masking noise similar to the noise made by the platform. This served to identify the time period in which the direction of perceived motion should be reported and to mask sound from the platform which could give magnitude cues.
The accuracy of the platform movement was tested over the range of movements tested. Using electronic calipers to measure the motion, the smallest 0.1-cm movements could be reproduced with a maximum error of <0.02 cm. The standard deviation of these multiple measurements was 0.008 cm.
Responses were collected using a three-button control box that the subject held. The center button was pressed by the subject to initiate each stimulus. The two buttons at either end were used to identify the perceived direction of motion as forward or backward.
Stimuli
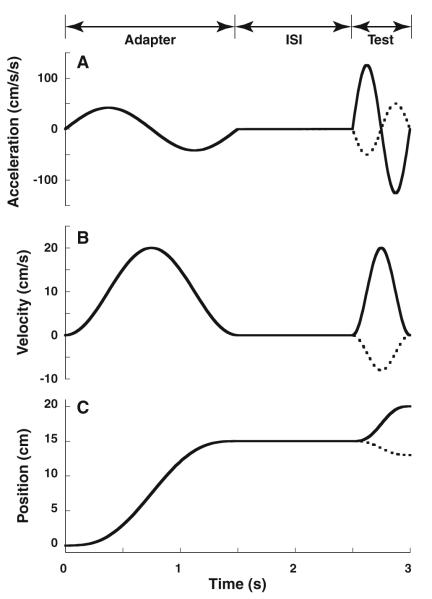
Both the adapter (the initial stimulus of constant amplitude) and test stimuli (second stimulus that is adjusted based on the responses) consisted of a sine wave in acceleration, which lasted 1.5 s for the adapter stimulus (0.66 Hz) or 0.5 s (2 Hz) for the test stimulus (Fig. 1; Eq. 1). A longer adapter was used because some preliminary data and results from visual MAE (Hershenson 1989) indicated that a longer adapting stimulus was likely to produce a stronger aftereffect. The test stimulus was shorter so it could be used to accurately probe a finer resolution of time points after the adapter. A fore–aft stimulus was used to minimize potential artifacts associated with eye movement caused by the vestibulo-ocular reflex. The stimuli can be described in the acceleration (a(t)), velocity (v(t)), or position (d(t)) domains given the frequency in Hz (f) and total displacement (D) (Eqs. 1-3). The motion platform required a position signal (d(t)) at a resolution of 60 Hz (Fig. 2; Eq. 3). These motion profiles were chosen because they contain no discontinuities in acceleration, velocity, or position, and they have previously been used for threshold determination (Benson et al. 1989; Grabherr et al. 2008).
| (1) |
| (2) |
| (3) |
Fig. 1.
The two-interval stimulus. The first interval (adapter) lasted 1.5 s during which the subject was moved 15 cm at a peak velocity of 20 cm/s. After the adapter, there was an inter-stimulus interval (ISI) during which no motion occurred. In this example, the ISI was 1 s, but it was varied between 0.5 and 3.0 s. The ISI was followed by a second-interval (test) stimulus lasting 0.5 s. The amplitude of the 0.5-s test stimulus was varied by the staircase. The largest test stimulus (solid line) is shown with a smaller test stimulus in the opposite direction (dashed). a: Acceleration, b: velocity, c: position
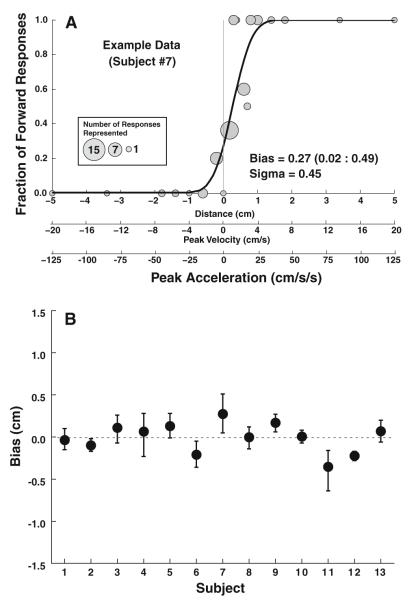
Fig. 2.
Single Interval data. a: Example data from a single subject (#7). Each gray circle represents a single-stimulus level with the diameter of the circle proportional to the number of responses at that level. Positive displacements represent forward motion. The mean of the cumulative distribution function represents the bias with the best fit to this data (solid line) and was 0.27 cm with a 95 % confidence interval of 0.02–0.49. The scale below allows the comparison of displacement, peak velocity, and peak acceleration of the test stimulus. b: Summary of single interval data for all subjects. Error bars represent 95 % confidence intervals (CI)
A small amount of mechanical oscillation was added to every test stimulus presentation. The movement was added only in sway (side to side), and its purpose was to create a small amount of noise and vibration to minimize non-vestibular cues in the event that no stimulus was delivered. This oscillation consisted of a 6-Hz sine wave (3 cycles during the 0.5-s test stimulus). The wave was multiplied by the first cycle of 1-Hz sine wave so that the intensity was largest in the middle of the trial with a maximum amplitude of 0.06 cm. This mechanical vibration had an effect only for small movements (<0.5 cm). The effect was to eliminate the possibility of subjects using subtle vibration cues to determine whether motion had occurred.
The head motion was measured with an accelerometer mounted to the platform and for some control experiments to a bite bar. The accelerometer verified that there was no continued vibration or motion persisting more than 150 ms after the adapting stimulus was delivered.
