Summary
Background and objectives
Peritonitis is a well known complication of peritoneal dialysis (PD), whereas in hemodialysis (HD), bacteremia can be life threatening. Whether patients undergoing PD have higher risk than HD patients for infection-related hospitalizations (IRH) remains unknown.
Design, setting, participants, & measurements
A propensity score–matched retrospective cohort of patients undergoing long-term dialysis between January 2001 and December 2007 was assembled. Propensity scores were calculated using multivariable (demographic characteristics, smoking, body mass index, comorbid conditions, and laboratory data) logistic regression to estimate probability of receiving PD versus HD. A comparison of IRH risk by dialysis modality was estimated using a counting-process survival model.
Results
A total of 910 pairs of patients were matched by propensity scores. During a median follow-up of 2.1 years (interquartile range, 1.1–3.5 years), 341 patients were hospitalized once for an infection, 123 twice, and 106 at least three times. PD was associated with an increased risk for IRH compared with HD (propensity-matched hazard ratio [HR], 1.52). PD was associated with a reduced risk for septicemia (HR, 0.31) and pneumonia (HR, 0.58) but also an increased risk for dialysis-related infectious hospitalizations (HR, 3.44), defined as all cases of peritonitis and vascular access-related bacteremia, but not all septicemia cases.
Conclusions
PD patients are at higher risk for IRH than are HD patients. This risk is mostly explained by dialysis-related infections. However, further studies are needed to evaluate whether the severity of those hospitalizations is similar and whether this increased risk for IRH is associated with worse outcomes.
Introduction
Long-term dialysis is associated with a high burden of morbidity necessitating frequent hospital admissions. In this population, infections are a common cause of hospitalizations and the second leading cause of death, after cardiovascular disease (1–6). Those admissions carry a high financial burden on health care systems, and the impact of infection is greater in the dialysis population than in the general population (3,7). Peritonitis is a well known complication of peritoneal dialysis (PD). Peritonitis can lead to significant changes in the peritoneal membrane characteristics, which in turn is a major cause of modality failure. On the other hand, patients receiving hemodialysis (HD) are at risk for infections associated with vascular access, including bloodstream infections, which can be life-threatening. Therefore, both dialysis modalities are associated with different types of serious infections. Because dialysis modality is often a patient’s choice when no contraindication exists for one specific modality, a patient should be well informed of the advantages and disadvantages of each modality. The importance of such discussions is even greater for subgroups of patients who are at increased risk for infection (e.g., immunocompromised patients).
Data from the U.S. Renal Data System (USRDS) suggest that HD patients have a higher risk for septicemia but fewer infection-related hospitalizations (IRHs) than PD patients (1). However, this disparity may be explained by case-mix differences because patients receiving each modality are usually very different. Unfortunately, existing studies on this issue were single-center or limited because of small sample size; therefore they had statistical power concerns (8,9). Therefore, how those risks balance between dialysis modalities has not been formally studied in a large dialysis population. It remains unknown whether patients undergoing PD are at higher risk for dialysis- and non–dialysis-associated IRH than are HD patients.
The objectives of this study were to describe and compare risk for dialysis- and non–dialysis-associated IRH among incident PD and HD patients using a propensity score–matched cohort.
Materials and Methods
Study Population and Data Sources
We obtained data from the Canadian Organ Replacement Register (CORR) and provincial health services administrative databases. All residents (approximately 8 million) in Quebec, Canada, are covered for medical and hospital services by a universal single-payer health care system (Régie de l’assurance maladie du Québec [RAMQ]). The RAMQ physician claims databases include all visits, diagnosis codes (International Classification of Diseases, Ninth Revision [ICD-9]), and procedures during inpatient or outpatient encounters. RAMQ also hosts the hospital discharge summary databases, providing admission and discharge dates, primary and secondary diagnoses (coded using ICD-9 or, after 2006, ICD-10), and procedures during the admission. Validation of discharge summary data in Quebec has shown high accuracy for many conditions (10–13).
CORR, a national information system for renal and extrarenal replacement therapies, collects longitudinal center and patient data from all dialysis units across Canada. Data elements include demographic characteristics, comorbid conditions, dialysis clinical data, outcome-related data (death and kidney transplant), and facility profiles.
In the study cohort, we included all adult patients who initiated long-term dialysis between January 1, 2001, and December 31, 2007. Data for at least 2 years before the start of dialysis was obtained from CORR and RAMQ. To be included, a patient had to be present in both CORR and RAMQ data sources. Patients were considered for inclusion at the date of initiation of long-term dialysis (index date). For patients who initiated dialysis during a hospitalization, the date of hospital discharge was their index date. We excluded patients who initiated long-term dialysis before January 1, 2001; had less than 90 days of dialysis after index date; or had a prior kidney transplant. The 90-day criterion was used to include only maintenance dialysis patients and to facilitate comparability with existing published studies in this area. Study patients were followed until kidney transplant, death, or end of the study period. Dialysis modality (PD or HD [home and in-center combined]) was attributed at 90 days according to physician claims at that moment and therefore may differ from modality at dialysis initiation.
