Summary
Background and objectives
Skepticism about performing renal biopsies is often because of uncertainty regarding risk of complications. The aim of this study was to evaluate safety and relevant complications of renal biopsies in pediatric and adult patients in a large national registry study.
Design, setting, participants, & measurements
Kidney biopsies reported in the Norwegian Kidney Biopsy Registry from 1988 to 2010 were included. Risk factors for major complications (blood transfusion and/or surgical or catheter intervention) were analyzed using logistic regression statistics.
Results
Of the 9288 biopsies included, 715 were from children, and 8573 were from adults (≥18 years). Median age was 49 years (range=2 weeks to 94 years). Gross hematuria appeared after biopsy in 1.9% of the patients; 0.9% of patients needed blood transfusion, and 0.2% of patients needed surgical intervention/catheterization. The frequencies were 1.9%, 0.9%, and 0.2% in adults and 1.7%, 0.1% and 0.1% in children, respectively; 97.9% of the biopsies were without complications. In unadjusted analyses, risk factors for major complications were age>60 years, estimated GFR<60 ml/min per 1.73 m2, systolic hypertension, acute renal failure, and smaller clinical center size (<30 biopsies/yr). Adjusted analyses (adjusted for age and/or estimated GFR) showed higher odds ratios (OR) only for smaller clinical center (OR=1.60 [1.02–2.50]) and low estimated GFR (estimated GFR=30–59 ml/min per 1.73 m2 [OR=4.90 (1.60–14.00)] and estimated GFR<30 ml/min per 1.73 m2 [OR 15.50 (5.60–43.00)]).
Conclusions
Percutaneous renal biopsy is a low-risk procedure in all ages. Reduced estimated GFR and smaller center size are associated with an increased risk of major complications.
Introduction
Renal biopsies have been performed for more than a century (1), and percutaneous biopsy methodology was established in the 1940s (2,3). The technical advances in imaging diagnostics and biopsy procedure have evolved from indirect visualization to real-time ultrasound guidance (4). Most centers use an automated spring-loaded biopsy device constructed in the end of the 1980s (5–7). As a consequence, the general safety and complication rate of the procedure has substantially improved. Kidney morphology is essential in the diagnosis and subsequent evaluation of disease activity (8,9). Indeed, knowledge of the morphologic changes in kidney biopsies has been shown to alter the treatment in a high percentage of the patients (10,11). Postbiopsy bleeding (hematoma and hematuria) is the primary complication of renal biopsies (12–14). However, most of the perirenal hematomas are minor and without clinical significance, and major complications are infrequent in both adults and children when traditional risk factors, like hypertension and bleeding diathesis, are respected (15,16).
In this study, we report the procedure-related complications and safety issues in the period from April of 1988 to November of 2010 from the Norwegian Kidney Biopsy Registry comprising 8573 adult and 715 pediatric cases.
Materials and Methods
Norwegian Kidney Biopsy Registry has registered clinical and histopathologic data for nearly all patients with a native renal biopsy in Norway (current population of 5 million inhabitants) since April of 1988. All included patients or their designees signed informed consent. The local nephrologists (26 different hospitals) report clinical data in a registry notification form, and histopathological data are reported by a limited group of nephropathologists that examines all the biopsies in one center (Haukeland University Hospital). Information regarding biopsy-related complications was lacking in 357 (3.7%) reports, and these cases were excluded from the study. The remaining 9288 cases were included for the statistical analysis. All cases were biopsied with percutaneous techniques: 99.7% were ultrasound-guided, and 0.3% were computer tomography-guided.
In the report forms, there were five complication alternatives: hematoma, gross hematuria, blood transfusion, surgery, and a free text field. Angiographic embolization in the free text field was included in the surgery group. The pathologist reported whether the biopsy was representative (i.e., containing sufficient or insufficient material for adequate evaluation). The term complications used in the current study was confined to gross hematuria, blood transfusions, and/or surgery/arterial embolization (i.e., hematoma was not regarded as a significant complication unless associated with one of the other complications listed). The term major complications was defined as blood transfusions and/or surgery/arterial embolization. The following variables were explored: age, estimated GFR (eGFR; calculated with the Modification of Diet in Renal Disease formula in adults [17] and the revised Schwartz formula in children [18]), systolic BP, proteinuria (u-protein≥0.3 g/d or reported proteinuria or nephritic syndrome), hematuria (u-dipstick≥1 or reported hematuria or nephritic syndrome), CKD stages 3–5 (eGFR<60 ml/min per 1.73 m2), nephrotic syndrome (u-protein≥3.0 g/d and s-albumin<35 g/L or clinician-marked nephrotic syndrome), acute renal failure, rapidly progressive glomerulonephritis, amyloidosis, biopsy needle size (gauge), number of needle passes, specialty of the doctor performing the biopsy, and center size (≥650 or <650 biopsies performed during the study period corresponding to ≥30 or <30 biopsies/yr).