The first block of trials was single interval with only the test stimulus and no adapting stimulus. The purpose of this block was to measure any pre-existing bias in vestibular perception. The maximum stimulus was 5 cm of motion during 0.5 s (2.0 Hz, peak velocity 20 cm/s, peak acceleration 126 cm/s2). Two staircases were interleaved. One staircase started with forward motion and the other with backward motion. This was done to eliminate a potential bias based on the initial test stimulus, and minimize the ability of subjects to identify patterns in the stimulus presentation. For each response in the direction of the staircase, the stimulus displacement was moved in the opposite direction. The test stimulus magnitude was varied on a continuum such that each staircase could step through zero. Thus, the staircases tended to deliver most stimuli in the range where subjects were equally likely to perceive a movement in either direction, and there were not necessarily equal numbers of test stimuli on either side of zero. The initial step size was 1.6 cm, but with each reversal in response direction, the step size decreased by half down to a minimum of 0.1 cm. The level was changed in a 1-up, 1-down manner, which converges on the mean of the psychometric function. This mean, also called the point of subjective equality (PSE), corresponds to the stimulus that is perceived as zero motion. Each staircase contained 25 stimulus presentations so that the block of single interval trials included 50 presentations of the test stimulus. If the subject did not respond with a perceived direction within 2 s, no response was recorded and the stimulus was represented when that staircase was active again.
For the remainder of trial blocks, a two-interval procedure was used to measure potential aftereffects. The adapting (first interval) stimulus was always 15 cm of motion over 1.5 s (0.66 Hz, peak velocity 20 cm/s, peak acceleration 42 cm/s2, Fig. 1), although the direction could be forward or backward. Forward and backward adapting stimuli were randomly interleaved such that within a trial block, 50 % of stimuli had a forward adapter and 50 % had a backward adapter. After an inter-stimulus interval (ISI) in which no motion occurred, a test stimulus (second interval) was delivered similar to that described above for the single interval condition. For each adapter, there were two staircases: one that started with 5 cm forward motion and the other that started with 5 cm backward motion. Thus, each of these trial blocks included 4 randomly interleaved staircases, each of which included 25 stimulus presentations. Thus, these aftereffect blocks included 100 stimulus presentations.
The single interval block was always run first. Each two-interval block of trials was limited to a single ISI, 0.5, 1.0, 1.5, or 3.0 s, and the order was varied between subjects.
Experimental procedure
Subjects were instructed that during the test stimulus of each trial, they would move either forward or backward. Afterward subjects pushed one of two buttons to indicate the direction of perceived movement. Subjects were encouraged to guess if uncertain. The experiment was practiced a few times in the light to ensure comprehension of the task prior to data collection in darkness.
Prior to stimulus delivery, the subject heard a 500-Hz, 0.125-s single tone to signal that the next stimulus was ready and the start button could be pressed. The stimulus was delivered immediately after the subject pressed the start button. After the stimulus was delivered, two 0.125-s tones were played in rapid succession to indicate that the stimulus had been delivered and to suggest that one of two response buttons should be pressed. These tones were played from speakers mounted to the motion platform to eliminate any potential auditory localization cues. When a response button was pressed, a key click sound was played which did not depend on the accuracy of the response, but indicated that the subject’s selection had been recognized by the program. If no response was entered within 2 s, a “timeout sound” was played (a low-frequency buzz). After either a response or timeout, the platform returned to the center starting position using a motion profile similar to the stimulus but taking 2 s.
Subjects
A total of 13 subjects (5 females) participated in the experiment. Ages ranged from 21 to 68 (41 ± 19, mean ± SD). All 5 blocks of trials were usually completed in a single session lasting about 90 min with breaks between blocks of trials. For two subjects, multiple sessions were required due to scheduling constraints. Two subjects (#3 and #5) were familiar with the design and purpose of the experiment. The other subjects had participated in previous experiments in the laboratory using the motion platform but were otherwise naïve to the design and purpose of the experiment. Subjects understood that the adapter stimulus could be followed by a test stimulus that was either forward or backward motion. Informed consent was obtained from all participants. The protocol was approved by the University of Rochester Research Science Review Board.
Subjects were screened prior to participation. The screening included caloric testing, an audiogram, visual acuity testing, and screening questions to rule out any known history of vestibular disease or cognitive deficit. Based on these results, the subjects had normal peripheral vestibular function and hearing.
Analysis
The percentage of forward responses for each stimulus level was plotted as a function of the stimulus used. A cumulative Gaussian function with confidence intervals was determined from those data points using a Monte Carlo maximum-likelihood criteria allowing for a small lapse rate which was fit to the data set with the constraint that lambda and gamma are equal and were within the range of 0.00–0.05 as previously described (Wichmann and Hill 2001a, b) and used by others (Fetsch et al. 2009; Mac-Neilage et al. 2010). Data from each subject were resampled and fit 2,000 times so that multiple estimates of the mean could be generated and 95 % CI determined. Sigma was also determined. In aftereffect blocks, the mean was calculated 2,000 times using random resampling of the data (Wichmann and Hill 2001b). This resampling was done with forward adapter stimuli creating a distribution of means and a degree of uncertainty in the determination of the true mean value. A similar procedure was done for the backward adapter stimuli. In cases in which there was no overlap between these two distributions, the p value was <0.0005 (1/2,000). Otherwise, the degree of overlap determined the p value such that if the two distributions were exactly the same, the p value would be 1.0. For this test, a p value of p = 0.01, that is, no more than 1 % of the means were in the overlapping area, was considered significant.
