Abstract
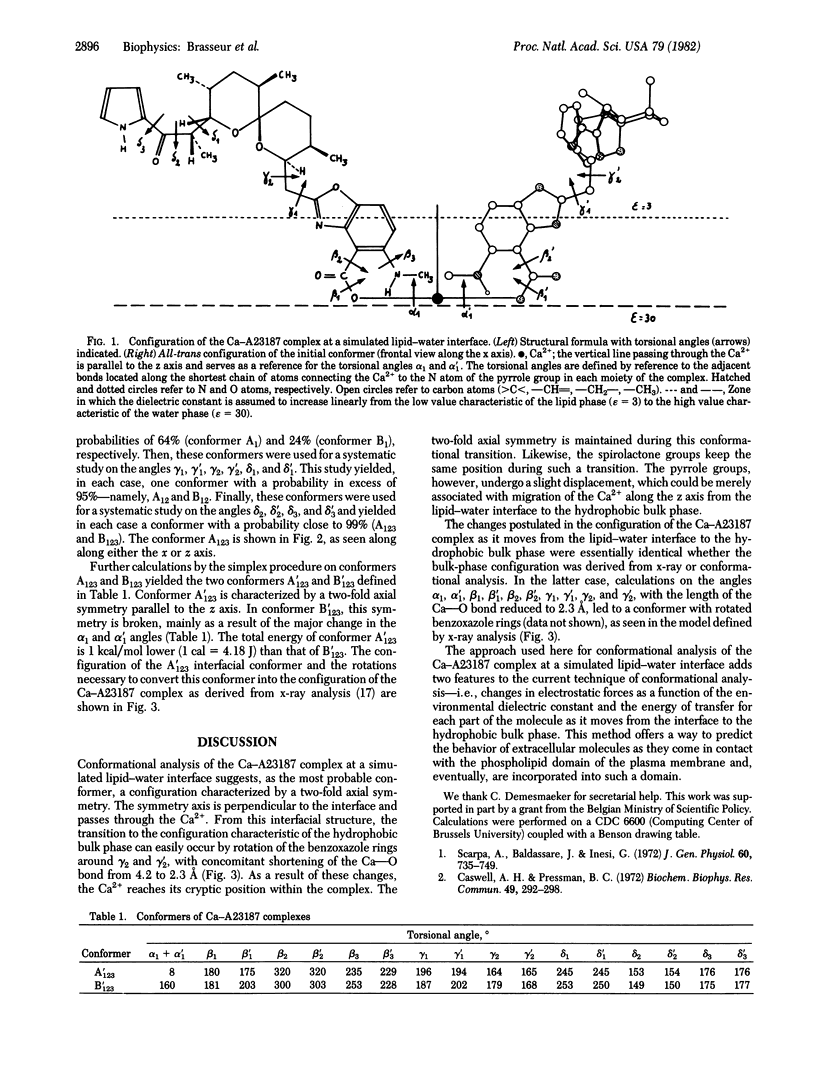
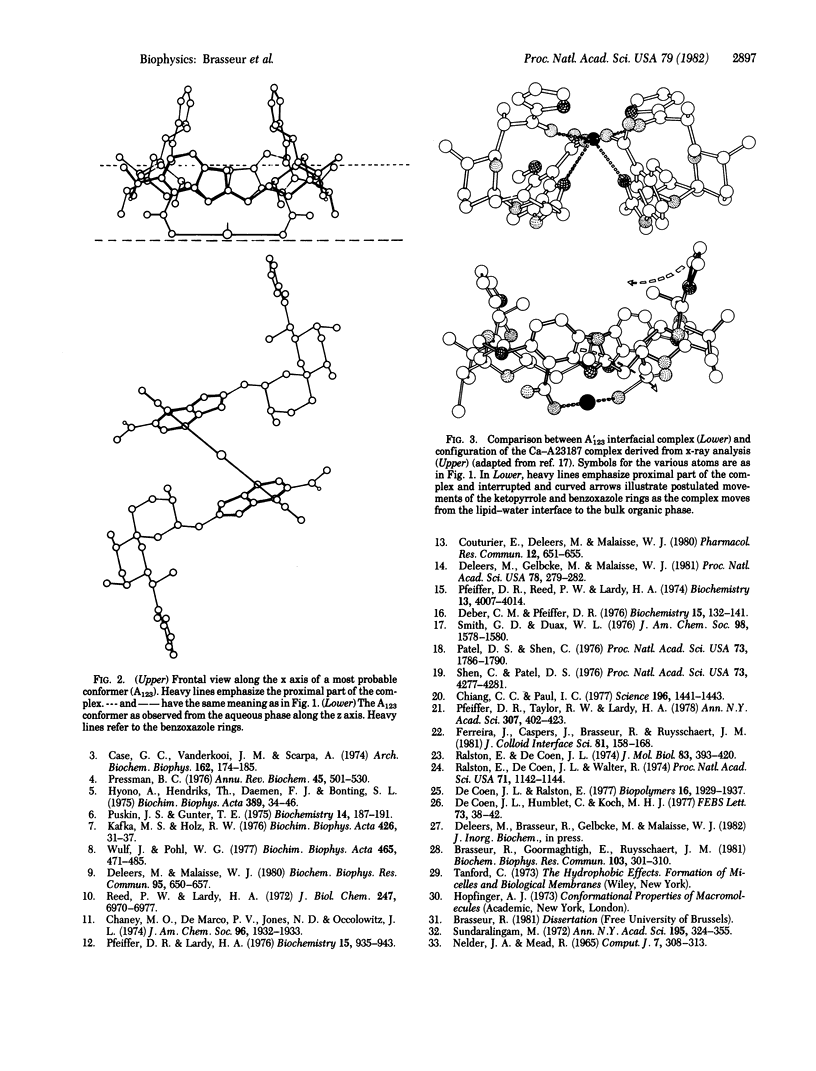
A possible conformation of the complex formed by one calcium ion and two molecules of the ionophore A23187 at a simulated lipid--water interface was predicted by a variant method for conformational analysis. This method takes into account, in addition to the Van der Waals energy, electrostatic interaction, and torsional potential, the alteration of electrostatic forces attributable to changes in dielectric constant at the interface and the transfer energy for each part of the complex as it moves through the lipid-water interface. The most probable conformer was characterized by a two-fold axial symmetry that was maintained during transition to the hydrophobic bulk conformation. Minor changes in the interfacial structure were sufficient to achieve the configuration characteristic of the hydrophobic bulk phase.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brasseur R., Goormaghtigh E., Ruysschaert J. M. Theoretical conformational analysis of phospholipids bilayers. Biochem Biophys Res Commun. 1981 Nov 16;103(1):301–310. doi: 10.1016/0006-291x(81)91693-4. [DOI] [PubMed] [Google Scholar]
- Case G. D., Vanderkooi J. M., Scarpa A. Physical properties of biological membranes determined by the fluorescence of the calcium ionophore A23187. Arch Biochem Biophys. 1974 May;162(1):174–185. doi: 10.1016/0003-9861(74)90116-7. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Pressman B. C. Kinetics of transport of divalent cations across sarcoplasmic reticulum vesicles induced by ionophores. Biochem Biophys Res Commun. 1972 Oct 6;49(1):292–298. doi: 10.1016/0006-291x(72)90043-5. [DOI] [PubMed] [Google Scholar]
- Chaney M. O., Demarco P. V., Jones N. D., Occolowitz J. L. Letter: The structure of A23187, a divalent cation ionophore. J Am Chem Soc. 1974 Mar 20;96(6):1932–1933. doi: 10.1021/ja00813a047. [DOI] [PubMed] [Google Scholar]
- Chiang C. C., Paul I. C. Monomeric forms of the acid ionophore lasalocid A (X-537A) from polar solvents. Science. 1977 Jun 24;196(4297):1441–1443. doi: 10.1126/science.867039. [DOI] [PubMed] [Google Scholar]
