Summary
Background and objectives
An interarm BP difference has been associated with atherosclerosis and adverse cardiovascular outcomes. This study investigated whether an interleg BP difference was associated with peripheral vascular disease and overall and cardiovascular mortality in hemodialysis patients.
Design, setting, participants, & measurements
This study enrolled 210 hemodialysis patients from December 2006 to January 2007. Bilateral leg BPs were measured simultaneously by an ankle-brachial index (ABI)–form device before hemodialysis.
Results
The mean follow-up period was 4.4±1.5 years. ABI <0.9 and high brachial-ankle pulse wave velocity were independently associated with an interleg difference in systolic BP of ≥15 mmHg or diastolic BP of ≥10 mmHg. Furthermore, this difference was an independent predictor for overall mortality (hazard ratio [HR], 3.36; 95% confidence interval [CI], 1.68–6.72; P<0.01) and cardiovascular mortality (HR, 4.84; 95% CI, 1.84–12.71; P<0.01) after adjustment for demographic, clinical, and biochemical parameters. After further adjustment for ABI <0.9 and brachial-ankle pulse wave velocity, the relation remained significant to overall mortality (HR, 2.91; 95% CI, 1.28–6.64; P=0.01) and cardiovascular mortality (HR, 3.15; 95% CI, 1.05–9.44; P=0.04).
Conclusions
A difference in systolic BP of ≥15 mmHg or diastolic BP of ≥10 mmHg between legs was associated with peripheral vascular disease and increased risk for overall and cardiovascular mortality in hemodialysis patients. Detection of an interleg BP difference may identify hemodialysis patients at increased risk of peripheral vascular disease and overall and cardiovascular mortality.
Introduction
A BP difference between arms is sometimes encountered in various populations, such as people with hypertension, diabetes, or peripheral vascular disease (1–4). An appreciation of the presence of an interarm BP difference is vital for the accurate diagnosis and management of hypertension in primary care. Several studies have reported that an interarm BP difference is associated with subclavian stenosis, peripheral artery disease, and pre-existing coronary artery disease (5–7). Furthermore, an interarm BP difference is strongly associated with increased cardiovascular mortality and all-cause mortality (2,7,8).
An interarm BP difference is also sometimes found in patients with CKD. Moreover, this difference is associated with increased all-cause mortality in this population (9). The importance of a BP difference between arms is already recognized (1–4). However, measurement of BP in the arm with blood access is inappropriate in hemodialysis patients. The hypotheses that an association exists between an interleg BP difference and that peripheral vascular disease and an interleg BP difference is a useful predictor for overall and cardiovascular mortality in hemodialysis patients have not been examined. Accordingly, in this study, we investigated the relationship between an interleg BP difference and peripheral vascular disease in hemodialysis patients. Furthermore, we also examined whether an interleg BP difference could predict overall and cardiovascular mortality in such patients.
Materials and Methods
Study Patients and Design
This study was conducted at one dialysis clinic in a regional hospital in Taiwan. All routine hemodialysis patients in this hospital were included except 6 patients refusing ankle-brachial index (ABI)–form device examinations, 4 patients with atrial fibrillation, 2 patients with bilateral below-knee amputation, and 13 patients with inadequate image visualization. Ultimately, we enrolled 210 patients (93 males and 117 females) from December 2006 to January 2007 and followed up until transfer, death, or March 2012. During the period of follow-up, 35 patients transferred to other hospitals. There was no significant difference in comorbidities, laboratory data, and medications between transfer and nontransfer patients except higher mean arterial pressure and shorter follow-up period in transfer patients. The protocol was approved by our institutional review board and all enrolled patients gave written informed consent.
Hemodialysis
All patients underwent routine hemodialysis three times a week using a Toray 321 machine (Toray Medical Company, Tokyo, Japan). Each hemodialysis session was performed for 3–4 hours using a dialyzer with a blood flow rate of 250–300 ml/min and dialysate flow of 500 ml/min.
