Summary
Background and objectives
Obstructive nephropathy is a leading cause of CKD in children. The assessment of severity of renal impairment and the prediction of which children will progress to renal failure are, however, challenging.
Design, Setting, Participants, & Measurements
This case-control study measured the urinary excretion of candidate biomarkers in 27 prevalent case-patients with posterior urethral valves (PUVs) and 20 age-matched controls, correlated their urinary concentration with GFR, and analyzed receiver-operating characteristic (ROC) curve and regression analyses to assess their performance as tests for low GFR.
Results
The median urinary protein-to-creatinine ratio was higher in children with PUV (45 g/mol; range, 5–361 g/mol) than in controls (7 g/mol; range, 3–43 g/mol) (P<0.01) and correlated inversely with renal function (r = −0.44; P<0.05). In whole urine, excretion of aquaporin-2 was significantly decreased, whereas that of TGFβ and L1 cell adhesion molecule (L1CAM) was significantly increased. Whole-urine TGFβ excretion correlated inversely with GFR (r = −0.53; P<0.05). As tests for low GFR, whole-urine TGFβ, L1CAM, and urinary protein-to-creatinine ratio performed best, with areas under the ROC curves of 0.788, 0.795, and 0.814, respectively. By linear regression analysis, whole-urine TGFβ, L1CAM, and urinary protein-to-creatinine ratio were associated with low GFR in the case-patients.
Conclusions
Candidate biomarkers of obstructive nephropathy can be readily measured in whole urine and in urine exosomes. In boys with PUV, these biomarkers correlate with GFR.
Introduction
Posterior urethral valves (PUVs) are the most common cause of lower urinary tract obstruction in males. The estimated incidence of PUV is 1:5000–1:8000 of live male births (1). The associated obstructive nephropathy and renal dysplasia are the leading cause of ESRD in surviving children (2). Approximately 20%–30% of affected children progress to ESRD in the first decade of life, and the majority progress to CKD (3–5).
Most cases of PUV are diagnosed by antenatal ultrasonographic findings and by postnatal confirmation by voiding cystourethrography or during cystoscopy (6). The assessment of the severity of kidney injury is more difficult. No available tests can predict which patients will progress to ESRD. However, the severity of functional renal impairment is directly related to structural damage of the developing kidneys. In our previous studies of congenital urinary tract obstruction in the human and monkey fetus, we observed pronounced injury, characterized by a paucity of collecting ducts, tubular dilatation and atrophy, and interstitial fibrosis deep into the medulla (7–9). To assess the extent of these injuries in humans, one would have to perform a kidney biopsy to obtain tissue for analysis. Given the inherent risks of this procedure in the fetus and younger child, surrogate markers of structural changes of obstructive nephropathy that can be measured in urine would be useful.
We have previously identified candidate proteins that are differentially expressed in the tissue of obstructed fetal kidneys compared with control kidneys (8,9). These included changes in scaffolding transmembrane epithelial proteins involved in cell-cell adhesion, with decreases in and alteration of the distribution of E-cadherin and β-catenin, increases in N-cadherin and L1 cell adhesion molecule (L1CAM) in injured collecting-duct cells, increases in the mesenchymal proteins vimentin and α-smooth muscle actin (α-SMA) in the renal interstitium (reflecting epithelial-mesenchymal transition and myofibroblast recruitment), decreases in proteins expressed specifically by differentiated principal and intercalated cells of the collecting duct (including aquaporin-2 [AQP2] and vacuolar-type H+-adenosine triphosphatase, respectively), decreases in expression of the flow-sensing protein transient receptor potential cation channel subfamily V member 4 (TRPV4) in the collecting duct, and increases in cytokines involved in fibrosis (in particular TGFβ in the tubular epithelium and surrounding renal interstitium).
The major aim of this work was to study the correlation of urinary biomarker excretion and kidney function in boys with PUV. Preliminary aims were to define the feasibility of measuring these candidate biomarkers in urine and to determine whether the excretion profile of boys with PUV differed from that of children who were normal or had minor kidney abnormalities.
Materials and Methods
The study was approved and consent was waived by the Ethics Committee of the University of British Columbia.
Identification of Case-Patients
Inclusion criteria for case-patients included boys, age 1–18 years, with the diagnosis of a PUV confirmed by ultrasonography and voiding cystourethrography. Exclusion criteria included inability to provide a urine sample, age under 1 year (to control for normal tubular maturation), requirement for dialysis, or a measured GFR (mGFR) of <15 ml/min per 1.73 m2. Twenty-seven children with PUV were identified from the British Columbia Children’s Hospital nephrology clinical database. Urinary studies were performed on all 27 patients. Of these 27 patients, 25 had mGFRs. Estimated GFR (eGFR) was also calculated in these case-patients using the revised bedside Schwartz equation (10,11).