The SPSS 17 package (SPSS Inc., Chicago, IL) was used for statistical analysis. Significance testing was performed by t test and Mann–Whitney test. Differences of proportions between patient groups were compared with the chi-squared test (Pearson). A P value of 0.05 was considered statistically significant, and all tests were two-tailed. Logistic regression analysis was performed for the analysis of risk factors, and both unadjusted analyses and analyses adjusted for categories of age (categorized as <18, 18–59, and ≥60 years) and eGFR (categorized as ≥60, 30–59, and <30 ml/min per 1.73 m2) were performed. Age was only adjusted for eGFR, and eGFR was only adjusted for age.
Results
The median age of the 9288 patients was 49 years (range=2 weeks to 94 years). There were 715 children (<18 years of age), with a mean age of 12.0 years (SD=4.9; range=2 weeks to 18 years); 8573 patients were adults (≥18 years), with a mean age of 50.6 years (SD=17.7; range=18–94 years). The clinical characteristics at the time of renal biopsy are shown in Table 1. The most common clinical sign was proteinuria, which was seen in approximately four of five of all the patients, and similar figures were seen in both adults and children (Table 1). Hematuria and nephrotic syndrome were more frequent in children than adults, and chronic renal failure and acute renal failure were more common in adults; 5.9% of the biopsies in the study period were rebiopsies.
Table 1.
Clinical characteristics at the time of renal biopsy given as percentages (total numbers in parentheses) separately for adults and children
| Clinical Characteristics | Total (%) (9288) | Adults (%) (8573) | Children (%) (715) | P Value |
|---|---|---|---|---|
| Proteinuria (>0.3 g/d)a | 81.0 (7522) | 81.1 (6955) | 79.3 (567) | 0.23 |
| Hematuriab | 68.8 (6393) | 68.4 (5862) | 74.3 (531) | 0.001c |
| CKD stages 3–5d | 61.6 (5726) | 64.9 (5568) | 22.1 (158) | <0.001c |
| Nephrotic syndromee | 28.7 (2667) | 27.8 (2380) | 40.1 (287) | <0.001c |
| Acute renal failure | 18.6 (1723) | 19.0 (1630) | 13.0 (93) | <0.001c |
| Rapidly progressive glomerulonephritis | 3.4 (314) | 3.3 (284) | 4.2 (30) | 0.21 |
u-Protein≥0.3 g/d or clinician marked proteinuria or nephritic syndrome.
u-Dipstick≥1 or clinician marked hematuria or nephritic syndrome.
Significant difference between adults and children.
CKD stages 3–5 indicate eGFR<60 ml/min per 1.73 m2.
u-Protein≥3.0 g/d and s-albumin<35 g/L or clinician marked nephrotic syndrome.
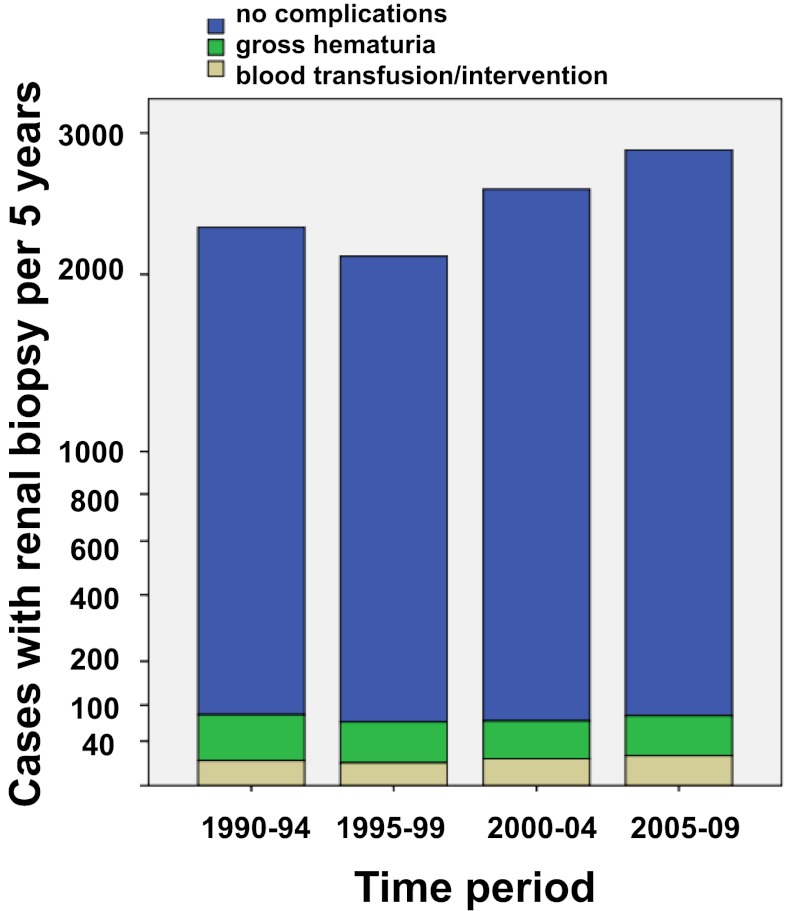
The biopsy-related complications are shown in Table 2. Serious complications were infrequent in both children and adults (Table 2). The total frequency of blood transfusion or intervention (surgery or angiography) was low (0.9% and 0.2% of all cases, respectively). There was a significantly lower frequency of blood transfusions in children (only one child) than adults. Less than 2% had macroscopic hematuria (Table 2), whereas hematoma was reported in 3.9% of all cases. The frequency of hematoma was significantly higher in children (8.1%) than adults (3.5%; P<0.001). During the last 5 years of the study period, the number of hematomas was higher (7.4% compared with 3.9% in the whole study period), and this change was more evident in children (18.9% compared with 8.1% in the whole study period). The registry did not specify the criteria for the diagnosis of postprocedural hematoma in terms of size or associated clinical symptoms. The total number of hematoma should, therefore, be taken with caution and may reflect an increasing use of systematic safety routines, including postbiopsy ultrasound examination, especially in children. The rates of gross hematuria, blood transfusion, and invasive procedures remained stable throughout the study period (Figure 1).
