Abstract
The posterior hypothalamus (PH) is known to reduce nociceptive pain, but the effect of PH stimulation on neuropathic pain is not known. Because neurons containing the neurotransmitter orexin-A are located in the PH in some strains of rat and intrathecal injection of orexin-A produces antinociception in a neuropathic pain model, we hypothesized that orexin-A from neurons in the PH modifies nociception in the spinal cord dorsal horn. To test this hypothesis, the cholinergic agonist carbachol or normal saline was microinjected into the PH of lightly anesthetized female Sprague-Dawley rats with chronic constriction injury (CCI) and foot withdrawal latencies (FWL) were measured. Carbachol-induced PH stimulation produced dose dependent antinociception as shown by significantly increased FWL compared to saline controls. To investigate the role of orexin-A in PH-induced antinociception, the orexin-1 receptor antagonist SB334867 or dimethyl sulfoxide (DMSO) for control, was given intrathecally following carbachol-induced PH stimulation. SB334867 decreased FWL compared to DMSO controls. These data are suggestive that stimulating the PH produces antinociception in a neuropathic pain model and that the antinociceptive effect is mediated in part by orexin-1 receptors in the spinal cord dorsal horn.
Keywords: Antinociception, neuropathic pain, orexin-A, orexin-1 receptor, posterior hypothalamus
INTRODUCTION
Stimulation of the hypothalamus using electrical current or excitatory drugs like the non-specific cholinergic muscarinic agonist carbamyl choline (carbachol) can elicit marked antinociception (Klamt and Prado, 1991, Franco and Prado, 1996, Holden et al., 1999, Holden and Naleway, 2001, Holden et al., 2002, Leone et al., 2004, Holden et al., 2005). Electrical stimulation of the posterior hypothalamus (PH) produces potent antinociception in acute pain (Rhodes and Liebeskind, 1978), while chemical stimulation produces antinociception on the foot withdrawal and tail flick tests (Jeong and Holden, 2005) and reduces facial pain in rats (Bartsch et al., 2004). In addition, destruction or deactivation of the PH increases pain responses (Millan et al., 1983, Manning and Franklin, 1998). Human studies demonstrate that electrical stimulation of the PH produces antinociception in patients with primary headaches (Franzini et al., 2003, Leone et al., 2005, Rasche et al., 2006). These findings are suggestive that the PH plays a role in antinociception in both animals and humans in acute pain.
The effect of PH stimulation on neuropathic pain, which alters the way nociception is processed, is not known. However, preliminary studies done in our lab showed that PH stimulation produced antinociception in the chronic constriction injury (CCI) model (Jeong and Holden, 2006). The CCI model induces a peripheral mononeuropathy through loose ligation of the common sciatic nerve and produces pain responses in rats that resemble clinical neuropathic pain, including hyperalgesia and allodynia (Bennett and Xie, 1988, Attal et al., 1990, Bennett, 1993, Baron et al., 1999). The neurotransmitters involved in this PH-induced antinociception have not been identified.
One such neurotransmitter may be the hypothalamic peptide orexin. Originally determined to be a regulator of feeding behaviors, two forms of orexin, orexin-A and orexin-B, have been identified (Sakurai et al., 1998). Although derived from the same precursor, prepro-orexin, they have different structures, and preferentially bind and activate the orexin-1 receptor (OX1R) and the orexin-2 receptor (OX2R), respectively. Axons containing orexin-A and orexin-B, and the receptors OX1R and OX2R, are densely located in the superficial lamina in the spinal cord, which is involved in nociception (Craig and Dostrovsky, 1999, Millan, 1999, van den Pol, 1999, Date et al., 2000, Bingham et al., 2001, Hervieu et al., 2001, Cluderay et al., 2002, Grudt et al., 2002, Guan et al., 2004). Spinally-projecting orexin-containing neurons have been observed in the hypothalamus (van den Pol, 1999), and cell bodies of orexin-A neurons are located in the PH of Sprague-Dawley rats (Sakurai et al., 1998, Chen et al., 1999, Cutler et al., 1999, Nambu et al., 1999, Briski and Sylvester, 2001, Baldo et al., 2003, Cheng et al., 2003b). Intrathecal administration of orexin-A profoundly attenuates the increased pain response following nerve injury (Yamamoto et al., 2003a, Suyama et al., 2004, Kajiyama et al., 2005). These findings are suggestive that orexin, and particularly orexin-A, may also play a role in nociceptive processing.
We propose that orexin-A from neurons in the PH modifies nociception in the spinal cord dorsal horn in the CCI model of neuropathic pain. To test this hypothesis, we microinjected carbachol into the PH of rats with CCI. One group of rats received pretreatment with atropine sulfate to determine specificity of action of the carbachol. In separate groups of rats we stimulated the PH and gave the specific orexin-A receptor antagonist, SB-334867 intrathecally. Nociceptive responses were measured using the foot withdrawal test, a valid and reliable test of nociception (Yeomans and Proudfit, 1994) that measures response times to a noxious thermal stimulus. These data have been published in part as an abstract (Jeong and Holden, 2006, 2007).
EXPERIMENTAL PROCEDURES
The Institutional Animal Care Committee at the University of Illinois at Chicago approved the experimental protocols used in this study. The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 90-23). All efforts were made to minimize animal suffering, reduce the numbers of animals used, and use alternatives to in vivo experiments.
Design
The pre-posttest experimental design with control groups was used for a series of behavioral experiments to test the functional effects of orexin-A in the PH on neuropathic pain in the spinal cord dorsal horn. Rats were randomly assigned either to the treatment or control group. Nociceptive modulation was manipulated through intracranial and intrathecal injections and outcomes measured through nociceptive testing using the foot withdrawal test.
Sample
Eighty-nine adult female Sprague-Dawley rats (230–300 gm; Charles River, Portage, MI) were used for behavioral experiments. All rats were maintained on a 12-hour light- dark cycle with free access to food and water. Each rat was only used once. To reduce the possibility of estrous cycle influence, rats were randomly assigned to either the treatment or control group, and no two rats were taken from the same cage on the same day.