Identification of Controls
Inclusion criteria for controls included boys and girls, age 1–18 years, who were attending the outpatient general pediatric nephrology clinic at British Columbia Children’s Hospital. Exclusion criteria included inability to provide a urine sample; age under 1 year of age; or an underlying diagnosis of nephrotic syndrome, primary tubular disorder, or significant hydronephrosis, which could potentially confound the interpretation of the protein studies.
Twenty control patients were enrolled and are called the “total control” group. Among these 20 patients, 2 had mild hydronephrosis (with no evidence of parenchymal damage), 2 had well controlled hypertension (1 with essential hypertension who was receiving a calcium-channel blocker, and the other with white coat hypertension who was not receiving treatment), 7 had a congenital anomaly of the kidney (2 with solitary kidneys, 4 with unilateral small kidneys, and 1 with a horseshoe kidney), and 3 had dysfunctional voiding. These 14 patients were called “non-normal” controls. The remaining 6 patients in the total control group were entirely normal, with no evidence of a kidney problem; these patients were called the “normal” controls. There was no formal prospective evaluation of kidney function in the control group; however, we identified previous serum creatinine measurements performed in these children as part of routine evaluations for other reasons. Of the 20 children, 15 had had previous serum creatinine measurements, which were all within the normal range for age, and normal eGFR (>90 ml/min per 1.73 m2) when calculated using the revised bedside Schwartz equation (10,11).
Collection and Processing of Urine Samples
Urine samples were collected during regular clinic visits. Protease inhibitors (protease inhibitor cocktail P2714; Sigma-Aldrich, Oakville, Ontario, Canada) were added immediately to prevent degradation of urinary proteins. All samples were subsequently frozen at −20°C. Before analyses, thawed urine samples were centrifuged for 10 minutes at 300 g and for 20 minutes at 10,000 g at 4°C to remove cellular debris. For enrichment of urinary proteins, 4 ml of urine supernatant was spun in an Amicon Ultra-4 concentrator (Millipore, Billerica, MA) at 4000 g for 20 minutes to reduce volumes to about 100 μl. This fraction was designated “whole urine” for this study. Urine exosomes were then collected by centrifugation of the supernatant at 200,000 g for 1 hour at 4°C using a Beckman SW 40 rotor (Beckman Instruments, Fullerton, CA) and then resuspended in PBS with protease inhibitors. Urinary proteins and exosome-associated protein concentrations were measured by NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Middletown, VA) at 280 nm. Total protein and creatinine were measured in unconcentrated urine using UPRO and CREA (isotope dilution mass spectrometry) reagent slide methods on the VITROS 5.1 FS analyzer (Ortho Clinical Diagnostics, Rochester, NY). Urine protein measurement is a dye-binding method (pyrocatechol-violet molybdate complex), and urine creatinine is measured by an enzymatic method (creatinine amidohydrolase), with calibration traceable to a gas chromatography–isotope dilution mass spectrometry reference method. Protein in the urine was normalized to creatinine concentration and expressed as the urinary protein-to-creatinine ratio.
Immunoblotting
Whole-urine and urine exosome samples were solubilized in 5× SDS-sample buffer, and equal amounts of urine and urinary exosome proteins were separated by SDS-PAGE. After fractionation by SDS-PAGE, proteins were transferred onto nitrocellulose membrane and were probed with the appropriate primary antibodies against E-cadherin (BD Transduction Laboratories, San Diego, CA); β-catenin (Cell Signaling Technology, Inc., Danvers, MA); vimentin, α-SMA, AQP2, and L1CAM (Sigma-Aldrich); and N-cadherin, TGFβ1, vacuolar-type H+-adenosine triphosphatase, and TRPV4 (Santa Cruz Biotechnology, Santa Cruz, CA). This was followed by incubation with the appropriate antirabbit and antimouse IgG horseradish peroxidase–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Detection was carried out with enhanced chemiluminescence (Sigma-Aldrich). Densitometry of the blots was performed with ImageJ software (National Institutes of Health, Bethesda, MD), and the concentration of the biomarker protein was then expressed in arbitrary units.
Determination of Measured GFR
GFR was measured by a two-point single injection of 99mTc-diethylenetriaminepentaacetic acid with correction for the early exponential phase using the Bröchner-Mortensen equation (12). CKD was classified into CKD stage 1 (GFR ≥ 90 ml/min per 1.73 m2), stage 2 (GFR, 60–89 ml/min per 1.73 m2), stage 3 (GFR, 30–59 ml/min per 1.73 m2), and stage 4 (GFR, 15–29 ml/min per 1.73 m2) (13).
Statistical Analyses
Continuous demographic data and urinary and exosome biomarker values for the different groups were expressed as medians and range. We log transformed the data, then performed two-sided t tests for significance. Statistically significant findings were defined as P<0.05.