Table 2.
Frequency of different complications given as percentages (total numbers in parentheses) separately for adults and children
| Type of Complication | All Patients (%) (9288) | Adults (%) (8573) | Children (%) (715) | P Value |
|---|---|---|---|---|
| Gross hematuria | 1.9 (178) | 1.9 (167) | 1.5 (11) | 0.44 |
| Blood transfusion | 0.9 (79) | 0.9 (78) | 0.1 (1) | 0.03a |
| Surgery/arterial embolization | 0.2 (18) | 0.2 (17) | 0.1 (1) | 0.73 |
| Complicationsb | 2.6 (215) | 2.7 (233) | 1.8 (13) | 0.15 |
| Major complicationsc | 0.9 (88) | 1.0 (86) | 0.3 (2) | 0.06 |
Significant difference between adults and children.
Complications were gross hematuria, blood transfusions, and/or surgery/arterial embolization.
Major complications were blood transfusions and/or surgery/arterial embolization.
Figure 1.
Kidney biopsy complications.
In the free text field, one patient was reported with arteriovenous fistula, one patient was reported with urosepsis and one patient was reported with ascites leakage. No patient was reported with death. A total of 97.9% of the patients (9092 patients) had no complications.
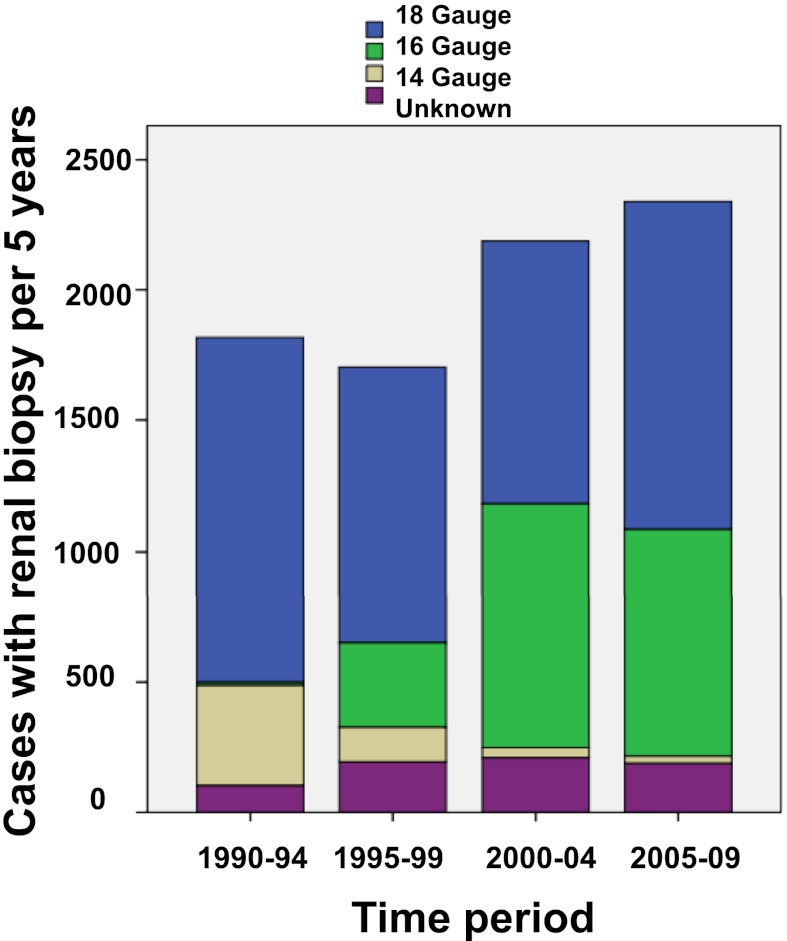
Figure 2 shows the change in biopsy needle size used in the study period. There was an overall shift from 14 to 16 gauge (G) size, and in the majority of the biopsies (82%), the needle size was 16 or 18 G (Table 3). A significantly higher rate of macroscopic hematuria was reported after use of 18 G compared with 16 G. Major complications (Table 3) were not related to needle size. The percentage of biopsies containing representative tissue was similar across all needle types (94.3%). The median number of glomeruli in the tissue samples per subject was 10 (range=0–86), and the number was lowest when an 18-G needle was used (9, range=0–76). In 3.0% of the biopsies, no glomeruli were found.