The repeated-measures analysis of variance (ANOVA) was used to compare the bias between subjects and test conditions for two-interval experiments. Factors included forward versus backward adapter, and ISI. The Wilcoxon signed-ranks test was used to compare biases with forward and backward adapters across subjects for each ISI. Statistical significance was defined as p < 0.05.
Results
The experiment was well tolerated by all subjects, and all were able to complete the 5 test conditions and identify the stimulus directions at the extremes of the range of test stimuli used reliably. It was found that in many subjects, perception of the test stimulus was biased based on the preceding adapter.
The bias was determined as the mean of the psychometric function for the single interval trial (Fig. 2). The bias was within 0.4 cm of zero in every subject with the average difference from zero 0.13 ± 0.11 cm (mean of absolute values ± SD, Fig. 2b). The mean bias corresponds with a peak velocity of 0.5 cm/s. The mean sigma was 0.29 ± 0.14 cm; as a measure of the threshold, this corresponds to a peak velocity of 1.2 ± 0.6 cm/s and peak acceleration of 7 ± 3 cm/s2.
The addition of an adapter stimulus influenced the perception of the test stimuli. The most common effect was for perception of the test stimulus to be influenced in the direction opposite the adapter which is referred to as an aftereffect. The data of a single subject who demonstrated a large aftereffect and a second subject with a small but significant effect are shown in Fig. 3a–c. Significant effects of the direction of the adapter were determined for each subject using the overlapping fits technique described in the “Materials and methods”. With a 0.5-s ISI, the adapter had a significant effect in 8 of the 13 subjects (Fig. 4). In 3 of these subjects (# 3, 6, and 11), the effect was atypical in that the perception of the test stimulus was biased in the same direction as the adapter—a priming effect. The possibility that the priming effect in these 3 subjects may have been due to reporting the motion of the adapter rather than the test stimulus was considered, but found to be unlikely: In subject #3, this test condition repeated on 3 occasions with similar results. Second, if the subject were only responding to the adapter stimulus, the test stimulus would have no effect and no PSE could be determined which was not the case in any of these subjects. In the remaining 5 subjects (# 4, 5, 7, 8, and 10), the perception of the test stimulus was biased in the direction opposite the adapter (an aftereffect).
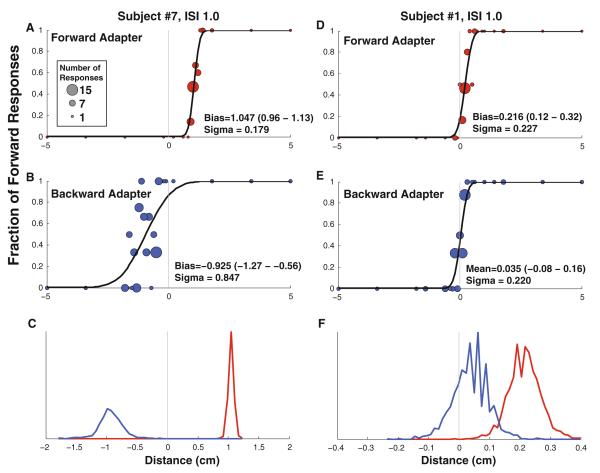
Fig. 3.
Vestibular perception following an adapting stimulus in two sample subjects. A circle is shown for each stimulus level presented with the number of stimuli represented proportional to the diameter of the data point. The test stimulus was delivered 1 s after the end of the adapter ended (ISI 1 s). Red symbols represent a forward adapter, and blue symbols represent a backward adapter. a, b: Exemplary data from subject #7 who had an usually large after effect. d, e Represent subject #10 who had a smaller aftereffect which remained significant. a, d: The adapter is forward motion. The mean is shifted to 1.05 cm for subject #7 and 0.22 for subject #10, indicating that after the adapter the subject would be more likely to perceive this forward motion as no movement. b, e: The adapter is backward motion. In both the cases, the mean is shifted in the negative direction relative to the forward adapter, indicating that small movements were much more likely to be perceived as forward movement. c, f: The cumulative distribution functions shown in the upper panels were refit to data that were randomly resampled ×2,000. These plots represent histograms of the biases determined from the resulting fits. In these plots, the scale of the X-axis has been changed relative to the other plots for clarity. In c, there was no overlap in the histograms indicating a highly significant difference (p < 0.0005). In f, the histograms overlapped and in 18 pairs of the 2,000 fits (p = 0.009), the bias with a backward adapter was found to be larger than with a forward adapter
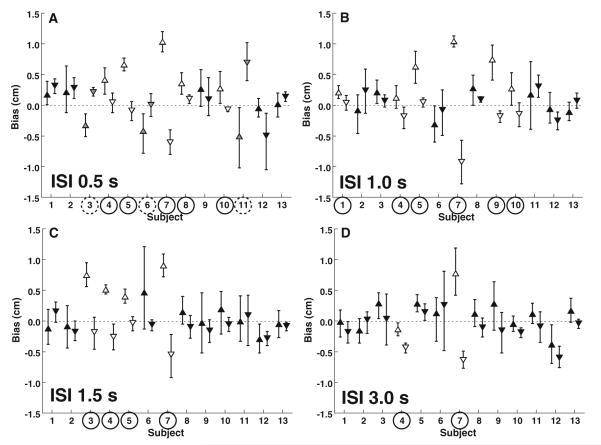
Fig. 4.
