Abstract
A series of ring-constrained (N)-methanocarba-5′-uronamide 2,N6-disubstituted adenine nucleosides have been synthesized via Mitsunobu condensation of the nucleobase precursor with a pseudosugar ring containing a 5′-ester functionality. Following appropriate functionalization of the adenine ring, the ester group was converted to the 5′-N-methylamide. The compounds, mainly 2-chloro substituted derivatives, were tested in both binding and functional assays at human adenosine receptors (ARs), and many were found to be highly potent and selective A3AR agonists. Selected compounds were compared in binding to the rat A3AR to assess their viability for testing in rat disease models. The N6-(3-chlorobenzyl) and N6-(3-bromobenzyl) analogues displayed Ki values at the human A3AR of 0.29 and 0.38 nM, respectively. Other subnanomolar affinities were observed for the following N6 derivatives: 2,5-dichlorobenzyl, 5-iodo-2-methoxybenzyl, trans-2-phenyl-1-cyclopropyl, and 2,2-diphenylethyl. Selectivity for the human A3AR in comparison to the A1AR was (fold): the N6-(2,2-diphenylethyl) analogue 34 (1900), the N6-(2,5-dimethoxybenzyl) analogue 26 (1200), the N6-(2,5-dichlorobenzyl) and N6-(2-phenyl-1-cyclopropyl) analogues 20 and 33 (1000), and the N6-(3-substituted benzyl) analogues 17, 18, 28, and 29 (700–900). Typically, even greater selectivity ratios were obtained in comparison with the A2A and A2BARs. The (N)-methanocarba-5′-uronamide analogues were full agonists at the A3AR, as indicated by the inhibition of forskolin-stimluated adenylate cyclase at a concentration of 10 µM. The N6-(2,2-diphenylethyl) derivative was an A3AR agonist in the (N)-methanocarba-5′-uronamide series, although it was an antagonist in the ribose series. Thus, many of the previously known groups that enhance A3AR affinity in the 9-riboside series, including those that reducing intrinsic efficacy, may be adapted to the (N)-methanocarba nucleoside series of full agonists.
Introduction
There are four subtypes of adenosine receptors (ARs): A1, A2A, A2B, and A3.1 Each of these subtypes is associated with specific experimental therapeutic modalities.2 Selective adenosine receptor antagonists and agonists are under development for treating such diverse conditions including inflammation, cardiac ischemia, stroke, asthma, diabetes, cardiac arrhythmias, and other disorders.3–7 Agonists selective for the A3AR are potentially useful for the treatment of stroke, neurodegenerative diseases, myocardial infarction, and cancer.4,5,8–10 In colon carcinoma, a cytostatic effect of an A3AR agonist appears to be related to its downstream activation of the Wnt pathway.10 A3AR antagonists are potentially useful for the treatment of glaucoma.11
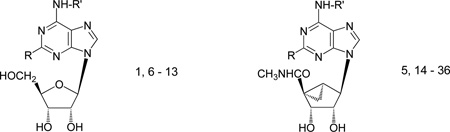
Prototypical high affinity ligands for the A3AR include the N6-(3-iodobenzyl) adenosine analogues 1 – 5 (Chart 1).12 The moderately A3AR-selective nucleoside IBMECA 2a (N6-(3-iodobenzyl)-5′-N-methylcarboxamidoadenosine) is a full agonist,13 which is currently in phase 2 clinical trials for the treatment of melanoma and colon carcinoma.10 Also, chronically administered 2a was neuroprotective in a model of ischemic brain damage in gerbils.5 The corresponding 2-chloro derivative Cl-IB-MECA 2b was the first highly selective A3AR agonist14 and has been shown to be cardioprotective in a variety of in vitro and in vivo models.4,15,16 In an SAR study of adenosine derivatives,13 the N6-(3-iodobenzyl) substituent was found to enhance the selectivity for the A3AR. Also, the 2-chloro modification has been found to enhance potency in a variety of AR agonists. When present as the sole modifications of adenosine, as in the 9-riboside derivatives 1a and 1b, they reduced the intrinsic efficacy at the A3AR, to the extent that 2-chloro-9-riboside 1b was an antagonist of this subtype. Rigidity introduced in the ribose moiety also tends to reduce A3AR efficacy, such as the (N)-methanocarba ring system, a bridged carbocyclic ring system present in the partial agonists 3 and 4 and in the full agonist 5.17,18 Structural features that reduced efficacy included N6-benzyl groups, small moieties at the 2-position, and sterically constrained moieties in the ribose region. For each of these efficacy-diminishing factors, the 5′-uronamide group tended to restore the observed loss of A3AR efficacy, as observed in the full agonism displayed by 2 and 5. There is still a need for the development of more highly selective A3AR agonists than the widely used A3AR agonists 2a and 2b, for which some literature reports have indicated limited pharmacological selectivity.19,25
Chart 1.
Chemical structures of adenosine derivatives used in the present study and containing a 9-ribose moiety (1), a 9-ribose-5′-N-methyluronamide moiety (2), or an (N)-methanocarba moiety (3 - 5).
The present study characterizes the SAR (structure activity relationship) of a broad class of highly potent and selective A3AR agonists based on compound 5. The principal design element of these agonists is a modified ribose moiety. The analogues contain the (N)-methanocarba (bicyclo[3.1.0]hexane) ring system, which is a rigid ribose substitute lacking the ether oxygen. This ring system maintains the 2′-exo-(N) ring-twist conformation of the ribose-like ring (pseudosugar moiety), which has been demonstrated to be favored in A3AR binding (more so than at other AR subtypes).20 These agonists also contain a flexible 5′-uronamide group, necessary to assure full activation of the A3AR.12 We utilized a new synthetic route for the synthesis of these derivatives21,22 which allows for a more versatile substitution of the adenine moiety than the previous synthetic route.23 In addition, we incorporated N6-substituents that have been shown previously to favor A3AR binding affinity—mainly N6-benzyl and N6-phenylethyl24 groups—and included new variations thereof. These derivatives were characterized biologically at the four human AR subtypes expressed in Chinese hamster ovary (CHO) cells25 in binding and functional assays to assess their selectivity within the same species. The results indicate that we achieved the design of many new, sterically-constrained and selective A3AR agonists with nanomolar and subnanomolar affinities. Several of these high affinity agonists were docked in the putative binding site of a rhodopsin26-based model of the human A3AR.18,24,27
RESULTS
Chemical Synthesis
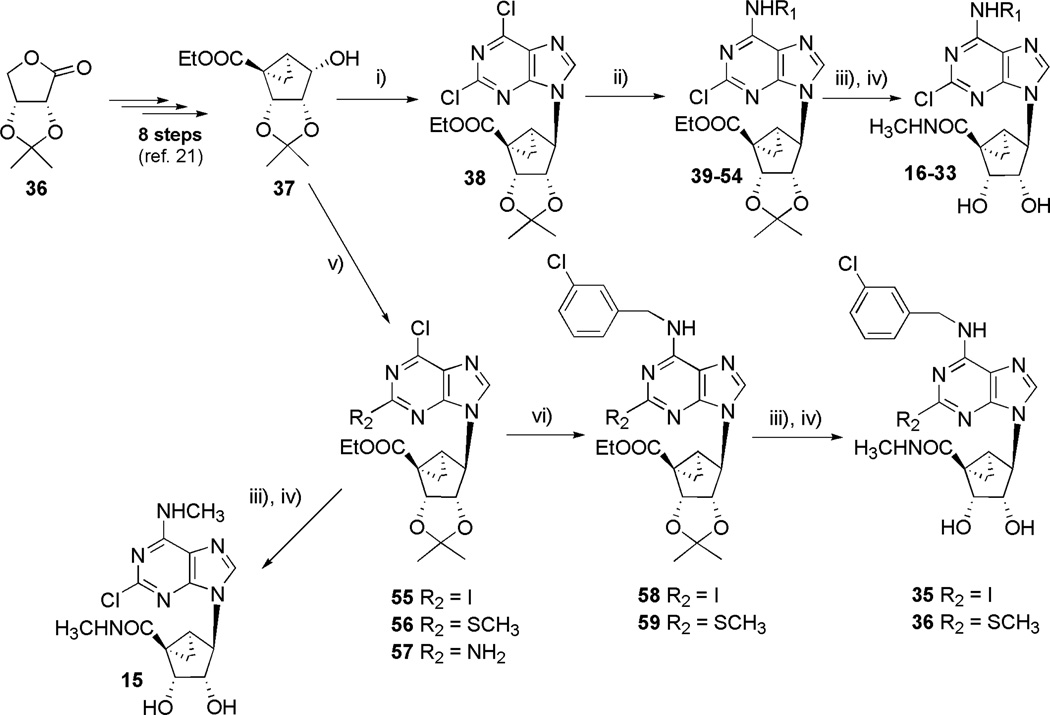
The novel derivatives 15 and 17 - 36 were synthesized as shown in Schemes 1 – 3. Most analogues contained a 2-chloro substituent, although 2-amino 15, 2-iodo 35, and 2-alkylthio 36 groups were also included.
Scheme 1.
Reagents and conditions: i) 2,6-dichloropurine, DIAD, TPP, THF, r.t.; ii) RNH2, TEA, MeOH; iii) 40% aq. MeNH2, MeOH; iv) 10% CF3COOH in MeOH, H2O, 70°C; v) 6-chloro-2-iodopurine or 6-chloro-2-methylthiopurine or 6-chloro-2-aminopurine, DIAD, TPP, THF, r.t.; vi) 3-chlorobenzylamine, TEA, MeOH.
Scheme 3.
Reagents and conditions: i) tert-butyl nitrite, methyl disulphide, acetonitrile, r.t. ii) aq. NaOH, i-PrOH,THF.
The general synthetic route follows the method of Joshi et al.21,22 The protected (N)-methanocarba ring system 37 containing a carbonyl group at the 5′-position was prepared in 8 steps from isopropylidene erythronolactone 36 (Scheme 1). Compound 37 and the nucleobase precursor, 2,6-dichloropurine, were condensed using a Mitsunobu coupling.28 Substitution of the 6-chloro of 38 was then carried out by treating with an excess of the appropriate amine, such as a substituted benzylamine, to provide the series of protected nucleoside 5′-esters 39 – 54. Following appropriate substitution at the N6 position, the 5′-esters were treated with an excess of methylamine, and the isopropylidene group was removed from the 2′- and 3′-hydroxyl groups upon acid treatment to provide the target compounds 16 - 33.
The various benzylamine derivatives used in this study, when not commercially available, were synthesized by the methods shown in Scheme 2. 2,5-Dichlorobenzylamine 64 and 2-chloro-5-iodobenzylamine 65 were prepared from the corresponding benzyl bromides. Compound 60 was prepared from 2-chloro-5-iodotoluene by bromination with N-bromosuccinimide in CCl4.29 60 and 61 were treated with potassium phthalimide in N,N-dimethylformamide at 60°C to obtain compounds 62 and 63 as white solids in 95% yield. Deprotection with hydrazine gave 64 and 65 in 80% yield.30
Scheme 2.
Reagents and conditions: i) NBS, benzoyl peroxide in dry CCl4, reflux, 3h; ii) potassium phthalimide, in dry DMF at 80°C, 3h; iii) NH2NH2, in EtOH; reflux, 24h; iv) CH3I, K2CO3 in DMF at r.t. overnight; v) AcONH4, NaCNBH3 in dry MeOH, 48h; vi) CuI, (PPh3)2PdCl2, in dry Et2NH; vii) benzyl bromide, K2CO3 in DMF at r.t. overnight; viii) LiAlH4, in dry THF, reflux, 3h; ix) (Boc)2O, 10% Et3N in MeOH, 45°C, 1 h; x) 2-bromoacetamide, K2CO3 in DMF at r.t. overnight; xi) 15% TFA in CH2Cl2, 45 min.
5-Iodo-2-methoxybenzylamine 68 was prepared starting from 5-iodosalicylaldehyde 66. Accordingly, compound 66 was methylated with methyl iodide in the presence of K2CO3 in N,N-dimethylformamide and subsequently, the formyl moiety was transformed to methylamino by reductive amination with NaCNBH4 in the presence of ammonium acetate.31
Compounds 72 and 73 were synthesized through a Sonogashira-type reaction in high yield using CuI and (Ph3P)PdCl2 as catalysts. 32 For the preparation of both 5-chloro-2-benzyloxybenzylamine 76 and 5-chloro-2-(aminocarbonylmethyloxy)benzylamine 80, 5-chloro-2-hydroxybenzamide 74 was used as starting material. Compound 74 was treated with benzyl bromide in the presence of K2CO3 in N,N-dimethylformamide to obtain 5-chloro-2-benzyloxybenzamide 75 in 98% yield. Finally, the amide 75 was reduced to the corresponding amine 76 with LiAlH4 in tetrahydrofuran.
Reduction of compound 74 with LiAlH4 in tetrahydrofuran gave 5-chloro-2-hydroxybenzylamine 77 in 88% yield. This preparation showed an improvement over the reported procedure, based on the hydrogenation of 5-chloro-2-hydroxybenzaldehyde oxime.33 The amine 77 was protected as a t-butylcarbamate 78, and the hydroxyl group was alkylated with 2-bromoacetamide in the presence of K2CO3 in N,N-dimethylformamide. The subsequent deprotection of the amino group with 15% trifluoroacetic acid in dichloromethane gave the final product 80 as a white solid.
Substitution at the 2-position by groups other than chloro was possible and accomplished by condensing the appropriate 2-substituted nucleobases with the (N)-methanocarba sugar moiety 37 to provide the protected nucleoside esters 55 and 56 Scheme 1). Successive treatment with 3-chlorobenzylamine and methylamine followed by subsequent acid hydrolysis afforded the 2-iodo and 2-thiomethyl analogues 35 and 36. Alternately, the 2-amino substituted analogue was obtained by first condensing the 2-amino substituted nucleobase with 37 followed by the direct interaction with excess methylamine and subsequent hydrolysis to afford 15.