Figure 2.
Kidney biopsy needle sizes.
Table 3.
Comparison of complications and number of glomeruli for different needle diameter
| Needle Thickness | 14 Gauge | 16 Gauge | 18 Gauge | Unknown | Total |
|---|---|---|---|---|---|
| N total | 858 | 2146 | 4998 | 744 | 8746 |
| Percent total | 9.8 | 24.5 | 57.1 | 8.5 | 100 |
| Percent (N) complicationsa | 2.6 (22) | 2.0 (43)b | 3.0 (151) | 2.3 (17) | 2.7 (233) |
| Percent (N) major complicationsc | 0.6 (5) | 1.2 (25) | 0.9 (46) | 0.8 (6) | 0.9 (82) |
| Percent (N) gross hematuria | 2.2 (19) | 1.0 (22)d | 2.4 (118) | 1.6 (12) | 2.0 (171) |
| Percent (N) blood transfusions | 0.2 (2) | 1.1 (23) | 0.9 (43) | 0.7 (5) | 0.8 (73) |
| Percent (N) surgery/angiographic embolization | 0.4 (3) | 0.2 (5) | 0.1 (7) | 0.3 (2) | 0.2 (17) |
| Percent representative tissue | 94.6 | 93.5 | 94.5 | 94.9 | 94.3 |
| Median number of glomeruli (range) | 12 (0–86)d | 12 (0–61)d | 9 (0–76) | 11 (0–69)d | 10 (0–86) |
Complications were gross hematuria, blood transfusions, and/or surgery/arterial embolization.
P<0.05 (P value compared with 18-gauge needle).
Major complications were blood transfusions and/or surgery/arterial embolization.
P<0.001 (P value compared with 18-gauge needle).
Analysis of the number of needle passes per biopsy procedure showed that one or two passes were performed in the majority of the patients (47.5% and 37.9%, respectively). The biopsy was performed by a nephrologist in 33.4% of the cases, and this number decreased during the study period (data not shown). Radiologists performed the biopsy in 53.5%, and in 13.1% of the cases, the nephrologist and the radiologist handled the procedure together.
Risk factors for major complications (blood transfusions, surgery, or angiographic embolization) are analyzed in Table 4. Older age, lower eGFR, higher systolic BP, acute renal failure, and smaller center sizes were all highly significant risk factors in the unadjusted analyses. The unadjusted relative risk of major complications was significantly lower in children; when corrected by eGFR level, the risk was similar in children and adults. The needle size, number of passes, and physician specialty did not influence the rate of major complications. The diagnosis of rapidly progressive glomerulonephritis or amyloidosis did not carry any increased procedural risk. A lower eGFR (CKD stages 3–5) and smaller center size (<30 biopsies/yr) were the only significant risk factors after adjustment for age and/or eGFR.
Table 4.
Odds ratios for major complications (blood transfusion, surgery, and/or arterial embolization)
| N Total | N (%) with Major Complications | Unadjusted Analyses | Adjusted Analysesa | |||
|---|---|---|---|---|---|---|
| Odds Ratio (CI) | P Value | Odds Ratio (CI) | P Value | |||
| Age (years) | ||||||
| <18 | 715 | 2 (0.3) | 0.40 (0.10–1.70) | 0.46 (0.06–3.40) | ||
| 18–59 | 5609 | 39 (0.7) | 1.00 (ref) | <0.001 | 1.00 (ref) | 0.34 |
| ≥60 | 2964 | 47 (1.6) | 2.30 (1.50–3.50) | 1.20 (0.80–2.00) | ||
| eGFR (ml/min per 1.73 m2) | ||||||
| ≥60 | 3307 | 4 (0.1) | 1.00 (ref) | 1.00 (ref) | ||
| 30–59 | 2307 | 14 (0.6) | 5.00 (1.70–15.00) | <0.001 | 4.90 (1.60–14.00) | <0.001 |
| <30 | 3309 | 66 (2.0) | 16.80 (6.10–46.00) | 15.50 (5.60–43.00) | ||
| Systolic BP | ||||||
| <140 | 4141 | 27 (0.7) | 1.00 (ref) | 1.00 (ref) | ||
| 140–159 | 2476 | 24 (1.0) | 1.50 (0.86–2.60) | 0.001 | 1.10 (0.61–1.90) | 0.29 |
| ≥160 | 2127 | 32 (1.5) | 2.30 (1.40–3.90) | 1.30 (0.77–2.30) | ||
| Proteinuria (g/d) | ||||||
| 0.3–0.99 | 2435 | 18 (0.7) | 1.00 (ref) | 1.00 (ref) | ||
| 1–2.99 | 1933 | 23 (1.2) | 1.60 (0.87–3.00) | 0.28 | 1.60 (0.84–3.00) | 0.14 |
| ≥3.0 | 3154 | 33 (1.0) | 1.40 (0.80–2.50) | 1.60 (0.89–2.90) | ||
| Acute renal failure | ||||||
| Yes | 1723 | 30 (1.7) | 2.29 (1.50–3.60) | <0.001 | 1.10 (0.69–1.80) | 0.48 |
| No | 7576 | 58 (0.8) | 1.00 (ref) | 1.00 (ref) | ||
| Rapidly progressive glomerulonephritis | ||||||
| Yes | 314 | 3 (1.0) | 1.00 (0.31–3.20) | 0.99 | 1.70 (0.54–5.50) | 0.41 |
| No | 8974 | 85 (1.0) | 1.00 (ref) | 1.00 (ref) | ||
| Amyloidosis | ||||||