Summary of 2I response per subject for each inter-stimulus interval (ISI). Upward pointing triangles represent trials with forward adapters, and downward pointing triangles represent backward adapter trials. Error bars represent ±95 % CI. Open triangles represent significant differences that are also marked with a circle around the subject number. Subject numbers circled with a solid line indicate a significant TAE such that the perception of the second stimuli was shifted in the opposite direction of the adapter. Circles with a dashed line and gray filled triangles indicate a significant perceptual shift such that perception of the second stimulus was shifted in the same direction as the adapter
At ISIs of 1 s and longer, all the trial blocks that demonstrated a significant effect with adapter had an aftereffect: The adapter influenced perception of the test stimulus in the opposite direction (Fig. 4b–d). At an ISI of 1 s, 6 subjects demonstrated a significant aftereffect, at 1.5 s 4 subjects did, and at 3 s only 2 subjects had an aftereffect. The number of subjects who demonstrated an aftereffect even if it was not significant was 7 at 0.5 s, 9 at 1.0 s, 10 at 1.5 s, and 11 at 3 s. The Wilcoxon matchedpairs signed-ranks test revealed a significant effect only at ISIs of 1.5 s (p = 0.02) and 3 s (p = 0.005).
The threshold of perception is often described as the sigma or standard deviation of the psychometric function. There were subtle effects in the width or sigma of the psychometric function across testing conditions (Fig. 5). For the 1I condition, the sigma averaged 0.29 ± 0.14 cm, which was equivalent to a threshold of 1.2 ± 0.6 cm/s or 7 ± 3 cm/s2. The sigma across 2I conditions averaged 0.46 ± 0.28 cm (mean ± SD); with the test stimulus used, this was equivalent to 1.8 ± 1.1 cm/s and 12 ± 7 cm/s2. A 2-way ANOVA with subjects and test conditions demonstrated that differences between subjects accounted for 26 % of the variation and differences between test conditions were 14 % of the variation. Here, test condition was significant (p = 0.01, F = 2.89) and the difference between subjects highly significant (p < 0.001, F = 3.44). When a 2-way ANOVA was used to examine subject and adapter direction, the subjects accounted for 31 % of the total variation (p < 0.001, F = 6.94) and adapter direction accounted for 6.7 % of the total variation (p = 0.02, F = 6.50). Overall, the sigma was wider for forward adapters (0.53 ± 0.29 cm) than for backward adapters (0.39 ± 0.25).
Fig. 5.
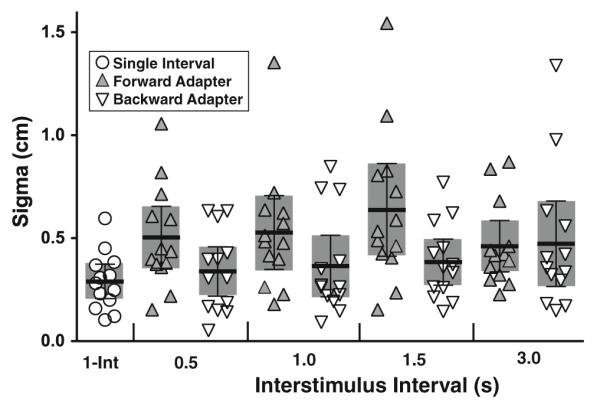
Sigma or width of the psychometric function by condition. Circles represent the 1I condition. 2I conditions are grouped by ISI for forward adapters (gray up pointing triangles) and backward adapters (open down pointing triangles). Dark bars represent the mean with error bars, and shaded area represents the 95 % CI of the mean
When the responses were aggregated across subjects, the mean bias in the single interval experiment was not significantly different from zero (one-sample t test, p > 0.5, Fig. 6). In two-interval stimulus presentations, some individuals demonstrated significant TAE at every ISI tested. When a 2-way ANOVA was used to examine the effect of adapter stimulus direction across the ISIs, the effect of the adapting stimulus was highly significant (p < 0.001, F = 17.3). This effect of the adapting stimulus remained highly significant even if the exemplary data of subject #7 were excluded (p = 0.006, F = 8.0). For ISI, the effect was not significant (p = 0.32, F = 1.14); for the interaction between ISI and adapter direction, the effect was also not significant (p > 0.5, F = 0.36). Due to the opposite directions of priming and aftereffects, there was no significant effect of adapter direction in the aggregate data at an ISI of 0.5 s (paired t test, p = 0.5). At an ISI of 1.0 s, the TAE was more consistent but still not significant in the mean data (p = 0.08); at the longer ISIs of 1.5 and 3.0 s, the TAE was significant in the aggregate data (p = 0.02 for both). Overall, the average bias was 0.19 cm after a forward adapter and −0.08 cm after a backward adapter.
Fig. 6.