The requisite 6-chloro-2-iodopurine was prepared by a literature procedure,35 while 6-chloro-2-methylthiopurine was prepared as delineated in Scheme 3. Accordingly, 6-chloro-2-methylthiopurin-9-yl-methyl 2,2-dimethylpropionate34 81 was diazotized with tert-butyl nitrite, and the resulting diazo intermediate was trapped with an excess of methyl disulfide to afford 82, which upon hydrolysis of the pivaloylmethyloxy protecting group with aqueous NaOH, gave the requisite 2-methylthiopurine 83.
Biological Activity
The AR binding affinities of both newly-synthesized (15 and 17 – 36) and known (1 – 14 and 16) nucleoside derivatives (Table 1) was investigated. The known adenosine agonist derivatives included 9-ribosides 1, 6 – 12, (N)-methanocarba derivatives 3 - 5, and 9-riboside 5′-N-methyluronamide analogues 2a, 2b, 14, and 16. Binding affinity at the human A3AR, and in selected cases the rat A3AR, was studied using the radioiodinated agonist 125I-N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide, 36 and binding at the human A1AR and A2AAR utilized the selective agonists [3H]R-N6-[phenylisopropyl]adenosine and [3H]2-[p-(carboxyethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine, respectively. Functional assays of the human A2BAR and A3AR consisted of measuring cyclic AMP production in intact CHO cells expressing the ARs (Table 2).25 The functional effect of A3AR was inhibition of forskolin-stimulated cAMP production, and the A2BAR effect was stimulation of basal cAMP production.
Table 1.
Potency of adenosine derivatives at human A1, A2A, A2B and A3ARs and the rat A3AR and maximal agonist effects at human A3ARs expressed in CHO cells.a
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | N6-R′ | C2-R | Ki (hA1AR) nM a or % displ. at 10 µM |
Ki (hA2AAR) nM a or % displ. at 10 µM |
% Activation (hA2BAR)b at 10 µM |
Ki (hA3AR) nMa |
% Activation (hA3AR)b at 10 µM |
Ki (rA3AR) |
| Riboside derivatives | ||||||||
| 6c | CH3 | H | 6000 ± 2000 | 17% | 16 | 9.3 ± 0.4 | 96 ± 3 | 6400 ± 1600d |
| 7c | CH3 | NH2 | 480 ± 20 | 15% | 11 | 39 ± 2 | 98 ± 3 | >10,000 |
| 1a c | 3-iodobenzyl | H | 7.4 ± 1.7 | 140 ± 20 | 58 | 5.8 ± 0.4 | 46 ± 8 | 9.5 ± 1.4 |
| 1b c | 3-iodobenzyl | Cl | 17 ± 2 | 200 ± 30 | 16 | 1.8 ± 0.1 | 0 | 2.7 ± 1.2 |
| 8 | 3-chlorobenzyl | H | 20 ± 3 | 1400 ± 200 | ND | 4.4 ± 1.7d | 80 ± 3d | 35 ± 20d |
| 9 c | 5-chloro-2- methyloxybenzyl |
H | 9.2 ± 0.5 | 400 ± 10 | 57 | 1.3 ± 0.2 | 53 ± 3 | ND |
| 10 c |
trans-2-phenyl-1- cyclopropyl |
H | 120 ± 30 | 2500 ± 700 | 26 | 0.86 ± 0.09 | 101 ± 5 | 400 ± 30 |
| 11d | 2-methylbenzyl | H | 39 ± 8 | 760 ± 110 | ND | 47 ± 11 | 100 ± 3 | 38 ± 15 |
| 12 d | 3-pyridylmethyl | H | 42 ± 4 | 2300 ± 120 | ND | 4.5 ± 1.1 | 100 ± 6 | 290 ± 70 |
| 13 f | 2,2-diphenylethyl | H | 50 ± 16 | 510 ± 50 | 70 | 3.9 ± 0.7 | 0 | 540 ± 200 |
|
5′-N-Methyluronamido riboside derivatives |
||||||||
| 2a | 3-iodobenzyl | H | 51 ± 5 | 2900 ± 600d | 0h | 1.8 ± 0.7d | 100 | 1.1 ± 0.3i |
| 2b | 3-iodobenzyl | Cl | 220 ± 20 | 5400 ± 2500d | 0h | 1.4 ± 0.3d | 100 ± 4 | 0.33 ± 0.08i |
| (N)-Methanocarba derivatives | ||||||||
| 3 | 3-iodobenzyl | H | 35 ± 3 | 860 ± 70 | ND | 9.2 ± 0.7d | 13 ± 1 | ND |
| 4 | 3-iodobenzyl | Cl | 65 ± 17 | 1600 ± 400 | ND | 1.9 ± 0.7d | 3 ± 2 | ND |
|
5′-N-Methyluronamido (N)-methanocarba derivatives |
||||||||
| 14 | CH3 | Cl | 2100 ± 1700 | 6% | ND | 2.2 ± 0.6 | 104 ± 7 | 160 ± 30 |
| 15 | CH3 | NH2 | 1600 ± 200 | 17% | ND | 1.6 ± 0.3 | 117 ± 21 | 0.87 ± 0.24 |
| 16 | 3-iodobenzyl | H | 700 ± 270 | 6200 ± 100 | ND | 2.4 ± 0.5e | 100e | ND |
| 5 | 3-iodobenzyl | Cl | 136 ± 22 g | 784 ± 97g | ND | 1.5 ± 0.2e | 100e | 1.1 ± 0.1 |
| 17 | 3-bromobenzyl | Cl | 270 ± 70 | 1300 ± 100 | 38 | 0.38 ± 0.11 | 100 ± 11 | 0.76 ± 0.08 |
| 18 | 3-chlorobenzyl | Cl | 260 ± 60 | 2300 ± 100 | 38 | 0.29 ± 0.04 | 103 ± 7 | 1.0 ± 0.10 |
| 19 | 3-fluorobenzyl | Cl | 640 ± 140 | 5100 ± 200 | 28 | 2.4 ± 0.1 | 101 ± 5 | 1.6 ± 0.1 |
| 20 | 2,5-dichloro-, benzyl |
Cl | 540 ± 70 | 1300 ± 100 | 32 | 0.56 ± 0.06 | 102 ± 3 | ND |
| 21 | 2-chloro-5- iodobenzyl |
Cl | 340 ± 20 | 480 ± 20 | 58 | 0.83 ± 0.19 | 105 ± 6 | ND |
| 22 | 5-chloro-2- methoxybenzyl |
Cl | 240 ± 50 | 1200 ± 100 | 37 | 1.5 ± 0.0 | 107 ± 15 | ND |
| 23 | 5-chloro-2- (aminocarbonyl- methyloxy)benzyl |
Cl | 81 ± 10 | 800 ± 70 | ND | 5.3 ± 0.4 | 106 ± 12 | ND |
| 24 | 5-chloro-2- benzyloxybenzyl |
Cl | 1200 ± 200 | 850 ± 160 | 49 | 5.2 ± 1.3 | 110 ± 10 | 11 ± 3 |
| 25 | 5-iodo-2- methoxybenzyl |
Cl | 200 ± 20 | 430 ± 30 | 46 | 0.58 ± 0.12 | 101 ± 10 | 0.87 ± 0.24 |
| 26 | 2,5-dimethoxy- benzyl |
Cl | 1600 ± 200 | 52% | 0 | 1.4 ± 0.2 | 107 ± 10 | ND |
| 27 | 2-methylbenzyl | Cl | 710 ± 300 | 3800 ± 200 | 24 | 3.7 ± 0.6 | 100 ± 3 | ND |
| 28 | 3-methylbenzyl | Cl | 450 ± 80 | 3700 ± 600 | 46 | 0.63 ± 0.18 | 106 ± 3 | ND |
| 29 | 3-(3-hydroxypropynyl) benzyl |
Cl | 2600 ± 300 | 56% | 28 | 2.9 ± 0.7 | 102 ± 5 | 1.6 ± 0.6 |
| 30 | 2-chloro-5-(3- hydroxypropynyl) benzyl |
Cl | 14% | 12% | 6 | 217 ± 27 | 108 ± 5 | ND |
| 31a | 4-aminobenzyl | Cl | 4100 ± 100 | 28% | 22 | 14 ± 3 | 98 ± 10 | ND |
| 31b | 4-amino-3- iodobenzyl |
Cl | 48 ± 2 | 1100 ± 100 | ND | 3.1 ± 0.1 | 103 ± 7 | ND |
| 32 | 3-pyridylmethyl | Cl | 2600 ± 1200 | 33% | 4 | 11 ± 3 | 106 ± 7 | ND |
| 33 |
trans-2-phenyl-1- cyclopropyl |
Cl | 770 ± 50 | 4800 ± 200 | 48 | 0.78 ± 0.06 | 110 ± 7 | ND |
| 34 | 2,2-diphenylethyl | Cl | 1300 ± 100 | 1600 ± 100 | 46 | 0.69 ± 0.02 | 107 ± 9 | 10 ± 4 |
| 35 | 3-chlorobenzyl | I | 2200 ± 600 | 43% | ND | 3.6 ± 0.8 | 107 ± 3 | 3.9 ± 0.4 |
| 36 | 3-chlorobenzyl | SCH3 | 610 ± 40 | 52% | ND | 1.5 ± 0.2 | 100 ± 4 | ND |
All AR experiments were performed using adherent CHO cells stably transfected with cDNA encoding the human or rat ARs. Percent activation of the human A3AR was determined at 10 µM. Binding at human A1 and A2AARs in this study was carried out as described in Methods using [3H]R-PIA or [3H]CGS 21680 as a radioligand. Values from the present study are expressed as mean ± s.e.m., n = 3–5.
Percent activity at 10 µM, relative to 10 µM Cl-IB-MECA (A3).
Data from Ohno et al. 18
Data from Lee et al. 23
Data from Tchilibon et al.24
Data from Jacobson et al.40
Data from de Zwart et al.54
Data from Kim et al.14
ND not determined.
Cristalli and coworkers37 and other investigators18,23,38 have established that N6-methylation of adenosine analogues tends to increase the human A3AR affinity and selectivity. In the (N)-methanocarba series, the N6-methyl-2-chloro derivative 14 was 3-fold more potent in A3AR binding in the present study than reported previously.23 The replacement of 2-chloro with a 2-amino group to provide 15 increased the human A3AR affinity slightly. Compound 15 was 24-fold more potent in A3AR binding than the 9-riboside equivalent 7.18
The 3-iodobenzyl group has long been known to enhance affinity and/or selectivity at the A3AR.13,14 In the (N)-methanocarba series, compound 16, reported previously,12,23 was confirmed to be selective for the human A3AR (300-fold in comparison to the human A1AR). Addition of the 2-chloro group resulting in the previously reported agonist 5 having slightly enhanced A3AR affinity, as was seen also for the pairs of compounds 1a and 1b and compounds 2a and 2b. Substitution at the 3-position with other halo atoms further increased human A3AR affinity (unlike the ribose 5′-uronamide case, in which 3-iodo was optimal13). The optimal human A3AR affinity in this series was achieved with the 3-chloro substitution in 18 with a Ki value of 0.29 nM. Compound 18 was selective for the human A3AR in comparison to the human A1 and A2AAR by 890- and 7800-fold, respectively. The A3AR selectivity of 18 in comparison to the A2BAR was >10,000-fold. There was a trend toward an increase of human A3AR affinity and a decrease of affinity at the other AR subtypes with decreasing size of the halo atom, except for the 3-fluoro analogue, which displayed a Ki value at the A3AR of 2.4 nM. The affinity of the 3-halobenzyl analogues at the rat A3AR was less variable than at the corresponding human receptor, and compounds 5 and 17 – 19 all displayed Ki values within the range of 0.8 – 1.6 nM. Dihalo substitution of the benzyl ring in the 2,5-dichloro analogue 20 and the 2-chloro-5-iodobenzyl analogue 21 maintained subnanomolar affinity at the human A3AR. The 5-chloro substitution combined with an ether group at the 2-position (i.e. methyl ether 22, aminocarbonylmethyl ether 23, or benzyl ether 24) also maintained moderately high affinity at the A3AR. The bulky benzyl ether 24 was 230-fold selective for the human A3AR in comparison to the A1AR. The 5-iodo equivalent of the 5-chloro-2-methoxy derivative 22, i.e. compound 25, was 3-fold more potent at the A3AR upon halogen exchange. However, the affinity enhancement observed upon replacing m-chloro with m-iodo was not general. For example, with the mono-substituted benzyl derivatives compounds 5 and 18, the 3-chloro analogue 18 was 5-fold more potent than the corresponding 3-iodo 5. Replacement of this 5-chloro atom in compound 22 with methoxy, e.g., 26, did not change the A3AR affinity; however, the A3AR selectivity improved.
The N6-(2-methylbenzyl) group is present in metrifudil 11, a nonselective adenosine agonist that previously had been in clinical trials for its hypotensive effect.39 In the 2-chloro-(N)-methanocarba series, this substitution in 27 provided high affinity at the A3AR and selectivity (190-fold in comparison to the A1AR). Placement of the methyl group at the 3-position in 28 enhanced the affinity at the A3AR by 3-fold to give a Ki value of 0.63 nM. Inclusion of a 3-hydroxypropynyl group at the meta position of the benzyl ring in 29 produced with high affinity binding to both the human and rat A3AR, but curiously when a 2-chloro substitution of the benzyl ring was included in 30 the affinity was significantly (76-fold) reduced.
The high affinity radioiodinated tracer used in this study for binding to the A3AR, I-AB-MECA, contains an N6-(4-aminobenzyl) group.32 Compound 31a in the present series of 2-chloro-(N)-methanocarba derivatives contained the same substituent group, and displayed a Ki value at the A3AR of 14 nM. The compound was A3AR selective by 300-fold in comparison to the A1AR. Following direct iodination of 31a to form 31b, the A3AR affinity increased by 4-fold, however the selectivity was greatly reduced.