IRH
We identified all admissions during study follow-up with an infection as primary diagnosis on the discharge sheet according to ICD-9 or ICD-10 (after 2006) codes. IRHs were categorized in eight mutually exclusive categories: abdominal, dialysis-related, genitourinary, musculoskeletal, pneumonia, septicemia, skin, and other infection (see Supplemental Table 1 for specific codes); dialysis-related infections included peritonitis, infections due to vascular device, implant, graft, or peritoneal dialysis catheter. Because it was impossible to determine whether a case of peritonitis was dialysis related or not, all peritonitis episodes were considered dialysis related.
Covariates
Covariates measured at baseline included demographic characteristics (RAMQ), body mass index (BMI; CORR), race (CORR), smoking (CORR), visit to a nephrologist in the 6–12 months before dialysis initiation (RAMQ), and hospitalization in the year prior (RAMQ). Comorbid conditions (listed in Table 1) were identified through ICD-9 codes from the RAMQ billing database in the 2 years before the index date. Because CORR also identifies comorbid conditions listed above at dialysis initiation by an indicator variable, we combined both sources to increase sensitivity. All laboratory data were obtained at dialysis initiation (CORR). Creatinine was used to calculate estimated GFR by applying the four-variable Modification of Diet in Renal Disease equation (14).
Table 1.
Baseline characteristics for the whole cohort and the propensity score–matched cohort
| Baseline Characteristics | Whole Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| HD (n=4933) | PD (n=925) | P Value | HD (n=910) | PD (n=910) | P Value | |
| Age (yr) | 65.9±14.0 | 58.4±14.7 | <0.001 | 58.5±16.4 | 58.8±14.5 | 0.72 |
| Female sex (%) | 39.3 | 41.3 | 0.27 | 40.9 | 41.2 | 0.89 |
| Race (%)a | ||||||
| Black | 4.3 | 3.1 | 0.31 | 3.9 | 3.1 | 0.43 |
| White | 88.8 | 88.0 | 86.0 | 88.0 | ||
| Other | 6.9 | 8.9 | 10.1 | 8.8 | ||
| BMI (kg/m2)b | 27.3±6.4 | 26.1±5.2 | <0.001 | 26.2±5.5 | 26.2±5.2 | 0.79 |
| Smoking (%) | 14.5 | 15.4 | 0.52 | 13.7 | 15.4 | 0.32 |
| Visit to a nephrologist 6–12 mo prior (%) | 59.7 | 71.8 | <0.001 | 71.3 | 71.3 | 1.00 |
| Hospitalization in prior year (%) | 62.9 | 81.0 | <0.001 | 82.3 | 80.7 | 0.37 |
| Comorbid conditions (%) | ||||||
| Cardiovascular disease | 55.6 | 40.0 | <0.001 | 39.7 | 40.7 | 0.67 |
| Cerebrovascular disease | 16.0 | 9.6 | <0.001 | 8.7 | 9.8 | 0.42 |
| Chronic pulmonary disease | 28.2 | 17.3 | <0.001 | 17.6 | 17.6 | 1.00 |
| Chronic liver disease | 3.5 | 1.8 | 0.01 | 2.9 | 1.9 | 0.16 |
| Congestive heart failure | 37.9 | 21.5 | <0.001 | 20.9 | 21.9 | 0.61 |
| Diabetes | 52.9 | 40.1 | <0.001 | 38.8 | 40.4 | 0.47 |
| Hyperlipidemia | 51.1 | 45.3 | 0.001 | 46.7 | 45.5 | 0.61 |
| Hypertension | 93.4 | 93.5 | 0.91 | 93.6 | 93.5 | 0.92 |
| Malignancy | 18.1 | 12.8 | <0.001 | 11.7 | 12.9 | 0.43 |
| Peripheral vascular disease | 36.0 | 23.5 | <0.001 | 25.2 | 23.7 | 0.48 |
| Laboratory data | ||||||
| Albumin (g/dl)c | 3.4±0.7 | 3.6±0.6 | <0.001 | 3.6±0.7 | 3.6±0.6 | 0.60 |
| Estimated GFR (ml/min per 1.73 m2)d | 9.1±4.1 | 8.8±3.9 | 0.01 | 8.8±3.5 | 8.8±3.9 | 0.85 |
| Hemoglobin (g/dl)e | 10.4±1.8 | 11.1±1.7 | <0.001 | 11.1±1.7 | 11.1±1.7 | 1.00 |
| Phosphorus (mg/dl)f | 5.8±2.1 | 5.4±1.6 | <0.001 | 5.5±1.6 | 5.4±1.6 | 0.46 |
| Urea (mg/dl)g | 87.4±33.2 | 83.4±30.0 | <0.001 | 84.1±29.1 | 83.5±30.1 | 0.71 |
Values expressed with a plus/minus sign are the mean ± SD. Albumin, multiply by 10 to convert g/dl to g/L; hemoglobin, multiply by 10 to convert g/dl to g/L; phosphorus, multiply by 0.3229 to convert mg/dl to mmol/L; urea, multiply by 0.357 to convert mg/dl to mmol/L. HD, hemodialysis; PD, peritoneal dialysis; BMI, body mass index.