- Couturier E., Deleers M., Malaisse W. J. Synergism between two distinct ionophores in translocating calcium from an aqueous to an organic environment. Pharmacol Res Commun. 1980 Jul;12(7):651–655. doi: 10.1016/s0031-6989(80)80102-0. [DOI] [PubMed] [Google Scholar]
- De Coen J. L., Humblet C., Koch M. H. Theoretical conformational analysis of Met-enkephalin. FEBS Lett. 1977 Jan 15;73(1):38–42. [PubMed] [Google Scholar]
- De Coen J. L., Ralston E. Theoretical conformational analysis of Asn1, Val5 angiotensin II. Biopolymers. 1977 Sep;16(9):1929–1943. doi: 10.1002/bip.1977.360160908. [DOI] [PubMed] [Google Scholar]
- Deber C. M., Pfieffer D. R. Ionophore A23187. Solution conformations of the calcium complex and free acid deduced from proton and carbon-13 nuclear magnetic resonance studies. Biochemistry. 1976 Jan 13;15(1):132–141. doi: 10.1021/bi00646a020. [DOI] [PubMed] [Google Scholar]
- Deleers M., Gelbcke M., Malaisse W. J. Formation of hybrid complexes between Ca and the ionophores bromolasalocid (Br-X537A) and A23187. Proc Natl Acad Sci U S A. 1981 Jan;78(1):279–282. doi: 10.1073/pnas.78.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleers M., Malaisse W. J. Ionophore-mediated calcium exchange diffusion in liposomes. Biochem Biophys Res Commun. 1980 Jul 31;95(2):650–657. doi: 10.1016/0006-291x(80)90835-9. [DOI] [PubMed] [Google Scholar]
- Hyono A., Hendriks T., Daemen F. J., Bonting S. L. Movement of calcium through artificial lipid membranes and the effects of ionophores. Biochim Biophys Acta. 1975 Apr 21;389(1):34–46. doi: 10.1016/0005-2736(75)90383-1. [DOI] [PubMed] [Google Scholar]
- Kafka M. S., Holz R. W. Ionophores X537A and A23187. Effects on the permeability of lipid bimolecular membranes to dopamine and calcium. Biochim Biophys Acta. 1976 Feb 19;426(1):31–37. doi: 10.1016/0005-2736(76)90426-0. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shen C. Structural and kinetic studies of lasalocid A (X537A) and its silver, sodium, and barium salts in nonpolar solvents. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1786–1790. doi: 10.1073/pnas.73.6.