Pearson correlation was performed to test for correlations between individual urinary biomarker proteins, urinary protein-to-creatinine ratio, and GFR. Proteins identified as clinically or statistically significant were then studied in multivariate analyses to explore the relationship of these proteins to mGFR. Linear regression was performed to explore the relationship of these individual proteins with mGFR as the dependent variable. Logistic regression determined the relationship of the variables with mGFR < 60 ml/min per 1.73 m2 (CKD stages 3 and 4).
In box plot graphs, boxes represent interquartile range for which the top, middle, and bottom lines are 75th percentile, median, and 25th percentile, respectively. Top and bottom lines extend to the furthest data point within 1.5 times the interquartile range. Open circle data points represent outlying values.
Receiver-operating characteristic (ROC) curves were generated to test the ability of each candidate biomarker to predict a low GFR. Sensitivities and specificities were calculated for urinary protein-to-creatinine ratio and other individual biomarker proteins at various cutoff values. All statistical analyses were performed using SPSS software, version 18.0 (SPSS Inc., Chicago, IL).
Regression analyses were used to predict low GFR. We included four variables in the models: whole-urine TGFβ, whole-urine L1CAM, exosome TGFβ, and urinary protein-to-creatinine ratio. These variables were chosen on the basis of the strength of their individual association with mGFR and their performance by ROC curve analysis. Skewed data were log transformed before analysis.
Results
We compared our 27 prevalent case-patients with PUV with the total, normal, and non-normal control groups (Table 1). Age did not significantly differ among the groups. Urinary protein-to-creatinine ratios in the normal, non-normal, and total control groups were similar and were significantly lower than in the case-patients (Tables 1 and 2). All 27 case-patients were male; in the total control group, 13 patients were female and 7 were male. Among the controls, boys and girls were similar in age and had similar excretion of biomarkers.
Table 1.
Patient demographic characteristics
| Characteristic | Total Controls | Total Controls (n=20) | Case-Patients (n=27) | |
|---|---|---|---|---|
| Non-Normal (n=14) | Normal (n=6) | |||
| Male (n) | 5 | 2 | 7 | 27 |
| Age (yr) | 7 (1–16) | 9 (2–16) | 7 (1–16) | 8 (1–18) |
| Urinary protein-to-creatinine ratio (g/mol)a | 8 (2–43) | 7 (4–22) | 7 (2–43) | 45 (5–361) |
| Measured GFR (ml/min per 1.73 m2)b | Not done | Not done | Not done | 64 (27–138) |
Values represent the median with the range in parentheses.
Normal, 0–22 g/mol (14).
For 25 of the 27 case-patients.
Table 2.
Whole-urine biomarker protein concentrations
| Biomarker | Total Controls (n=20) | Total Controls (n=20) | Case-Patients (n=27) | ||||
|---|---|---|---|---|---|---|---|
| Non-Normal (n=14) | Normal (n=6) | ||||||
| Hydronephrosis (n=2) | Hypertension (n=2) | Congenital Anomaly (n=7) | Dysfunctional Voiding (n=3) | ||||
| AQP2 | 4.5a (3.4–5.7) | 4.5a (4.5–4.6) | 3.5a (0.3–6.4) | 2.9 (0.5–2.9) | 4.5a (1.1–8.2) | 3.7a (0.3–8.2) | 0.3 (0.2–8.5) |
| VATPase | 3.1 (1.9–4.2) | 10.4 (7.8–13.0) | 13.7 (2.1–35.2) | 42.8a (31.7–64.2) | 8.8 (3.0–43.9) | 8.8 (1.9–64.2) | 13.0 (1.3–49.4) |
| TGFβ | 4.5 (1.3–7.6) | 3.1 (2.2–4.0) | 1.6 (1.0–6.9) | 6.5 (2.40–7.1) | 1.7a (1.1–5.0) | 2.2a (1.0–7.6) | 4.1 (0.6–14.7) |
| N-cadherin | 9.3a (8.9–9.8) | 26.3 (9.9–42.8) | 3.1 (0.5–18.1) | 3.3 (1.8–27.5) | 5.9 (0.4–13.8) | 8.0 (0.3–42.8) | 2.8 (0.5–17.0) |
| TRPV4 | 2.5 (1.7–3.4) | 1.9 (0.7–3.2) | 3.3 (0.9–7.7) | 1.9 (1.6–3.2) | 3.0 (1.2–5.2) | 2.1 (0.7–7.7) | 2.0 (0.5–10.7) |
| L1CAM | 5.9 (4.3–7.5) | 2.9 (0.8–5.0) | 5.7 (2.1–10.7) | 1.9 (1.7–8.5) | 2.1a (1.8–3.9) | 4.1 (0.8–10.7) | 4.1 (0.9–11.8) |
| Urinary protein-to-creatinine ratio | 5.5a (5.1–8.1) | 5.7a (3.9–7.5) | 7.4a (2.4–39.4) | 12.9 (5.4–42.9) | 6.6a (3.6–22) | 7.2a (2.4–42.9) | 44.8 (5.2–361) |
Values represent the median with the range in parentheses. AQP2, aquaporin-2; VATPase, vacuolar-type H+-adenosine triphosphatase; TRPV4, transient receptor potential cation channel subfamily V member 4; L1CAM, L1 cell adhesion molecule.