Race was missing in 2.7% of patients.
BMI value was missing in 6.2% of patients.
An albumin value was missing in 10.2% of patients.
A creatinine value was missing in 9.9% of patients.
A hemoglobin value was missing in 10.5% of patients.
A phosphorus value was missing in 16.4% of patients.
A urea value was missing in 13.9% of patients.
Statistical Analyses
Descriptive baseline data are presented using mean ± SD or median and interquartile range (IQR), as appropriate. Baseline characteristics between PD and HD patients were compared by the t test, Mann-Whitney U test, or chi-squared proportion test, as appropriate. Hospitalization rates were calculated by dividing the number of admissions by the total patient-years of follow-up. Because having multiple short hospitalizations may not be worse than having one long hospitalization, we also calculated median length of stay (LOS) and ratio of LOS and total person-year (as a measure of proportion of time hospitalized during follow-up).
Propensity scores were calculated using multivariable logistic regression to estimate probability of receiving PD versus HD. All covariates described previously were included in the propensity score model. Propensity scores were then used to match PD patients to HD patients 1:1 using a greedy nearest-neighbor matching algorithm. Missing data for BMI and laboratory data were imputed using a multiple imputation technique (five imputations).
To evaluate risk for IRH associated with dialysis modality, we conducted two different models: (1) an unadjusted counting-process survival model by Prentice, Williams and Peterson (PWP-CP) using a propensity score–matched cohort, and (2) a multivariate PWP-CP model using the whole cohort sample (15). Conditional survival models such as PWP-CP are appropriate when a given patient can have multiple events of the same type but the risk for those events changes after a patient experiences an event (15). For example, during follow-up a patient may be hospitalized twice for an infection-related condition (same type of event) but is probably at higher risk to be hospitalized again after being hospitalized once (15). First five events were considered in the PWP-CP model. Statistical interaction was tested for selected subgroups (age, sex, BMI, race, cardiovascular disease) by adding a multiplicative term in the model. All analyses were done using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC).
Sensitivity Analyses
Retrospective studies are prone to bias. Therefore, we conducted several sensitivity analyses to explore robustness of our results: (1) We built a fully adjusted model using the matched cohort to control for residual confounding; (2) we conducted models without imputing missing value (complete case analysis); (3) we conducted an analysis in which follow-up was terminated at the first claim for the opposite dialysis modality because patients may switch between dialysis modalities; (4) we conducted a sensitivity analysis in which follow-up was limited to 2 years because rates of IRH may vary by dialysis vintage; (5) we evaluated risk for IRH such that IRHs were identified using all diagnoses (primary and secondary), and not only the primary diagnosis; (6) we combined septicemia and dialysis-related infections as a single category because septicemia may originate from vascular access or peritonitis, among other causes; (7) we redid the analyses by starting follow-up at 90 days after dialysis initiation because rates of infections in the first 3 months may be very different because of temporary catheter use; and (8) we repeated all analyses using a Cox regression model limited to the first IRH.
Ethical Considerations
This study was approved by the Government of Quebec ethics committee (Commission d’accès à l’information) and Maisonneuve-Rosemont Hospital ethics committee, and informed consent was waived.