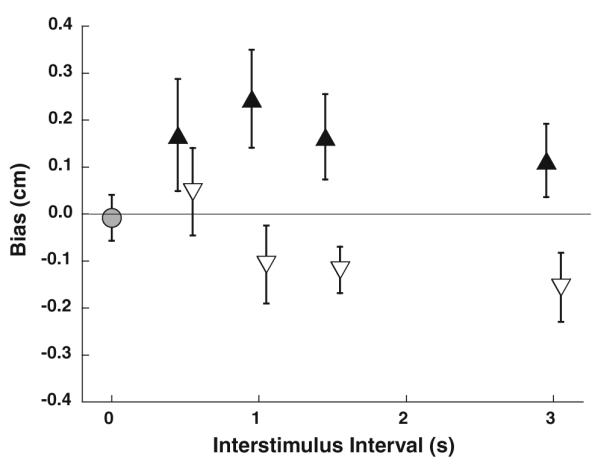
Summary of data from all 13 subjects. The single interval experiment is marked with a gray circle. Two-interval experiments are marked with triangles. Filled upward pointing triangles represent stimulus presentations with forward adapters, and open downward pointing triangles represent stimulus presentations with backward adapters. Error bars represent ±1 SEM
Discussion
This paper describes a novel aftereffect in vestibular perception. The tendency was to perceive the test stimulus in the direction opposite the adapting stimulus, an effect in the same direction as aftereffects described in other sensory systems. This result is similar to aftereffects seen in visual motion (Wade 1994; Anstis et al. 1998), sound intensity (Reinhardt-Rutland 1998), proprioception (Seizova-Cajic et al. 2007), and others.
Although the TAE described here is similar in direction to aftereffects in other sensory stimuli, the finding is somewhat unexpected for the vestibular system. Prior studies of rotation have demonstrated perception of continued rotation in the same direction, suggesting this perception is driven by the same pathways that control eye movements (Bertolini et al. 2011). The TAE described is otolith mediated and the effects of velocity storage are not seen with non-rotatory stimuli such as that studied here. Furthermore, velocity storage usually requires an order of magnitude longer period of motion than the 1.5-s adapter used here (Young and Oman 1969). Thus, the TAE seems unrelated to velocity storage.
Interestingly, velocity storage is also absent in roll (Bertolini et al. 2008), but a phenomenon analogous to aftereffects has been observed. In aviation circles, this is known as the “Gillingham illusion”(Lyons et al. 1994). In a flight simulator- (Nooij and Groen 2011) and aircraft-based (Ercoline et al. 2000) experiment, subjects inappropriately attempted to counter a previous roll motion with a motion in the same direction in more than two-thirds of trials. This result suggests that after the initial roll, it is common to have a false perception of roll in the opposite direction. This finding provides evidence against the hypothesis that vestibular perception is linked to the concurrent vestibular eye movements (Bertolini et al. 2011), although neither the current experiment nor the roll experiments study a situation where eye movement would be expected to persist after the initial stimulation.
There has been some work on the effects of vestibular stimulation on visual MAE. In these experiments, MAE was studied in conditions where the subject is stationary and also with conditions where there was congruent vestibular motion (Wallach and Flaherty 1975; Harris et al.1981). When an expanding visual stimulus was shown during forward movement (Harris et al. (1981) also tested backward motion but demonstrated no effect), the subsequent MAE is significantly diminished. These experiments do not directly test for a TAE since it is the visual MAE that is nulled and the vestibular stimulus is always paired with a visual stimulus. However, the finding that when the TAE and MAE are combined, there is a decreased MAE suggests that a pure TAE may be in the opposite direction of the MAE. This hypothesis is based on the perceptual summation of MAE and TAE effects analogous to the Bayesian integration seen with many other sensory modalities (Angelaki et al. 2009). The results of the current experiments that directly measure the TAE suggest this is not the case. There may be multiple reasons for this: One is that in these prior experiments the vestibular stimulus was much longer than that used in the current experiment and occurred in both directions, even though it was paired with a visual stimulus in only one direction, and another possibility is that the interaction between visual MAE and TAE cannot be described as a simple summation, which is suggested by the interaction occurring in forward but not backward motion (Harris et al. 1981).
When the test stimulus was in close proximity to the adapting stimulus, there were a minority of subjects (# 3, 6, and 11) who demonstrated a significant priming effect in which the test stimulus was biased in the same direction as the adapter. Such a priming effect has also been demonstrated for short duration (<0.16 s) visual stimuli with a short ISI (≤0.5 s) (pinkus and Pantle 1997; Kanai and Verstraten 2005). In the visual system, the priming effect is not seen for stimuli as long as those used in this current experiment where a MAE is observed (Kanai and Verstraten 2005). The visual and vestibular systems are known to have different temporal dynamics with regard to perception (Barnett-Cowan and Harris 2009), so we should not expect TAE to have the same temporal dynamics as MAE. In the current experiments, a priming effect was observed in only a small subset of subjects and was limited to an ISI of 0.5 s. Although the dynamics of the TAE are clearly different, the priming effect seen here may represent an analogous phenomenon. It is possible that the current priming effect is the result of the known phase lag in central vestibular neurons that respond to linear acceleration (Tomlinson et al. 1996; Angelaki and Dickman 2000), which may allow the adapting stimulus to be confused with the subsequent test stimulus when the interval between them is short.
The otolith system has a well-known frequency dependence which has been described for the linear vestibuloocular reflex (paige and Tomko 1991), as well as for the perception of translation (Soyka et al. 2011). An analogous frequency dependence has also been described for the perception of yaw rotation (Grabherr et al. 2008). All of these studies predict a near flat frequency response over the range of frequencies used in this study (0.66 and 2.0 Hz). Thus, it is unlikely that the difference in the frequency of the adapter and test stimulus is responsible for the observed aftereffect.