Procedures
Surgical procedure for CCI
All rats received either CCI or sham surgery as follows: following deep pentobarbital anesthesia (50 mg/kg; intraperitoneal injection), and using aseptic technique, the common sciatic nerve was exposed at the level of the middle of the thigh by blunt dissection through the biceps femoris. Proximal to the sciatic trifurcation, about 7 mm of nerve was freed of adhering tissue and four ligatures (4.0 chromic gut), spaced 1 mm apart, were tied loosely around the nerve. The length of the nerve affected was 4 to 5 mm long. The ligatures were placed in a manner that lightly constricted the diameter of the nerves when viewed with 40X magnification, which retards but does not arrest circulation through the superficial epineural vasculature, and produces a small, brief twitch in the muscle surrounding the exposure. The incision was closed in layers. Rats were observed daily for signs of neuropathic pain, including limping and guarding of the affected limb, and for lack of grooming, social inactivity, and weight loss of greater than 20%. The procedure of sham surgery was identical except that ligation of sciatic nerve was not done.
Analgesiometric testing
Pain responses were measured by the foot withdrawal test. The hairy surface of the hind feet was blackened with India ink to facilitate more uniform heating of the skin surface and a focused beam of high intensity light was directed at the lateral aspect of the hind paws. The time interval between the onset of skin heating and withdrawal was measured electronically. The cutoff was 8 seconds to prevent burning of the skin. The low cutoff was 1 second. Foot withdrawal latencies were then measured at 5-minute intervals for 50 minutes after microinjection or until they return to baseline.
Experiment 1: Stimulation of the PH
Two weeks after neuropathic surgery when peak neuropathic responses were observed, rats were lightly anesthetized with intraperitoneal pentobarbital (35 mg/kg) and immobilized in a stereotaxic frame after shaving the scalp. The midline scalp was infused with bupivicaine (0.25%; 0.10 ml, which provides local anesthetic relief for approximately 24 hr; (James Artwhol, DVM, University of Illinois at Chicago Biological Resources Laboratory, personal communication). An incision was made along the midline of the scalp, and a burr hole was drilled into the skull to allow insertion of a microinjection guide cannula into the PH to a location defined by the following stereotaxic coordinates: −3.1 mm from bregma, lateral −0.5 mm, vertical +2.1 mm, incisor bar set at −2.5 mm. A 23-gauge stainless steel guide cannula was lowered into the region of the left PH through a burr hole to the predetermined position. A 32-gauge stainless steel microinjection cannula was connected to a 10 μl syringe by a length of PE-10 polyethylene tubing. This tubing was filled with a solution of the non-selective cholinergic agonist, carbamylcholine chloride (carbachol; Sigma, St. Louis, MO, USA) which is also known to depolarize orexin neurons (Yamanaka et al., 2003, Bayer et al., 2005). Carbachol was dissolved in physiological saline and filtered through a 0.2 μl syringe filter prior to use. The injection cannula was then inserted into the guide cannula, and extended 1 mm beyond the cannula edge. After a baseline the foot withdrawal latency was taken and recorded at 1 minute prior to microinjection, the carbachol was microinjected in a volume of 0.5 μl over a 1-minute period using an electronic syringe pump. The microinjector was left in place for an additional 60 seconds prior to removal to reduce the flow of drug up the guide cannula. For control, 0.5 μl of normal saline was injected to the PH in place of carbachol.
To determine the optimal effect of carbachol on the PH, a dose response study was done in which carbachol was microinjected in a dose of either 62.5, 125, or 250 nmol in a volume of 0.5 μl normal saline and foot withdrawal latencies measured. The 125 nmol dose was determined to provide optimum antinociception and was used in all other experiments. To determine receptor specificity for carbachol microinjected in the PH, a separate group of rats was pretreated in the PH with the muscarinic antagonist, atropine sulfate (14 nmol/0.5 μl normal saline; Sigma, St. Louis, MO, USA) to block the cholinergic effect. One minute later, the optimal dose of carbachol was microinjected into the PH. Foot withdrawal latencies were measured as described previously.
Experiment 2: The role of orexin in PH-induced antinociception
To determine whether the antinociceptive effect of PH stimulation was mediated by spinally projecting orexin-A neurons, an intrathecal catheter was inserted through an incision in the cisterna magna of rats and the tip position over the lumbar enlargement of the dorsal spinal cord (Yaksh and Rudy, 1976), as follows: rats were anesthetized with pentobarbital (35 mg/Kg) and mounted in a stereotaxic instrument. A midline incision was made over the occiput of the skull, extending from a line between the ears to a point approximately 2 cm caudal and a small incision was then made in the atlanto-occipital membrane. Keeping the angle of the catheter parallel with the dorsal surface of the brainstem, a 32-gauge intrathecal catheter was carefully advanced to a position 8 cm caudal of the cisterna at the level of lumbar enlargement (Yaksh and Rudy, 1976). The cannula was connected by PE-50 tubing to a 100 μl syringe in an electronic syringe pump. The rats were then prepared for intracerebral injection as described above. A baseline foot withdrawal latency was taken, and 1 minute later the optimal dose of carbachol or normal saline was microinjected into the PH. Three foot withdrawal measurements were taken at 5-minute intervals. One of three doses of the orexin-1 receptor antagonist, SB-334867, 15, 30, or 60 nmol in a total volume of 15μl of dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) was then administrated intrathecally over 1 minute and withdrawal latencies were measured 1 minute postinjection, and then every 5 minutes until the latencies returned to baseline (Yamamoto et al., 2002, Cheng et al., 2003a, Yamamoto et al., 2003b)
Additional groups of neuropathic rats were prepared only for intrathecal cannulation as described above. A baseline measurement was taken. One minute later, either 15, 30, or 60 nmol of SB-334867 in 15μl of DMSO was injected intrathecally similar to experiment 2, but without PH activation. Fifteen μl of DMSO was used as control. Foot withdrawal latencies were then measured at 5-minute intervals for 50 minutes.