Results
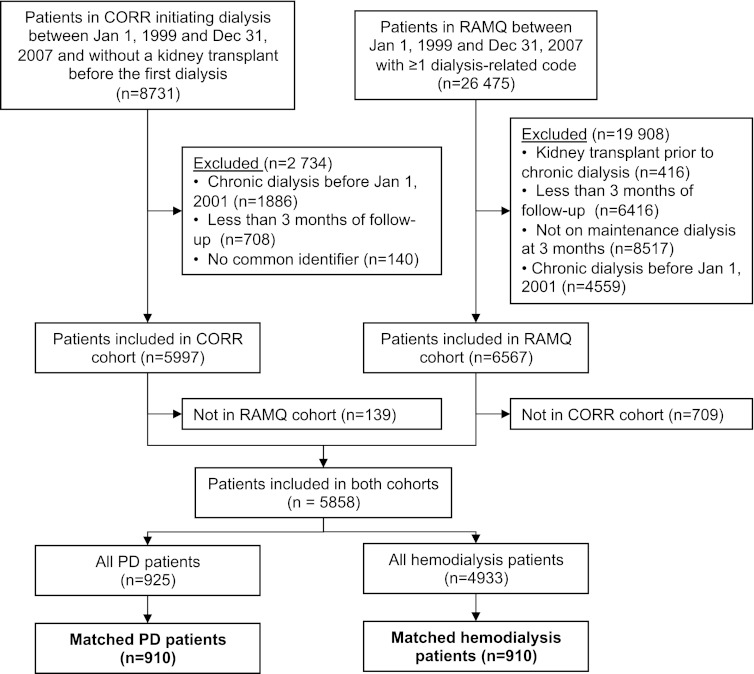
The whole cohort included 5858 patients (925 PD and 4933 HD patients) who were followed for a median of 2.0 years (IQR, 1.0–3.4 years). Derivation of the cohort is detailed in Figure 1. We were able to match by propensity scores 910 pairs of patients, who were followed for a median time of 2.1 years (IQR, 1.1–3.5). Baseline characteristics for the whole and propensity score–matched cohorts are detailed in Table 1. As expected, baseline characteristics were well balanced between PD and HD patients in the matched cohort.
Figure 1.
Derivation of cohort. Despite meeting all inclusion criteria according to Régie de l’assurance maladie du Québec (RAMQ) data, some RAMQ cohort patients (n=709) were excluded because they were not registered in Canadian Organ Replacement Register (CORR) (n=593) or had been excluded according to CORR data (kidney transplant [n=40], dialysis initiation before January 2001 [n=19], or <3 months of follow-up [n=57]). Similarly, some patients were excluded from CORR (n=139) because they had no service in RAMQ (n=4) or were excluded on the basis of RAMQ data (kidney transplant [n=30], <3 months of follow-up or dialysis [n=87], or dialysis initiation before January 2001 [n=18]). PD, peritoneal dialysis.
For the whole sample, we identified 2906 IRHs (2267 among HD patients and 639 among PD patients) during the study period. Among the matched cohort, 341 patients were hospitalized once for an infection, 123 twice, and 106 at least three times. Rates of hospitalization for the matched cohort are given in Table 2. The proportions of all hospitalizations that were related to infection were 12% for HD, and 21% for PD. Propensity-matched IRH rates were almost twice as high for PD than for HD (0.29 versus 0.17/person-year). This disparity appeared to be mainly explained by dialysis-related and abdominal infections. On the other hand, cardiovascular and other hospitalization rates were similar between both modalities.
Table 2.
Propensity-matched hospitalization rates and length of stay (n=1820)
| Variable | Hemodialysis | Peritoneal Dialysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | Rate per Person-Year | LOS | Patients (n) | Rate per Person-Year | LOS | ||||
| Median (IQR) (d) | LOS/Follow-up per 1000 | Median (IQR) (d) | LOS/Follow-up per 1000 | ||||||
| All causes | 3147 | 1.39 | 3 (1–9) | 37.5 | 2994 | 1.38 | 4 (2–10) | 39.7 | |
| Cardiovascular | 754 | 0.33 | 5 (2–11) | 10.2 | 667 | 0.31 | 6 (2–15) | 12.0 | |
| Infection | 383 | 0.17 | 8 (4–17) | 7.0 | 631 | 0.29 | 7 (4–14) | 10.3 | |
| Abdominal | 27 | 0.012 | 9 (3–14) | 0.60 | 39 | 0.018 | 7 (4–18) | 0.55 | |
| Dialysis-related | 94 | 0.041 | 7 (4–15) | 1.5 | 401 | 0.185 | 6 (4–13) | 5.9 | |
| Genitourinary | 23 | 0.010 | 5 (3–8) | 0.27 | 20 | 0.009 | 6 (5–13) | 0.25 | |
| Musculoskeletal | 15 | 0.007 | 20 (13–44) | 0.48 | 17 | 0.008 | 9 (1–21) | 1.2 | |
| Pneumonia | 51 | 0.022 | 7 (5–12) | 0.78 | 27 | 0.012 | 7 (4–16) | 0.40 | |
| Septicemia | 69 | 0.030 | 10 (5–18) | 1.2 | 17 | 0.008 | 6 (4–13) | 0.48 | |
| Skin | 29 | 0.013 | 9 (5–21) | 0.59 | 28 | 0.013 | 7 (3–14) | 0.37 | |
| Other infection | 75 | 0.033 | 9 (4–17) | 1.6 | 82 | 0.038 | 8 (4–13) | 1.2 | |
| Other | 2010 | 0.89 | 2 (1–7) | 20.3 | 1696 | 0.78 | 3 (1–8) | 17.4 | |
LOS, length of stay; IQR, interquartile range; LOS/follow-up, ratio of total length-of-stay and total follow-up.