Assessment of ABI and Brachial-Ankle Pulse Wave Velocity
The values of ABI and brachial-ankle pulse wave velocity (PWV) were measured 10–30 minutes before hemodialysis. The ABI and brachial-ankle PWV were measured using an ABI-form device, which automatically and simultaneously measured BPs in both arms and ankles using an oscillometric method (10–16). Occlusion and monitoring cuffs were placed tightly around the upper arm without blood access and both sides of the lower extremities in the supine position. ABI was calculated by the ratio of the ankle systolic BP (SBP) divided by the arm SBP and the lower value of the ankle SBP was used for the calculation. For measuring brachial-ankle PWV, pulse waves obtained from the brachial and tibial arteries were recorded simultaneously and the transmission time, which was defined as the time interval between the initial increase in brachial and tibial waveforms, was determined. The transmission distance from the arm to each ankle was calculated according to body height. The brachial-ankle PWV value was automatically computed as the transmission distance divided by the transmission time. After obtaining bilateral brachial-ankle PWV values, the higher one was used for later analysis. The ABI and brachial-ankle PWV measurements were done once in each patient. Because the ABI and brachial-ankle PWV were noninvasive and reliable diagnostic tools for peripheral vascular disease (11,13), patients with ABI <0.9 or increased brachial-ankle PWV were considered to have peripheral vascular disease in this study.
Collection of Demographic, Medical, and Laboratory Data
Demographic and medical data, including age, sex, smoking history (ever versus never), and comorbid conditions, were obtained from medical records and interviews with patients. The body mass index was calculated as the ratio of weight in kilograms divided by the square of height in meters. Laboratory data were measured from fasting blood samples using an autoanalyzer (D-68298 Mannheim COBAS Integra 400; Roche Diagnostics GmbH). High-sensitivity C-reactive protein (CRP) (Dade Behring Marburg GmbH) was measured by commercially available kits. Blood samples were obtained within 1 month of enrollment. Kt/V was evaluated monthly as a marker of dialysis efficiency and was determined according to the Gotch procedure (17). In addition, information regarding patient medications, including aspirin, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, β-blocker, calcium channel blockers, and hepatic hydroxymethyl glutaryl–CoA reductase inhibitors (statins), during the study period was obtained from medical records.
Statistical Analyses
Statistical analysis was performed using SPSS 15.0 software for Windows (SPSS Inc, Chicago, IL). Data are expressed as percentages, mean ± SD, or median (25th–75th percentile) for interleg BP difference, duration of dialysis, triglyceride, and high-sensitivity CRP. The differences between groups were checked by the chi-squared test for categorical variables, by the independent t test for continuous variables with approximately-normal distribution, or by the Mann–Whitney U test for continuous variables with skewed distribution. Multiple logistic regression analysis was used to identify the factors associated with an interleg difference in SBP of ≥15 mmHg or diastolic BP (DBP) of ≥10 mmHg. Significant variables in univariate analysis were selected for multivariate analysis. Time to overall and cardiovascular mortality and covariates of risk factors were modeled using the Cox proportional hazards model. The association between an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg and overall and cardiovascular mortality was assessed by a modified stepwise procedure in four modeling steps. The first model consisted of age and sex. The second model consisted of adding clinical risk factors. The third step was adding biochemical factors. The final step was entering ABI <0.9 and brachial-ankle PWV into the model. A significant improvement in model prediction was based on the −2 log-likelihood ratio statistic, which followed a difference in likelihood ratio, and the P value was based on the incremental value compared with the previous model. A difference was considered significant if the P value was <0.05.