Histology
At the end of each experiment, the animals were deeply anesthetized with sodium pentobarbital. The brains were removed and placed in a formalin fixative overnight, followed by 20% sucrose solution. Microinjection sites relative to the PH were verified by cutting 40-μm transverse brain sections, rinsed three times in cold phosphate buffered saline (PBS; 10 mM), mounted on gelatin-coated slides, stained with 0.05% neutral red, and coverslipped. Placement of the microinjection cannula was determined by plotting the most ventral portion of the cannula tip in serial sections by brightfield microscopy. Tracings of the appropriate sections were then made using the Neurolucida Imaging System (Microbrightfield, Colchester, VT, USA) and compared to the coordinates described by Paxinos and Watson (1998). The data were excluded when the cannula was not in the PH. For nerve histology, the sciatic nerve was exposed after completing experimental procedures and observed for two constrictions or a region of nearly uniform thinning, a pronounced swelling just proximal to the constricted region, and a distinct, but smaller, swelling distally.
Statistical analysis
Data were analyzed using the computer program SPSS/PC, version 15.0 for Windows. To determine whether the neuropathic paw manifested thermal hyperalgesia, the foot withdrawal latencies difference between the neuropathic and the sham paws was tested two-way repeated measure analysis of variance (ANOVA). The level for statistical significance was set at alpha = 0.05. Statistical comparisons of the left foot withdrawal latencies among treatment and control groups across time points were made using means and standard errors of the means, and two-way repeated measure ANOVA. Dunnett’s test was used for post hoc comparisons.
RESULTS
Of the 89 rats used, 19 were excluded. Three rats showed self-mutilation; five rats did not exhibit neuropathic behaviors on the left affected foot; and 11 rats showed misplacement of either intracranial, intrathecal, or both cannulae.
Neuropathic pain behaviors
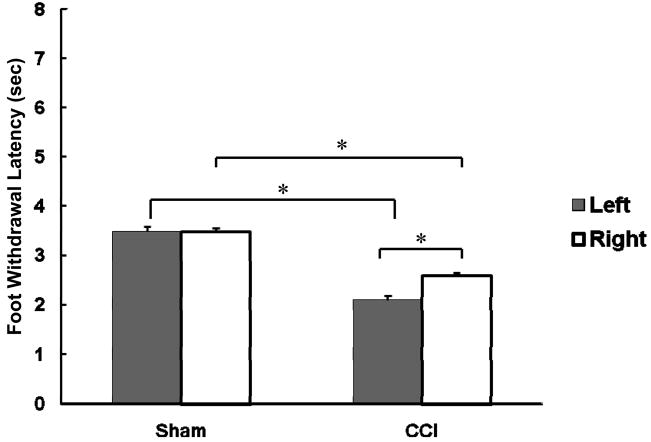
Rats that received neuropathic surgery on the left hind paw showed limping and a mild degree of foot-drop on the affected paw. Rats also raised the affected hind paw from the floor and held it in a protected position, similar to that observed by Bennett and Xie (1988). There was neither weight loss nor general weakness. To confirm a decrease in foot withdrawal latency on the left neuropathic paw as an indication of hyperalgesia, we compared the baseline latencies between the neuropathic and sham paws. As shown in Figure 1, the left neuropathic paw showed significantly shorter latencies compared to the sham paw (p < 0.05; 2.06±0.07 and 3.46±0.09, respectively), indicating the presence of hyperalgesia in the affected paw. We also observed significantly decreased response latencies on the right, unligated paw for CCI rats compared to the sham right paw (2.60 ±0.16 and 3.48 ±0.12 sec, respectively). Therefore, we used separate groups of rats as sham surgery controls rather than using the unoperated right paw of CCI rats as control.
Figure 1.
Comparisons of baseline foot withdrawal latencies between sham surgery and CCI groups for the left and right paws. The asterisk (*) shows statistically significant difference between groups (p < 0.05).
The effect of PH stimulation on neuropathic pain
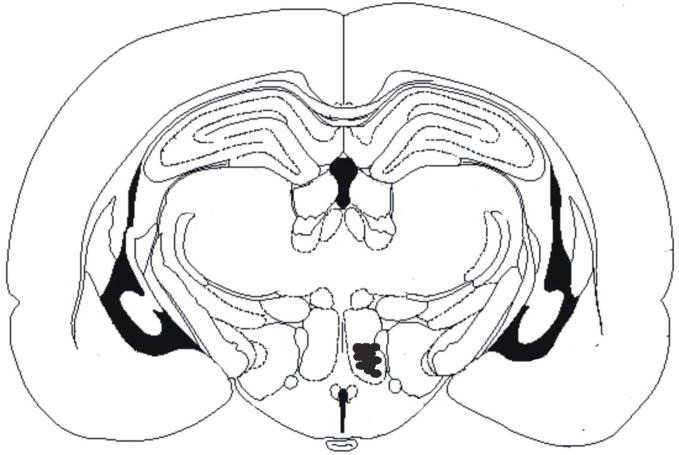
To determine whether carbachol microinjection into the PH produces antinociception in neuropathic pain, carbachol or saline was microinjected into the PH at the locations shown in Figure 2 and the foot withdrawal latencies were measured. Because other areas in the brain are known to produce antinociception, we excluded data from those rats with microinjections outside the PH.
Figure 2.
Representative sample of microinjection sites in CCI rats receiving125 nmol of carbachol. Mean response latencies were taken 10 minutes post injection and are represented as follows: filled circle, 6.1–8.0 seconds; open square, 4.1–6.0 seconds; open reverse triangle, 2.1–4.0 seconds. Abbreviations: 3V, 3rd ventricle; CA1, field CA1 of hippocampus; CA2, field CA2 of hippocampus; CA3, field CA3 of hippocampus; D3V, dorsal 3rd ventricle; f, fornix; LH, lateral hypothalamic area; LV, lateral ventricle; PH, posterior hypothalamic area. Stereotaxic coordinates are −3.1 mm from bregma, lateral −0.5 mm, vertical +2.1 mm, incisor bar set at −2.5 mm.