More diverse aromatic substitution at the 6-position was present in compounds 32– 34. N6-(3-pyridylmethyl)adenosine 12 displayed a relatively high affinity at the A3AR.17 Unlike the various N6-benzyl analogues, the 3-pyridylmethyl substitution in the (N)-methanocarba analogue 32 resulted in a 2.5-fold reduction in affinity in comparison to the simple 9-riboside 12. The trans-2-phenyl-1-cyclopropyl analogue 33 (Ki 0.78 nM) was equipotent to the 9-riboside 10, while the 2,2-diphenylethyl analogue 34 (Ki 0.69 nM) was 6-fold more potent than the corresponding 9-riboside 13. In the case of 34, applying the (N)-methanocarba 5′-uronamide modification converted an A3AR antagonist 13 into a full agonist.
High selectivity for the A3AR was achieved for many of the (N)-methanocarba analogues. Selectivity for the A3AR in comparison to the A1AR was (fold): the N6-(2,2-diphenylethyl) analogue 34 (1900), the N6-(2,5-dimethoxybenzyl) analogue 26 (1200), the N6-(2,5-dichlorobenzyl) and N6-(2-phenyl-1-cyclopropyl) analogues 20 and 33 (1000), and the N6-(3-substituted benzyl) analogues 17, 18, 28, and 29 (700–900).
The N6-moiety providing the highest affinity at the human A3AR was introduced into several analogues bearing substituents at the 2-position of the adenine ring. Although the 2-chloro group provided some enhancement of affinity over no substituent, the 2-iodo 35 and 2-methylthio 36 analogues were 12- and 5-fold less potent, respectively, than the corresponding 2-chloro analogue 18. Nevertheless, the selectivity for the human A3AR in comparison to the human A1AR remained high (590- and 390-fold, respectively).
Molecular Modeling
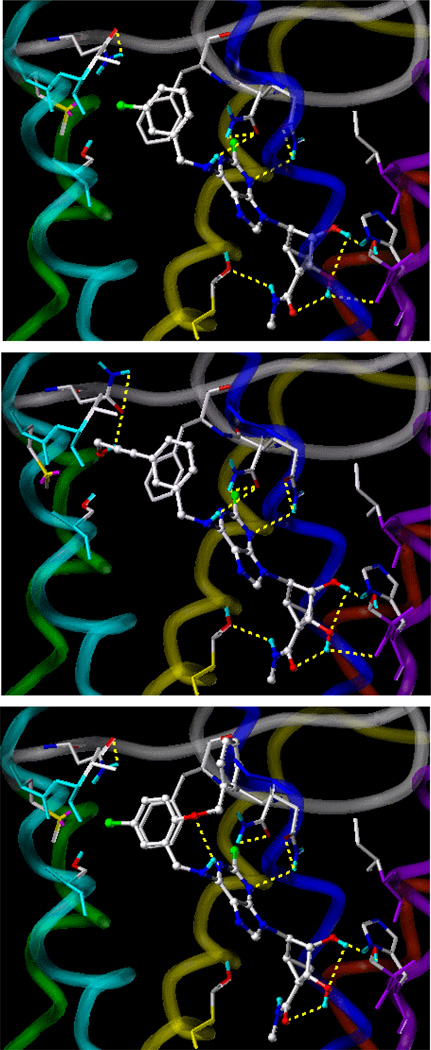
To determine an energetically favorable binding region and orientation of compound 18, the previously reported hA3AR complex with agonist 2b was used as the starting geometry for the ribose binding position.12 Different positions of the N6-substituent were examined with the ribose moiety anchored in the putative binding site, and the resulting energies of complex with different conformers were compared. The optimized docking mode of compound 18 is shown in Figure 1A. Residues that were within 5 Å proximity to the ligand in this putative binding site were the same residues for docked A3AR agonists containing a ribose moiety.12, 62
Figure 1.
The complex of the hA3AR with (A) 18, (B) 24, and (C) 29 in the putative binding site. The nucleosides are displayed as ball-and-stick models, and the side chains of hA3AR are shown as stick models. The H-bonding between ligand and hA3AR is displayed in yellow. The A3AR is represented by a tube model with a different color for each TM domain (TM3 in yellow, TM4 in green, TM5 in cyan, TM6 in blue, TM7 in purple). Residues that interacted with ligand in the putative binding site were; T94 (3.36), N150 (EL2), Q167 (EL2), F168 (EL2), S181 (5.42), M177 (5.38), V178 (5.39), N250 (6.55), I268 (7.39), S271 (7.42), and H272 (7.43).
As shown in Figure 1, the purine ring of 18 was surrounded by a hydrophobic pocket defined by L91 (3.33) and L246 (6.51). In addition, certain orientations placed atoms within H-bonding distance, such as the exocyclic amine acting as donor and the side chain carbonyl group of N250 (6.55) at 2.7 Å, and between the purine N3 atom and the side chain of Q167 (EL2) at 2.8 Å. The Cl atom at the C-2 position was surrounded by hydrophobic residues, L91 (3.33), the methyl group of T87 (3.29) and the aromatic ring of F168 in EL2, and was also proximal to the hydrophilic residue Q167 (EL2). The 2′-OH group of the ribose moiety was within H-bonding distance from the backbone carbonyl group of I268 (7.39) at 2.8 Å and the side chain of H272 (7.43) at 2.5 Å. The 3′-OH group could form an H-bond with the backbone carbonyl group of S271 (7.42) at 2.7 Å. Thus, there were some difference in putative H-bonding of the hydroxyl groups in the (N)-methanocarba analogues compared with 9-ribosides such as 2b, which displayed a putative H-bond between the 3′-OH group and H272 (7.43).12,14 Also, putative H-bonding was observed between the 5′-amide nitrogen and T94 (3.36) at 2.7 Å and between the 5′-carbonyl group and 3′-hydroxyl group at 2.3 Å. For binding at the N6-position, the benzyl moiety showed an additional hydrophobic interaction with F168 (EL2), and the chloro atom of the benzyl substituent was in proximity to M177 (5.38). This putative hydrophobic interaction was consistent with the high binding affinity of 28, which has a 3-methyl substituent on the benzyl ring. The receptor docking model suggested that 18 displayed more favorable van der Waals and electrostatic interactions than with the complexes of the N6-5-chloro-2-benzyloxybenzyl derivative 24 and the N6-3-(3-hydroxypropynyl)benzyl analogue 29.63,64
Discussion
Activation of the A3AR is increasingly associated with critical biological processes, such as its action on T-cells,41 dendritic cells,42 mast cells,43–45 natural killer cells,46 epithelial cells,47 and in the brain.48,49 This was accomplished by designing agonist nucleosides, with an awareness of the structural determinants of full agonism and of the preference for the (N)-ribose ring conformation in binding to the A3AR. The goal was to prepare novel, fully efficacious A3AR agonists having high affinity and selectivity. The (N)-methanocarba substitution of the ribose ring, resulting in rigidification of the pseudosugar moiety in a 2′-exo (N)-envelope conformation,20 preserves or enhances affinity at the A3AR to a greater degree than at other AR subtypes. However, the (N)-methanocarba substitution alone also appeared to reduce A3AR efficacy; thus, 3 and 4 were partial agonists. Therefore, we have included in these analogues the 5′-uronamido modification, which restores the loss of intrinsic efficacy observed with the (N)-methanocarba ring system and with various adenine substitutions (see below),12 without reducing the associated A3AR selectivity. The N-methyl 5′-uronamide was used, since this corresponds to the optimal 5′-substitution observed for the ribose series.13
At the N6-position and 2-position of the adenine ring, we have incorporated groups known to be associated with high A3AR potency, for example substituted N6-benzyl groups.13 These groups also tend to enhance A3AR potency in both human and rat, unlike the N6-methyl group, which favors human but not rat A3ARs.18 The N6-(3-iodobenzyl) group itself may lead to a reduction of efficacy, even in the absence of multiple substitutions of the adenine moiety. In the presence of an N6-substitution, such as 3-iodobenzyl or cyclopentyl, a 2-chloro group in adenosine analogues, in certain cases, leads to further reduced efficacy at the A3AR.9 The N6-substituted 2-chloroadenosine analogues, however, were full agonists when combined with the 5′-uronamido modification in the present (N)-methanocarba series.
The meta-position of the N6-benzyl ring in the ribose series is known as a favorable site for substitution. In general, analogues that were substituted at both 5- and 2-positions in the present study were highly potent and selective A3AR agonists. The comparable compounds with or without a 2-chloro group on the benzyl ring were generally similar in A3AR affinity (cf. m-iodo analogues 5 and 21 and m-chloro analogues 18 and 20).
A study of novel binary drugs50 that activate both A1 and A3ARs has concluded that a linear propynyl group favors A3AR selectivity when placed at the 3-position of the N6-benzyl ring in the ribose series. We wished to test if this finding extended to the (N)-methanocarba series. Compound 29 was similar in A3AR binding affinity (Ki 2.9 nM) to the other N6-benzyl substituted analogues. The loss of affinity upon 2-chloro substitution of the same N6-benzyl moiety in 30 (Ki 220 nM) may be explained in terms of restricted rotation of this ring due to the propynyl group, which might place the 2-chloro atom in an unfavorable position.
Several analogues were included that contain a substituted N6-(2-phenylethyl) moiety, known to produce high A3AR affinity. However, as confirmed in the present structural series, such modifications favor human but not rat A3ARs. The adenosine derivative (N6-(1S,2R)-(2-phenyl-1-cyclopropyl)adenosine), with a Ki value in binding to the hA3AR of 0.63 nM (38-fold more potent than the 1R,2S isomer) and high agonist potency,17, 24 constitutes a lead for the development of N6 derivatives of adenosine with high hA3AR affinity. We have utilized this modification in its trans, racemic form (as occurs in analogue 10 in Table 1), in the design of the highly hA3AR selective agonist 33. We have not resolved the diastereomers of 33, but it is likely that the 1S,2R-form is the more potent isomer.24 The N6-(2,2-diphenylethyl) analogue 34 was a full agonist and the most selective for the hA3AR in the present series. This suggests that the loss of A3AR efficacy seen in related nucleosides23 can be overcome and that further derivatization of the N6-(2-phenylethyl) moiety might provide useful A3AR agonists.
Other reported A3AR agonists include the 3′-amino-3′-deoxy derivatives reported by DeNinno and coworkers.51,52 We have examined whether N6 groups found to favor A3AR affinity in that structural series, such as the N6-[5-chloro-2-(aminocarbonylmethoyloxy)benzyl] group in 23, also led to high affinity within the present series. Indeed, 23 and the 2-benzyloxy ether 24 were highly potent at the human A3AR. Thus, sterically bulky substitution at the 2-position of the benzyl group of the (N)-methanocarba nucleosides is tolerated at the A3AR. Furthermore, we provided a molecular model that assigns a putative binding region for this moiety. The 3′-amino-3′-deoxy modification of the ribose reported previously in A3AR ligands was not included in the present study. Other adenosine A3AR agonists are based on 5′-methylthio, 5′-vinyl, and similar groups on the ribose moiety,38,52 however, these groups were also not included in the present study, in favor of those incorporating the 5′-uronamide group.
In conclusion, many of the previously known substituent groups that enhance A3AR affinity, while in some cases reducing intrinsic efficacy in the 9-riboside series, may be adapted to the (N)-methanocarba-5′-uronamide nucleoside series of full agonists. The affinity and selectivity of the agonists in the (N)-methanocarba-5′-uronamide series often exceeded those of the corresponding ribosides. According to rhodopsin-based modeling, the binding regions of both the N6-arylalkyl and the ribose (or pseudosugar) moieties were shown to be similar, but not identical, in the 9-riboside and (N)-methanocarba-5′-uronamide series. The putative orientation of the N6-benzyl group within the binding site depended on whether bulky substituent groups were present. An additional potential advantage of the (N)-methanocarba nucleosides may be avoiding metabolic transformations to which 9-ribosides would be subject,20 which is a topic for future studies. It will now be useful to examine these agonists in other pharmacological and disease models4,10,15 in order to determine the generality of the selectivity for the A3AR and to further validate the therapeutic utility of these selective agonists.
Experimental Procedures
Chemical Synthesis
Materials and instrumentation
Compound 11 was purchased from Sigma (St. Louis, MO), and compounds 3 and 4 were prepared as reported.13, 14 Compounds 9 and 10 were prepared as reported.18, 24
Reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO). 1H NMR spectra were obtained with a Varian Gemini 300 spectrometer using CDCl3, CD3OD or DMSO-d6 as solvents. The chemical shifts are expressed as ppm downfield from TMS. All melting points were determined with a Thomas-Hoover apparatus (A. H. Thomas Co.) and are uncorrected.
Purity of compounds was checked using a Hewlett–Packard 1100 HPLC equipped with a Luna 5µ RP-C18(2) analytical column (250 X 4.6 mm; Phenomenex, Torrance, CA). System A: linear gradient solvent system: H2O/CH3CN from 95/5 to 20/80 in 20 min; the flow rate was 1 mL/min. System B: linear gradient solvent system: 5 mM TBAP/CH3CN from 80/20 to 20/80 in 20 min, then isocratic for 2 min; the flow rate was 1 mL/min. Peaks were detected by UV absorption with a diode array detector. All derivatives tested for biological activity showed >96% purity in the HPLC systems.
TLC analysis was carried out on aluminum sheets precoated with silica gel F254 (0.2mm) from Aldrich. Low-resolution mass spectrometry was performed with a JEOL SX102 spectrometer with 6-kV Xe atoms following desorption from a glycerol matrix or on LC/MS 1100 Agilent, 1100 MSD, with Waters Atlantis Column C18. High resolution mass measurements were performed on a proteomics optimized Q-TOF-2 (Micromass-Waters) using external calibration using polyalanine. Observed mass accuracies are those expected based on known instruments performance as well as the trends in observed masses of standard compounds measured at intervals during the series of measurements. Reported masses are observed masses uncorrected for this time dependent drift in mass accuracy.