| Yes | 255 | 3 (1.2) | 1.21 (0.38–3.90) | 0.75 | 0.92 (0.28–3.00) | 0.88 |
| No | 6256 | 61 (1.0) | 1.00 (ref) | 1.00 (ref) | ||
| Needle size (gauge) | ||||||
| 14 | 858 | 5 (0.6) | 0.63 (0.25–1.60) | 0.79 (0.31–2.00) | ||
| 16 | 2146 | 25 (1.2) | 1.30 (0.78–2.10) | 0.55 | 1.40 (0.85–2.30) | 0.92 |
| 18 | 4998 | 46 (0.9) | 1.00 (ref) | 1.00 (ref) | ||
| Unknown | 744 | 6 (0.8) | 0.88 (0.37–2.10) | 0.93 (0.39–2.20) | ||
| Number of needle passes | ||||||
| 1 | 698 | 6 (0.9) | 1.60 (0.40–6.40) | 1.80 (0.44–7.10) | ||
| 2 | 557 | 3 (0.5) | 1.00 (ref) | 0.18 | 1.00 (ref) | 0.35 |
| 3 | 158 | 4 (2.5) | 4.80 (1.10–22.00) | 4.00 (0.87–18.00) | ||
| ≥4 | 56 | 1 (1.8) | 3.40 (0.34–33.00) | 2.80 (0.28–28.00) | ||
| Specialty | ||||||
| Nephrologist | 2975 | 22 (0.7) | 1.00 (ref) | 1.00 (ref) | ||
| Radiologist | 4766 | 50 (1.0) | 1.40 (0.86–2.40) | 0.17 | 1.10 (0.65–1.80) | 0.66 |
| Both | 1163 | 30 (2.6) | 1.50 (0.76–3.00) | 1.20 (0.58–2.30) | ||
| Center size (biopsies in study period) | ||||||
| ≥650 | 4873 | 32 (0.7) | 1.00 (ref) | 1.00 (ref) | ||
| <650 | 4415 | 56 (1.3) | 1.90 (1.30–3.00) | 0.003 | 1.60 (1.02–2.50) | 0.04 |
CI, confidence interval; eGFR, estimated GFR.
Corrected for categories of age and eGFR. Age is only corrected for eGFR, and eGFR is only corrected for age.
Discussion
The main finding in the present study is that a renal biopsy should be regarded as a safe procedure (both in adults and children) provided that general contraindications are respected, and the overall rate of major complications was less than 1% (Table 2). Of note, the relative risk was even lower in children, and only 2 of 715 children (0.3%) were affected. Importantly, the lowest complication rates were seen in centers performing more than 30 biopsies/yr, indicating the best clinical routines occur when biopsies are done approximately on a weekly basis.
To our knowledge, the current study is the only nationwide registry-based and largest report of renal biopsy complications, and our findings confirm the general impression of low complication rates and improved safety of modern renal biopsy procedures (19). Similar low complication rates have been reported in recent studies (16,19,20) in contrast to earlier studies (reporting 5%–7% complication rates) (21–25). The crucial safety evaluation of kidney biopsies is characterized by the overall risk and frequency of major complications, which in our study, are defined as need of blood transfusion and/or surgery or catheter-based intervention (embolization) secondary to bleeding, and they should not be jeopardized by procedural events of minor clinical relevance. Therefore, the description of kidney biopsy complications should be restrained to clinically meaningful complications, especially because there often is an unfounded skepticism toward biopsies because of many reports of frequent occurrence of clinically less relevant events, like microscopic hematuria, transient gross hematuria, and asymptomatic minor perirenal hematoma. The latter should, in fact, be regarded as an epiphenomenon because of the high incidence (reported in up to 60%–90% of cases after examination prospectively with ultrasonography or computertomography). Of note, several authors found that a hematoma alone is not a reliable predictor of a serious adverse outcome (7,16,26,27). Perirenal hematomas should not be regarded as a clinical significant complication in the absence of significant bleeding or persisting pain necessitating delayed hospitalization. The number of hematomas in the present study was significantly higher in children, probably because of a more systematic use of routine ultrasonographic examination, and this finding must, therefore, be interpreted with caution. Such a view is supported by several studies. The work by Kersnik Levart et al. (16) found mostly small perinephritic hematoma in 63.2% of 87 children, and 10 of the hematomas (11.5%) were more than 2 cm in diameter. Other authors report that the majority of hematomas are less than 2 cm, and about 1%–2% are described as symptomatic (15,28,29).