Median LOS of IRH was similar between HD and PD (Table 2), suggesting that the higher IRH rate did not consist of shorter hospitalizations. However, LOS for septicemia, dialysis-related infections, and musculoskeletal infections appeared shorter for PD patients, suggesting that those infections were more severe among HD patients.
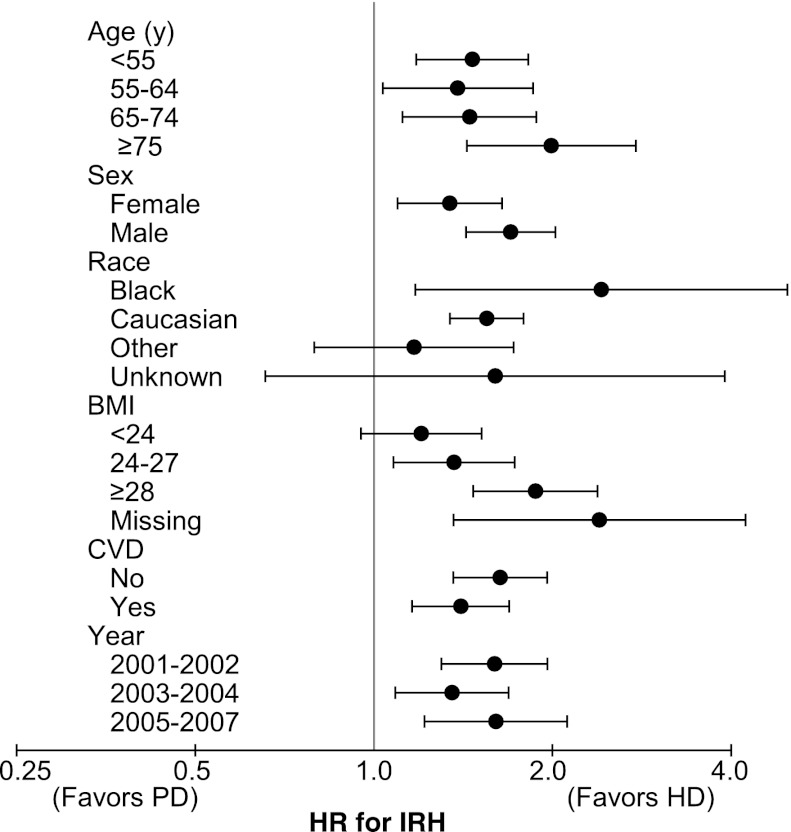
According to the main model using the matched cohort, PD was associated with an increased risk for IRH compared with HD (unadjusted hazard ratio [HR], 1.52; 95% confidence interval [CI], 1.34–1.74). As shown in Figure 2, this increased IRH risk associated with PD was constant across most subgroups (age, sex, race, cardiovascular disease, and year of dialysis initiation), and interaction terms were not significant. IRH risk associated with PD appeared to increase with BMI (P for interaction = 0.04). When the whole sample was used, results were similar (Table 3). Adjustment for potential confounders increased the risk estimate from 1.38 (95% CI, 1.26–1.51) to 1.52 (95% CI, 1.38–1.68).
Figure 2.
Risk for infection-related hospitalizations associated with dialysis modality for different subgroups (matched cohort). Hemodialysis is the reference group. Calendar years are years of dialysis initiation. The x-axis is on a logarithmic scale where 1 represents no effect. Error bars represent 95% confidence intervals. BMI, body mass index in kg/m2; CVD, cardiovascular disease; HD, hemodialysis; HR, hazard ratio; IRH, infection-related hospitalization.
Table 3.