Bromomethyl-2-chloro-5-iodobenzene (60)
A mixture of 2-chloro-5-iodo-toluene (0.5 g, 1.98 mmol), N-bromosuccinimide (0.422 g, 2.35 mmol) and benzoyl peroxide (21.5 mg, 0.089 mmol) in dry CCl4 (5 mL) was stirred and heated to reflux for 3 h. After cooling, the mixture was filtered, and the red filtrate was washed with a saturated solution of sodium thiosulfate (2 × 10 mL). The organic phase was washed with brine, dried over Na2SO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (petroleum ether), to give 60 as a white solid (361 mg, 55%). 1H NMR (CDCl3, 300 MHz) δ 7.76 (d, J= 2.4 Hz, 1H), 7.56 (dd, J= 8.7, 2.1 Hz, 1H), 7.12 (d, J= 8.4 Hz, 1H), 4.49 (s, 2H). MS (m/e) (positive FAB) 332.1 (M + H)+, m.p. 94–95 °C.
N-(2-Chloro-5-iodobenzyl) phthalimide (63)
Bromomethyl-2-chloro-5-iodo-benzene (60) (400 mg, 1.2 mmol) and potassium phthalimide (693 mg, 1.5 mmol) were stirred in dry DMF (20 mL) and heated to 80°C for 3 hours. After cooling, the suspension was filtered and concentrated in vacuo, and the residue was partitioned between water (30 mL) and Et2O (30 mL). The aqueous phase was extracted with ether (20 mL × 3). The combined organic phases were dried over Na2SO4, filtered and concentrated to give 63 (455 mg, 95%). 1H NMR (CDCl3, 300 MHz) δ 7.92-7.86 ( (m, 2H), 7.78-7.75 (m, 2H), 7.54-7.50 (m, 2H), 7.10 (d, J= 8.4 Hz, 1H), 4.93 (s, 2H). MS (m/e) (API-ES) 397.9 (M)+, m.p. 134–136°C.
N-(2,5-Dichlorobenzyl phthalimide) (62)
Compound 62 was prepared by the same procedure as compound 63. 1H NMR (CDCl3, 300 MHz) δ 7.93−7.89 (m, 2H), 7.79-7.75 (m, 2H), 7.32 (d, J= 6 Hz, 1H), 7.21-7.16 (m, 2H), 4.96 (s, 2H). MS (m/e) (API-ES) 306.12 (M)+, m.p. 145–146°C.
2-Chloro-5-iodo-benzylamine hydrochloride (65)
Compound 63 (350mg, 0.88 mmol) was dissolved in dry EtOH (15 mL) and hydrazine (0.1 mL) was added. The stirred mixture was refluxed for 24 h, cooled and the EtOH evaporated. The residue was dissolved in Et2O (3 mL) and treated with HCl / Et2O. The precipitated hydrochloride salt was filtered and triturated with dry Et2O (3 × 2mL), obtaining 200 mg of product (yield 75%). 1H NMR (D2O, 300 MHz) 7.88 (s, 1H), 7.80 (dd, J= 8.4, 1.8 Hz, 1H), 7.31 ( (d, J=7.8 Hz, 1H), 4.27 (s, 2H). MS (m/e) (positive FAB) 268.1 (M−Cl)+, m.p. 207–210°C.
2,5-Dichlorobenzylamine hydrochloride (64)
64 was prepared by the same procedure as compound 65. 1H NMR (D2O, 300 MHz) δ 7.59−7.47 (m, 3H), 4.33 (s, 2H). MS (m/e) (positive FAB) 177.1. (M−Cl)+, m.p. >220°C.
2-Methoxy-5-iodobenzaldehyde (67)
5-Iodo-salicylaldehyde (1.0 g, 4.0 mmol) was dissolved in DMF (10 mL) and to the stirred solution K2CO3 (0.828 g, 6.0 mmol) and CH3I (1.14 g, 8.0 mmol) were added. The mixture was stirred at room temperature overnight. The suspension was concentrated in vacuo, and the residue was partitioned between water (30 mL) and Et2O (30 mL). The aqueous phase was separated and extracted with ether (20 mL × 2). The combined organic phases were dried over Na2SO4, filtered and concentrated to give 67 (1.02 g, 98%). 1H NMR (CDCl3, 300 MHz) δ 10.34 (s, 1H), 8.09 (s, 1H), 7.82-7.78 (m, 1H), 7.26 (s, 1H), 6.78 (d, J= 8.7 Hz, 1H), 3.91 (s, 3H). MS (m/e) (positive FAB) 262.1 (M+H)+, m.p. 140–142°C.
2-Methoxy-5-iodobenzylamine (68)
To a stirred solution of 67 (500 mg, 1.9 mmol) and ammonium acetate (1.5 g, 19.4 mmol) in dry methanol (6 mL) NaCNBH3 (170 mg, 2.66 mmol) was added in one portion. The resulting mixture was stirred at room temperature for 48 h. Concentrated HCl was added until pH< 2. The methanol was evaporated and the resulting white residue was dissolved in water (10 mL) and washed with Et2O (2 × 10 mL). The aqueous phase was then basified with aqueous KOH (45%), saturated with NaCl and extracted with CH2Cl2 (10 mL × 4). The combined organic phases were dried over Na2SO4, filtered and concentrated to give 68 as a yellow oil (200 mg, 40%). 1H NMR (CDCl3, 300 MHz) δ 7.55−7.45 (m, 2H), 6.46- 6.58 (m, 1H), 3.82-3.72 (m, 5H), 1.63 (br s, 2H). MS (m/e) (positive FAB) 264.1 (M+H)+, m.p. 95–97°C.
3-(3-Hydroxypropynyl)-benzylamine (72)
Cuprous iodide (1.06 mg, 0.0056 mmol) was added to a mixture of (PPh3)2PdCl2 (7.84 mg, 0.011 mmol) and 3-iodobenzylamine (262 mg, 1.12 mmol) in dry diethylamine (7 mL) under a nitrogen atmosphere. Then a solution of propargyl alcohol (41.2 µL, 0.73 mmol) in dry diethylamine (3 mL) was added. The resulting solution was stirred at room temperature for 3 h. The solvent was concentrated in vacuo and the residue was partitioned between water (20 mL) and CHCl3 (20 mL). The aqueous phase was separated and extracted with CHCl3 (20 mL × 2). The combined organic phases were dried over Na2SO4, filtered and concentrated. The residue was purified by PTLC (chloroform/methanol 9:1) to give 72 (106 mg, 90% yield). 1H NMR (CDCl3, 300 MHz) δ 7.39−7.26 (m, 4H), 4.48 (s, 2H), 3.85 (s, 2H), 1.73 (br s, 2H). MS (m/e) (positive FAB) 162.1 (M+H)+.
2-Chloro-5-(3-hydroxypropynyl)-benzylamine (73)
Compound 73 was prepared by the same procedure as compound 72, using compound 65 as starting material. 1H NMR (CDCl3, 300 MHz) δ 7.48 (s, 1H), 7.28−7.25 (m, 3H), 4.48 (s, 2H), 3.92 (s, 2H), 1.92 (br s, 2H). MS (m/e) (positive FAB) 196.1 (M+H)+.
5-Chloro-2-(methoxybenzyl)-benzamide (75)
5-Chloro-2-hydroxy-benzamide (1.5 g, 8.7 mmol) was dissolved in DMF (120 mL) and to the stirred solution K2CO3 (1.38 g, 10 mmol) and benzylbromide (1.71 g, 10 mmol) were added. The mixture was stirred at room temperature overnight. The suspension was concentrated in vacuo, and the residue was partitioned between water (30 mL) and Et2O (30 mL). The aqueous phase was separated and extracted with ether (20 mL × 2). The combined organic phases were dried over Na2SO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (petroleum ether/ ethyl acetate 90/10), to give 75 as a white solid (2.15 g, 95%). 1H NMR (CDCl3, 300 MHz) δ 8.20 (d, J= 3 Hz, 1H), 7.67 (br s, 1H), 7.44−7.38 (m, 6H), 7.99 (d, J= 9 Hz, 1H), 5.85 (br s, 1H), 5.17 (s, 2H). MS (m/e) (positive FAB) 262.1 (M+H)+. m.p. 110-112°C.
5-Chloro-2-(methoxybenzyl)-benzylamine hydrochloride (76)
To a suspension of LiAlH4 (280 mg, 7.37 mmol) in dry tetrahydrofuran (20 mL) under nitrogen atmosphere, a solution of 75 (1.0 g, 3.83 mmol) in THF (10 mL) was added and the mixture was refluxed for 3 h. After cooling, the excess of LiAlH4 was destroyed with a saturated solution of sodium sulfate. The mixture was filtered on MgSO4 and the filtrate concentrated in vacuo. The residue was partitioned between water (30 mL) and CH2Cl2 (30 mL). The aqueous phase was separated and extracted with CH2Cl2 (20 mL × 2). The combined organic phases were dried over Na2SO4, filtered and concentrated. The residue was dissolved in Et2O (5 mL) and treated with HCl / Et2O. The precipitated hydrochloride salt was filtered and triturated with dry Et2O (3 × 2mL), obtaining 760 mg of product (yield 70%). 1H NMR (CD3OD, 300 MHz) δ 7.50−7.33 (m, 7H), 7.15 (d, J= 9 Hz, 1H), 5.22 (s, 2H), 4.13 (s, 2H). MS (m/e) (API-ES) 248 (M-Cl)+. m.p 119-121°C.
5-Chloro-2-hydroxy-benzylamine (77)
To a suspension of LiAlH4 (437 mg, 11.5 mmol) in dry tetrahydrofuran (30 mL) under nitrogen atmosphere, a solution of 5-chloro-2-hydroxybenzamide 74 (1.5 g, 5.7 mmol) in THF (10 mL) was added and the mixture was refluxed for 3 h. After cooling, the excess of LiAlH4 was destroyed with a saturated solution of sodium sulfate. The mixture was filtered on MgSO4 and the filtrate concentrated in vacuo. The residue was partitioned between water (30 mL) and CH2Cl2 (30 mL). The aqueous phase was separated and extracted with CH2Cl2 (20 mL × 2). The combined organic phases were dried over Na2SO4, filtered and concentrated. The obtained solid was recrystallized from ethanol obtaining 787 mg of pure product (yield 88%). 1H NMR (CDCl3, 300 MHz) δ 7.10 (dd, J= 6.6, 2.7 Hz, 1H), 6.94 (d, J= 2.4 Hz, 1H), 6.76 (d, J= 8.7 Hz, 1H), 4.10 (br s, 2H), 1.61 (br s, 2H). MS (m/e) (API-ES) 158 (M +H)+, m.p. 160-162°C.
(5-Chloro-2-hydroxy-benzyl)-carbamic acid tert-butyl ester (78)
Compound 77 (300 mg, 1.9 mmol) was dissolved in a dry solution at 10% of triethylamine in MeOH (12 mL) and a solution of di-t-butylcarbamate (1 M in THF, 3.8 mL, 3.8 mmol) was added. The solution was stirred at 45°C for 1 h and then concentrated in vacuo. The residue was purified by flash chromatography on silica gel (petroleum ether/ ethyl acetate 90/10), to give 78 as a white solid (350 mg, 80%). 1H NMR (CDCl3, 300 MHz) δ 9.02 (br s, 1H), 7.10 (dd, J= 8.4, 2.4 Hz, 1H), 6.94 (d, J= 2.4 Hz, 2H), 6.76 (d, J= 8.7 Hz, 1H), 5.23 (br s, 1H), 4.17 (d, J= 6.9 Hz, 2H), 1.44 (s, 9H). MS (m/e) (API-ES) 258 (M +H)+, m.p 124–126°C.
(2-Carbamoylmethoxy-5-chloro-benzyl)-carbamic acid tert-butyl ester (79)
5-Chloro-2-hydroxy-benzyl)-carbamic acid tert-butyl ester 78 (210 mg, 0.8 mmol) was dissolved in DMF (6 mL) and to the stirred solution K2CO3 (138 mg, 1 mmol) and 2-bromoacetamide (138 mg, 1 mmol) were added. The mixture was stirred at room temperature overnight. The suspension was concentrated in vacuo, and the residue was partitioned between water (20 mL) and CH2Cl2 (10 mL). The aqueous phase was separated and extracted with CH2Cl2 (20 mL × 2). The combined organic phases were dried over Na2SO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (petroleum ether/ ethyl acetate 80/20), to give 79 as a white solid (226 mg, 90%). 1H NMR (CDCl3, 300 MHz) δ 7.88 (br s, 1H), 7.27-7.18 (m, 2H), 6.76 (d, J= 8.7 Hz, 1H), 5.54 (br s, 1H), 4.78 ( br s, 1H), 4.47 (s, 1H), 4.36 (d, J= 6.3 Hz), 1.43 (s, 9H). MS (m/e) (API-ES) 337.0 (MNa)+. m.p 142–143°C.
5-Chloro-2-(aminocarbonyl-methyloxy)-benzylamine (80)
Compound 79 (200 mg, 0.63 mmol) was treated with a solution at 15% of TFA in CH2Cl2 at room temperature for 45 min. The solution was concentrated in vacuo and water (10 mL) was added. The aqueous phase was washed with Et2O, basified with aqueous 2N NaOH, and extracted with CHCl3 (10 mL × 3) obtaining 130 mg of a white solid (80), (yield 96%). 1H NMR (CDCl3, 300 MHz) δ 8.37 (br s, 1H), 7.29-7.22 (m, 2H), 6.82 (d, J= 9.3 Hz, 1H), 5.44 (br s, 1H), 4.60 (s, 2H), 3.89 (s, 2H). MS (m/e) (API-ES) 215 (M+H)+, m.p. 135–137°C.