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer D. R., Lardy H. A. Ionophore A23187: the effect of H+ concentration on complex formation with divalent and monovalent cations and the demonstration of K+ transport in mitochondria mediated by A23187. Biochemistry. 1976 Mar 9;15(5):935–943. doi: 10.1021/bi00650a001. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Reed P. W., Lardy H. A. Ultraviolet and fluorescent spectral properties of the divalent cation ionophore A23187 and its metal ion complexes. Biochemistry. 1974 Sep 10;13(19):4007–4014. doi: 10.1021/bi00716a029. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Puskin J. S., Gunter T. E. Electron paramagnetic resonance of copper ion and manganese ion complexes with the ionophore A23187. Biochemistry. 1975 Jan 14;14(1):187–191. doi: 10.1021/bi00672a031. [DOI] [PubMed] [Google Scholar]
- Ralston E., De Coen J. L. Folding of polypeptide chains induced by the amino acid side-chains. J Mol Biol. 1974 Mar;83(3):393–420. doi: 10.1016/0022-2836(74)90287-3. [DOI] [PubMed] [Google Scholar]
- Ralston E., De Coen J. L., Walter R. Tertiary structure of H-Pro-Leu-Gly-NH2, the factor that inhibits release of melanocyte stimulating hormone, derived by conformational energy calculations. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1142–1144. doi: 10.1073/pnas.71.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Scarpa A., Baldassare J., Inesi G. The effect of calcium ionophores on fragmented sarcoplasmic reticulum. J Gen Physiol. 1972 Dec;60(6):735–749. doi: 10.1085/jgp.60.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Patel D. J. Free acid, anion, alkali, and alkaline earth complexes of lasalocid a (X537A) in methanol: structural and kinetic studies at the monomer level. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4277–4281. doi: 10.1073/pnas.73.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaralingam M. Discussion paper: molecular structures and conformations of the phospholipids and sphingomyelins. Ann N Y Acad Sci. 1972 Jun 20;195:324–355. [PubMed] [Google Scholar]
- Wulf J., Pohl W. G. Calcium ion-flux across phosphatidylcholine membranes mediated by ionophore A23187. Biochim Biophys Acta. 1977 Mar 17;465(3):471–485. doi: 10.1016/0005-2736(77)90266-8. [DOI] [PubMed] [Google Scholar]