Results
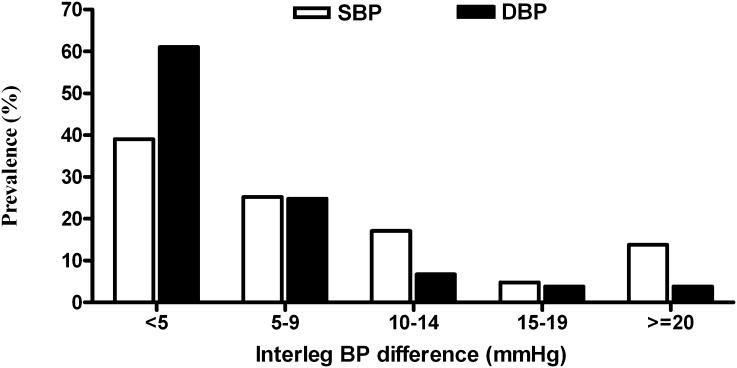
The mean age of the 210 patients was 59.3±13.1 years. The prevalence of interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg was 26.2%. The comparison of baseline characteristics between patients with and without an interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg is shown in Table 1. Compared with patients with an interleg SBP difference <15 mmHg and DBP <10 mmHg, patients with an interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg were found to have an older age, higher prevalence of diabetes mellitus (DM), higher prevalence of a history of hypertension, higher pulse pressure, higher prevalence of ABI <0.9 (P<0.001), higher brachial-ankle PWV (P<0.001), lower HDL cholesterol, and lower creatinine. The value of ABI was lower (0.92±0.21 and 1.14±0.12, respectively) and brachial-ankle PWV (2246.7±688.9 and 1802.1±430.5 cm/s, respectively) was higher in patients with an interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg (both P<0.001). Figure 1 illustrates the distribution of the interleg SBP and DBP differences in study patients. There were 18.6% and 13.8% of patients with an interleg difference in SBP of ≥15 mmHg and DBP of ≥10 mmHg, respectively.
Table 1.
Comparison of baseline characteristics between patients with and without an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg
| Characteristics | All Patients (n=210) | Interleg SBP Difference <15 mmHg and DBP <10 mmHg (n=155) | Interleg SBP Difference ≥15 mmHg or DBP Difference ≥10 mmHg (n=55) |
|---|---|---|---|
| Difference in SBP (mmHg) | 6.5 (3–12) | 5 (3–8) | 20 (8–35)a |
| Difference in DBP (mmHg) | 3 (2–6) | 3 (1–4.5) | 10 (5–15.25)a |
| Age (yr) | 59.3±13.1 | 57.6±13.5 | 64.0±10.9b |
| Male sex | 44.3 | 43.9 | 45.5 |
| Duration of dialysis (yr) | 5.5 (3.4–8.4) | 5.6 (3.4–8.5) | 4.7 (3.2–8.1) |
| Smoking history | 25.2 | 24.5 | 27.3 |
| Diabetes mellitus | 38.6 | 31.6 | 58.2b |
| Hypertension | 71.0 | 67.1 | 81.8b |
| Coronary artery disease | 28.6 | 25.8 | 36.4 |
| Cerebrovascular disease | 9.0 | 7.1 | 14.5 |
| Mean arterial pressure (mmHg) | 101.5±17.1 | 100.5±16.4 | 104.4±18.7 |
| Pulse pressure (mmHg) | 66.1±17.2 | 62.6±15.0 | 75.7±19.4a |
| ABI <0.9 | 13.8 | 3.2 | 43.6a |
| Brachial-ankle PWV (cm/s) | 1919.1±545.9 | 1802.1±430.5 | 2246.7±688.9a |
| Body mass index (kg/m2) | 23.9±3.6 | 23.8±3.5 | 24.0±3.9 |
| Laboratory parameters | |||
| Albumin (g/dl) | 3.83±0.28 | 3.85±0.27 | 3.79±0.32 |
| Fasting glucose (mg/dl) | 119.3±54.2 | 115.0±52.6 | 131.6±57.2 |
| Triglyceride (mg/dl) | 132 (91.75–213.25) | 130 (86–203) | 137 (97–218) |
| Total cholesterol (mg/dl) | 184.5±41.8 | 187.1±43.2 | 177.4±36.8 |
| HDL cholesterol (mg/dl) | 46.5±14.7 | 48.6±15.4 | 40.0±10.1a |
| LDL cholesterol (mg/dl) | 88.1±26.5 | 88.7±27.4 | 86.4±24.1 |
| Hemoglobin (g/dl) | 9.9±1.1 | 9.9±1.1 | 10.1±1.2 |
| Creatinine (mg/dl) | 10.3±2.3 | 10.5±2.3 | 9.7±2.1b |
| Phosphorous (mg/dl) | 4.9±1.2 | 4.8±1.2 | 5.0±1.2 |