General procedure for the synthesis of compounds 39–54 and 16–33
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Bromobenzylamino)-2-chloro-purin-9-yl]-2',3'-Oisopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (39)
3-Bromobenzylamine hydrochloride (122 mg, 0.55 mml) was added to a solution of 38 (50 mg, 0.12 mmol) and triethylamine (1 mL) in methanol (3 mL). The mixture was stirred at room temperature for 3 h. Then it was concentrated in vacuo to dryness and the residue was purified by PTLC (chloroform/methanol 15:1) to give 39 (55 mg, 82%). 1H NMR (CDCl3, 300 MHz) δ 7.70 (s, 1H), 7.52 (s, 1H), 7.31-7.18 (m, 2H), 6.18 (br s, 1H), 5.87 (d, J= 7.2 Hz, 1H), 4.85-4.71 (m, 4H), 4.32-4.16 (m, 2H), 2.24-2.19 (m, 1H), 1.75-1.70(m, 1H), 1.55-1.49 (m, 4H), 1.35(t, J = 7.8, 3H), 1.29 (s, 3H). MS (m/e) (positive FAB) 564.1 (M+H)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Fluorobenzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (40)
1H NMR (CDCl3, 300 MHz) δ 7.65 (s, 1H), 7.34-7.26 (m, 1H), 7.15-6.57 (m, 3H), 6.22 (br s, 1H), 5.87 (d, J= 8.4 Hz, 1H), 4.85 (br s, 3H), 4.72 (d, J= 7.2 Hz, 1H), 4.34-4.17 (m, 2H), 3.48 (d, J= 5.1 Hz, 1H), 2.24-2.18 (m, 1H), 1.75-1.69 (m, 1H), 1.55 (s, 3H), 1.34 (t, J = 7.8, 3H), 1.29 (s, 3H). MS (m/e) (ASI-ES) 502.1 (M+H)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Chlorobenzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (41)
1H NMR (CDCl3, 300 MHz) 1H NMR (CDCl3, 300 MHz) δ 1.20-1.42 (m, 3H), 1.44-1.83 (m, 8H), 2.20-2.26 (m, 1H), 4.05-4.38 (m, 2H), 4.82-4.94 (m, 3H), 5.38 (d, J = 4.5 Hz, 1H), 5.86 (d, J = 8 Hz, 1H), 6.15 (br s, 1H), 7.21-7.40 (m, 4H), 7.67 (s, 1H), MS (m/e) (positive-FAB) 518.1 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2,5-Dichlorobenzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (42)
1H NMR (CDCl3, 300 MHz) δ 7.68 (s, 1H), 7.51 (s, 1H), 7.33 (d, J= 8.7 Hz, 1H), 7.23-7.19 (m, 1H), 6.28 (br s, 1H), 5.57 (d, J= 8.4 Hz, 1H), 4.85 (br s, 3H), 4.7 (d, J= 6 Hz, 1H), 4.32-4.16 (m, 2H), 2.21-2.19 (m, 1H), 1.75-1.70 (m, 1H), 1.55-1.50 (m, 4H), 1.35 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 554.1 (M+H)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2-Chloro-5-iodo-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (43)
1H NMR (CDCl3, 300 MHz) δ 7.85 (s, 1H), 7.67 (s, 1H), 7.57-7.53 (dd, J= 8.1, 1.8 Hz, 1H), 7.12 (d, J= 8.4 Hz, 1H), 6.32 (br s, 1H), 5.58 (d, J= 8.4 Hz, 1H), 4.85 (br s, 3H), 4.73 (d, J= 6 Hz, 1H), 4.33-4.14 (m, 2H), 2.24-2.19 (m, 1H), 1.75-1.70 (m, 1H), 1.55-1.50 (m, 4H), 1.35 (t, J = 7.8 Hz, 3H), 1.29 (s, 3H). MS (m/e) (positive FAB) 644.1 (M)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(5-Chloro-2-mehoxybenzylamino)-2-chloro-purin-9-yl]-2',3'-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (44)
1H NMR (CDCl3, 300 MHz) δ 1.11-1.23 (m, 6H), 1.36-1.81 (m, 5H), 1.09-1.23 (m, 1H), 3.86 (s, 3H), 4.08-4.41 (m, 2H), 4.60-4.83 (m, 3 H), 5.30 (s, 1H), 5.85 (d, J = 4.5 Hz, 1H), 6.21-6.29 (m, 1H), 6.81 (d, J = 12 Hz, 1H), 7.21-7.42 (m, 3H), 7.67 (br s, 1H), MS (m/e) (positive-FAB) 548.1 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(5-Chloro-2-(aminoycarbonylmethyloxy)-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (45)
1H NMR (CDCl3, 300 MHz) δ 7.66 (s, 1H), 7.36 (s, 1H), 7.30-7.27 (m, 1H), 7.07-7.05 (m, 1H), 6.81 (d, J= 8.7 Hz, 1H), 6.12 (br s, 1H), 5.85 (d, J= 8.4 Hz, 1H), 5.65 (br s, 1H), 5.01-4.85 (br s, 3H), 4.73 (d, J= 6 Hz, 1H), 4.50 (s, 1H), 4.34-4.21 (m, 2H), 2.23-2.18 (m, 1H), 1.74-1.69 (m, 1H), 1.55-1.50 (m, 4H), 1.34 (t, J = 7.8 Hz, 3H), 1.29 (s, 3H). MS (m/e) (API-ES) 591.1 (M+H)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(5-Chloro-2-benzyloxy-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (46)
1H NMR (CDCl3, 300 MHz) δ 7.62 (s, 1H), 7.39-7.30 (m, 6H), 7.18 (dd, J= 8.7, 2.7 Hz, 1H), 6.86 (d, J= 8.7 Hz, 1H), 6.33 (br s, 1H), 5.86 (d, J= 8.4 Hz, 1H), 5.81 (s, 2H), 4.85 (br s, 3H), 4.70 (d, J= 6 Hz, 1H), 4.32-4.19 (m, 2H), 2.23-2.18 (m, 1H), 1.74-1.69 (m, 1H), 1.55-1.50 (m, 4H), 1.34 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 624.2 (M+H)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(5-Iodo-2-methoxy-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (47)
1H NMR (CDCl3, 300 MHz) δ 7.71-7.64 (m, 2H), 7.57-7.53 (m, 2H), 6.21 (br s, 1H), 5.57 (d, J= 8.4 Hz, 1H), 4.84 (s, 2H), 4.75-4.69 (m, 2H), 4.32-4.20 (m, 2H), 3.85 (s, 3H), 2.23-2.18 (m, 1H), 1.74-1.69 (m, 1H), 1.55-1.50 (m, 4H), 1.34 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 640.1 (M+H)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2,5-Dimethoxy-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (48)
1H NMR (CDCl3, 300 MHz) δ 7.62 (s, 1H), 7.03 (s, 1H), 7.79 (s, 2H), 6.39 (br s, 1H), 5.57 (d, J= 8.4 Hz, 1H), 4.83-4.69 (m, 4H), 4.31-4.19 (m, 2H), 3.83 (s, 3H), 3.76 (s, 3H), 2.21-2.17 (m, 1H), 1.73-1.68 (m, 1H), 1.55-1.49 (m, 4H), 1.35 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 544.2 (M+H)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2-Methyl-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (49)
1H NMR (CDCl3, 300 MHz) δ 7.58 (s, 1H), 7.32 (d, J= 6.9 Hz, 1H), 7.23-7.16 (m, 2H), 6.07 (br s, 1H), 5.57 (d, J= 8.4 Hz, 1H), 4.83-4.69 (m, 4H), 4.32-4.16 (m, 2H), 2.37 (s, 3H), 2.23-2.18 (m, 1H), 1.74-1.69 (m, 1H), 1.55-1.50 (m, 4H), 1.35 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 498.3 (M)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Methyl-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (50)
1H NMR (CDCl3, 300 MHz) δ 7.56 (s, 1H), 7.26-7.09 (m, 4H), 6.27 (br s, 1H), 5.87 (d, J= 8.4 Hz, 1H), 4.83-4.71 (m, 4H), 4.30-4.20 (m, 2H), 2.33 (s, 3H), 2.22-2.17 (m, 1H), 1.74-1.69 (m, 1H), 1.55-1.50 (m, 4H), 1.34 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 498.3 (M)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-(3-Hydroxypropynyl)-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (51)
1H NMR (CDCl3, 300 MHz) δ 7.56 (s, 1H), 7.38 (s, 1H), 7.34-7.26 (m, 2H), 6.73 (br s, 1H), 5.87 (d, J= 8.4 Hz, 1H), 4.82-4.70 (m, 4H), 4.46 (d, J= 6 Hz, 2H), 4.32-4.20 (m, 2H), 2.41 (br s, 1H), 2.23-2.18 (m, 1H), 1.75-1.70 (m, 1H), 1.55-1.50 (m, 4H), 1.35 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 538.2 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2-Chloro-5-(3-hydroxypropynyl)-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (52)
1H NMR (CDCl3, 300 MHz) δ 7.63 (s, 1H), 7.48 (s, 1H), 7.32-7.22 (m, 2H), 6.63 (br s, 1H), 5.87 (d, J= 8.4 Hz, 1H), 4.88-4.79 (m, 3H), 4.71 (d, J= 4.8 Hz, 1H), 4.45 (d, J= 6 Hz, 2H), 4.32-4.19 (m, 2H), 2.35 (br s, 1H), 2.23-2.18 (m, 1H), 1.75-1.68 (m, 2H), 1.55 (s, 3H), 1.35 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 572.1 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(4-Amino-benzylamino)-2-chloro-purin-9-yl]-2',3'-O-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (53)
1H NMR (CDCl3, 300 MHz) δ 7.59 (s, 1H), 7.107 (d, J= 8.1 Hz, 2H), 6.65 (d, J= 8.1 Hz, 2H), 6.12 (br s, 1H), 5.87 (d, J= 8.4 Hz, 1H), 4.84 (s, 1H), 4.73-4.58 (m, 3H), 4.33-4.20 (m, 2H), 3.17 (br s, 2H), 2.26-2.18 (m, 1H), 1.75-1.69 (m, 1H), 1.56-1.50 (m, 4H), 1.35 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 499.2 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Pyridylmethylamino)-2-chloro-purin-9-yl]-2',3'-Oisopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (54)
1H NMR (CDCl3, 300 MHz) δ 8.66 (br s, 1H), 8.54 (br s, 1H), 7.73 (d, J= 6.6 Hz, 1H), 7.67 (s, 1H), 7.34-7.27 (m, 1H) 6.26 (br s, 1H), 5.88 (d, J= 8.4 Hz, 1H), 4.85 (br s, 3H), 4.72 (d, J= 4.8 Hz, 1H), 4.45 (d, J= 6 Hz, 2H), 4.33-4.20 (m, 2H), 2.24-2.18 (m, 1H), 1.75-1.70 (m, 2H), 1.56 (s, 3H), 1.35 (t, J = 7.8 Hz, 3H), 1.28 (s, 3H). MS (m/e) (positive FAB) 485.2 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Bromobenzylamino)-2-chloro-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methyl amide (17)
The ester 39 (45 mg, 0.08 mmol) was dissolved in methanol (3 mL) and treated with an aqueous solution of methylamine (1 mL, 40%). This mixture was stirred at room temperature overnight, then the solvent was evaporated to dryness, and the white residue was purified by PTLC (chloroform/methanol 9:1) to give the uronamide 17 (19.6 mg, 40%). 1H NMR (CDCl3, 300 MHz) δ 7.68 (s, 1H), 7.51 (s, 1H), 7.31-7.19 (m, 2H), 6.83 (br s, 1H), 6.30 (br s, 1H), 5.67 (d, J= 6.9 Hz, 1H), 4.79-4.78 (m, 4H), 2.92 (d, J=4.5 Hz, 3H), 2.07-2.02 (m, 1H), 1.70-1.65 (m, 2H), 1.55 (s, 3H), 1.27 (s, 3H). MS (m/e) (positive FAB) 549.1 (M+1)+.