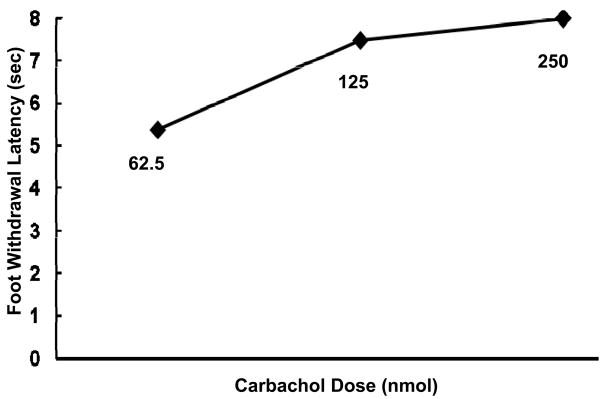
Figure 3 shows the dose response for three carbachol doses, taken at 10 minutes post injection, the optimal time of effect. Both the 125 and 250 nmol doses of carbachol significantly increased the foot withdrawal latencies as compared to saline controls (p < 0.05) while 62.5 nmol of carbachol did not significantly increase latencies (p > 0.05). We chose the 125 nmol dose for use in further experiments because it was the smallest dose that produced the greatest effect.
Figure 3.
Foot withdrawal latencies of three different doses of carbachol on PH stimulation for the left CCI foot. Data indicate mean foot withdrawal latency of each dose at ten minutes post-injection.
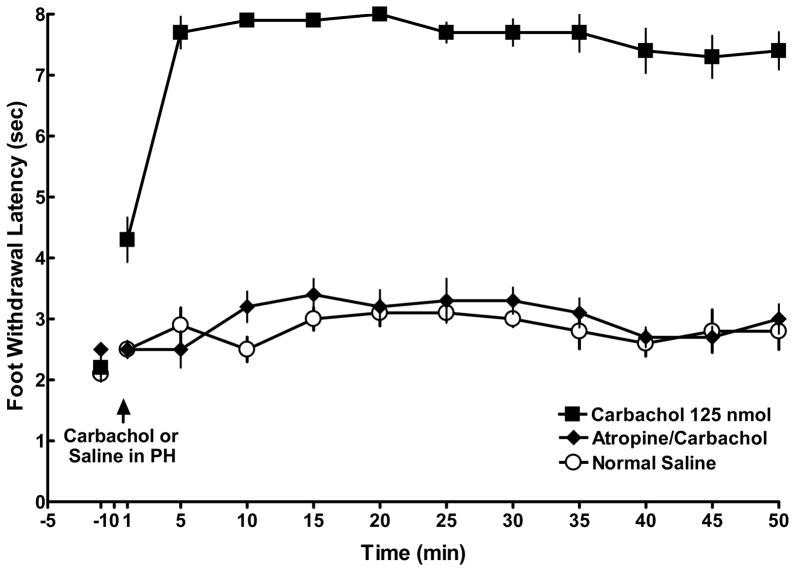
In rats with CCI ligation, microinjection of 125 nmol carbachol in the PH produced a significant increase in nociceptive responses compared to rats receiving saline in the PH (Figure 4; 7.9 ±0. 1 and 2.5± 0.2 sec, respectively, p < 0.05). This carbachol-induced antinociceptive effect occurred within 5 min post injection, went almost to cut off, and was maintained for over 50 min. This antinociceptive effect was blocked by pretreatment of the PH with atropine sulfate. As seen in Figure 4, foot withdrawal latencies in those rats receiving atropine were not different from rats in the saline control group (p > 0.5), indicating that carbachol acts at muscarinic receptors to produce PH-induced antinociception.
Figure 4.
The effect of carbachol-induced PH stimulation on the left CCI foot. Following a baseline response latency measurement at −1 minute, 125 nmol of carbachol (closed squares; n = 5/ group) was microinjected into the PH and produced robust antinociception compared to rats given normal saline (open circles; n = 5/ group) in the PH. Pretreatment with atropine sulfate at time −1 minute (closed diamonds; n = 5/ group) blocked carbachol-induced antinociception. Symbols equal the mean ± SEM. Standard error bars may be obscured by the symbols.
The effect of intrathecal administration of the OX1R antagonist, SB-334867, on PH-induced antinociception in neuropathic pain
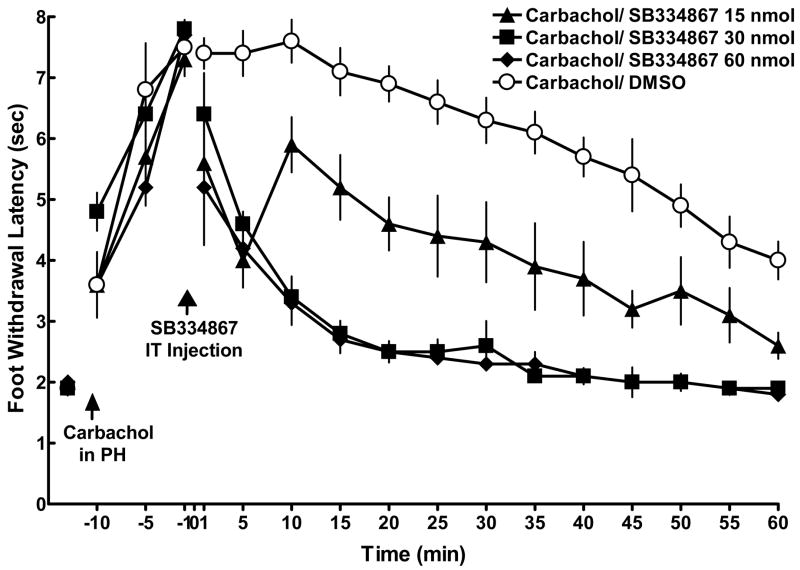
To determine whether PH-induced antinociception was mediated by orexin-A acting on OX1R in the dorsal horn, carbachol (125 nmol/0.5μl of normal saline) was microinjected into the PH following a baseline measurement at –15 minutes and antinociception was obtained. The OX1R antagonist, SB-334867, was then injected intrathecally in doses of 15, 30, or 60 nmol in 15 μl of DMSO. DMSO was used as control. All doses of SB-334867 significantly decreased the foot withdrawal latencies as compared with DMSO controls (p < 0.05; Figure 5). Both the 30 and 60 nmol of SB-334867 decreased foot withdrawal latencies to the baseline level, suggesting that these doses both completely blocked antinociception induced by PH stimulation with carbachol. The 15 nmol dose also significantly decreased withdrawal latencies, but not to the level of controls, suggesting that only a partial blockade of PH-induced antinociception occurred at this lowest dose.