| Calcium-phosphorous product (mg2/dl2) | 47.6±12.0 | 47.7±11.8 | 49.3±12.4 |
| Uric acid (mg/dl) | 7.6±1.5 | 7.6±1.5 | 7.8±1.7 |
| High-sensitivity CRP (mg/L) | 0.28 (0.11–0.74) | 0.25 (0.1–0.61) | 0.35 (0.16–0.85) |
| Kt/V (Gotch) | 1.30±0.24 | 1.31±0.24 | 1.27±0.25 |
| Cardio-thoracic ratio >50% | 44.3 | 40.6 | 54.5 |
| Medications | |||
| Aspirin use | 12.4 | 11.6 | 14.8 |
| ACEI and/or ARB use | 19.6 | 20.6 | 16.7 |
| β-blocker use | 19.0 | 19.5 | 17.1 |
| Calcium channel blocker use | 35.9 | 36.1 | 35.2 |
| Statin use | 28.7 | 28.4 | 29.6 |
Data are presented as percentages, mean ± SD, and median (interquartile range), unless otherwise indicated. SBP, systolic BP; DBP, diastolic BP; ABI, ankle-brachial index; PWV, pulse wave velocity; CRP, C-reactive protein; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
P<0.001 compared with patients with interleg SBP difference <15 mmHg and DBP <10 mmHg.
P<0.05 compared with patients with interleg SBP difference <15 mmHg and DBP <10 mmHg.
Figure 1.
The distribution of the interleg BP difference in study patients. There were 18.6% and 13.8% of patients with an interleg difference in systolic BP (SBP) of ≥15 mmHg and diastolic BP (DBP) of ≥10 mmHg, respectively.
Determinants of an Interleg SBP Difference ≥15 mmHg or DBP Difference ≥10 mmHg
Table 2 shows the determinants of an interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg in our study patients. In the univariate regression analysis, an interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg was found to be significantly associated with old age, DM, hypertension, high pulse pressure, ABI <0.9, high brachial-ankle PWV, low HDL cholesterol, and low creatinine. In the multivariate analysis, ABI <0.9 (odds ratio [OR], 18.35; P<0.01), high brachial-ankle PWV (per 10 cm/s) (OR, 1.01; P<0.01), and low HDL cholesterol (OR, 0.96; P=0.01) were independently associated with an interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg.
Table 2.
Determinants of an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg
| Parameter | Multivariate Analysis | |
|---|---|---|
| OR (95% CI) | P | |
| Age (per 1 yr) | 0.99 (0.96–1.03) | 0.63 |
| Diabetes mellitus | 0.77 (0.31–1.90) | 0.57 |
| Hypertension | 1.31 (0.48–3.56) | 0.59 |
| Pulse pressure (per 1 mmHg) | 1.02 (0.99–1.05) | 0.14 |
| ABI <0.9 | 18.35 (5.30–63.59) | <0.01 |
| Brachial-ankle PWV (per 10 cm/s) | 1.01 (1.01–1.02) | <0.01 |
| HDL cholesterol (per 1 mg/dl) | 0.96 (0.92–0.99) | 0.01 |
| Creatinine (per 1 mg/dl) | 0.99 (0.81–1.21) | 0.89 |
OR, odds ratio; CI, confidence interval; SBP, systolic BP; DBP, diastolic BP; ABI, ankle-brachial index; PWV, pulse wave velocity.
To avoid colinearity, we also used the higher value of the ankle SBP to calculate the ABI and still found that ABI <0.9 (5.7%) was significantly associated with an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg (OR, 6.63; P=0.01) after the multivariate analysis.