This intermediate (18 mg, 0.03 mmol) was treated with a solution of trifluoroacetic acid in MeOH (5 mL, 10%) and H2O (0.5 mL) and the mixture was heated at 70°C for 3 h. The solution was cooled and the solvent removed to dryness by coevaporation with toluene in vacuo. The white residue was purified by PTLC (chloroform/methanol 9:1) to give the final product 17 (10 mg, 70%). 1H NMR (CDCl3, 300 MHz) δ 7.83 (s, 1H), 7.52-7.50 (m, 1H), 7.43-7.39 (m, 1H), 7.31-7.17 (m, 2H), 6.90 (br s, 1H), 6.63 (br s, 1H), 4.95-4.78 (m, 5H), 4.07 (d, J= 6Hz, 1H), 2.90 (d, J= 5.1 Hz, 3H), 2.24-2.19 (m, 1H), 1.72 (br s, 2H), 1.36-1.32 (m, 1H). HRMS (M+1)+: calculated 507.0547, found 507.0686. HPLC (System A) 20.4 min (99%) (System B), 12.9 min (99%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Chlorobenzylamino)-2-chloro-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (18)
1H NMR (CDCl3, 300 MHz) δ 1.17-1.39 (m, 1H), 1.45-1.61 (m, 1H), 2.01-2.09 (m, 1H), 2.81 (d, J=4.5 Hz, 1H), 3.91-4.08 (m, 2H), 4.78-5.05 (m, 5H), 6.71 (br s, 1H), 7.04 (br s, 1H), 7.11-7.29 (m, 4H), 7.74 (s, 1H), HRMS (M+1)+: calcd 463.0974, Found 463.1052. HPLC (System A) 14.8 min (99%) (System B), 12.5 min (99%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Fluorobenzylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (19)
7.84 (s, 1H), 7.34-7.26 (m, 1H), 7.15-6.95 (m, 3H), 6.86 (br s, 1H), 6.56 (br s, 1H), 4.88-4.80 (m, 4H), 4.08 (d, J= 6Hz, 1H), 2.91 (d, J= 5.1 Hz, 3H), 2.27-2.23 (m, 1H), 1.78 (br s, 2H), 1.36-1.32 (m, 1H). HRMS (M+1)+: calculated 447.1347, found 447.1378. HPLC (System A) 13.6 min (99%) (System B), 16.8 min (98%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2,5-Dichlorobenzylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methyl amide (20)
1H NMR (CDCl3, 300 MHz) δ 7.94 (s, 1H), 7.60 (s, 1H), 7.43 (d, J= 8.4 Hz, 1H), 7.34-7.30 (m, 1H), 7.04 (br s, 1H), 6.61 (br s, 1H), 5.12-4.92 (m, 1H), 431-4.22 (m, 1H), 3.03 (d, J= 5.1 Hz, 3H), 2.31-2.27 (m, 1H), 1.54-1.46 (m, 1H). HRMS (M+1)+: calculated 497.0662, found 497.0797. HPLC (System A) 17.2 min (98%) (System B), 13.8 min (97%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2-Chloro-5-iodo-benzylamino)-2-chloro-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (21)
1H NMR (CDCl3, 300 MHz) δ 7.83 (m, 2H), 7.55 (d, J= 8.4 Hz, 1H), 7.12 (d, J= 8.4 Hz, 1H), 6.86 (br s, 1H), 6.43 (br s, 1H), 4.96-4.80 (m, 3H), 4.13-4.08 (m, 2H), 2.91 (d, J= 5.1 Hz, 3H), 2.24-2.19 (m, 1H), 1.72 (br s, 2H), 1.36-1.32 (m, 1H). HRMS (M+1)+: calculated 589.0018, found 589.0250. HPLC (System A) 16.6 min (98%) (System B), 14.6 min (97%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(5-Chloro-2-methoxy-benzylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (22)
1H NMR (CDCl3, 300 MHz) δ 1.12-1.42 (m, 2H), 2.21-2.27 (m, 1H), 2.91 (d, J = 4.8 Hz, 3H), 3.86 (s, 3H), 4.01-4.22 (m, 2H), 4.65-4.83 (m, 3H), 4.95-5.01 (m, 1H), 6.42 (br s, 1H), 6.81 (d, J = 12Hz, 1H), 6.95 (br s, 1H), 7.21-7.43 (m, 3H), 7.81 (s, 1H). HRMS (M+1)+: calculated 493.1080, found 493.1158. HPLC (System A) 15.1 min (99%) (System B), 12.9 min (98%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(5-Chloro-2-(aminocarbonylmethyloxy)-benzylamino)-2-chloro-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (23)
1H NMR (CD3OD, 300 MHz) δ 8.04 (s, 1H), 7.38 (s, 1H), 7.25 (d, J=8.4 Hz, 1H), 6.94 (d, J= 8.4 Hz, 1H), 5.07 (d, J= 6.3 Hz, 1H) 4.59 (s, 2H), 4.99 (d, J= 6.3 Hz, 1H) 2.99-2.68 (m, 5H), 2.05-2.02 (m, 1H), 1.38-1.34 (m, 1H). HPLC (System A) 12.3 min (97%) (System B), 9.1 min (98%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(5-Chloro-2-benzyloxy-benzylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (24)
1H NMR (CDCl3, 300 MHz) δ 7.80 (s, 1H), 7.41-7.31 (m, 6H), 7.18 (dd, J= 8.7, 2.7 Hz, 1H), 6.90-6.84 (m, 2H), 6.57 (br s, 1H), 5.09 (s, 2H), 4.87-4.77 (m, 5H), 4.07 (d, J= 6Hz, 1H), 2.90 (d, J= 5.1 Hz, 3H), 2.54-2.21 (m, 1H), 1.92 (br s, 2H), 1.36-1.32 (m, 1H). HRMS (M+1)+: calculated 569.1471, found 569.1625. HPLC (System A) 17.2 min (98%) (System B), 16.7 min (98%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(5-Iodo-2-methoxy-benzylamino)-2-chloro-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (25)
1H NMR (CDCl3, 300 MHz) δ 7.79 (s, 1H), 7.67 (s, 1H), 7.54 (dd, J= 8.7, 2.7 Hz, 1H), 6.94 (br s, 1H), 6.65 (d, J= 8.7 Hz, 1H), 6.48 (br s, 1H),4.97-4.57 (m, 5H), 4.07 (m, 1H), 3.85 (s, 3H), 2.91 (d, J= 5.1 Hz, 3H), 2.24-2.19 (m, 1H), 1.94-1.85 (br s, 2H), 1.38-1.33 (m, 1H). HRMS (M+1)+: calculated 585.0514 found 585.0639. HPLC (System A) 18.7 min (98%) (System B), 13.9 min (99%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2,5-Dimethoxy-benzylamino)-2-chloro-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (26)
1H NMR (CDCl3, 300 MHz) δ 7.77 (s, 1H), 6.99-6.97 (m, 2H), 6.78-6.75 (m, 2H), 6.90 (br s, 1H), 6.63 (br s, 1H), 4.91 (d, J= 6.3 Hz, 1H), 4.77-4.74 (m, 3H), 4.05 (d, J= 6Hz, 1H), 3.82 (s, 3H), 3.75 (s, 3H), 2.90 (d, J= 5.1 Hz, 3H), 2.21-2.15 (m, 3H), 1.36-1.32 (m, 1H). HRMS (M+1)+: calculated 489.1653 found 489.1771. HPLC (System A) 17.5 min (98%) (System B), 11.3 min (98%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2-Methyl-benzylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (27)
1H NMR (CDCl3, 300 MHz) δ 7.76 (s, 1H), 7.31 (d, J= 6.9 Hz, 1H), 7.22-7.14 (m, 3H), 6.97 (br s, 1H), 6.20 (br s, 1H), 5.0 7 (br s, 1H), 4.79 (br s, 2H), 4.34 (br s, 1H), 4.11 (d, J= 6Hz, 1H), 3.14 (br s, 1H), 2.92 (d, J= 5.1 Hz, 3H), 2.37 (s, 3H), 2.19-2.14 (m, 3H), 1.97 (br s, 1H), 1.38-1.33 (m, 1H). HRMS (M+1)+: calculated 443.1598 found 443.1704. HPLC (System A) 14.3 min (99%) (System B), 12.1 min (99%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Methyl-benzylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (28)
1H NMR (CDCl3, 300 MHz) δ 7.79 (s, 1H), 7.23-7.09 (m, 4H), 6.93 (br s, 1H), 6.42 (br s, 1H), 4.95 (d, J= 6.3 Hz, 1H), 4.79 4.70 (m, 3H), 4.09 (d, J= 6Hz, 1H), 2.91 (d, J= 5.1 Hz, 3H), 2.33 (s, 3H), 2.22-2.17 (m, 1H), 1.97 (br s, 2H), 1.37-1.31 (m, 1H). HRMS (M+1)+: calculated 443.1598 found 443.1633. HPLC (System A) 14.5 min (99%) (System B), 12.0 min (99%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-(3-Hydroxypropynyl)-benzylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (29)
1H NMR (CDCl3, 300 MHz) δ 8.02 (s, 1H), 7.70 (s, 1H), 7.61 (br s, 1H), 7.33-7.22 (m, 2H), 5.62 (br s, 1H), 5.20 (br s, 1H), 5.11-5.03 (m, 1H), 4.94 (br s, 1H), 4.80 (s, 1H), 4.67 (d, J= 6Hz, 1H), 4.45-4.31 (m, 1H), 3.96 (br s, 1H), 2.97 (d, J= 5.1 Hz, 3H), 2.27-2.24 (m, 1H), 2.07 (br s, 1H), 1.36-1.32 (m, 1H). HRMS (M+1)+: calculated 483.1547 found 483.1596. HPLC (System A) 16.8 min (97%) (System B), 9.2 min (98%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(2-Chloro-5-(3-hydroxypropynyl)-benzylamino)-2-chloro-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (30)
1H NMR (CDCl3, 300 MHz) δ 7.93 (s, 1H), 7.75 (s, 1H), 7.38-7.26 (m, 2H), 5.37-5.26 (m, 1H), 4.80-4.75 (m, 2H), 4.65-4.59 (m, 1H), 4.50-4.41 (m, 2H), 4.02 (br s, 1H), 3.72-3.70 (m, 1H), 3.10-3.07 (m, 1H), 2.99 (d, J= 5.1 Hz, 3H), 2.29-2.23 (m, 1H), 2.01 (br s, 1H), 1.36-1.32 (m, 1H). HRMS (M+1)+: calculated 517.1158 found 517.1278. HPLC (System A) 17.3 min (98%) (System B), 11.1 min (98%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(4-Amino-benzylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (31a)
1H NMR (CD3OD, 300 MHz) δ 8.02 (s, 1H), 7.15 (d. J= 8.1 Hz, 2H), 6.70 (d. J= 8.1 Hz, 2H), 5.09 (d, J= 6.6 Hz, 1H), 4.81 (s, 1H), 4.61 (s, 2H), 4.02 (d, J= 6.6 Hz, 1H), 2.88 (s, 3H), 2.07-2.05 (m, 1H), 1.40-1.35 (m, 1H). HRMS (M+1)+: calculated 444.1551 found 444.1688. HPLC (System A) 10.8 min (98%) (System B), 7.1 min (98%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Pyridylmethylamino)-2-chloro-purin-9-yl]- 2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (32)
1H NMR (CD3OD, 300 MHz) δ 8.57 (s, 1H), 8.37 (s, 1H), 7.99 (s, 1H), 7.85 (d. J= 8.1 Hz, 1H), 7.38-7.34 (m, 1H), 5.05 (d, J= 6.6 Hz, 1H), 4.76 ( br s, 3H), 3.97 (d, J= 6.6 Hz, 1H), 2.26 (s, 3H), 2.04-1.99 (m, 1H), 1.35-1.295 (m, 2H). HRMS (M+1)+: calculated 430.1394 found 430.1467. HPLC (System A) 9.9 min (99%) (System B), 2.7 min (97%).
6-Chloro-2-methylthiopurin-9-ylmethyl 2,2-dimethylpropionate (82)
To a stirred solution of 2-amino-6-chloropurin-9-yl-methyl 2,2-dimethylpropionate 81 (0.566 g, 2 mmol) in acetonitrile (2 mL) was added methyl disulfide (0.94 g, 10 mmol), and tert-butyl nitrite (90%, 1.14 g, 10 mmol) and the resulting reaction mixture was stirred at room temperature for 8 h. The reaction mixture was concentrated in vacuo, and the resulting crude product was subjected to silica gel column chromatography (AcOEt/petroleum ether = 1/10), which furnished 82 (0.364 g, 55%): 1H NMR (CDCl3, 300 MHz) δ 8.19 (s, 1H), 6.12 (s, 2H), 2.65 (s, 3H), 1.17 (s, 9H). MS (m/e) (positive-FAB) 315 (M+1)+.
6-Chloro-2-methylthiopurine (83)
To a solution of 6-chloro-2-methylthiopurin-9-ylmethyl 2,2-dimethylpropionate (82) (0.314 g, 1 mmol) in i-PrOH (10 mL) and THF (25 mL) was added 2N aq NaOH (2 mL) and the resulting reaction mixture was stirred at room temperature for 12 hr. The reaction mixture was concentrated under reduced pressure and the residue obtained was purified by silica gel column chromatography (AcOEt/ petroleum ether = 3/7) which afforded 83 (0.112 g, 56%): 1H NMR (DMSO-d6, 300 MHz) δ 8.54 (s, 1H), 2.58 (s, 3H) MS (m/e) (positive-FAB) 200.9 (M+1)+.
(1'S,2'R,3'S,4'S,5'S)-4'-[6-Chloro-2-iodo-purin-9-yl]-2',3'-isopropylidenebicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (55)
To a solution of triphenylphosphine (0.262 g, 1 mmol) and 6-chloro-2-iodopurine (0.175 g, 0.63 mmol) in THF (3 mL) was added DIAD (0.202 g, 1 mmol), and the reaction mixture was stirred for 10 min. A solution of the alcohol 37 (0.121 g, 0.5 mmol) in THF was added to the reaction mixture, and the mixture was further stirred for 10 h. The solvent was removed in vacuo, and the residue obtained was purified by silica gel column chromatography (AcOEt/ petroleum ether = 4/6) which afforded 55 (0.136 g, 54%): 1H NMR (CDCl3, 300 MHz) δ 1.30-136 (m, 4H), 1.57 (s, 6H), 1.75-180 (m, 1H), 2.12-2.22 (m, 1H), 4.1-4.2 (m, 2H), 4.75 (d, J = 14 Hz, 1H), 4.92 (s, 1H), 5.85 (d, J = 15 Hz, 1H), 7.98 (s, 1H), MS (m/e) (positive-FAB) 505.0 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-Chloro-2-methylthio-purin-9-yl]-2',3'-isopropylidenebicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (56)
To a solution of triphenylphosphine (0.262 g, 1 mmol) and 6-chloro-2-methylthiopurine 83 (0.125 g, 0.63 mmol) in THF (3 mL) was added DIAD (0.202 g, 1 mmol) and the reaction mixture was stirred for 10 min. After which time alcohol 37 (0.121 g, 0.5 mmol) in THF was added and the reaction mixture was further stirred for 10 hr. The solvent was removed in vacuo, and the residue obtained was purified by silica gel column chromatography (AcOEt/petroleum ether = 4/6) which afforded 56 (0.112 g, 53%): 1H NMR (CDCl3, 300 MHz) δ 1.25-1.55 (m, 4H), 1.56 (s, 6H), 1.75-1.82 (m, 1H), 2.25-2.32 (m, 1H), 2.64 (s, 3H), 4.18-4.29 (m, 2H), 4.82 (d, J= 12 Hz, 1H), 4.92 (s, 1H), 5.85 (d, J = 11Hz, 1H), 7.90 (s, 1H), MS (m/e) (positive-FAB) 425.10 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-Chloro-2-amino-purin-9-yl]-2',3'-isopropylidenebicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (57)
To a solution of triphenylphosphine (0.262 g, 1 mmol) and 2-amino-6-chloropurine (0.169 g, 1 mmol) in THF (3 mL) was added DIAD (0.202 g, 1 mmol) and the reaction mixture was stirred for 10 min. After which time alcohol 37 (0.121 g, 0.5 mmol) in THF was added to the reaction mixture was further stirred for 10 h. The solvent was removed in vacuo, and the residue obtained was purified by silica gel column chromatography (AcOEt/ petroleum ether = 5/5) which afforded 57 (0.041 g, 21%): 1H NMR (CDCl3, 300 MHz) δ 1.20-1.55 (m, 6H), 1.52 (m, 4H), 1.75-1.85 (m, 1H), 2.12-2.24 (m, 1H), 4.11-4.24 (m, 2H), 4.67 (d, J = 11 Hz, 1H), 4.77 (s, 1H), 5.23 (br s, 2H), 5.88 (d, J = 12 Hz, 1H), 7.72 (s, 1H), MS (m/e) (positive-FAB) 394.2 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Chlorobenzylamino)-2-iodo-purin-9-yl]-2',3'-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (58)
1H NMR (CDCl3, 300 MHz) δ 1.11-1.22 (m, 4H), 1.23-1.79 (m, 7H), 2.18-2.24 (m, 1H), 4.10-4.21 (m, 2H), 4.66-4.87 (m, 3H), 5.30 (s, 1H), 5.85 (d, J = 4.5 Hz, 1H), 6.05-6.16 (m, 1H), 7.21-7.26 (m, 4H), 7.58 (s, 1H), MS (m/e) (positive-FAB) 610.1 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Chlorobenzylamino)-2-iodo-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (35)
1H NMR (CDCl3, 300 MHz) δ 1.15-1.22 (m, 1H), 1.65-1.70 (m, 1H), 2.08-2.13 (m, 1H), 2.94 (d, J = 4.5 Hz, 3H), 4.02-4.23 (m, 2H), 4.61-4.82 (m, 3H), 5.02-5.12 (m, 1H), 6.22-6.31 (m, 1H), 6.61-6.72 (m, 1H), 7.11-7.21 (m, 4H), 7.76 (s, 1H), MS (m/e) (positive-FAB) 515.2 (M+1)+. HPLC (System A) 15.7 min (98%) (System B), 13.5 min (99%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Chlorobenzylamino)-2-methylthio-purin-9-yl]-2',3'-isopropylidene-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (59)
1H NMR (CDCl3, 300 MHz) δ 1.15-1.40 (m, 7H), 1.42-1.81 (m, 5H), 2.53 (s, 3H), 4.15-4.38 (m, 2H), 4.78-4.92 (m, 4H), 5.84 (d, J = 8.5 Hz), 6.02 (br s, 1H), 7.19-7.40 (m, 4H), 7.56 (s, 1H), MS (m/e) (positive-FAB) 555.0 (M+1)+.