Risk for infection-related hospitalizations using whole cohort
| Variable | Adjusted Hazard Ratio (95% CI)a |
|---|---|
| Age (by 10 yr) | 0.99 (0.96–1.02) |
| Male sex (versus female) | 1.03 (0.95–1.11) |
| Race | |
| Black | 1.03 (0.85–1.26) |
| White | 1.00 (Reference). |
| Other | 0.97 (0.84–1.11) |
| BMI (by 1 kg/m2) | 1.00 (0.96–1.03) |
| PD (versus HD) | 1.52 (1.38–1.68) |
| Smoking (versus nonsmoking) | 0.92 (0.83–1.03) |
| Nephrologist visit 6–12 mo prior (versus none) | 0.91 (0.84–0.99) |
| Hospitalization in prior year (versus none) | 1.01 (0.93–1.11) |
| Comorbid conditions | |
| Cardiovascular disease | 1.03 (0.93–1.12) |
| Cerebrovascular disease | 0.95 (0.85–1.06) |
| Chronic pulmonary disease | 1.24 (1.14–1.36) |
| Chronic liver disease | 1.19 (0.96–1.48) |
| Congestive heart failure | 1.02 (0.93–1.12) |
| Diabetes | 1.21 (1.10–1.32) |
| Hyperlipidemia | 0.95 (0.88–1.04) |
| Hypertension | 0.99 (0.84–1.17) |
| Malignancy | 1.08 (0.97–1.20) |
| Peripheral vascular disease | 1.11 (1.02–1.22) |
| Laboratory data | |
| Albumin (by 1 g/dL) | 0.94 (0.87–1.02) |
| Estimated GFR (by 1 mL/min per 1.73 m2) | 1.02 (1.00–1.03) |
| Hemoglobin (by 1 g/dL) | 0.99 (0.97–1.01) |
| Phosphorus (by 1 mg/dL) | 1.29 (1.04–1.59) |
| Urea (by 10 mg/dL) | 0.98 (0.86–1.12) |
n=5858. CI, confidence interval; BMI, body mass index; PD, peritoneal dialysis; HD, hemodialysis.
Model adjusted for age, sex, race, BMI, dialysis modality, smoking, visit to a nephrologist 6–12 months prior, hospitalization in year prior, all comorbid conditions, and all laboratory data.
According to the model using the whole sample, other factors associated with an increased risk for IRH were chronic pulmonary disease, diabetes, peripheral vascular disease, and higher phosphorus level (Table 3).
Risk for different subtypes of IRH and other types of hospitalizations are given in Table 4. With use of the propensity-matched cohort, PD was associated with an increased risk for dialysis-related hospitalizations (HR, 3.44; 95% CI, 2.72–4.34) and a reduced risk for septicemia (HR, 0.31; 95% CI, 0.18–0.52) and pneumonia (HR, 0.58; 95% CI, 0.37–0.94). When septicemia and dialysis-related infections were combined, PD remained associated with a higher risk (HR, 2.19; 95% CI, 1.82–2.64). PD patients had similar risks for all-cause hospitalization (HR, 1.04; 95% CI, 0.98–1.10) and cardiovascular hospitalization (HR, 0.99; 95% CI, 0.89–1.11) compared with HD.
Table 4.
Risk for hospitalizations subtypes associated with peritoneal dialysis versus hemodialysis
| Variable | Unadjusted HR from Model Using Matched Cohort (95% CI)a | Adjusted HR from Model Using Whole Sample (95% CI)b |
|---|---|---|
| All causes | 1.04 (0.98–1.10) | 1.02 (0.98–1.07) |
| Cardiovascular | 0.99 (0.89–1.11) | 0.99 (0.91–1.08) |
| Infection | 1.52 (1.34–1.74) | 1.52 (1.38–1.68) |
| Abdominal | 1.47 (0.90–2.41) | 1.26 (0.91–1.75) |
| Dialysis-related | 3.44 (2.72–4.34) | 3.47 (2.93–4.11) |
| Genitourinary | 0.95 (0.52–1.74) | 0.85 (0.50–1.46) |
| Musculoskeletal | 1.31 (0.62–2.75) | 1.27 (0.62–2.58) |
| Pneumonia | 0.58 (0.37–0.94) | 0.54 (0.37–0.80) |
| Septicemia | 0.31 (0.18–0.52) | 0.30 (0.18–0.51) |
| Skin | 1.01 (0.60–1.70) | 1.18 (0.78–1.79) |
| Other infection | 1.13 (0.82–1.55) | 1.01 (0.78–1.31) |
| Other | 0.92 (0.86–0.98) | 0.91 (0.86–0.97) |
HR, hazard ratio; CI, confidence interval.
n=1820. Reference is hemodialysis.
n=5858. Model adjusted for age, sex, race, body mass index, dialysis modality, smoking, visit to a nephrologist 6–12 months prior, hospitalization in year prior, all comorbid conditions, and all laboratory data. Reference is hemodialysis.
Fully adjusting the matched cohort model led to results almost identical to those seen with the unadjusted model (HR, 1.57; 95% CI, 1.37–1.80). For the adjusted model using the whole sample, the HR for IRH associated with PD was similar without imputation (1.60; 95% CI, 1.43–1.78). Limiting follow-up to a first claim for the opposite dialysis modality (HR, 1.69; 95% CI, 1.46–1.96) or to 2 years in the matched cohort (HR, 1.55; 95% CI, 1.32–1.81) yielded results very similar to those derived from using total follow-up. Excluding the first 90 days after dialysis initiation also produced similar results (HR, 1.58; 95% CI, 1.37–1.81). Using both primary and secondary diagnoses to identify infections led to a slightly lower risk (HR, 1.25; 95% CI, 1.14–1.36). Restricting the analysis to the first IRH using a Cox regression model gave similar results: HR of 1.59 for the whole sample adjusted (95% CI, 1.38–1.85) and 1.69 for the matched cohort unadjusted (95% CI, 1.43–2.00).