Significantly different than in case-patients.
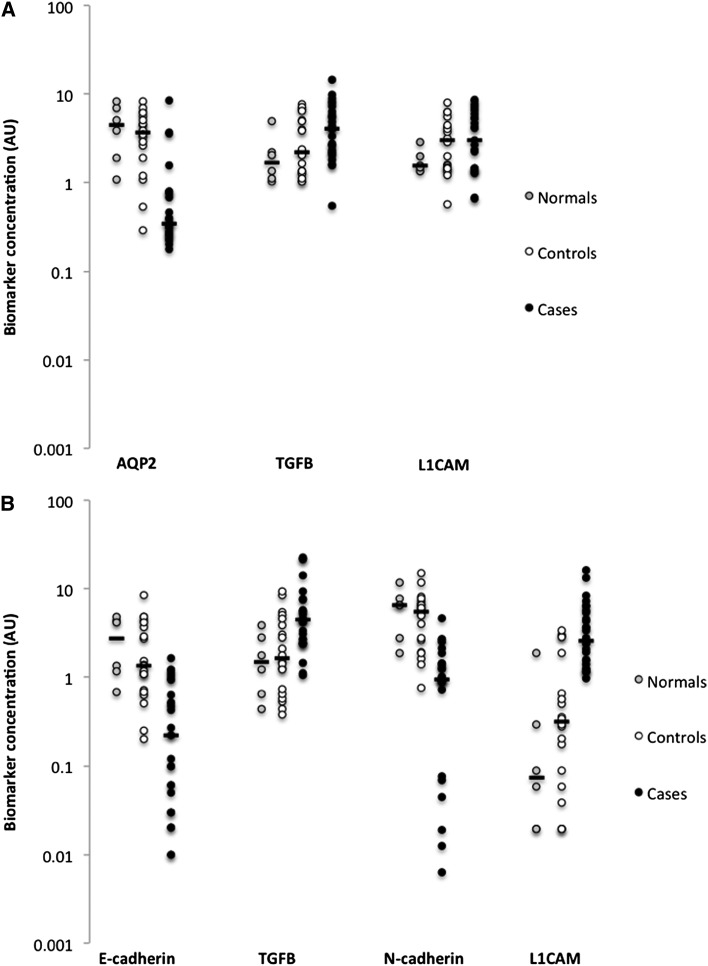
Profiles of excretion of whole-urine and exosome biomarker proteins were similar in the normal control and total control groups. For whole-urine biomarker proteins, the case-patients excreted significantly less AQP2 (P<0.001) and significantly more TGFβ (P<0.05) than both control groups and significantly more L1CAM than normal controls (P<0.05) (Table 2 and Figure 1A). For exosome biomarker protein excretion, the case-patients excreted significantly less E-cadherin (P<0.01) and N-cadherin (P<0.01) and significantly more TGFβ (P<0.05) and L1CAM (P<0.01) than both the normal and non-normal control groups (Table 3 and Figure 1B). We could not detect E-cadherin and β-catenin in whole-urine samples or vimentin or α-SMA in either protein fraction.
Figure 1.
Excretion of biomarker proteins. (A) Whole-urine aquaporin-2 (AQP2) was decreased (P<0.01) and TGFβ was increased (P<0.05) in case-patients with posterior urethral valves (PUVs) compared with values in both normal and control groups, whereas excretion of L1 cell adhesion molecule (L1CAM) was increased in case-patients compared with excretion in the normal group (P<0.05). (B) Exosome E-cadherin (P<0.01) and N-cadherin (P<0.01) were decreased and TGFβ (P<0.05) and L1CAM (P<0.01) were increased in case-patients with PUV compared with values in both normal and control groups. Dots are individual data points, horizontal bars represent the median values, and all data are plotted on a log-transformed y-axis. AU, arbitrary unit.
Table 3.