In the present large study, gross hematuria was seen in less than 2%, and the benign course supports the view that such events should not be considered serious complications unless bleeding is of a magnitude causing a significant decline in hemoglobin concentration or delayed hospitalization. The great majority of the patients (97.9%) had neither macroscopic hematuria nor blood transfusion or surgery/embolization. These findings are in line with other studies (27,30–32).
In the current study, the risk factors for major complications were older age, low eGFR, systolic hypertension, acute renal failure, and smaller center size (<30 biopsies/yr) in the unadjusted analyses. Only low eGFR and smaller center size were associated with increased risk in the adjusted analyses. In fact, the relative risk was 16 times higher when eGFR was below 30 ml/min per 1.73 m2 (Table 4). Although elevated risk has been shown previously, the increased risk incurred by kidney failure may be underscored in many reports (14,21,33,34), and the work by Whittier and Korbet (25) found that serious complications were about two times as common in patients with serum creatinine above 5.0 mg/dl. It is conceivable that this observation is associated with the general increased bleeding tendency seen in kidney failure patients (33). Similarly, acute renal failure more than doubled the risk of major complications in our study, and the reason for this finding is probably also related to increased bleeding tendency (Table 4). As a consequence, careful recognition of reduced kidney function before the procedure is important. To minimize biopsy risk, meticulous control of clinical routines (to avoid inappropriate use of anticoagulant medication 1 week before as well as after the biopsy procedure) is mandatory, especially in patients with reduced eGFR.
An overview of studies from many centers shows the wide variation in complication rates (27). In the current nationwide registry study, we found the lowest complication rates in the most active and experienced centers (e.g., university hospitals performing biopsies, on average, on a weekly basis or >30 biopsies/yr). This finding is a reassuring observation that strengthens the importance of introducing adequate training programs (27) and supports the view that modern biopsy procedures are safe in experienced hands. Furthermore, the general low complication rate indicates similar adequate clinical practice among our centers. This finding is in contrast to two recent nationwide surveys from the United Kingdom (19) and France (35) showing relatively great variability in clinical practice and complication rates among centers and highlighting the importance of establishing safe procedural standards.
Although there has been a change in practice (fewer patients with isolated microscopic hematuria and more elderly patients undergo a biopsy), the total frequency of renal biopsies in Norway has remained stable for the last 20 years and was 10.9/100,000 in 2009 (13/100,000 in adults and 3.8/100,000 in children). Other reports have shown a range of biopsy frequencies between 3.3 and 23 per 100,000 inhabitants (36,37).
In many centers, the numbers of kidney biopsies are relatively low in children because of a traditional view of many nephrologists and pediatricians that the procedure is associated with an unacceptable high complication risk. General skepticism to the routine use of anesthesia is probably also contributing to the restraint of kidney biopsies. In our cohort, kidney biopsies in children below the age of 15 years were usually performed with short-lasting general anesthesia (sedation) without intubation. Our experience supports the findings in the work by Mauer and Drummond (38) that general anesthesia often can be avoided in patients above the age of 12 years (9). No complications caused by general anesthesia were reported in our study. Hence, the present encouraging findings should contribute to attenuating unfounded skeptic attitudes against kidney biopsies in children as long as general clinical safety routines are respected (14).
Valid biomarkers are generally lacking in many renal diseases, and several important studies have shown the role of kidney morphology as an important prognostic and therapeutic guide in common renal diseases (e.g., IgA nephritis [39], lupus nephritis [40], and diabetes nephropathy [41]); potential prognostic capacity has been shown in orphan diseases like Fabry disease (42). Furthermore, modern therapies of kidney diseases include potent and potentially toxic drug intervention in many common as well as a rapidly increasing number of rare diseases identified by genetic molecular techniques. The latter is mirrored by an increasing general focus from biotechnology companies on development of orphan drugs (43). Importantly, the Food and Drug Administration and the European Medical Association have recently presented guidelines that highlight the importance of meticulous safety and follow-up control of tolerance and side effects of new medical treatment in children (44). Hence, this fundamental change of attitude should also be reflected in the use of evidence-based risk evaluation when it comes to indications of renal biopsy.
It has been suggested that the complication rate is lower with the use of thin biopsy needles (27,45). However, valid comparative studies on needle size and complications in native kidneys are lacking. In the current study, the shift of needle size from 14 to 16 G over time (Figure 2) and the dominance of 16- and 18-G needles (Table 3) may be seen as a result of higher focus, in general, on minimizing risk factors and the acceptance of less tissue per needle pass. The median number of glomeruli per subject in our study (10, range=0–86) was comparable with the median number in other studies (7) and significantly higher by use of both 14- and 16-G needles compared with 18-G needles (P<0.001). However, the percentage of biopsies characterized as representative tissue was in the same level irrespective of needle size. Surprisingly, there was a significantly higher rate of gross hematuria by use of 18 compared with 16 G. This finding has not been reported previously and may be because of the tendency of thinner needles to deviate from the lower pole sector in a proximal direction (where vessel density is higher), with subsequent bleeding into the pelvis. Of note, serious complications, like surgery/embolization and blood transfusions, were of the same order in all needle sizes (Tables 3 and 4).