Discussion
This retrospective study compared the risk for IRH between PD and HD incident patients after matching for numerous comorbid conditions and laboratory data and using recurrent hospitalizations. We found a 52% increased risk for overall IRH among PD patients compared with HD patients. The increased risk appeared to be mostly explained by a higher risk for dialysis-related hospitalizations, including those for peritonitis.
Using USRDS data, one study found a cumulative incidence of septicemia-related admission at 7 years of 11.7% in HD patients and 9.4% in PD patients (16). According to USRDS annual report, PD patients have a greater adjusted (age, sex, race, and primary diagnosis) rate of infection-related hospitalization than HD patients (1). However, this difference may be explained by different distribution of other baseline characteristics of these two groups. With use of the same data but matching HD patients to PD patients, rates of infection-related hospitalizations remained higher among PD patients, but differences were smaller (1). According to USRDS unadjusted rates, abdominal infections were the only major organ site where PD had higher rates than HD (1).
In a small single-center study, PD patients had higher risk for peritonitis and other serious infections but lower risk for bacteremia, cellulitis, and pneumonia compared with HD patients (8). However, PD was not associated with an overall increased rate of admissions for infections in multivariate models, in part because of the lack of statistical power (8). A recent Canadian study showed no effect of dialysis modality on IRH risk, but the sample size was very small (9). The fact that our results showed a higher risk than previously reported may be explained by improved control of confounding and by a different dialysis population. Inclusion of the first 90 days after dialysis initiation may also be a factor, but sensitivity analysis excluding that period produced similar results.
All-cause hospitalization risk was not associated with PD. This suggests that the higher risk for IRH is partly compensated by a reduced risk for other causes of hospitalization.
Pathogenesis of infection in the HD population has been summarized recently (17,18). Few studies are aimed at determining the risk factors for IRH. Some reported risk factors are age (6,8,16), diabetes (2,6,8,16,19), serum albumin level (2,6,8,16), temporary catheters (16), arteriovenous grafts (16), black race (8,16), no insurance (16), decreased hematocrit levels (20), higher use of erythropoietin (20), high comorbidity score (2), smoking (21), pulmonary disease (6), and corticosteroid use (21). We found an increased risk only with diabetes. We did not find an increased risk with smoking, but the effect may be captured by chronic pulmonary disease. In our study, low serum albumin tended to be associated with an increased risk, but this finding was not statistically significant. Increased risk for IRH with peripheral vascular disease is probably explained by limb and foot infections. Although BMI had no effect on the overall IRH risk, the risk associated with PD tended to be higher among patients with higher BMI. Higher BMI has been reported as a risk factor for peritonitis among PD patients (22).
Despite improved connection of the PD catheter to the solution bags, resulting in a decline in admissions for peritonitis in the last decade among PD patients, overall IRH rates increased during the same period (1). Although not as strong as in-hospital mortality, LOS may be considered a marker of hospitalization severity. Except for septicemia, dialysis-related infections, and musculoskeletal infections, LOS for other causes of IRH was similar between HD and PD. A recent study showed a higher risk for death from infection among PD patients compared with HD patients after 6 months of treatment (23). IRH was also reported as increasing short-term risk for a cardiovascular event in older patients undergoing dialysis (24).
Our study has several strengths. First, we used linked data sets that allow matching a large cohort of HD patients to PD patients using considerably more covariates than noted in previous reports, thereby reducing confounding and improving statistical power. Second, we used recurrent hospitalizations. Third, we were able to follow patients in their first 90 days after dialysis initiation. This period, which probably presents high risk, is often excluded from other studies because hospitalizations are not covered in other data sets. Finally, a universal healthcare setting allowed inclusion of all age groups and nearly eliminated selection bias.
Our study does have some limitations. First, dialysis modality was captured early after dialysis initiation and may have changed during follow-up, leading to misclassification. However, limiting follow-up at modality switch showed similar results. Cause of hospitalization was categorized using ICD codes, which are not as reliable as chart reviews. Because of inclusion of patients from a whole province, chart reviews were not feasible. However, misclassification of outcomes is expected to be nondifferential and therefore should affect only rates but not comparison between dialysis modalities. Septicemia included bacteremia; therefore, some septicemia cases were probably related to vascular access. Unfortunately, type of vascular access was not available in our data. However, PD was still associated with a higher risk when septicemia and dialysis-related infections were combined. Because we evaluated only IRH, we do not know how dialysis modalities compare with regard to risk for infections not necessitating hospitalizations. Because hospitalization is a marker of disease severity, we believe we identified the most clinically important events. Despite numerous covariates, residual confounding cannot be excluded. Because indication or patient preference for PD may vary by geographic regions, our population of PD patients may be different from those in other regions. However, we believe that the relatively young population with a high prevalence of diabetes described in Table 1 is representative of the North American PD population as described in USRDS and previous studies (1,8,9). Finally, by excluding patients with less than 90 days of follow-up, our results may not apply to patients undergoing long-term dialysis who were rapidly transplanted or died in the first 90 days of dialysis.