Risk of Overall Mortality
The mean follow-up period was 4.4±1.5 years in all patients. During the period of follow-up, 41 deaths were recorded in these 210 patients (19.5%), including fatal cardiovascular events (n=22), malignancy (n=3), infectious disease (n=10), gastrointestinal bleeding (n=2), and others (n=4). Table 3 displays the HRs of an interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg for overall mortality with and without adjustment for demographic, clinical, biochemical risk factors as well as ABI <0.9 and brachial-ankle PWV. An interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg was associated with overall mortality in the age- and sex-adjusted model (HR, 3.30; 95% confidence interval [CI], 1.76–6.18; P<0.01) and in the multivariable model adjusting for age, sex, DM, and a history of hypertension, coronary artery disease, and cerebrovascular disease (HR, 3.09; 95% CI, 1.62–5.91; P<0.01). This relation remained significant after further adjustment for mean arterial pressure, pulse pressure, albumin, log triglyceride, total cholesterol, hemoglobin, creatinine, phosphorous, calcium-phosphorous product, and log high-sensitivity CRP (HR, 3.36; 95% CI, 1.68–6.72; P<0.01). This relation was attenuated after further adjustment for ABI <0.9 and brachial-ankle PWV, but still remained significant (HR, 2.91; 95% CI, 1.28–6.64; P=0.01).
Table 3.
Relation of an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg to overall and cardiovascular mortality using the Cox proportional hazards model
| Interleg BP Difference | Overall Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Unadjusted | 3.94 (2.11–7.35) | <0.01 | 5.73 (2.40–13.68) | <0.01 |
| Age and sex adjusted | 3.30 (1.76–6.18) | <0.01 | 4.71 (1.96–11.30) | <0.01 |
| Multivariate adjusted model 1 | 3.09 (1.62–5.91) | <0.01 | 4.92 (1.98–12.24) | <0.01 |
| Multivariate adjusted model 2 | 3.36 (1.68–6.72) | <0.01 | 4.84 (1.84–12.71) | <0.01 |
| Multivariate adjusted model 3 | 2.91 (1.28–6.64) | 0.01 | 3.15 (1.05–9.44) | 0.04 |
Multivariate model 1 is adjusted for age, sex, diabetes mellitus, hypertension, coronary artery disease, and cerebrovascular disease. Multivariate model 2 comprises model 1 as well as mean arterial pressure, pulse pressure, albumin, log triglyceride, total cholesterol, hemoglobin, creatinine, phosphorous, calcium-phosphorous product, and log high-sensitivity CRP. Multivariate model 3 comprises model 2 as well as ABI <0.9 and brachial-ankle PWV. HR, hazard ratio; CI, confidence interval; SBP, systolic BP; DBP, diastolic BP; ABI, ankle-brachial index; PWV, pulse wave velocity; CRP, C-reactive protein.
Risk of Cardiovascular Mortality
Twenty-two cardiovascular deaths were documented during the follow-up period, including heart failure (n=14), myocardial infarction (n=5), and ventricular fibrillation (n=3). A Cox proportional hazards regression analysis of an interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg for cardiovascular mortality is shown in Table 3. An interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg was associated with cardiovascular mortality in the multivariable model after adjustment for age and sex (HR, 4.71; 95% CI, 1.96–11.30; P<0.01) and in the multivariable model after adjustment for age, sex, DM, a history of hypertension, coronary artery disease, and cerebrovascular disease (HR, 4.92; 95% CI, 1.98–12.24; P<0.01). The relation was still significant after further adjustment for mean arterial pressure, pulse pressure, albumin, log triglyceride, total cholesterol, hemoglobin, creatinine, phosphorous, calcium-phosphorous product, and log high-sensitivity CRP (HR, 4.84; 95% CI, 1.84–12.71; P<0.01). This relation was attenuated after further adjustment for ABI <0.9 and brachial-ankle PWV, but still remained significant (HR, 3.15; 95% CI, 1.05–9.44; P=0.04).
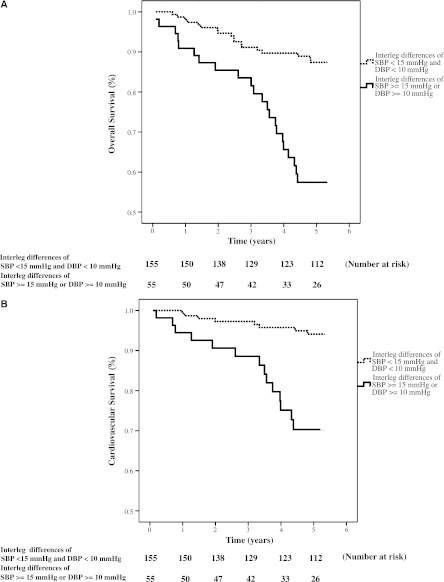
Figure 2 illustrated the Kaplan-Meier curves for overall and cardiovascular survival (both log-rank P<0.01) in all patients subdivided according to interleg SBP difference ≥15 mmHg or DBP difference ≥10 mmHg.