Interestingly, we did not observe any influence of number of needle passes on the rate of serious complications. The reason for this finding is not known, but it may be related to restraint with multiple needle insertions in high-risk patients and children. In our study, the majority of the biopsy cases (85.4%) had one or two needle passes, and the percentage of patients with three or more passes was low (14.6%). This finding is in contrast to the recent British audit, where the standard target of >80% needing three or less passes was achieved in 86.4% (19). No difference in complication rate was seen between different medical specialists. In our study, most biopsies were performed by a radiologist, whereas 33.4% were done by a nephrologist; the latter percentage has been decreasing over the last decade as reported previously, and it represents an educational challenge in nephrology (27).
Limitations of this study are potential underreporting, reporting bias, and lack of detailed information about other potential complications not prespecified in the registry report form. All GFR analyses were based on eGFR, although imprecision of eGFR is well known in subgroups of patients.
In conclusion, we have shown that kidney biopsies are safe procedures with low complication rates in children and adults when contraindications are respected. Lower eGFR and biopsies performed in smaller centers are risk factors.
Disclosures
None.
Acknowledgments
The authors are grateful to the Norwegian doctors who reported data to the Norwegian Kidney Biopsy Registry.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Nephrology and the Percutaneous Renal Biopsy: A Procedure in Jeopardy of Being Lost Along the Way,” on pages 1545–1547.
References
- 1.Cameron JS, Hicks J: The introduction of renal biopsy into nephrology from 1901 to 1961: A paradigm of the forming of nephrology by technology. Am J Nephrol 17: 347–358, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Iversen P, Brun C: Aspiration biopsy of the kidney. Am J Med 11: 324–330, 1951 [DOI] [PubMed] [Google Scholar]
- 3.Alwall N: Aspiration biopsy of the kidney, including i.a. a report of a case of amyloidosis diagnosed through aspiration biopsy of the kidney in 1944 and investigated at an autopsy in 1950. Acta Med Scand 143: 430–435, 1952 [PubMed] [Google Scholar]
- 4.Donovan KL, Thomas DM, Wheeler DC, Macdougall IC, Williams JD: Experience with a new method for percutaneous renal biopsy. Nephrol Dial Transplant 6: 731–733, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto M, Fujisawa S, Sudo M: Percutaneous renal biopsy well-visualized by orthogonal ultrasound application using linear scanning. Clin Nephrol 30: 106–110, 1988 [PubMed] [Google Scholar]
- 6.Wiseman DA, Hawkins R, Numerow LM, Taub KJ: Percutaneous renal biopsy utilizing real time, ultrasonic guidance and a semiautomated biopsy device. Kidney Int 38: 347–349, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Riehl J, Maigatter S, Kierdorf H, Schmitt H, Maurin N, Sieberth HG: Percutaneous renal biopsy: Comparison of manual and automated puncture techniques with native and transplanted kidneys. Nephrol Dial Transplant 9: 1568–1574, 1994 [PubMed] [Google Scholar]
- 8.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R: Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 361: 40–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M, International Diabetic Nephopathy Study Group : The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 54: 2164–2171, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Richards NT, Darby S, Howie AJ, Adu D, Michael J: Knowledge of renal histology alters patient management in over 40% of cases. Nephrol Dial Transplant 9: 1255–1259, 1994 [PubMed] [Google Scholar]
- 11.Stratta P, Canavese C, Marengo M, Mesiano P, Besso L, Quaglia M, Bergamo D, Monga G, Mazzucco G, Ciccone G: Risk management of renal biopsy: 1387 cases over 30 years in a single centre. Eur J Clin Invest 37: 954–963, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Shidham GB, Siddiqi N, Beres JA, Logan B, Nagaraja HN, Shidham SG, Piering WF: Clinical risk factors associated with bleeding after native kidney biopsy. Nephrology (Carlton) 10: 305–310, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Soares SM, Fervenza FC, Lager DJ, Gertz MA, Cosio FG, Leung N: Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: Single-center experience in 101 patients. Am J Kidney Dis 52: 1079–1083, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Buxo JA, Donadio JV, Jr: Complications of percutaneous renal biopsy: An analysis of 1,000 consecutive biopsies. Clin Nephrol 4: 223–227, 1975 [PubMed] [Google Scholar]
- 15.Mendelssohn DC, Cole EH: Outcomes of percutaneous kidney biopsy, including those of solitary native kidneys. Am J Kidney Dis 26: 580–585, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Kersnik Levart T, Kenig A, Buturović Ponikvar J, Ferluga D, Avgustin Cavić M, Kenda RB: Real-time ultrasound-guided renal biopsy with a biopsy gun in children: Safety and efficacy. Acta Paediatr 90: 1394–1397, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain F, Mallik M, Marks SD, Watson AR, British Association of Paediatric Nephrology : Renal biopsies in children: Current practice and audit of outcomes. Nephrol Dial Transplant 25: 485–489, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Bohlin AB, Edström S, Almgren B, Jaremko G, Jorulf H: Renal biopsy in children: Indications, technique and efficacy in 119 consecutive cases. Pediatr Nephrol 9: 201–203, 1995 [DOI] [PubMed] [Google Scholar]
