Abstract
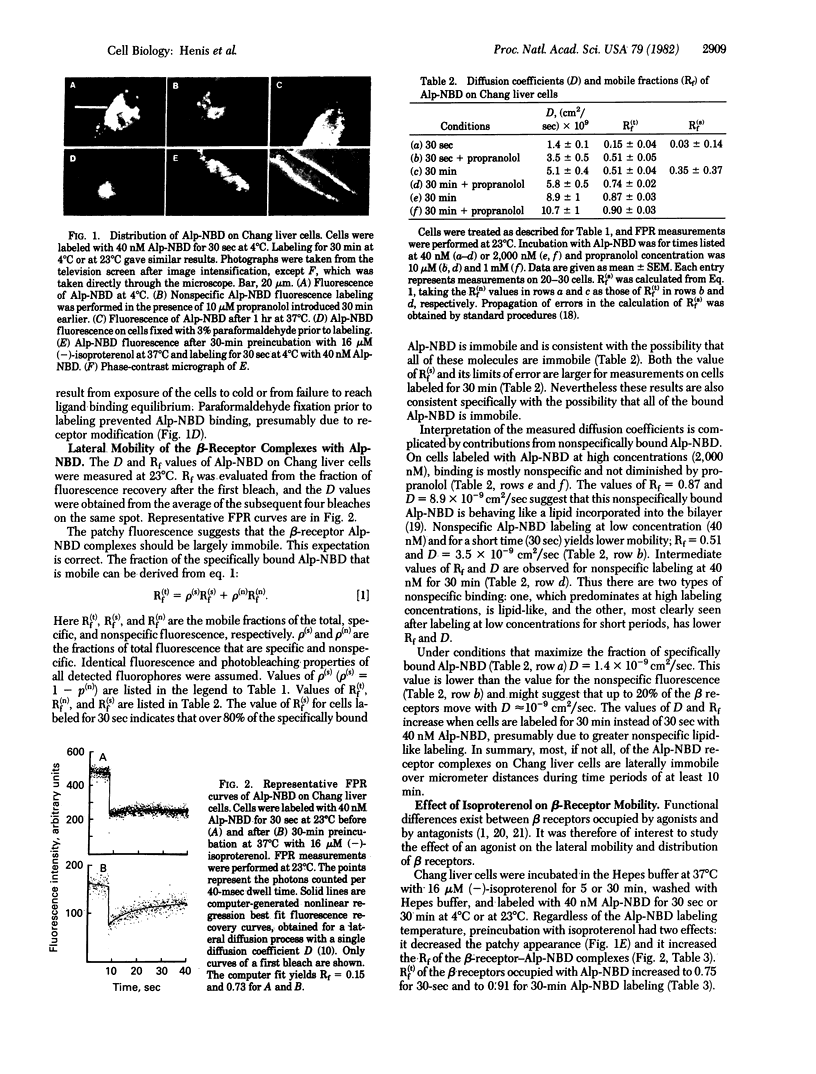
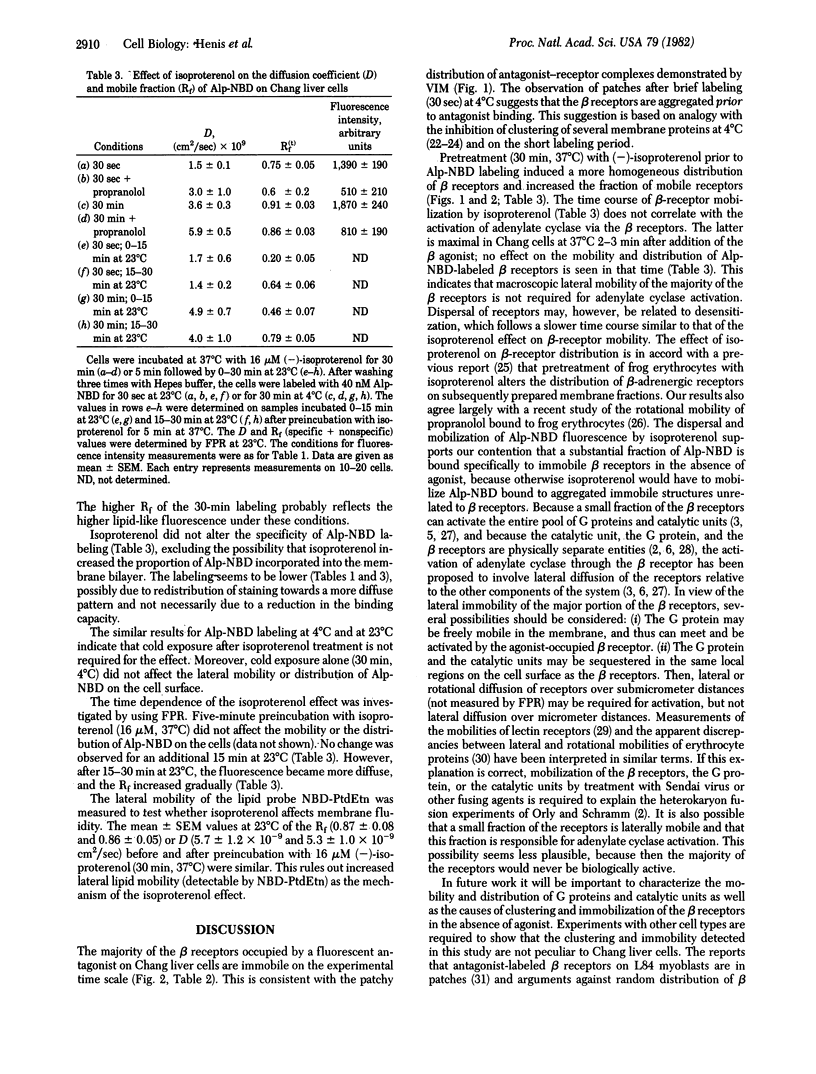
We have studied the lateral mobility and distribution of beta receptors on Chang human liver cells by fluorescence photobleaching recovery and video intensification microscopy. The beta receptors were labeled with the fluorescent antagonist 7-(2-allylphenoxy)-2,2-dimethyl-6-hydroxy-1-(4-nitrobenzo-2-oxa-1,3-diazolyl)-1 ,4-diazaheptane (Alp-NBD). Sixty to 75% of the staining was specific (displaceable by unlabeled antagonists). Most of the antagonist-occupied beta receptors were immobile, because only 15-25% of their fluorescence recovered on the experimental time scale at 23 degrees C. This immobility correlates with the clustered distribution of Alp-NBD--beta-receptor complexes at 4 degrees C and 37 degrees C. The beta receptors appear to be aggregated prior to antagonist binding, because visible patches were observed immediately after labeling for 30 sec at 4 degrees C. Preincubation at 37 degrees C with (--)-isoproterenol, a beta agonist, prior to Alp-NBD labeling induced a time-dependent release of the beta receptors to a more homogeneous distribution and increased the mobile fraction to 70-80% (lateral diffusion coefficient = 1.4 X 10(-9) cm2/sec at 23 degrees C). This is not due to an effect on membrane fluidity, because the diffusion coefficient of a lipid probe was not altered. The time course of agonist-induced beta-receptor mobilization correlates with receptor loss and adenylate cyclase desensitization but is much slower than adenylate cyclase activation. This indicates that adenylate cyclase activation by beta receptors does not require macroscopic lateral mobility of the majority of the beta receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas D., Yaffe D., Skutelsky E. Ultrastructural probing of beta-adrenoreceptors on cell surfaces. FEBS Lett. 1978 Nov 1;95(1):173–176. doi: 10.1016/0014-5793(78)80077-5. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakardjieva A., Galla H. J., Helmreich E. J. Modulation of the beta-receptor adenylate cyclase interactions in cultured Chang liver cells by phospholipid enrichment. Biochemistry. 1979 Jul 10;18(14):3016–3023. doi: 10.1021/bi00581a017. [DOI] [PubMed] [Google Scholar]