Figure 2.
Overall and cardiovascular survival curves. Kaplan–Meier analyses of (A) overall and(B) cardiovascular survival (both log-rank P<0.001) in all patients subdivided according to an interleg difference in systolic BP (SBP) of ≥15 mmHg or diastolic BP (DBP) of ≥10 mmHg.
Predictive Values of an Interleg Difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg, ABI <0.9, and Brachial-Ankle PWV in Relation to Overall and Cardiovascular Mortality
The incremental values of an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg, ABI <0.9, and brachial-ankle PWV in the prediction of overall and cardiovascular mortality are shown in Table 4. The addition of an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg to a model adjusted for demographic, clinical, and biochemical risk factors significantly improved the prognostic values of overall (P<0.01) and cardiovascular mortality (P<0.01). Similarly, the addition of ABI <0.9 to the same model also significantly improved the prognostic values of overall (P=0.03) and cardiovascular mortality (P<0.01). However, brachial-ankle PWV had no significant incremental values in predicting overall (P=0.65) and cardiovascular mortality (P=0.62).
Table 4.
Predictive values of interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg, ABI <0.9, and brachial-ankle PWV in relation to overall and cardiovascular mortality
| Parameters | Overall Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|
| Difference in Likelihood Ratio | P | Difference in Likelihood Ratio | P | |
| Interleg BP difference | 11.70 | <0.01 | 10.68 | <0.01 |
| ABI <0.9 | 4.67 | 0.03 | 8.30 | <0.01 |
| Brachial-ankle PWV | 0.87 | 0.65 | 0.24 | 0.62 |
P value was based on the incremental value compared with the previous model, which was adjusted for age, sex, diabetes mellitus, hypertension, coronary artery disease, cerebrovascular disease, mean arterial pressure, pulse pressure, albumin, log triglyceride, total cholesterol, hemoglobin, creatinine, phosphorous, calcium-phosphorous product, and log high-sensitivity CRP. SBP, systolic BP; DBP, diastolic BP; ABI, ankle-brachial index; PWV, pulse wave velocity; CRP, C-reactive protein.
Discussion
In this study, using a simultaneous measurement technique, we evaluated the association between an interleg BP difference and peripheral vascular disease and whether an interleg BP difference could predict overall and cardiovascular mortality in hemodialysis patients over a follow-up period of 4.4 years. We found that an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg was independently associated with ABI <0.9 and high brachial-ankle PWV and increased overall and cardiovascular mortality in patients with hemodialysis. The addition of an interleg BP difference and ABI <0.9 but not brachial-ankle PWV to a traditional model adjusted for demographic, clinical, and biochemical risk factors could significantly improve the predictive values for overall and cardiovascular mortality.
The ABI <0.9 and brachial-ankle PWV are markers of peripheral artery occlusive disease and arterial stiffness, respectively (18–22). Lower ABI is reported to be associated with generalized atherosclerosis (e.g., common carotid artery intima-media thickness and the degree of stenosis in the intracranial internal carotid artery and middle cerebral artery) (23,24). Previous studies had also showed low ABI or ABI <0.9 had a significant correlation with the interarm difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg (1,6,25). Our study also revealed that ABI <0.9 and high brachial-ankle PWV were significantly associated with an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg. Thus, measuring bilateral leg BP in hemodialysis patients may be helpful in detection of existing peripheral artery occlusive disease or increased arterial stiffness.
