Abstract
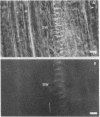
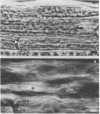
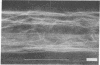
The distribution of F-actin in the complex tissues of a higher plant organ has been visualized by fluorescence labeling the roots of the conifers Chamaecyparis obtusa and Pseudotsuga menziesii with F-actin-specific fluorescent dye-conjugated phallicidin. F-actin is present in the parenchymatous cells of the vascular tissue. Some vascular parenchyma cells possess larger numbers of F-actin-containing structures (microfilament bundles) than are known to exist in any other higher plant cell. Tissue type appears to be an important determinant of the presence or absence of F-actin in a cell. For example, in contrast to vascular cells, cortical cells show no indication of fluorescence labeling of F-actin after incubation with fluorescent phallicidin. Cytoplasmic streaming is seen only in vascular cells and in a pattern that reflects the intracellular distribution of F-actin.
Keywords: 7-nitrobenz-2-oxa-1,3-diazole-phallicidin fluorescent labeling; cytoplasmic streaming; microfilament bundle
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. S., Allen R. D. Cytoplasmic streaming in green plants. Annu Rev Biophys Bioeng. 1978;7:497–526. doi: 10.1146/annurev.bb.07.060178.002433. [DOI] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R. 7-Nitrobenz-2-oxa-1,3-diazole (NBD)--phallacidin: synthesis of a fluorescent actin probe. Anal Biochem. 1981 Jan 1;110(1):31–38. doi: 10.1016/0003-2697(81)90107-x. [DOI] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J. S. The identification of F actin of the pollen tube and protoplast of Amaryllis belladonna. Exp Cell Res. 1974 Oct;88(2):435–439. doi: 10.1016/0014-4827(74)90269-9. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Multigene families encoding actin and tubulin. Cell. 1981 Apr;24(1):6–7. doi: 10.1016/0092-8674(81)90494-3. [DOI] [PubMed] [Google Scholar]
- Kersey Y. M., Hepler P. K., Palevitz B. A., Wessells N. K. Polarity of actin filaments in Characean algae. Proc Natl Acad Sci U S A. 1976 Jan;73(1):165–167. doi: 10.1073/pnas.73.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas J. P., Lafountain J. Protoplasmic streaming, cytochalasin B, and growth of the pollen tube. Tissue Cell. 1972;4(1):11–14. doi: 10.1016/s0040-8166(72)80002-8. [DOI] [PubMed] [Google Scholar]
- Nagai R., Rebhun L. I. Cytoplasmic microfilaments in streaming Nitella cells. J Ultrastruct Res. 1966 Mar;14(5):571–589. doi: 10.1016/s0022-5320(66)80083-7. [DOI] [PubMed] [Google Scholar]
- Nothnagel E. A., Barak L. S., Sanger J. W., Webb W. W. Fluorescence studies on modes of cytochalasin B and phallotoxin action on cytoplasmic streaming in Chara. J Cell Biol. 1981 Feb;88(2):364–372. doi: 10.1083/jcb.88.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. P., Thimann K. V. Intracellular fibers in oat coleoptile cells and their possible significance in cytoplasmic streaming. Proc Natl Acad Sci U S A. 1966 Sep;56(3):888–894. doi: 10.1073/pnas.56.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy M. V., Mühlethaler K. Cytoplasmic microfilaments in plant cells. J Ultrastruct Res. 1972 Jan;38(1):46–62. doi: 10.1016/s0022-5320(72)90083-4. [DOI] [PubMed] [Google Scholar]
- Seagull R. W., Heath I. B. The effects of tannic acid on the in vivo preservation of microfilaments. Eur J Cell Biol. 1979 Dec;20(2):184–188. [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- Williamson R. E. Cytoplasmic streaming in Chara: a cell model activated by ATP and inhibited by cytochalasin B. J Cell Sci. 1975 May;17(3):655–668. doi: 10.1242/jcs.17.3.655. [DOI] [PubMed] [Google Scholar]
- Worley J. F. Rotational streaming in fiber cells and its role in translocation. Plant Physiol. 1968 Oct;43(10):1648–1655. doi: 10.1104/pp.43.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Nagai R. Motile apparatus in Vallisneria leaf cells. I. Organization of microfilaments. J Cell Sci. 1981 Apr;48:193–205. doi: 10.1242/jcs.48.1.193. [DOI] [PubMed] [Google Scholar]