- Barovsky K., Brooker G. (-)-[125I]-iodopindolol, a new highly selective radioiodinated beta-adrenergic receptor antagonist: measurement of beta-receptors on intact rat astrocytoma cells. J Cyclic Nucleotide Res. 1980;6(4):297–307. [PubMed] [Google Scholar]
- Cherksey B. D., Zadunaisky J. A., Murphy R. B. Cytoskeletal constraint of the beta-adrenergic receptor in frog erythrocyte membranes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6401–6405. doi: 10.1073/pnas.77.11.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J., Bürkli A., Busslinger M., Schneider G., Parish G. R. Rotational diffusion of band 3 proteins in the human erythrocyte membrane. Nature. 1976 Sep 30;263(5576):389–393. doi: 10.1038/263389a0. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Citri Y., Schramm M. Resolution, reconstitution and kinetics of the primary action of a hormone receptor. Nature. 1980 Sep 25;287(5780):297–300. doi: 10.1038/287297a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Elson E. L. Inhibition of the mobility of mouse lymphocyte surface immunoglobulins by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1072–1076. doi: 10.1073/pnas.78.2.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe R., Crowther A. F., Stephenson J. S., Rao B. S., Smith L. H. Beta-adrenergic blocking agents. I. Pronethalol and related N-alkyl and N-aralkyl derivatives of 2-amino-1-(2-naphythyl)ethanol. J Med Chem. 1968 Sep;11(5):1000–1008. doi: 10.1021/jm00311a020. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A., Shechter Y., Neufeld E. J., Schlessinger J. Mobility, clustering, and transport of nerve growth factor in embryonal sensory cells and in a sympathetic neuronal cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3469–3473. doi: 10.1073/pnas.77.6.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M. E., Ross E. M., Gilman A. G. beta-Adrenergic receptor: ligand binding properties and the interaction with adenylyl cyclase. Adv Cyclic Nucleotide Res. 1977;8:1–83. [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey J. V., Tate R., Mullikin D., Lefkowitz R. J. Regulation of adenylate cyclase-coupled beta adrenergic receptor binding sites by beta adrenergic catecholamines in vitro. Mol Pharmacol. 