Previous studies reported that a difference in SBP of ≥10 mmHg or ≥15 mmHg or DBP of ≥10 mmHg between arms was strongly associated with increased cardiovascular events and all-cause mortality in hypertensive patients (2,7,8). Agarwal et al. also evaluated the prognostic importance of interarm SBP difference in chronic renal failure patients and found that an interarm SBP difference of ≥10 mmHg conferred an increased overall mortality (9). Our study also showed that an interleg BP difference was significantly correlated with poor overall and cardiovascular survival in hemodialysis patients. One potential explanation is that unequal limb atherosclerosis might be the cause contributing to an interarm or interleg BP difference, and the interarm or interleg BP difference might then have prognostic value for overall and cardiovascular mortality as worsened atherosclerosis (8,26). In fact, our study showed that an interleg difference in SBP of ≥15 mmHg or DBP of ≥10 mmHg was significantly associated with peripheral vascular disease indicated by ABI <0.9 and increased brachial-ankle PWV. Furthermore, even after adjusting ABI <0.9 and brachial-ankle PWV, the relation between an interleg BP difference and overall and cardiovascular mortality still remained significant. Hence, some nonatherosclerotic mechanisms might be responsible for the correlation between an interleg BP difference and mortality. Further studies are required to elucidate the mechanisms. However, many unfavorable survival factors in our patients with an interleg BP difference, such as old age, DM, hypertension, wide pulse pressure, low HDL cholesterol, and low creatinine, might partially explain the association between an interleg BP difference and overall and cardiovascular mortality in this study.
Previous reports demonstrated that a lower ABI was more prevalent in the ESRD population and was responsible for significant morbidity and mortality in these patients (14–16,27,28). ABI measurement has been proposed as a screening test, but is not routinely undertaken in hemodialysis patients. Its measurement requires time, specialized equipment, experience, and training (29), whereas bilateral leg BP measurement in hemodialysis patients can be easily done in the supine position before, during, or after hemodialysis. Furthermore, our study demonstrated that an interleg BP difference was a useful prognostic factor in hemodialysis patients. Therefore, routine BP measurements in both legs should be taken in patients receiving hemodialysis.
In this study, BP was determined using a noninvasive cuff-oscillometric method, but not an invasive method. The oscillometric method was not a popular one to measure BP in daily clinical practice. Hence, although the measurement device used in this study could automatically and simultaneously measure the four-limb BPs, our results might be changed if a conventional mercury sphygmomanometer was used. In addition, although bilateral arm BP measurement is inappropriate in hemodialysis patients, it is easier to obtain in other populations; thus, the interarm BP difference should be still a more suitable prognostic factor than the interleg BP difference in patients without hemodialysis. Hence, the clinical utility of interleg BP difference in nonhemodialysis patients may be limited.
Our results demonstrated that an interleg BP difference was significantly correlated with ABI <0.9 and high brachial-ankle PWV. Moreover, this difference could predict poor overall and cardiovascular survival in hemodialysis patients. Detection of an interleg BP difference may provide a simple method of identifying patients at increased risk of peripheral vascular disease and overall and cardiovascular mortality in hemodialysis patients.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Clark CE, Campbell JL, Evans PH, Millward A: Prevalence and clinical implications of the inter-arm blood pressure difference: A systematic review. J Hum Hypertens 20: 923–931, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Clark CE, Campbell JL, Powell RJ: The interarm blood pressure difference as predictor of cardiovascular events in patients with hypertension in primary care: Cohort study. J Hum Hypertens 21: 633–638, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Clark CE, Greaves CJ, Evans PH, Dickens A, Campbell JL: Inter-arm blood pressure difference in type 2 diabetes: A barrier to effective management? Br J Gen Pract 59: 428–432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aboyans V, Criqui MH, McDermott MM, Allison MA, Denenberg JO, Shadman R, Fronek A: The vital prognosis of subclavian stenosis. J Am Coll Cardiol 49: 1540–1545, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL: Association of a difference in systolic blood pressure between arms with vascular disease and mortality: A systematic review and meta-analysis. Lancet 379: 905–914, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Clark CE, Campbell