In the aggregate data and several individual subjects, there was a clear TAE. However, in this experiment, not all subjects demonstrated a TAE. This suggests that it may be weaker than visual MAE, but it may also be due to the nature of the vestibular stimulus delivered. In the current laboratory, the amount of displacement is limited which also imposes practical limits on the duration and velocity of the stimulus that can be delivered. In these experiments, fore–aft motion was chosen to minimize any potential effects of eye movement. However, sensitivity to acceleration is better with inter-aural motion than with the fore–aft direction at both the peripheral (Fernandez and Goldberg 1976) and central (Gu et al. 2010) levels, although others have found similar sensitivities in both inter-aural and fore–aft motion (Benson et al. 1986). Thus, it is possible that a greater fraction of individuals would have demonstrated a stronger TAE if a larger, higher velocity, or different direction stimuli were delivered but the stimulus parameters that may produce a stronger TAE are not well understood.
In the visual system, a constant velocity stimulus provides continued stimulation. However, the otoliths are acceleration sensors so a constant velocity stimulus after the initial acceleration would not provide any stimulation. Furthermore, for stimuli that begin and end with the subject stationary, such as those used in the current experiments, acceleration and deceleration must be balanced. The finding that these aftereffects occur using such a stimulus is evidence that these aftereffects are a central phenomena that fits with evidence demonstrating that otolith signals are integrated to velocity prior to central processing (Angelaki and Dickman 2000).
One potential purpose of the TAE described here would be allowing to calibrate the vestibular responses over time. The assumption that the time-averaged response represents no motion allows an offset in responses to be nulled. Such a purpose has been implicated for visual MAE (Ullman and Schechtman 1982; Anstis et al. 1998). There is also evidence that the vestibular system adapts to chronic stimulation (Miles and Eighmy 1980) or sensory conflict with vision (Cohen et al. 1992; Crane and Demer 2000). Perceptual adaptation has also been observed with prolonged microgravity during spaceflight (Clement et al. 2001). With training adaptation to electrical stimulation can be shortened to a few minutes (Merfeld et al. 2006). A similar effect has been observed in regular and irregular otolith afferents of the squirrel monkey after 90s of adaptation to centrifugal forces (Fernandez and Goldberg 1976). It is possible that an analogous effect also occurs after shorter during stimuli but it is not clear that vestibular stimulation occurring over time periods such as the 1.5-s stimulus used here is enough to induce significant adaptation. Although very short periods of adaptation have been described in the visual system (Glasser et al. 2011), we know of no analogous vestibular mechanism. It is imaginable that such a mechanism would be important for vestibular activity occurring over a long period of time, but such a mechanism is less likely for the relatively short (1.5-s) period of adaptation described here. These aftereffects may also be a result of coding optimization as hypothesized for a purpose for visual MAEs (Barlow 1990). Because the dynamic range of neurons is limited, adjusting the response after a period of stimulation can allow a wider dynamic range; if this occurs, it could better explain TAE after a shorter period of adaptation such as those observed.
The stimulus used in this study is not purely vestibular and also included an auditory and somatosensory component. It is not believed that the auditory cues provided cues to direction but they did provide cues to the stimulus magnitude. Masking noise was used in an attempt to eliminate any effects of noise. The adapting stimulus had a peak acceleration of 42 cm/s2, which is above the threshold for translation for those with bilateral vestibular loss (Walsh 1961; Gianna et al. 1995), suggesting that sensation of pressure on the body could indicate the direction of motion. Although all the subjects included in this study had healthy vestibular function, it is possible that extra-vestibular cues influenced the TAE described here and there is evidence that the proprioceptive system can produce aftereffects (Seizova-Cajic et al. 2007).
It is most likely that the vestibular system and specifically the otoliths played the primary role in the TAE described here. Although the adapting stimulus had a vestibular and somatosensory component, the test stimuli were near the vestibular threshold minimizing somatosensory influence (Walsh 1961). The level of certainty in perceiving the test stimulus (sigma) suggested that it was primarily a function of the vestibular system since these thresholds were below that previously reported for translation in patients with bilateral vestibular loss (Walsh 1961). Although it is not possible to rule out a somatosensory component of the TAE in this data, such an effect would have to occur across somatosensory and vestibular modalities and hence be multisensory and central in origin.
The thresholds found in this study, 7 ± 3 cm/s2 for a 1I stimulus and 12 ± 7 cm/s2 after an adapting stimulus, are in line with those described previously. Prior reports that did not include an adapter stimulus have reported linear translation thresholds of 6.3 cm/s2 (MacNeilage et al. 2010) and 5.7 cm/s2 (Benson et al. 1986), which are very close to the value reported here. The addition of an adapter stimulus increased the threshold in addition to causing a bias. An analogous decrease in sensitivity after a large stimulus has been described in the auditory system (Reinhardt-Rutland 1998; Eatock 2000) and for the somatosensory system (Gescheider and Wright 1969; Lundstrom 1986). Central mechanisms have also been described by which neural circuits change from a stimulus detection toward a stimulus discrimination paradigm after stimulation causing a decrease in detection threshold (Wang et al. 2010). Thus, it is not surprising to find similar behavior in the vestibular system. There was a trend toward higher thresholds after a forward adapter than after a backward adapter but this was likely due to 2 individuals with extreme data (Fig. 5). This may be related to the previously described effect where some subjects have vestibular thresholds that are significantly directionally asymmetric (Roditi and Crane 2012).