Figure 5.
Intrathecal injection of OX1R antagonist SB-334867blocked PH-induced antinociception in CCI rats. Following a baseline measurement at −15 min, carbachol (125 nmol) was injected into the PH and three foot withdrawal latencies were taken at −10, −5 and −1 minute. At time 0, the OX1R antagonist, SB-334867 (15, 30, or 60 nmol; closed triangles, squares, and diamonds, respectively; n = 5/group), was injected intrathecally. All three dose of SB-334867 produced a significant decrease in foot withdrawal latencies as compared to DMSO controls (open circles). Symbols equal the mean ± SEM. Standard error bars may be obscured by the symbol.
To verify that blockade of carbachol-induced antinociception by SB-334867 was not due to a volume injected in the intrathecal space, and to determine whether the orexin-A pathway is tonically active in the CCI model of neuropathic pain, SB-334867 was injected intrathecally in CCI rats without prior PH stimulation. None of the three doses of SB-334867 produced a significant difference in left foot withdrawal latencies compared to controls (p > 0.05).
DISCUSSION
In the present study, we demonstrated that carbachol-induced PH stimulation produced a robust antinociceptive response in female rats with CCI compared to rats given saline in the PH. This antinociceptive response was mediated by cholinergic receptors in the PH, and blocked by intrathecal administration of the OX1R antagonist, SB-334867. While these findings are novel and are suggestive of a direct projection from orexin-containing neurons in the PH to the dorsal horn, we cannot say with certainty that such a connection exists in female Sprague Dawley rats. A number of studies identify orexin-immunoreactive neurons in the PH (Sakurai et al., 1998, Chen et al., 1999, Cutler et al., 1999, Nambu et al., 1999, Briski and Sylvester, 2001, Baldo et al., 2003, Cheng et al., 2003b), but while spinally projecting orexin-immunoreactive neurons have been identified in the LH (van den Pol, 1999), there is no anatomical evidence to date that shows definitive projection of PH orexin-containing neurons to the dorsal horn. It is also possible that carbachol in the PH stimulated non-orexin neurons that project to orexin-containing neurons elsewhere, such as the lateral hypothalamus (Baldo et al, 2003; Briski and Sylvester, 2001, vanden Pol, 1999), which could then project to the dorsal horn. Further anatomical studies are needed to determine the mechanisms involved in PH-induced antinociception.
Our use of female Sprague-Dawley rats in this study is meaningful because no reports that examine the role of orexin in female neuropathic pain exist in the literature. Two studies used male rats (Yamamoto et al., 2003a, Kajiyama et al., 2005) while one study did not state the sex of the rats that they used (Suyama et al., 2004). Researchers have a tendency to avoid using females because of a possible effect of estrous cycle on variability (Mogil and Chanda, 2005), although variance between subjects in male rodents is also possible. We attempted to control for between group variability induced by estrous cycle in this study by random assignment to either treatment or control group and random testing through the rat estrous cycle. However, we did not determine estrous cycle and cannot completely rule out the effect of estrus on our findings. Further work to determine this possibility, as well as male/female differences, needs to be done.
We observed thermal hyperalgesia on the left CCI paw of lightly anesthetized female Sprague-Dawley rats compared to the left sham paw, a finding consistent with those of others who have used female (Jevtovic-Todorovic et al., 1998, Wagner et al., 1998) and male Sprague Dawley rats with the CCI model (Bennett and Xie, 1988, Mao et al., 1995, Ren et al., 1995, Yamazaki et al., 2001, Hama, 2002, Ro et al., 2004). Our baseline measurement for the present study was several seconds lower than that of other studies. We used a lower baseline in order to make comparisons with our acute studies that use similar baseline latencies. While it is possible that this lower baseline produced artificially flattened response latencies in rats with CCI, we do not think that is the case. Our cutoff for the low measurement was 1 sec. The mean foot withdrawal measurement for left ligated rats was 2.13 with a small standard error. This finding does not suggest a flattened baseline reading. Furthermore, while we showed a significant decrease in withdrawal latencies for left ligated rats as compared to controls, our purpose was not to measure the hyperalgesic effect of sciatic nerve ligation per se, but to show antinociception in rats with sciatic nerve ligation. While our baselines may not be comparable to those of other investigators, they show a hyperalgesic response in CCI rats, and are appropriate for the present study.
Bennett and Xie (1988) indicated that the CCI model does not affect the contralateral paw in male Sprague-Dawley rats and that each animal can serve as its own control. In our experiments, the baseline foot withdrawal latency of the contralateral paw was longer than that of the ipsilateral paw, but shorter than the sham group (Figure 1). The difference between the left and right CCI baselines is only about 0.5 second, and while statistically significant, it is unclear whether this finding has behavioral significance. Immunocytochemical changes have been observed in the dorsal horn cord contralateral to the side of CCI ligation, including the neuronal activation marker Fos, as well as the transcription factor cyclic adenosine monophosphate response element binding protein, which is thought to be involved in central sensitization. These markers are significantly increased in the contralateral spinal cord dorsal horn, although significantly less so as compared to the ipsilateral side (Kajander et al., 1996, Yamazaki et al., 2001, Song et al., 2005). Other studies indicate that the contralateral paw in the CCI model shows no increased response to a pain stimulus, even in the presence of immunocytochemical changes in the contralateral dorsal horn (Mao et al., 1995, Whiteside and Munglani, 2001, Leiphart et al., 2003, Ro et al., 2004). Because it is not clear whether ligating the left sciatic nerve in the CCI model actually affects the contralateral paw, we chose to use a separate group of control rats rather than using each rat as its own control.
To identify the role of dorsal horn OX1R in antinociception produced by PH stimulation in neuropathic pain, we used a selective OX1R antagonist, SB-334867. Our findings agree with Kajiyama and colleagues (2005), who showed that 50 μg of SB-334867 given intrathecally block antinociception in diabetic neuropathic pain, and others who blocked orexin-A induced antinociception in incision and inflammatory pain with 30 nmol of SB-334867 (Yamamoto et al., 2002, Cheng et al., 2003a, Yamamoto et al., 2003b).