Exosome biomarker protein concentrations
| Biomarker | Total Controls (n=20) | Total Controls (n=20) | Case-Patients (n=27) | ||||
|---|---|---|---|---|---|---|---|
| Non-Normal (n=14) | Normal (n=6) | ||||||
| Hydronephrosis (n=2) | Hypertension (n=2) | Congenital Anomaly (n=7) | Dysfunctional Voiding (n=3) | ||||
| E-cadherin | 1.1a (0.7–1.5) | 3.8a (2.7–4.8) | 1.4a (0.3–8.4) | 0.5 (0.2–1.1) | 2.7a (0.7–4.4) | 1.4a (0.2–8.4) | 0.2 (0–1.2) |
| B-catenin | 0.9 (0.6–1.3) | 1.0 (0.4–1.7) | 1.0 (0.6–2.2) | 0.9 (0.8–1.3) | 1.2 (0.3–4.9) | 0.9 (0.4–4.9) | 2.1 (0–9.7) |
| AQP2 | 3.4 (0.7–6.1) | 5.9 (0.6–11.2) | 6.4 (0.7–12.6) | 3.6 (1.1–31.4) | 3.8 (1.5–31.4) | 4.3 (0.6–83.5) | 2.7 (1.1–21.9) |
| VATPase | 3.0 (1.6–4.5) | 2.1 (1.0–3.3) | 4.1 (0–20.1) | 8.3 (6.3–16.5) | 4.6 (2.7–10.1) | 4.3 (0–20.1) | 4.1 (0.4–25.3) |
| TGFβ | 2.2 (1.3–3.0) | 4.5 (0.5–8.4) | 2.1 (0.6–9.3) | 0.7 (0.4–4.6) | 1.5a (0.4–3.8) | 1.8a (0.4–14.2) | 4.5 (1.1–22.6) |
| N-cadherin | 5.8a (5.0–6.5) | 5.9a (3.9–7.9) | 4.9a (1.6–14.7) | 1.4 (0.8–6.0) | 6.4a (1.8–11.3) | 5.4a (0.8–14.7) | 0.9 (0–4.6) |
| L1CAM | 1.7 (0.3–3.1) | 1.5 (0–3.0) | 0.5 (0–3.5) | 0.4a (0.2–0.6) | 0.1a (0–1.9) | 0.3a (0–3.5) | 2.7 (1–16.8) |
| TRPV4 | 0.6 (0.2–1.0) | 0.4 (0–0.8) | 1.1a (0–3.9) | 2.1a (1.2–2.3) | 2.3 (1.0–5.7) | 1.3a (0–5.7) | 3.2 (0.7–12.9) |
Values represent the median with the range in parentheses. AQP2, aquaporin-2; VATPase, vacuolar-type H+-adenosine triphosphatase; L1CAM, L1 cell adhesion molecule; TRPV4, transient receptor potential cation channel subfamily V member 4.
Significantly different than in case-patients.
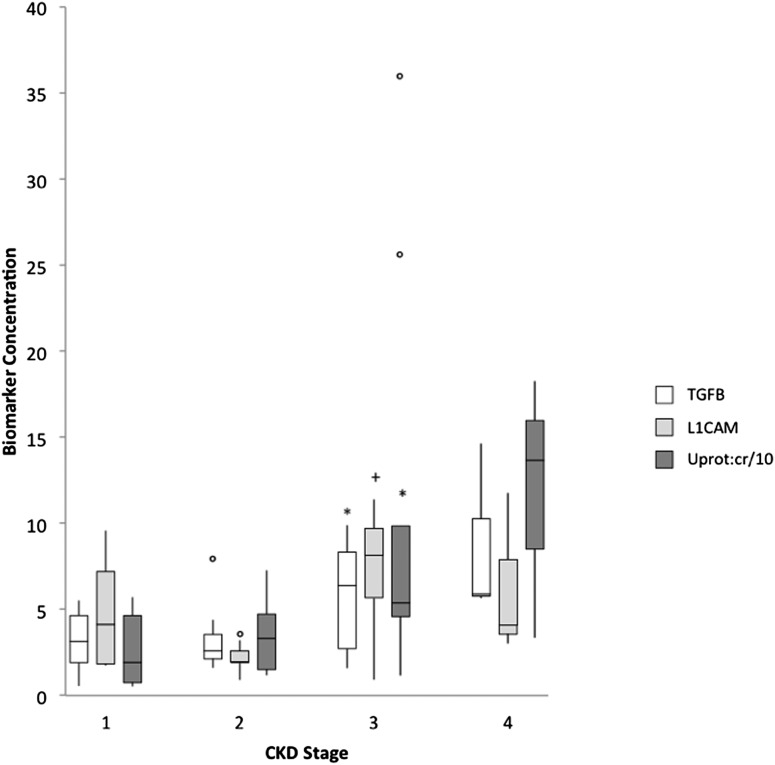
In addition to more proteinuria in case-patients than controls, urinary protein-to-creatinine ratio in the case-patients increased proportionately to the decrease in renal function: from 19.2 (CKD stage 1) to 33.2 (CKD stage 2) to 53.8 (CKD stage 3) to 136.6 g/mol (CKD stage 4) (Figure 2). Whole-urine TGFβ and L1CAM also increased with increasing CKD stage. Similarly, urinary protein-to-creatinine ratio and whole-urine TGFβ correlated inversely with measured GFR (r=−0.44 and −0.53, respectively); in contrast, as would be expected, eGFR correlated significantly with measured GFR (r=0.6) (Table 4).