The study of MAE has proven to be a good model for understanding motion perception in the visual system (Wade 1994; Anstis et al. 1998; Thompson and Burr 2009). The current study reports an analogous effect for the vestibular system. The TAE phenomena may provide a new tool for studying vestibular perception and visual–vestibular integration.
Acknowledgments
This work was funded by a grant from the National Institute on Deafness and Other Communication Disorders K23 DC011298. Additional support was provided by the American Otological Society and a grant from the Triological Society. Technical support was provided by Shawn Olmstead-Leahey.
References
- Addams R. An account of a peculiar optical phenomenon seen after having looked at a moving body etc. Mag J Sci. 1834;5:373–374. 3rd series. [Google Scholar]
- Andersen GJ, Braunstein ML. Induced self-motion in central vision. J Exp Psychol Hum Percept Perform. 1985;11:122–132. doi: 10.1037//0096-1523.11.2.122. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol. 2000;84:2113–2132. doi: 10.1152/jn.2000.84.4.2113. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Gu Y, Deangelis GC. Multisensory integration: psychophysics, neurophysiology, and computation. Curr Opin Neurobiol. 2009;19:1–7. doi: 10.1016/j.conb.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstis S, Verstraten FA, Mather G. The motion aftereffect. Trends Cogn Sci. 1998;2:111–117. doi: 10.1016/s1364-6613(98)01142-5. [DOI] [PubMed] [Google Scholar]
- Barlow HB. A theory about the functional role and synaptic mechanism of visual aftereffects. In: Blakemore C, editor. Vision: coding and efficiency. Cambridge University Press; Cambridge: 1990. pp. 363–375. [Google Scholar]
- Barnett-Cowan M, Harris LR. Perceived timing of vestibular stimulation relative to touch, light and sound. Exp Brain Res. 2009;198:221–231. doi: 10.1007/s00221-009-1779-4. doi:10.1007/s00221-009-1779-4. [DOI] [PubMed] [Google Scholar]
- Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med. 1986;57:1088–1096. [PubMed] [Google Scholar]
- Benson AJ, Hutt EC, Brown SF. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med. 1989;60:205–213. [PubMed] [Google Scholar]
- Bertolini G, Bockisch CJ, Straumann D, Zee DS, Ramat S. Do humans show velocity-storage in the vertical rVOR? Prog Brain Res. 2008;171:207–210. doi: 10.1016/S0079-6123(08)00628-6. doi:10.1016/S0079-6123(08)00628-6. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, Palla A. Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol. 2011;105:209–223. doi: 10.1152/jn.00154.2010. doi:10.1152/jn.00154.2010. [DOI] [PubMed] [Google Scholar]
- Bestelmeyer PE, Rouger J, DeBruine LM, Belin P. Auditory adaptation in vocal affect perception. Cognition. 2010;117:217–223. doi: 10.1016/j.cognition.2010.08.008. doi:10.1016/j.cognition.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Clement G, Moore ST, Raphan T, Cohen B. Perception of tilt (somatogravic illusion) in response to sustained linear acceleration during space flight. Exp Brain Res. 2001;138:410–418. doi: 10.1007/s002210100706. [DOI] [PubMed] [Google Scholar]
- Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res. 1992;90:526–538. doi: 10.1007/BF00230935. [DOI] [PubMed] [Google Scholar]
- Crane BT, Demer JL. Effect of adaptation to telescopic spectacles on the initial human horizontal vestibuloocular reflex. J Neurophysiol. 2000;83:38–49. doi: 10.1152/jn.2000.83.1.38. [DOI] [PubMed] [Google Scholar]
- Eatock RA. Adaptation in hair cells. Annu Rev Neurosci. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. doi:10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- Ercoline WR, Devilbiss CA, Yauch DW, Brown DL. Post-roll effects on attitude perception: “the Gillingham Illusion”. Aviat Space Environ Med. 2000;71:489–495. [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976;39:970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- Fetsch CR, Turner AH, Deangelis GC, Angelaki DE. Dynamic re-weighting of visual and vestibular cues during self-motion perception. J Neurosci. 2009;29:15601–15612. doi: 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gescheider GA, Wright JH. Effects of vibrotactile adaptation on the perception of stimuli of varied intensity. J Exp Psychol. 1969;81:449–453. doi: 10.1037/h0027888. [DOI] [PubMed] [Google Scholar]
- Gianna CC, Heimbrand S, Nakamura T, Gresty MA. Thresholds for perception of lateral motion in normal subjects and patients with bilateral loss of vestibular function. Acta Otolaryngol Suppl. 1995;520(Pt 2):343–346. doi: 10.3109/00016489509125266. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The perception of the visual world. Houghton Mifflin; Boston: 1950. [Google Scholar]
- Glasser DM, Tsui JM, Pack CC, Tadin D. Perceptual and neural consequences of rapid motion adaptation. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1101141108. doi:10.1073/pnas.1101141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Gu Y, Fetsch CR, Adeyemo B, Deangelis GC, Angelaki DE. Decoding of MSTd population activity accounts for variations in the precision of heading perception. Neuron. 2010;66:596–609. doi: 10.1016/j.neuron.2010.04.026. doi: 10.1016/j.neuron.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LR, Morgan MJ, Still AW. Moving and the motion after-effect. Nature. 1981;293:139–141. doi: 10.1038/293139a0. [DOI] [PubMed] [Google Scholar]