1976 May;12(3):409–419. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Coverstone M., Lefkowitz R. J. Identification of adenylate cyclase-coupled beta-adrenergic receptors in frog erythrocytes with (minus)-[3-H] alprenolol. J Biol Chem. 1975 Jul 10;250(13):4869–4876. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orly J., Schramm M. Coupling of catecholamine receptor from one cell with adenylate cyclase from another cell by cell fusion. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4410–4414. doi: 10.1073/pnas.73.12.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Pfeuffer T. Guanine nucleotide-controlled interactions between components of adenylate cyclase. FEBS Lett. 1979 May 1;101(1):85–89. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., Hollenberg M. D., Bennett V., Cuatrecasas P. Topographic separation of adenylate cyclase and hormone receptors in the plasma membrane of toad erythrocyte ghosts. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2860–2864. doi: 10.1073/pnas.74.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki W. L., Brooker G. [125I]Iodohydroxybenzylpindolol binding sites on intact rat glioma cells. Evidence for beta-adrenergic receptors of high coupling efficiency. J Biol Chem. 1978 Aug 10;253(15):5418–5425. [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Coupling of a single adenylate cyclase to two receptors: adenosine and catecholamine. Biochemistry. 1978 Sep 5;17(18):3811–3817. doi: 10.1021/bi00611a021. [DOI] [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978 Sep 5;17(18):3795–3795. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The visualization of fluorescent proteins in living cells by video intensification microscopy (VIM). Cell. 1978 Mar;13(3):501–507. doi: 10.1016/0092-8674(78)90323-9. [DOI] [PubMed] [Google Scholar]
- Zagyansky Y., Jard S. The effect of amphotericin B on lectin-induced aggregation of cell surface receptors. Exp Cell Res. 1981 Apr;132(2):387–397. doi: 10.1016/0014-4827(81)90114-2. [DOI] [PubMed] [Google Scholar]