Figure 2.
Relationship between CKD stage, proteinuria, and urinary biomarker proteins in case-patients with obstructive nephropathy due to posterior urethral valves. Whole-urine analysis demonstrates increasing urine TGFβ, L1 cell adhesion molecule (L1CAM), and urinary protein-to-creatinine ratio (Uprot:cr) with advancing CKD stage. *P<0.05 versus CKD stage 1 and 2; +P<0.05 versus CKD stage 2. CKD stage based on GFR in ml/min per 1.73 m2: stage 1, ≥90; stage 2, 60–89; stage 3, 30–59; stage 4, 15–29.
Table 4.
Correlation (r) of whole-urine proteins with measured GFR and age among case-patients
| Biomarker | Urinary Protein-to-Creatinine Ratio | Measured GFR | Age |
|---|---|---|---|
| AQP2 | −0.15 | −0.17 | 0.16 |
| VATPase | 0.42a | −0.29 | −0.28 |
| TGFβ | 0.5a | −0.53a | −0.07 |
| N-cadherin | 0.19 | 0.09 | 0 |
| TRPV4 | 0.5a | −0.15 | 0.35 |
| L1CAM | 0.34 | −0.32 | 0.09 |
| eGFR | −0.18 | 0.6a | −0.44a |
| Urinary protein-to-creatinine ratio | −0.44a | −0.26 |
AQP2, aquaporin-2; VATPase, vacuolar-type H+-adenosine triphosphatase; TRPV4, transient receptor potential cation channel subfamily V member 4; L1CAM, L1 cell adhesion molecule; eGFR, estimated GFR.
Significant correlation (P<0.05).
There were no significant correlations between age and urinary protein-to-creatinine ratio (Table 4) or between age and any of the urinary biomarkers (Tables 4 and 5). There was also no relationship between age and mGFR in our case-patients.
Table 5.
Correlation (r) of exosome proteins with measured GFR and age among case-patients
| Biomarker | Urinary Protein-to-Creatinine Ratio | Measured GFR | Age |
|---|---|---|---|
| E-cadherin | −0.3 | −0.11 | 0.05 |
| B-catenin | −0.17 | 0.11 | 0.18 |
| AQP2 | 0.44a | −0.06 | 0.04 |
| VATPase | 0.48a | −0.33 | 0 |
| TGFβ | −0.09 | 0.11 | −0.1 |
| N-cadherin | −0.15 | −0.04 | 0.22 |
| L1CAM | −0.1 | −0.15 | −0.06 |
| TRPV4 | 0 | −0.1 | 0.39 |
AQP2, aquaporin-2; VATPase, vacuolar-type H+-adenosine triphosphatase; L1CAM, L1 cell adhesion molecule; TRPV4, transient receptor potential cation channel subfamily V member 4.
Significant correlation (P<0.05).
ROC curve analyses demonstrated that whole-urine TGFβ, L1CAM, urinary protein-to-creatinine ratio, exosome TGFβ, and eGFR were the best tests of low GFR, with AUCs of 0.788, 0.795, 0.814, 0.654, and 0.976, respectively (Tables 6 and 7).
Table 6.
Whole-urine protein receiver-operating characteristic curve analysis
| Protein per Level | Sensitivity | Specificity | AUC (95% CI) |
|---|---|---|---|
| AQP2 | 0.497 (0.26–0.73) | ||
| 0.21 | 0.917 | 0.077 | |
| 0.37 | 0.5 | 0.538 | |
| 1.19 | 0.167 | 0.615 | |
| VATPase | 0.692 (0.48–0.91) | ||
| 1.59 | 0.917 | 0.154 | |
| 13.73 | 0.667 | 0.769 | |
| 29.3 | 0.167 | 0.923 | |
| TGFβ | 0.788 (0.60–0.98) | ||
| 1.59 | 0.917 | 0.077 | |
| 2.70 | 0.833 | 0.615 | |
| 7.60 | 0.333 | 0.923 | |
| N-cadherin | 0.5 (0.26–0.74) | ||
| 1.66 | 0.833 | 0.231 | |
| 2.69 | 0.667 | 0.538 | |
| 6.28 | 0.167 | 0.769 | |
| TRPV4 | 0.599 (0.37–0.83) | ||
| 0.62 | 0.917 | 0.077 | |
| 2.31 | 0.5 | 0.692 | |
| 8.60 | 0.083 | 0.923 | |
| L1CAM | 0.795 (0.61–0.98) | ||
| 1.32 | 0.917 | 0.077 | |
| 2.82 | 0.833 | 0.692 | |
| 6.06 | 0.417 | 0.846 | |
| Urinary protein-to-creatinine ratio | 0.814 (0.65–0.98) | ||
| 11.55 | 0.917 | 0.231 | |
| 43.50 | 0.833 | 0.692 | |
| 69.25 | 0.417 | 0.923 | |
| eGFR | |||
| 36.0 | 0.417 | 1.00 | 0.976 (0.80–1.00) |
| 59.0 | 0.75 | 1.00 | |
| 94.5 | 1.0 | 0.25 |
All protein levels are expressed in arbitrary units, urinary protein-to-creatinine as g/mol, and eGFR as ml/min per 1.73 m2. AUC, area under the receiver-operating characteristic curve; CI, confidence interval; AQP2, aquaporin-2; VATPase, vacuolar-type H+-adenosine triphosphatase; TRPV4, transient receptor potential cation channel subfamily V member 4; L1CAM, L1 cell adhesion molecule.