- Hershenson M. Duration, time constant, and decay of the linear motion aftereffect as a function of inspection duration. Percept Psychophys. 1989;45:251–257. doi: 10.3758/bf03210704. [DOI] [PubMed] [Google Scholar]
- Hess BJ, Angelaki DE. Inertial vestibular coding of motion: concepts and evidence. Curr Opin Neurobiol. 1997;7:860–866. doi: 10.1016/s0959-4388(97)80147-x. [DOI] [PubMed] [Google Scholar]
- Johansson G. Studies on visual perception of locomotion. Perception. 1977;6:365–376. doi: 10.1068/p060365. [DOI] [PubMed] [Google Scholar]
- Kanai R, Verstraten FA. Perceptual manifestations of fast neural plasticity: motion priming, rapid motion aftereffect and perceptual sensitization. Vision Res. 2005;45:3109–3116. doi: 10.1016/j.visres.2005.05.014. doi:10.1016/j.visres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Lundstrom RJ. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to vibration. Scand J Work Environ Health. 1986;12:413–416. [PubMed] [Google Scholar]
- Lyons TJ, Ercoline WR, Freeman JE, Gillingham KK. Classification problems of U.S. Air Force spatial disorientation accidents, 1989–9191. Aviat Space Environ Med. 1994;65:147–152. [PubMed] [Google Scholar]
- MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE. Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci. 2010;30:9084–9094. doi: 10.1523/JNEUROSCI.1304-10.2010. doi:10.1523/JNEUROSCI.1304-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng. 2006;53:2362–2372. doi: 10.1109/TBME.2006.883645. doi:10.1109/TBME.2006.883645. [DOI] [PubMed] [Google Scholar]
- Miles FA, Eighmy BB. Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol. 1980;43:1406–1425. doi: 10.1152/jn.1980.43.5.1406. [DOI] [PubMed] [Google Scholar]
- Nooij SA, Groen EL. Rolling into spatial disorientation: simulator demonstration of the post-roll (Gillingham) illusion. Aviat Space Environ Med. 2011;82:505–512. doi: 10.3357/asem.2946.2011. [DOI] [PubMed] [Google Scholar]
- Ohmi M, Howard IP. Effect of stationary objects on illusory forward self-motion induced by a looming display. Perception. 1988;17:5–11. doi: 10.1068/p170005. [DOI] [PubMed] [Google Scholar]
- Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol. 1991;65:1170–1182. doi: 10.1152/jn.1991.65.5.1170. [DOI] [PubMed] [Google Scholar]
- Pinkus A, Pantle A. Probing visual motion signals with a priming paradigm. Vision Res. 1997;37:541–552. doi: 10.1016/s0042-6989(96)00162-9. [DOI] [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1979;35:229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Reinhardt-Rutland AH. Increasing-loudness aftereffect following decreasing-intensity adaptation: spectral dependence in interotic and monotic testing. Perception. 1998;27:473–482. doi: 10.1068/p270473. [DOI] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol. 2012 doi: 10.1007/s10162-012-0318-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizova-Cajic T, Smith JL, Taylor JL, Gandevia SC. Proprioceptive movement illusions due to prolonged stimulation: reversals and aftereffects. PLoS ONE. 2007;2:e1037. doi: 10.1371/journal.pone.0001037. doi:10.1371/journal.pone.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno T, Ito H, Sunaga S. Vection aftereffects from expanding/contracting stimuli. Seeing perceiving. 2010;23:273–294. doi: 10.1163/187847510x532667. [DOI] [PubMed] [Google Scholar]
- Soyka F, Robuffo Giordano P, Beykirch K, Bulthoff HH. Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2011;209:95–107. doi: 10.1007/s00221-010-2523-9. doi:10.1007/s00221-010-2523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P, Burr D. Visual aftereffects. Current biology: CB. 2009;19:R11–R14. doi: 10.1016/j.cub.2008.10.014. doi:10.1016/j.cub.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, McConville KM, Na EQ. Behavior of cells without eye movement sensitivity in the vestibular nuclei during combined rotational and translational stimuli. J Vestib Res. 1996;6:145–158. [PubMed] [Google Scholar]
- Ullman S, Schechtman G. Adaptation and gain normalization. Proceedings of the Royal Society of London. Series B, Containing papers of a biological character. Royal Soc. 1982;216:299–313. doi: 10.1098/rspb.1982.0076. [DOI] [PubMed] [Google Scholar]
- Wade NJ. A selective history of the study of visual motion aftereffects. Perception. 1994;23:1111–1134. doi: 10.1068/p231111. [DOI] [PubMed] [Google Scholar]
- Wallach H, Flaherty EW. A compensation for field expansion caused by moving forward. Percept Psychophys. 1975;17:445–449. [Google Scholar]
- Walsh EG. Role of the vestibular apparatus in the perception of motion on a parallel swing. J Physiol. 1961;155:506–513. doi: 10.1113/jphysiol.1961.sp006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Webber RM, Stanley GB. Thalamic synchrony and the adaptive gating of information flow to cortex. Nat Neurosci. 2010;13:1534–1541. doi: 10.1038/nn.2670. doi:10.1038/nn.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001a;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001b;63:1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- Young LR, Oman CM. Model for vestibular adaptation to horizontal rotation. Aerosp Med. 1969;40:1076–1080. [PubMed] [Google Scholar]