The exact mechanisms of orexin-A and OX1R in the spinal cord in pain modulation are not yet clear. Orexin-A is Gq protein coupled, increases synaptic activity and depolarizes postsynaptic neurons (Sutcliffe and de Lecea, 2000, Eriksson et al., 2001, Kukkonen et al., 2002, Liu et al., 2002, Hoang et al., 2003, Dong et al., 2006) and therefore is likely to activate neurons. OX1R are probably not on primary afferent terminals, excitatory interneuron, or projection neurons, which would increase nociceptive transmission when excited, a finding not in line with our observations (Figure 4). Based on our findings, OX1R are more likely to activate GABA, glycine or enkephalin containing neurons in the dorsal horn which are inhibitory in nature (Tsuruhara and Takahashi, 1987, Atsumi et al., 1993, Fleming and Todd, 1994, Jonas et al., 1998, Kerchner et al., 2001, Kodama and Kimura, 2002, Liu et al., 2002, Moore et al., 2002, Baba et al., 2003, Peever et al., 2003, Dergacheva et al., 2005, Ataka and Gu, 2006). Although there is no evidence for colocalization of OX1R on GABA, glycine, or enkephalin neurons to date, based on the antinociceptive role demonstrated in the present study, and the excitatory nature of orexin-A, it is likely that orexin-A neurons in the PH have an indirect inhibitory role on nociception in the dorsal horn by activating inhibitory interneurons, which then inhibit primary afferent terminals or projection neurons.
Intrathecal administration of the OX1R antagonist SB-334867 given in the absence of PH stimulation produced no effect in our study. This finding is consistent with the work of others that show that the orexin-A pathway is not tonically active in neuropathic pain (Kajiyama et al., 2005), acute nociceptive pain (Bingham et al., 2001, Yamamoto et al., 2002), or inflammatory pain using carrageenan or formalin injection (Yamamoto et al., 2002, Yamamoto et al., 2003b). While intraperitoneal administration of SB-334867 alone increases hyperalgesic and allodynic effects in the mouse carrageenan model (Bingham et al., 2001), intraperitoneal injection introduces the possibility that peripheral OX1Rs are involved and they may produce a different response than central receptors.
Implications for Clinical Practice
The findings of this study help to understand how the body produces endogenous analgesia. Ultimately, the results of this study could lead to the development of better pharmaceutical agents that make use of the endogenous systems and the development of non-pharmaceutical interventions that recruit the hypothalamus to activate the PH-orexin-A pathway.
CONCLUSION
In summary, PH stimulation with carbachol decreased CCI neuropathic pain as demonstrated by increased foot withdrawal latencies. Pretreatment with atropine sulfate blocked PH-induced antinociception. Intrathecal application of an OX1R antagonist significantly decreased foot withdrawal latencies compared to DMSO controls, while the OX1R antagonist given alone had no effect in neuropathic pain. These findings are suggestive that cholinergic muscarinic receptors mediate activation of PH neurons and activated PH neurons produced antinociception in part via a descending orexin-A pathway that may be direct or indirect. Following PH stimulation, orexin-A is released in the spinal cord dorsal horn and binds to spinal OX1R to produce antinociception, probably through actions on inhibitory interneurons.
Acknowledgments
This work was supported by Midwest Nursing Research Society/American Nurses Foundation grant and USPHS grant HHS NR04778 from the National Institute of Nursing Research at the National Institutes of Health.
List of Abbreviations
- ANOVA
Analysis of Variance
- CCI
Chronic Constriction Injury
- DMSO
Dimethyl Sulfoxide
- GABA
Gamma-Aminobutyric Acid
- LH
Lateral Hypothalamus
- OX1R
Orexin-1 Receptor
- OX2R
Orexin-2 Receptor
- PBS
Phosphate Buffered Saline
- PH
Posterior Hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Younhee Jeong, Kyunghee University, 1 Hoegi-dong, Dongdaemun-gu, Seoul, 130-701, Korea (ROK), Phone : 82-2-961-2210, Fax : 82-2-961-9398, Email : yjeong2@khu.ac.kr.
Janean E. Holden, The University of Michigan, 400 N. Ingalls, Room 2340, Ann Arbor, MI 48109-5482, Phone: 734-763-0011, Fax: 734 936-5525, Email: holdenje@umich.edu.
References
- Ataka T, Gu JG. Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Mol Pain. 2006;2:36. doi: 10.1186/1744-8069-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi S, Sakamoto H, Kawate T, Zhai XY. Substance P-containing primary afferent receives inhibitory modulation directly from enkephalin- and GABA-containing interneurons in the dorsal horn of the chicken. Regul Pept. 1993;46:410–412. doi: 10.1016/0167-0115(93)90103-f. [DOI] [PubMed] [Google Scholar]
- Attal N, Jazat F, Kayser V, Guilbaud G. Further evidence for pain-related behaviours in a model of unilateral peripheral mononeuropathy. Pain. 1990;41:235–251. doi: 10.1016/0304-3959(90)90022-6. [DOI] [PubMed] [Google Scholar]
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Baron R, Levine JD, Fields HL. Causalgia and reflex sympathetic dystrophy: does the sympathetic nervous system contribute to the generation of pain? 1999;22:678–695. doi: 10.1002/(sici)1097-4598(199906)22:6<678::aid-mus4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Levy MJ, Knight YE, Goadsby PJ. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain. 2004;109:367–378. doi: 10.1016/j.pain.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, Jones BE. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. An animal model of neuropathic pain: a review. Muscle & Nerve. 1993;16:1040–1048. doi: 10.1002/mus.880161007. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bingham S, Davey PT, Babbs AJ, Irving EA, Sammons MJ, Wyles M, Jeffrey P, Cutler L, Riba I, Johns A, Porter RA, Upton N, Hunter AJ, Parsons AA. Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 2001;92:81–90. doi: 10.1016/s0304-3959(00)00470-x. [DOI] [PubMed] [Google Scholar]