Table 7.
Exosome protein receiver-operating characteristic curve analysis
| Protein per Level | Sensitivity | Specificity | AUC (95% CI) |
|---|---|---|---|
| E-cadherin | 0.436 (0.20–0.67) | ||
| 0.03 | 0.75 | 0.077 | |
| 0.49 | 0.417 | 0.615 | |
| 1.16 | 0.083 | 0.846 | |
| B-catenin | 0.449 (0.22–0.68) | ||
| 0.02 | 0.75 | 0.231 | |
| 2.11 | 0.417 | 0.615 | |
| 5.58 | 0.083 | 0.846 | |
| AQP2 | 0.583 (0.35–0.81) | ||
| 1.43 | 0.917 | 0.231 | |
| 2.58 | 0.667 | 0.538 | |
| 6.79 | 0.333 | 0.923 | |
| VATPase | 0.615 (0.38–0.85) | ||
| 2.05 | 0.833 | 0.077 | |
| 5.04 | 0.667 | 0.615 | |
| 16.40 | 0.333 | 0.846 | |
| TGFβ | 0.654 (0.43–0.88) | ||
| 2.42 | 0.917 | 0.308 | |
| 3.80 | 0.75 | 0.615 | |
| 8.50 | 0.167 | 0.769 | |
| N-cadherin | 0.532 (0.30–0.76) | ||
| 0.03 | 0.917 | 0.054 | |
| 0.92 | 0.583 | 0.538 | |
| 2.53 | 0.167 | 0.923 | |
| L1CAM | 0.519 (0.28–0.75) | ||
| 1.2 | 0.917 | 0.077 | |
| 2.08 | 0.667 | 0.462 | |
| 8.07 | 0.083 | 0.923 | |
| TRPV4 | 0.436 (0.18–0.69) | ||
| 1.03 | 0.917 | 0.077 | |
| 2.71 | 0.5 | 0.385 | |
| 6.82 | 0.25 | 0.923 |
All protein levels are expressed in arbitrary units. AUC, area under the receiver-operating characteristic curve; CI, confidence interval; AQP2, aquaporin-2; VATPase, vacuolar-type H+-adenosine triphosphatase; L1CAM, L1 cell adhesion molecule; TRPV4, transient receptor potential cation channel subfamily V member 4.
Using linear regression, we found urinary protein-to-creatinine ratio, whole-urine TGFβ, whole-urine L1CAM, and exosome TGFB correlated with mGFR in our case-patients (Table 8). According to logistic regression, none of the markers were associated with a low GFR.
Table 8.
Multivariable linear regression models
| Model | R2 | P Value |
|---|---|---|
| Urinary protein-to-creatinine ratio, whole-urine TGFβ, whole-urine L1CAM, exosome TGFβ | 0.47 | 0.009 |
| Urinary protein-to-creatinine ratio, whole-urine TGFβ, whole-urine L1CAM | 0.47 | 0.003 |
| Urinary protein-to-creatinine ratio, whole-urine TGFβ | 0.44 | 0.002 |
L1CAM, L1 cell adhesion molecule.
Discussion
In this study, we have demonstrated that proteins that are differentially expressed in kidneys in models of obstructive nephropathy can be measured in whole urine and in urine exosomes. In recent years, urinary proteomics–based approaches have emerged as tools for earlier detection of kidney disease, improved assessment of the severity of disease, or more precise monitoring of response to therapy (15). Urine exosomes are particularly well suited for the study of relevant biomarkers. They contain cell membrane and cytosolic proteins that are specific for each type of epithelial cell facing the urinary space, and they reflect the physiologic or pathophysiologic state of their cells of origin (16,17).
The candidate biomarker proteins in this study were chosen with a biologic rationale based on our previous description of tubulo-interstitial disease in fetal animal and human studies (8,9). In that regard we demonstrated altered excretion of AQP2 and TGFβ in whole urine and of E-cadherin, TGFβ, N-cadherin, and L1CAM in urinary exosomes in our case-patients with obstructive nephropathy due to PUV compared with controls. Whereas levels of urinary TGFβ and L1CAM increased with increasing CKD stage in our case-patients, TGFβ levels showed the strongest correlation with mGFR.