JL, Powell RJ, Thompson JF: The inter-arm blood pressure difference and peripheral vascular disease: Cross-sectional study. Fam Pract 24: 420–426, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Clark CE, Taylor RS, Shore AC, Campbell JL: The difference in blood pressure readings between arms and survival: Primary care cohort study. BMJ 344: e1327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark CE, Powell RJ: The differential blood pressure sign in general practice: Prevalence and prognostic value. Fam Pract 19: 439–441, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Bunaye Z, Bekele DM: Prognostic significance of between-arm blood pressure differences. Hypertension 51: 657–662, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S: Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis 166: 303–309, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y: Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 25: 359–364, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama H, Shoji T, Kimoto E, Shinohara K, Tanaka S, Koyama H, Emoto M, Nishizawa Y: Pulse wave velocity in lower-limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb 10: 253–258, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Chen JH, Chen SC, Liu WC, Su HM, Chen CY, Mai HC, Chou MC, Chang JM: Determinants of peripheral arterial stiffness in patients with chronic kidney disease in southern Taiwan. Kaohsiung J Med Sci 25: 366–373, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Chen SC, Chang JM, Hwang SJ, Chen JH, Lin FH, Su HM, Chen HC: Comparison of ankle-brachial index and brachial-ankle pulse wave velocity between patients with chronic kidney disease and hemodialysis. Am J Nephrol 29: 374–380, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Chen SC, Chang JM, Hwang SJ, Tsai JC, Liu WC, Wang CS, Lin TH, Su HM, Chen HC: Ankle brachial index as a predictor for mortality in patients with chronic kidney disease and undergoing haemodialysis. Nephrology (Carlton) 15: 294–299, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Chen SC, Chang JM, Hwang SJ, Tsai JC, Wang CS, Mai HC, Lin FH, Su HM, Chen HC: Significant correlation between ankle-brachial index and vascular access failure in hemodialysis patients. Clin J Am Soc Nephrol 4: 128–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotch FA: Evolution of the single-pool urea kinetic model. Semin Dial 14: 252–256, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Fishbane S, Youn S, Kowalski EJ, Frei GL: Ankle-arm blood pressure index as a marker for atherosclerotic vascular diseases in hemodialysis patients. Am J Kidney Dis 25: 34–39, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ: Edinburgh Artery Study: Prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 20: 384–392, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Newman AB, Tyrrell KS, Kuller LH: Mortality over four years in SHEP participants with a low ankle-arm index. J Am Geriatr Soc 45: 1472–1478, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S: Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension 39: 10–15, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lehmann ED: Clinical value of aortic pulse-wave velocity measurement. Lancet 354: 528–529, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Hayashi C, Ogawa O, Kubo S, Mitsuhashi N, Onuma T, Kawamori R: Ankle brachial pressure index and carotid intima-media thickness as atherosclerosis markers in Japanese diabetics. Diabetes Res Clin Pract 66: 269–275, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Sodhi HS, Shrestha SK, Rauniyar R, Rawat B: Prevalence of peripheral arterial disease by ankle-brachial index and its correlation with carotid intimal thickness and coronary risk factors in Nepalese population over the age of forty years. Kathmandu Univ Med J (KUMJ) 5: 12–15, 2007 [PubMed] [Google Scholar]
- 25.Kimura A, Hashimoto J, Watabe D, Takahashi H, Ohkubo T, Kikuya M, Imai Y: Patient characteristics and factors associated with inter-arm difference of blood pressure measurements in a general population in Ohasama, Japan. J Hypertens 22: 2277–2283, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ono K, Tsuchida A, Kawai H, Matsuo H, Wakamatsu R, Maezawa A, Yano S, Kawada T, Nojima Y: Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol 14: 1591–1598, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Chen SC, Chang JM, Hwang SJ, Wang CS, Liu WC, Chen JH, Su HM, Chen HC: An association between ankle-brachial index below 0.9 and arteriovenous fistula failure in diabetic patients with hemodialysis. Clin Nephrol 72: 501–502, 2009 [DOI] [PubMed] [Google Scholar]
- 28.O’Hare A, Johansen K: Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol 12: 2838–2847, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Ray SA, Srodon PD, Taylor RS, Dormandy JA: Reliability of ankle:brachial pressure index measurement by junior doctors. Br J Surg 81: 188–190, 1994 [DOI] [PubMed] [Google Scholar]