We have shown that PD patients are at higher risk for IRH than HD patients. This risk is mostly explained by dialysis-related infections. However, further studies are needed to evaluate whether severity of those hospitalizations is similar and whether this increased risk for IRH is associated with worse outcomes.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by a Fonds de la recherche en santé du Québec operating grant. J.P.L. is supported by a KRESCENT New Investigator Award. E.R. is a Senior Investigator of the Fonds de Recherche en Santé du Québec.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00440112/-/DCSupplemental.
References
- 1.U.S. Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 2.Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ, HEMO Study Group : Impact of dialysis dose and membrane on infection-related hospitalization and death: Results of the HEMO Study. J Am Soc Nephrol 14: 1863–1870, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Depner TA, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ, HEMO Study Group : The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol Dial Transplant 20: 1180–1186, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chavers BM, Solid CA, Gilbertson DT, Collins AJ: Infection-related hospitalization rates in pediatric versus adult patients with end-stage renal disease in the United States. J Am Soc Nephrol 18: 952–959, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Rayner HC, Pisoni RL, Bommer J, Canaud B, Hecking E, Locatelli F, Piera L, Bragg-Gresham JL, Feldman HI, Goodkin DA, Gillespie B, Wolfe RA, Held PJ, Port FK: Mortality and hospitalization in haemodialysis patients in five European countries: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 19: 108–120, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Dalrymple LS, Johansen KL, Chertow GM, Cheng SC, Grimes B, Gold EB, Kaysen GA: Infection-related hospitalizations in older patients with ESRD. Am J Kidney Dis 56: 522–530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Renal Data System : USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 8.Aslam N, Bernardini J, Fried L, Burr R, Piraino B: Comparison of infectious complications between incident hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol 1: 1226–1233, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Williams VR, Quinn R, Callery S, Kiss A, Oliver MJ: The impact of treatment modality on infection-related hospitalization rates in peritoneal dialysis and hemodialysis patients. Perit Dial Int 31: 440–449, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Levy AR, Mayo NE, Grimard G: Rates of transcervical and pertrochanteric hip fractures in the province of Quebec, Canada, 1981-1992. Am J Epidemiol 142: 428–436, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Mayo NE, Goldberg MS, Levy AR, Danys I, Korner-Bitensky N: Changing rates of stroke in the province of Quebec, Canada: 1981-1988. Stroke 22: 590–595, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Jollis JG, Ancukiewicz M, DeLong ER, Pryor DB, Muhlbaier LH, Mark DB: Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med 119: 844–850, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M: Using medical services claims to assess injuries in the elderly: Sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol 53: 183–194, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Greene T, Kusek J, Beck G: A simplified equation to predict glomerular filtration rate from serum creatinine. [Abstract] J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 15.Prentice RL, Williams BJ, Peterson AV: On the regression analysis of multivariate failure time data. Biometrika 68: 373–379, 1981 [Google Scholar]
- 16.Powe NR, Jaar B, Furth SL, Hermann J, Briggs W: Septicemia in dialysis patients: Incidence, risk factors, and prognosis. Kidney Int 55: 1081–1090, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Lafrance JP, Rahme E, Lelorier J, Iqbal S: Vascular access-related infections: Definitions, incidence rates, and risk factors. Am J Kidney Dis 52: 982–993, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Jaber BL: Bacterial infections in hemodialysis patients: Pathogenesis and prevention. Kidney Int 67: 2508–2519, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Shah BR, Hux JE: Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 26: 510–513, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Nissenson AR, Dylan ML, Griffiths RI, Yu HT, Dubois RW: Septicemia in patients with ESRD is associated with decreased hematocrit and increased use of erythropoietin. Clin J Am Soc Nephrol 1: 505–510, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Franklin J, Lunt M, Bunn D, Symmons D, Silman A: Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis 66: 308–312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald SP, Collins JF, Rumpsfeld M, Johnson DW: Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 24: 340–346, 2004 [PubMed] [Google Scholar]
- 23.Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown FG, Bannister KM, Wiggins KJ: Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am J Kidney Dis 53: 290–297, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Dalrymple LS, Mohammed SM, Mu Y, Johansen KL, Chertow GM, Grimes B, Kaysen GA, Nguyen DV: Risk of cardiovascular events after infection-related hospitalizations in older patients on dialysis. Clin J Am Soc Nephrol 6: 1708–1713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.