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-(3-Chlorobenzylamino)-2-methylthio-purin-9-yl]-2',3'-dihydroxy-bicyclo[3.1.0]hexane-1'-carboxylic acid ethyl ester (36)
1H NMR (CDCl3, 300 MHz) δ 1.12–1.18 (m, 1H), 1.62-176 (m, 1H), 2.22-2.29 (m, 1H), 2.51 (s, 3H), 2.90 (d, J = 3.5 Hz, 3H), 4.16 (d, J = 4.5 Hz, 1H), 4.65-4.98 (m, 4H), 6.21-6.32 (m, 1H), 6.71-6.81 (m, 1H), 7.11-7.42 (m, 4H), 7.72 (s, 1H), MS (m/e) (positive-FAB) 475.1 (M+1). HPLC (System A) 15.4 min (98%) (System B), 13.1 min (99%).
(1'S, 2'R, 3'S, 4'S, 5'S)-4'-[6-Methylamino-2-amino-purin-9-yl]-2',3'-dihydroxybicyclo[3.1.0]hexane-1'-carboxylic acid methylamide (15)
To a stirred solution of compound 57 (0.039, 0.1 mmol) in MeOH was added aq. CH3NH2 solution (0.5 mL, 40%) and the reaction mixture was stirred for 12 h. It was concentrated to dryness, and the residue was dissolved in the mixture containing 10% trifluoroacetic acid /MeOH (4 mL) and H2O (0.5 mL) was heated at 70°C for 3 h. The solvent was removed, and the residue was dried by coevaporation with dry toluene. The residue was purified using preparative TLC (CHCl3- MeOH, 80:20) to afford 15 (0.011 g, 36%): 1H NMR (DMSOd6, 300 MHz) δ 1.11-1.23 (m, 1H), 1.45-1.58 (m, 1H), 1.62-1.78 (m, 1H), 2.65 (d, J = 3.5 Hz, 3H), 2.91 (br s, 3H), 3.18 (d, J = 4 hz, 1H), 3.84-3.88 (m, 1H), 4.56 (s, 1H), 4.75 (d, J = 10 Hz, 1H), 5.08 (t, J = 4.5 Hz, 1H), 5.24 (d, J = 6.5 Hz), 5.82 (br s, 2H), 7.1 (br s, 1H), 7.55- 7.75 (m, 2H), MS (m/e) (positive-FAB) 334.1 (M+1). HPLC (System A) 6.6 min (98%) (System B), 5.0 min (98%).
Pharmacological Methods
[125I]N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide (I-AB-MECA; 2000 Ci/mmol), [3H]R-PIA (R-N6-[phenylisopropyl]adenosine, 34 Ci/mmol), [3H]CGS21680 (2-[p-(2-carboxyethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine, 47 Ci/mmol) and [3H]cyclic AMP (40 Ci/mmol) were from Amersham Pharmacia Biotech (Buckinghamshire, UK).
Cell culture and membrane preparation
CHO (Chinese hamster ovary) cells expressing the recombinant human A3AR25 were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin, 2 µmol/ml glutamine and 800 µ g/ml geneticin. The CHO cells expressing rat A3ARs were cultured in DMEM and F12 (1:1). Cells were harvested by trypsinization. After homogenization and suspension, cells were centrifuged at 500 g for 10 min, and the pellet was re-suspended in 50 mM Tris·HCl buffer (pH 8.0) containing 10 mM MgCl2, 1 mM EDTA and 0.1 mg/ml CHAPS. The suspension was homogenized with an electric homogenizer for 10 sec, and was then re-centrifuged at 20,000 g for 20 min at 4°C. The resultant pellets were resuspended in buffer in the presence of 3 Units/ml adenosine deaminase, and the suspension was stored at −80°C until the binding experiments. Striatal and forebrain tissues from Wistar rats were homogenized in ice-cold 50 mM Tris·HCl buffer, pH 7.4, using an electric homogenizer. The homogenate was centrifuged at 20,000 g for 10 min at 4°C, and the pellet was washed in fresh buffer. The final pellet was stored at −80°C until the binding experiments. The protein concentration was measured using the Bradford assay.55
Binding assay using [125I]4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide
Each tube in the competitive binding assay36 contained 100 µl membrane suspension (20 µg protein), 50 µl [125I]4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide (1.0 nM), and 50 µl of increasing concentrations of the test ligands in Tris·HCl buffer (50 mM, pH 8.0) containing 10 mM MgCl2, 1 mM EDTA. Nonspecific binding was determined using 10 µ M of 5′-N-ethylcarboxamidoadenosine in the buffer. The mixtures were incubated at 37°C for 60 min. Binding reactions were terminated by filtration through Whatman GF/B filters under reduced pressure using a MT-24 cell harvester (Brandell, Gaithersburgh, MD, USA). Filters were washed three times with 9 ml ice-cold buffer. Radioactivity was determined in a Beckman 5500B γ-counter.
Cyclic AMP accumulation assay
Intracellular cyclic AMP levels were measured with a competitive protein binding method.56,57 CHO cells that expressed recombinant human and rat A3ARs were harvested by trypsinization. After centrifugation and resuspension in medium, cells were plated in 24-well plates in 1.0 ml medium. After 24 hr, the medium was removed and cells were washed three times with 1 ml DMEM, containing 50 mM HEPES, pH 7.4. Cells were then treated with agonists and/or test compounds in the presence of rolipram (10 µ M) and adenosine deaminase (3 units/mL). After 45 min forskolin (10 µ M) was added to the medium, and incubation was continued an additional 15 min. The reaction was terminated by removing the supernatant, and cells were lysed upon the addition of 200 µ L of 0.1 M ice-cold HCl. The cell lysate was resuspended and stored at −20°C. For determination of cyclic AMP production, protein kinase A (PKA) was incubated with [3H]cyclic AMP (2 nM) in K2HPO4/EDTA buffer (K2HPO4, 150 mM; EDTA, 10 mM), 20 µ L of the cell lysate, and 30 µ L 0.1 M HCl or 50 µ L of cyclic AMP solution (0–16 pmol/200 µ L for standard curve). Bound radioactivity was separated by rapid filtration through Whatman GF/C filters and washed once with cold buffer. Bound radioactivity was measured by liquid scintillation spectrometry.
Statistical analysis
Binding and functional parameters were calculated using Prism 5.0 software (GraphPAD, San Diego, CA, USA). IC50 values obtained from competition curves were converted to Ki values using the Cheng-Prusoff equation.58 Data were expressed as mean ± standard error.
Molecular modeling
All calculations were performed on a Silicon Graphics Octane workstation (300 MHz MIPS R12000 (IP30) processor). All ligand structures were modified from the lowest energy conformation of Cl-IB-MECA12 using the “Sketch Molecule” of SYBYL 6.9.59 In all cases, MMFF force field60 and charge were applied using distance-dependent dielectric constants and conjugate gradient method until the terminal gradient reached 0.05 kcal/mol/Å.
A human A3AR model (PDB code: 1o74) constructed by homology to the x-ray structure of bovine rhodopsin with 2.8 Å resolution26 was used for the docking study. Compound 18, 24, and 29 in Table 2 were docked within the human A3AR model. The atom types of all ligands were manually assigned with Amber all-atom force field,61 and their charges were calculated before docking. The starting geometry of ligand conformation was chosen from the human A3AR complex model with Cl-IB-MECA, which was already validated by point-mutation.12 The ribose binding position of this series was fixed, using an atom-by-atom fitting method for the carbon atoms of the ribose ring. To determine the binding region of N6-derivatives, the flexible bond of N6-C-Car-Car angle important for the interaction of benzyl substituent was variously searched in the putative binding cavity, rotating by 60° increments. The same values of C5-C6-N6-C (234°) and C6-N6-C-Car (282°) angles from the Cl-IB-MECA complex were used, and the χ (O4′-C1′-N9-C8) angle was also fixed in an anti-conformation. Several starting conformations without any steric bump were selected for further optimization. The initial structures of all complexes were optimized using the Amber force field with fixed dielectric constant of 4.0 and terminating gradient of 0.1 kcal • mol−1 • Å−1.
Acknowledgements
We thank Dr. Victor Marquez (NCI, Frederick, MD) and Brian A. Harris (NIDDK) for helpful discussions and Dr. D. Eric Anderson and Dr. Victor Livengood (NIDDK) for mass spectral determinations. We are grateful to the Cystic Fibrosis Foundation for support provided to Susanna Tchilibon. B.V. Joshi thanks Gilead Sciences (Foster City, CA) for financial support.