- Briski KP, Sylvester PW. Hypothalamic orexin-A-immunpositive neurons express Fos in response to central glucopenia. Neuroreport. 2001;12:531–534. doi: 10.1097/00001756-200103050-00020. [DOI] [PubMed] [Google Scholar]
- Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett. 1999;260:161–164. doi: 10.1016/s0304-3940(98)00977-x. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Chou RC, Hwang LL, Chiou LC. Antiallodynic effects of intrathecal orexins in a rat model of postoperative pain. J Pharmacol Exp Ther. 2003a;307:1065–1071. doi: 10.1124/jpet.103.056663. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Kuchiiwa S, Gao HZ, Kuchiiwa T, Nakagawa S. Morphological study of orexin neurons in the hypothalamus of the Long-Evans rat, with special reference to co-expression of orexin and NADPH-diaphorase or nitric oxide synthase activities. Neurosci Res. 2003b;46:53–62. doi: 10.1016/s0168-0102(03)00026-9. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Craig AD, Dostrovsky JO. Medulla to thalamus. In: Wall PD, Melzack R, editors. Textbook of pain. Edinburgh: Churchill Livingstone; 1999. pp. 183–214. [Google Scholar]
- Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, Arch JR, Wilson S, Buckingham RE, Evans ML, Leslie RA, Williams G. Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 1999;20:1455–1470. doi: 10.1016/s0196-9781(99)00157-6. [DOI] [PubMed] [Google Scholar]
- Date Y, Mondal MS, Matsukura S, Nakazato M. Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neurosci Lett. 2000;288:87–90. doi: 10.1016/s0304-3940(00)01195-2. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Huang ZG, Bouairi E, Stephens C, Gorini C, Mendelowitz D. Hypocretin-1 (orexin-A) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. J Pharmacol Exp Ther. 2005;314:1322–1327. doi: 10.1124/jpet.105.086421. [DOI] [PubMed] [Google Scholar]
- Dong HL, Fukuda S, Murata E, Zhu Z, Higuchi T. Orexins increase cortical acetylcholine release and electroencephalographic activation through orexin-1 receptor in the rat basal forebrain during isoflurane anesthesia. Anesthesiology. 2006;104:1023–1032. doi: 10.1097/00000542-200605000-00019. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AA, Todd AJ. Thyrotropin-releasing hormone- and GABA-like immunoreactivity coexist in neurons in the dorsal horn of the rat spinal cord. Brain Res. 1994;638:347–351. doi: 10.1016/0006-8993(94)90670-x. [DOI] [PubMed] [Google Scholar]
- Franco AC, Prado WA. Antinociceptive effects of stimulation of discrete sites in the rat hypothalamus: evidence for the participation of the lateral hypothalamus area in descending pain suppression mechanisms. Braz J Med Biol Res. 1996;29:1531–1541. [PubMed] [Google Scholar]
- Franzini A, Ferroli P, Leone M, Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003;52:1095–1099. discussion 1099–1101. [PubMed] [Google Scholar]
- Grudt TJ, van den Pol AN, Perl ER. Hypocretin-2 (orexin-B) modulation of superficial dorsal horn activity in rat. J Physiol. 2002;538:517–525. doi: 10.1113/jphysiol.2001.013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, Wang QP, Hori T, Takenoya F, Kageyama H, Shioda S. Ultrastructure of orexin-1 receptor immunoreactivities in the spinal cord dorsal horn. Peptides. 2004;25:1307–1311. doi: 10.1016/j.peptides.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Hama AT. Capsaicin-sensitive primary afferents mediate responses to cold in rats with a peripheral mononeuropathy. Neuroreport. 2002;13:461–464. doi: 10.1097/00001756-200203250-00020. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90:693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- Holden JE, Farah EN, Jeong Y. Stimulation of the lateral hypothalamus produces antinociception mediated by 5-HT1A, 5-HT1B and 5-HT3 receptors in the rat spinal cord dorsal horn. Neuroscience. 2005;135:1255–1268. doi: 10.1016/j.neuroscience.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Holden JE, Naleway E. Microinjection of carbachol in the lateral hypothalamus produces opposing actions on nociception mediated by alpha(1)- and alpha(2)-adrenoceptors. Brain Res. 2001;911:27–36. doi: 10.1016/s0006-8993(01)02567-7. [DOI] [PubMed] [Google Scholar]
- Holden JE, Schwartz EJ, Proudfit HK. Microinjection of morphine in the A7 catecholamine cell group produces opposing effects on nociception that are mediated by alpha1- and alpha2-adrenoceptors. Neuroscience. 1999;91:979–990. doi: 10.1016/s0306-4522(98)00673-3. [DOI] [PubMed] [Google Scholar]
- Holden JE, Van Poppel AY, Thomas S. Antinociception from lateral hypothalamic stimulation may be mediated by NK(1) receptors in the A7 catecholamine cell group in rat. Brain Research. 2002;953:195–204. doi: 10.1016/s0006-8993(02)03285-7. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Holden JE. Neuroscience. Washington, D.C.: 2005. Stimulation of the Posterior Hypothalamus Produces Antinociception in Acute Pain Models in Rats. [Google Scholar]
- Jeong Y, Holden JE. Neuroscience. Atlanta, GA: 2006. The role of orexin-1 receptors in posterior hypothalamic-induced antinociception. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Holden JE. Orexin-A is involved in posterior hypothalmic-induced analgesia in a neuropathic pain model. Western Journal of Nursing Research 2007 [Google Scholar]
- Jevtovic-Todorovic V, Wozniak DF, Powell S, Nardi A, Olney JW. Clonidine potentiates the neuropathic pain-relieving action of MK-801 while preventing its neurotoxic and hyperactivity side effects. Brain Res. 1998;781:202–211. doi: 10.1016/s0006-8993(97)01247-x. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Madsen AM, Iadarola MJ, Draisci G, Wakisaka S. Fos-like immunoreactivity increases in the lumbar spinal cord following a chronic constriction injury to the sciatic nerve of rat. Neurosci Lett. 1996;206:9–12. doi: 10.1016/0304-3940(96)12447-2. [DOI] [PubMed] [Google Scholar]