- 21.al Rasheed SA, al Mugeiren MM, Abdurrahman MB, Elidrissy AT: The outcome of percutaneous renal biopsy in children: An analysis of 120 consecutive cases. Pediatr Nephrol 4: 600–603, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Abdurrahman MB: Percutaneous renal biopsy in a developing country: Experience with 300 cases. Ann Trop Paediatr 4: 25–30, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Edelmann CM, Jr, Greifer I: A modified technique for percutaneous needle biopsy of the kidney. J Pediatr 70: 81–86, 1967 [DOI] [PubMed] [Google Scholar]
- 24.Carvajal HF, Travis LB, Srivastava RN, De Beukelaer MM, Dodge WF, Dupree E: Percutaneous renal biopsy in children—an analysis of complications in 890 consecutive biopsies. Tex Rep Biol Med 29: 253–264, 1971 [PubMed] [Google Scholar]
- 25.Whittier WL, Korbet SM: Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol 15: 142–147, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Doyle AJ, Gregory MC, Terreros DA: Percutaneous native renal biopsy: Comparison of a 1.2-mm spring-driven system with a traditional 2-mm hand-driven system. Am J Kidney Dis 23: 498–503, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Korbet SM: Percutaneous renal biopsy. Semin Nephrol 22: 254–267, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Eiro M, Katoh T, Watanabe T: Risk factors for bleeding complications in percutaneous renal biopsy. Clin Exp Nephrol 9: 40–45, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Zhang PP, Ge YC, Li SJ, Xie HL, Li LS, Liu ZH: Renal biopsy in type 2 diabetes: Timing of complications and evaluating of safety in Chinese patients. Nephrology (Carlton) 16: 100–105, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Parrish AE: Complications of percutaneous renal biopsy: A review of 37 years’ experience. Clin Nephrol 38: 135–141, 1992 [PubMed] [Google Scholar]
- 31.Hergesell O, Felten H, Andrassy K, Kühn K, Ritz E: Safety of ultrasound-guided percutaneous renal biopsy-retrospective analysis of 1090 consecutive cases. Nephrol Dial Transplant 13: 975–977, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Manno C, Strippoli GF, Arnesano L, Bonifati C, Campobasso N, Gesualdo L, Schena FP: Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int 66: 1570–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Ferguson JH, Lewis JH, Zucker MB: Bleeding tendency in uremia. Blood 11: 1073–1076, 1956 [PubMed] [Google Scholar]
- 34.Escolar G, Díaz-Ricart M, Cases A: Uremic platelet dysfunction: Past and present. Curr Hematol Rep 4: 359–367, 2005 [PubMed] [Google Scholar]
- 35.Bollée G, Martinez F, Moulin B, Meulders Q, Rougier JP, Baumelou A, Glotz D, Subra JF, Ulinski T, Vrigneaud L, Brasseur J, Martin L, Daniel L, Kourilsky O, Deteix P, Sie P, Ronco P, Houillier P: Renal biopsy practice in France: Results of a nationwide study. Nephrol Dial Transplant 25: 3579–3585, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Briganti EM, Dowling J, Finlay M, Hill PA, Jones CL, Kincaid-Smith PS, Sinclair R, McNeil JJ, Atkins RC: The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant 16: 1364–1367, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Schena FP, The Italian Group of Renal Immunopathology : Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. Nephrol Dial Transplant 12: 418–426, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Mauer M, Drummond K: The early natural history of nephropathy in type 1 diabetes: I. Study design and baseline characteristics of the study participants. Diabetes 51: 1572–1579, 2002 [DOI] [PubMed] [Google Scholar]
- 39.D’Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M, International Society of Nephrology Working Group on the Classification of Lupus Nephritis. Renal Pathology Society Working Group on the Classification of Lupus Nephritis : The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65: 521–530, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noël LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA, Renal Pathology Society : Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21: 556–563, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Fogo AB, Bostad L, Svarstad E, Cook WJ, Moll S, Barbey F, Geldenhuys L, West M, Ferluga D, Vujkovac B, Howie AJ, Burns A, Reeve R, Waldek S, Noël LH, Grünfeld JP, Valbuena C, Oliveira JP, Müller J, Breunig F, Zhang X, Warnock DG, all members of the International Study Group of Fabry Nephropathy (ISGFN) : Scoring system for renal pathology in Fabry disease: Report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant 25: 2168–2177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson CA: Companies look for profit in orphan drugs. Am J Health Syst Pharm 67: 1892–1896, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Borrell Fontelles J, Pekkarinen M: Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. Available at http://ec.europa.eu/health/files/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf Accessed July 14, 2012
- 45.Piotto GH, Moraes MC, Malheiros DM, Saldanha LB, Koch VH: Percutaneous ultrasound-guided renal biopsy in children—safety, efficacy, indications and renal pathology findings: 14-year Brazilian university hospital experience. Clin Nephrol 69: 417–424, 2008 [DOI] [PubMed] [Google Scholar]