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Klotz K-N, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Yao L, Burbiel JC, Maass A, Müller CE. Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Expert Opin. Emerging Drugs. 2003;8:537–576. doi: 10.1517/14728214.8.2.537. [DOI] [PubMed] [Google Scholar]
- 3.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 4.Liang BT, Jacobson KA. A physiological role of the adenosine A3 receptor: sustained cardioprotection. Proc. Natl. Acad. Sci. USA. 1998;95:6995–6999. doi: 10.1073/pnas.95.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Lubitz DKJE, Lin RC-S, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral ischemia. Eur. J. Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada H, Asano O, Hoshino Y, Yoshikawa S, Matsukura M, Kabasawa Y, Niijima J, Kotake Y, Watanabe N, Kawata T, Inoue T, Horizoe T, Yasuda N, Minami H, Nagata K, Murakami M, Nagaoka J, Kobayashi S, Tanaka I, Abe S. 2-Alkynyl-8-aryl-9-methyladenines as novel adenosine receptor antagonists: Their synthesis and structure-activity relationships toward hepatic glucose production induced via agonism of the A2B receptor. J. Med. Chem. 2001;44:170–179. doi: 10.1021/jm990499b. [DOI] [PubMed] [Google Scholar]
- 7.Wu L, Belardinelli L, Zablocki JA, Palle V, Shryock JC. A partial agonist of the A1-adenosine receptor selectively slows AV conduction in guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H334–H343. doi: 10.1152/ajpheart.2001.280.1.H334. [DOI] [PubMed] [Google Scholar]
- 8.Fedorova IM, Jacobson MA, Basile A, Jacobson KA. Behavioral characterization of mice lacking the A3 adenosine receptor: Sensitivity to hypoxic neurodegeneration. Cell. Mol. Neurobiol. 2003;23:431–447. doi: 10.1023/A:1023601007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J. Infect. Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 10.Ohana G, Bar-Yehuda S, Arich A, Madi L, Dreznick Z, Rath-Wolfson L, Silberman D, Slosman G, Fishman P. Inhibition of primary colon carcinoma growth and liver metastasis by the A3 adenosine receptor agonist CF101. Br. J. Cancer. 2003;89:1552–1558. doi: 10.1038/sj.bjc.6601315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avila MY, Stone RA, Civan MM. Invest. Ophthalmol. Vis. Sci. 2002;43:3021–3026. [PubMed] [Google Scholar]
- 12.Gao ZG, Kim S-K, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. Structural determinants of A3 adenosine receptor activation: Nucleoside ligands at the agonist/antagonist boundary. J. Med. Chem. 2002;45:4471–4484. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallo-Rodriguez C, Ji X-D, Melman N, Siegman BD, Sanders LH, Orlina J, Fischer B, Pu Q-L, Olah ME, van Galen PJM, Stiles GL, Jacobson KA. Structure-activity relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J. Med. Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HO, Ji X-d, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3-adenosine receptors. J. Med. Chem. 1994;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tracey WR, Magee WP, Oleynek JJ, Hill RJ, Smith AH, Flynn DM, Knight DR. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2780. doi: 10.1152/ajpheart.00411.2003. [DOI] [PubMed] [Google Scholar]
- 16.Kodani E, Bolli R, Tang XL, Auchampach JA. Protection of IB-MECA against myocardial stunning in conscious rabbits is not mediated by the A1 adenosine receptor. Basic Res Cardiol. 2001;96:487–496. doi: 10.1007/s003950170031. [DOI] [PubMed] [Google Scholar]
- 17.Gao ZG, Blaustein J, Gross AS, Melman N, Jacobson KK. N6-Substituted adenosine derivatives: Selectivity, efficacy, and species differences at A3 adenosine receptors. Biochem. Pharmacol. 2003;65:1675–1684. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohno M, Gao ZG, Van Rompaey P, Tchilibon S, Kim SK, Harris BA, Blaustein J, Gross AS, Duong HT, Van Calenbergh S, Jacobson KA. Modulation of adenosine receptor affinity and intrinsic efficacy in nucleosides substituted at the 2-position. Bioorganic Med. Chem. 2004;12:2995–3007. doi: 10.1016/j.bmc.2004.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannon JP, Bray-French KM, Phillips RM, Fozard JR. Further pharmacological characterization of the adenosine receptor subtype mediating inhibition of oxidative burst in human isolated neutrophils. Drug Devel. Res. 1998;43:214–224. [Google Scholar]
- 20.Jacobson KA, Ji X-d, Li A-H, Melman N, Siddiqui MA, Shin K-J, Marquez VE, Ravi RG. Methanocarba analogues of purine nucleosides as potent and selective adenosine receptor agonists. J. Med. Chem. 2000;43:2196–2203. doi: 10.1021/jm9905965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi BV, Marquez VE, Fettinger JC, Jacobson KA. A new synthetic route to (N)methanocarba nucleosides acting as A3 adenosine receptor agonists. 227th ACS National Meeting; Aug. 2002; Boston, MA. Abstract MEDI 256. [Google Scholar]
- 22.Joshi BV, Moon HR, Fettinger JC, Marquez VE, Jacobson KA. A new synthetic route to (N)-methanocarba nucleosides designed as A3 adenosine receptor agonists. Synthesis. doi: 10.1021/jo0487606. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, Ravi RG, Ji X-d, Marquez VE, Jacobson KA. Ring-constrained (N)methanocarba-nucleosides as adenosine receptor agonists: Independent 5′-uronamide and 2′-deoxy modifications. Biorg. Med. Chem. Lett. 2001;11:1333–1337. doi: 10.1016/s0960-894x(01)00213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchilibon S, Kim SK, Gao ZG, Harris BA, Blaustein J, Gross AS, Melman N, Jacobson KA. Exploring distal regions of the A3 adenosine receptor binding site: Sterically-constrained N6-(2-phenylethyl)adenosine derivatives as potent ligands. Bioorganic Med. Chem. 2004;12:2021–2034. doi: 10.1016/j.bmc.2004.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klotz K-N, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ. Comparative pharmacology of human adenosine receptor subtypes – characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1998;357:1–9. doi: 10.1007/pl00005131. [DOI] [PubMed] [Google Scholar]
- 26.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp TE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 27.Gao Z-G, Kim S-K, Gross AS, Chen A, Blaustein JB, Jacobson KA. Identification of essential residues involved in the allosteric modulation of the human A3 adenosine receptor. Mol. Pharm. 2003;63:1021–1031. doi: 10.1124/mol.63.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsunobu O. The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformationof natural products. Synthesis. 1981;1:1–28. [Google Scholar]
- 29.Wilson AA, Dannals RF, Ravert HT, Frost JJ, Wagner HN., Jr Potent muscarinic cholinergic receptor antagonist. J. Med. Chem. 1989;32:1057–1062. doi: 10.1021/jm00125a021. [DOI] [PubMed] [Google Scholar]
- 30.Treu M, Frohlich J, Jordis U. Preparation of shortened norbelladine analogs. Molecules. 2002;7:743–750. [Google Scholar]
- 31.Williams RM, Ehrlich PP, Zhai W, Hendrix J. A new synthetic approach to 1-(Hydroxymethyl)-8-methoxy-1, 2,3,4-tetrahydroisoqyuinolin-4-one. J. Org. Chem. 1989;52:2615–2617. [Google Scholar]
- 32.Sonogashira K, Tohda Y, Hagihara N. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975:4467–4470. [Google Scholar]
- 33.Tucker TJ, Brady SF, Lumma WC, Lewis SD, Gardell SJ, Naylor-Olsen AM, Yan Y, Sisko JT, Stauffer KJ, Lucas BJ, Lynch JJ, Cook JJ, Stranieri MT, Holahan MA, Lyle EA, Baskin EP, Chen IW, Dancheck KB, Krueger JA, Cooper CM, Vacca JP. Design and synthesis of a series of potent and orally bioavailable noncovalent thrombin inhibitors that utilize nonbasic groups in the P1 position. J. Med. Chem. 1998;41:3210–3219. doi: 10.1021/jm9801713. [DOI] [PubMed] [Google Scholar]
- 34.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a Northern conformation: Enhanced potency as P2Y1 receptor antagonists. J. Med. Chem. 2003;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brun V, Legraverend M, Grierson DS. Cyclin-dependent kinase (CDK) inhibitors: development of a general strategy for the construction of 2,6,9-trisubstituted purine libraries. Part 1. Tetrahedron Lett. 2001;42:8161–8164. [Google Scholar]
- 36.Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL. [125I]AB-MECA, a high affinity radioligand for the rat A3 adenosine receptor. Mol. Pharmacol. 1994;45:978–982. [PMC free article] [PubMed] [Google Scholar]
- 37.Volpini R, Costanzi S, Lambertucci C, Taffi S, Vittori S, Klotz KN, Cristalli G. N6-alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A3 receptor and a starting point for searching A2B ligands. J. Med. Chem. 2002;45:3271. doi: 10.1021/jm0109762. [DOI] [PubMed] [Google Scholar]
- 38.Mogensen JP, Roberts SM, Bowler AN, Thomsen C, Knutsen LJ. The synthesis of new adenosine A3 selective ligands containing bioisosteric isoxazoles. Bioorg. Med. Chem. Lett. 1998;8:1767–1770. doi: 10.1016/s0960-894x(98)00302-3. [DOI] [PubMed] [Google Scholar]
- 39.Wildbrandt R, Frotscher U, Freyland M, Messerschmidt W, Richter R, Schulte-Lippern M, Zschaege B. Treatment of glomerulonephritis with metrifudil. Preliminary Report. Med. Klin. (Munich) 1972;67:1138–1140. [PubMed] [Google Scholar]
- 40.Jacobson KA, Kim HS, Ravi G, Kim SK, Lee K, Chen A, Chen W, Kim SG, Barak D, Liang BT, Gao Z-G. Engineering of A3 adenosine and P2Y receptors and their ligands. Drug Devel. Res. 2003;58:330–339. doi: 10.1002/ddr.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gessi S, Varani K, Merighi S, Cattabriga E, Avitabile A, Gavioli R, Fortini C, Leung E, Lennan SM, Borea PA. Expression of A3 adenosine receptors in human lymphocytes: Up-regulation in T cell activation. Mol. Pharmacol. 2004;65:711–719. doi: 10.1124/mol.65.3.711. [DOI] [PubMed] [Google Scholar]
- 42.Fossetta J, Jackson J, Deno G, Fan X, Du XK, Bober L, Soudé-Bermejo A, Bouteiller OD, Caux C, Lunn C, Lundell D, Palmer RK. Pharmacological analysis of calcium responses mediated by the human A3 adenosine receptor in monocyte-derived dendritic cells and recombinant cells. Mol. Pharmacol. 2003;63:342–350. doi: 10.1124/mol.63.2.342. [DOI] [PubMed] [Google Scholar]
- 43.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. Activation of murine lung mast cells by the adenosine A3 receptor. J. Immunol. 2003;171:338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 44.Tilley SL, Tsai M, Williams CM, Wang ZS, Erikson CJ, Galli SJ, Koller BH. Identification of A3 receptor- and mast cell-dependent and -independent components of adenosine-mediated airway responsiveness in mice. J. Immunol. 2003;171:331–337. doi: 10.4049/jimmunol.171.1.331. [DOI] [PubMed] [Google Scholar]
- 45.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ. Res. 2003;92:485–492. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- 46.Harish A, Hohana G, Fishman P, Arnon O, Bar-Yehuda S. A3 adenosine receptor agonist potentiates natural killer cell activity. Int. J. Oncol. 2003;23:1245–1249. [PubMed] [Google Scholar]
- 47.Shi C, Szczesniak A, Mao L, Jollimore C, Coca-Prados M, Hung O, Kelly M. A3 adenosine and CB1 receptors activate a PKC-sensitive Cl- current in human nonpigmented ciliary epithelial cells via a Gβ,γ-coupled MAPK signaling pathway. Br. J. Pharmacol. 2003;139:475–486. doi: 10.1038/sj.bjp.0705266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaar R, Lamperti ED, Toselli PA, Ravid K. Activity of the A3 adenosine receptor gene promoter in transgenic mice: characterization of previously unidentified sites of expression. FEBS Lett. 2002;532:267–272. doi: 10.1016/s0014-5793(02)03612-8. [DOI] [PubMed] [Google Scholar]
- 49.Rubaj A, Zgodzinski W, Sieklucka-Dziuba M. The influence of adenosine A3 receptor agonist: IB-MECA, on scopolamine- and MK-801-induced memory impairment. Behav. Brain Res. 2003;141:11–17. doi: 10.1016/s0166-4328(02)00314-5. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson KA, Xie R, Young L, Chang L, Liang BT. A novel pharmacological approach to treating cardiac ischemia: binary conjugates of A1 and A3 adenosine receptor agonists. J. Biol. Chem. 2000;275:30272–30279. doi: 10.1074/jbc.M001520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeNinno MP, Masamune H, Chenard LK, DiRico KJ, Eller C, Etienne JB, Tickner JE, Kennedy SP, Knight DR, Kong J, Oleynek JJ, Tracey WR, Hill RJ. 3′-Aminoadenosine-5′-uronamides: discovery of the first highly selective agonist at the human adenosine A3 receptor. J. Med. Chem. 2003;46:353. doi: 10.1021/jm0255724. [DOI] [PubMed] [Google Scholar]
- 52.Etienne JB, DeNinno MP, Eller C, Oleynek JJ, Hill RJ, Masamune H, DiRico KJ, Tickner JE, Chenard LK. Highly selective adenosine-3 (A3) agonists as agents for peri-operative cardioprotection. 224th ACS National Meeting; Aug. 2002; Boston, MA. Abstract MEDI 291. [Google Scholar]
- 53.van Tilburg EW, von Frijtag Drabbe Künzel J, de Groote M, Vollinga RC, Lorenzen A, IJzerman AP. N6,5′-Disubstituted adenosine derivatives as partial agonists for the human adenosine A3 receptor. J. Med. Chem. 1999;42:1393–1400. doi: 10.1021/jm981090+. [DOI] [PubMed] [Google Scholar]
- 54.de Zwart M, Link R, von Frijtag Drabbe Künzel JK, Cristalli G, Jacobson KA, Townsend-Nicholson A, IJzerman AP. A screening of adenosine analogues on the human adenosine A2B receptor as part of a search for potent and selective agonists. Nucleos. Nucleotid. 1998;17:969–986. doi: 10.1080/07328319808004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 56.Nordstedt C, Fredholm BB. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal. Biochem. 1990;189:231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 57.Post SR, Ostrom RS, Insel PA. Biochemical methods for detection and measurement of cyclic AMP and adenylyl cyclase activity. Methods Mol. Biol. 2000;126:363–374. doi: 10.1385/1-59259-684-3:363. [DOI] [PubMed] [Google Scholar]
- 58.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 59.Sybyl Molecular Modeling System, version 6.9. Tripos Inc.; 1969 South Hanley Rd., St. Louis, Missouri 63144, USA: [Google Scholar]
- 60.Halgren TA, MMFF VII. Characterization of MMFF94, MMFF94s, and other widely available force fields for conformational energies and for intermolecular-interaction energies and geometries. J. Comput. Chem. 1999;20:730–748. doi: 10.1002/(SICI)1096-987X(199905)20:7<730::AID-JCC8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 61.Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Jr, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A second generation force field for the simulation of proteins, nucleic acids and organic molecules. J. Am. Chem. Soc. 1995;117:5179–5197. [Google Scholar]
- 62.Residues that were within 5 Å proximity to the ligand in this putative binding site were: L91 (3.33), T94 (3.36), H95 (3.37), N150 (EL2), Q167 (EL2), F168 (EL2), S181 (5.42), M177 (5.38), V178 (5.39), F182 (5.43), W243 (6.48), L246 (6.51), S247 (6.52), N250 (6.55), I268 (7.39), S271 (7.42), and H272 (7.43)
- 63.Because of intramolecular H-bonding of the benzyloxy and exocyclic amino groups of the 5-chloro-2-benzyloxybenzyl derivative 24, there was no H-bonding at N6H with N250 (6.55). In addition, no H-bonding at the 3′-OH and 5′-NH groups was shown in its docking complex. Compared to 18, the binding of the N6-benzyl group in 24 was more shifted toward TM4, and the additional benzyl substituent at 2-position of its benzyl ring was directed towards upper region of TM6 and EL2, which accommodated the bulky substituent. The movement of the N6-benzyl binding region resulted in weak H-bonding interactions at the hydroxyl and 5′-uronamide groups of methanocarba ring, thus decreasing the binding affinity.
- 64.The docking complex of the N6-3-(3-hydroxypropynyl)benzyl analogue 29 displayed additional H-bonding of the terminal hydroxyl group with the side chain of N150 in EL2. Thus, the extended hydroxypropynyl group penetrated through a hydrophobic region in proximity to the N6-benzyl ring, and its hydroxyl group entered a hydrophilic region.