- Kajiyama S, Kawamoto M, Shiraishi S, Gaus S, Matsunaga A, Suyama H, Yuge O. Spinal orexin-1 receptors mediate anti-hyperalgesic effects of intrathecally-administered orexins in diabetic neuropathic pain model rats. Brain Res. 2005;1044:76–86. doi: 10.1016/j.brainres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Wang GD, Qiu CS, Huettner JE, Zhuo M. Direct presynaptic regulation of GABA/glycine release by kainate receptors in the dorsal horn: an ionotropic mechanism. Neuron. 2001;32:477–488. doi: 10.1016/s0896-6273(01)00479-2. [DOI] [PubMed] [Google Scholar]
- Klamt JG, Prado WA. Antinociception and behavioral changes induced by carbachol microinjected into identified sites of the rat brain. Brain Res. 1991;549:9–18. doi: 10.1016/0006-8993(91)90593-k. [DOI] [PubMed] [Google Scholar]
- Kodama T, Kimura M. Arousal effects of orexin-A correlate with GLU release from the locus coeruleus in rats. Peptides. 2002;23:1673–1681. doi: 10.1016/s0196-9781(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Holmqvist T, Ammoun S, Akerman KE. Functions of the orexinergic/hypocretinergic system. Am J Physiol Cell Physiol. 2002;283:C1567–1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- Leiphart JW, Dills CV, Levy RM. Decreased spinal alpha2a- and alpha2c-adrenergic receptor subtype mRNA in a rat model of neuropathic pain. Neurosci Lett. 2003;349:5–8. doi: 10.1016/s0304-3940(03)00610-4. [DOI] [PubMed] [Google Scholar]
- Leone M, Franzini A, D'Andrea G, Broggi G, Casucci G, Bussone G. Deep brain stimulation to relieve drug-resistant SUNCT. Ann Neurol. 2005;57:924–927. doi: 10.1002/ana.20507. [DOI] [PubMed] [Google Scholar]
- Leone M, May A, Franzini A, Broggi G, Dodick D, Rapoport A, Goadsby PJ, Schoenen J, Bonavita V, Bussone G. Deep brain stimulation for intractable chronic cluster headache: proposals for patient selection. Cephalalgia. 2004;24:934–937. doi: 10.1111/j.1468-2982.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, Franklin KB. Morphine analgesia in the formalin test: reversal by microinjection of quaternary naloxone into the posterior hypothalamic area or periaqueductal gray. Behav Brain Res. 1998;92:97–102. doi: 10.1016/s0166-4328(97)00130-7. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Phillips LL, Lu J, Mayer DJ. Increases in protein kinase C gamma immunoreactivity in the spinal cord dorsal horn of rats with painful mononeuropathy. Neurosci Lett. 1995;198:75–78. doi: 10.1016/0304-3940(95)11975-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Progress in Neurobiology. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Przewlocki R, Millan MH, Herz A. Evidence for a role of the ventro-medial posterior hypothalamus in nociceptive processes in the rat. Pharmacology, Biochemistry and Behavior. 1983;18:901–907. doi: 10.1016/s0091-3057(83)80013-6. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press, Inc; 1998. [Google Scholar]
- Peever JH, Lai YY, Siegel JM. Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol. 2003;89:2591–2600. doi: 10.1152/jn.00968.2002. [DOI] [PubMed] [Google Scholar]
- Rasche D, Foethke D, Gliemroth J, Tronnier VM. Deep brain stimulation in the posterior hypothalamus for chronic cluster headache Case report and review of the literature. Schmerz. 2006 doi: 10.1007/s00482-005-0462-3. [DOI] [PubMed] [Google Scholar]
- Ren K, Thomas DA, Dubner R. Nerve growth factor alleviates a painful peripheral neuropathy in rats. Brain Res. 1995;699:286–292. doi: 10.1016/0006-8993(95)00920-l. [DOI] [PubMed] [Google Scholar]
- Rhodes DL, Liebeskind JC. Analgesia from rostral brain stem stimulation in the rat. Brain Res. 1978;143:521–532. doi: 10.1016/0006-8993(78)90362-1. [DOI] [PubMed] [Google Scholar]
- Ro LS, Li HY, Huang KF, Chen ST. Territorial and extra-territorial distribution of Fos protein in the lumbar spinal dorsal horn neurons in rats with chronic constriction nerve injuries. Brain Res. 2004;1004:177–187. doi: 10.1016/j.brainres.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Song XS, Cao JL, Xu YB, He JH, Zhang LC, Zeng YM. Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol Sin. 2005;26:789–798. doi: 10.1111/j.1745-7254.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J Neurosci Res. 2000;62:161–168. doi: 10.1002/1097-4547(20001015)62:2<161::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Suyama H, Kawamoto M, Shiraishi S, Gaus S, Kajiyama S, Yuge O. Analgesic effect of intrathecal administration of orexin on neuropathic pain in rats. In Vivo. 2004;18:119–123. [PubMed] [Google Scholar]
- Tsuruhara H, Takahashi T. Glial and potassium responses to an afferent volley: suppression by enkephalin in the rat spinal cord in vitro. Neurosci Lett. 1987;76:291–295. doi: 10.1016/0304-3940(87)90417-4. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain. 1998;74:35–42. doi: 10.1016/S0304-3959(97)00148-6. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Munglani R. Cell death in the superficial dorsal horn in a model of neuropathic pain. J Neurosci Res. 2001;64:168–173. doi: 10.1002/jnr.1062. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Chiba T. Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br J Pharmacol. 2002;137:170–176. doi: 10.1038/sj.bjp.0704851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Saito O, Shono K, Aoe T, Chiba T. Anti-mechanical allodynic effect of intrathecal and intracerebroventricular injection of orexin-A in the rat neuropathic pain model. Neurosci Lett. 2003a;347:183–186. doi: 10.1016/s0304-3940(03)00716-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Saito O, Shono K, Hirasawa S. Activation of spinal orexin-1 receptor produces anti-allodynic effect in the rat carrageenan test. Eur J Pharmacol. 2003b;481:175–180. doi: 10.1016/j.ejphar.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Maeda T, Someya G, Wakisaka S. Temporal and spatial distribution of Fos protein in the lumbar spinal dorsal horn neurons in the rat with chronic constriction injury to the sciatic nerve. Brain Res. 2001;914:106–114. doi: 10.1016/s0006-8993(01)02783-4. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Characterization of the foot withdrawal response to noxious radiant heat in the rat. Pain. 1994;59:85–94. doi: 10.1016/0304-3959(94)90051-5. [DOI] [PubMed] [Google Scholar]