TGFβ, not surprisingly, was one of the most informative proteins excreted in urine and associated with exosomes. It is a multifunctional protein that controls proliferation, differentiation, and many other functions of the cell and plays an important role in the development of tissue fibrosis (18,19). TGFβ is a useful noninvasive diagnostic biomarker of upper and lower urinary tract obstruction (20–22). The increased levels of urinary TGFβ seen in our study probably reflect the extent of fibrosis in the kidneys of patients with PUV. Furthermore, increasing levels of TGFβ in whole urine of our case-patients were associated with a decrease in mGFR, the only protein studied that demonstrated this significant relationship.
Whole-urine L1CAM excretion was increased in our case-patients and correlated with GFR. This may be explained by the fact that L1CAM is a membrane glycoprotein normally expressed at the basolateral membrane of the collecting-duct epithelial cell, which with injury is translocated to the apical membrane (23,24).
We studied other potentially important biomarkers, including AQP2, a water channel (25); E-cadherin, a cell adhesion molecule (8,9,26,27); and N-cadherin, another member of the cadherin family (9,28,29). Although the excretion of these candidate proteins was altered in our case-patients, they did not correlate with renal function or with a reduced GFR.
Proteinuria has been described in children with PUV, but its relationship to long-term kidney outcome is unclear (30). In our case-patients, we demonstrated a correlation between urinary protein-to-creatinine ratio and mGFR: as proteinuria increased, GFR decreased. In addition, as a biomarker of low GFR, the urinary protein-to-creatinine ratio performed best, as demonstrated by ROC curve analysis. Although proteinuria has been shown to predict renal failure in select groups of adult patients, its ability to aid in prognosticating renal outcome and CKD progression in adults with glomerular disease and in children in general is limited (31). Therefore, other biomarkers either alone or in combination will be needed.
We also studied the performance of eGFR as a biomarker of low GFR in our case-patients. Its AUC was excellent, with high specificity, but at lower GFRs (<40 ml/min per 1.73 m2) the eGFR had sensitivities of 0.50 or less (Table 6). In other words, the eGFR had a high false-negative rate and overestimated the mGFR. It is not known whether eGFR is a valid measure or reflection of kidney injury, and it is unclear whether it can predict, alone or in combination with other biomarkers, the progression of disease or long-term outcome in PUV.
An important limitation of this study is its small sample size; we therefore may have missed important associations of candidate proteins with changes in GFR. In addition, future prospective studies with more patients are required to test the ability of these select biomarkers to predict progression and long-term outcomes in PUV. This is also a cross-sectional study, whereby only associations of the biomarkers with GFR could be drawn, emphasizing the need for larger prospective studies. Finally, future studies will be needed to measure these proteins in patients who have other significant kidney diseases, in particular those with CKD and low GFR, to determine whether these candidate biomarkers are specific to PUV.
In conclusion, we have identified and measured candidate biomarkers in the urine of patients with PUV; we have demonstrated that these children have significant differences in the excretion of many of these proteins compared with children from the clinic who are normal or have minor kidney abnormalities; and we have identified urinary protein-to-creatinine ratio, whole-urine TGFβ, and L1CAM as having the best correlation with GFR and the best performance as biomarkers.
Disclosures
None.
Acknowledgment
We thank Ruth Milner for her help with statistical analysis.
This work was supported by the British Columbia Provincial Renal Agency and the Transplant Research Foundation of British Columbia.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Krishnan A, de Souza A, Konijeti R, Baskin LS: The anatomy and embryology of posterior urethral valves. J Urol 175: 1214–1220, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA: Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant 11: 366–373, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Drozdz D, Drozdz M, Gretz N, Möhring K, Mehls O, Schärer K: Progression to end-stage renal disease in children with posterior urethral valves. Pediatr Nephrol 12: 630–636, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Lal R, Bhatnagar V, Mitra DK: Long-term prognosis of renal function in boys treated for posterior urethral valves. Eur J Pediatr Surg 9: 307–311, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Lopez Pereira P, Espinosa L, Martinez Urrutina MJ, Lobato R, Navarro M, Jaureguizar E: Posterior urethral valves: prognostic factors. BJU Int 91: 687–690, 2003 [DOI] [PubMed] [Google Scholar]
- 6.de Bruyn R, Marks SD: Postnatal investigation of fetal renal disease. Semin Fetal Neonatal Med 13: 133–141, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Matsell DG, Tarantal AF: Experimental models of fetal obstructive nephropathy. Pediatr Nephrol 17: 470–476, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Butt MJ, Tarantal AF, Jimenez DF, Matsell DG: Collecting duct epithelial-mesenchymal transition in fetal urinary tract obstruction. Kidney Int 72: 936–944, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Trnka P, Hiatt MJ, Ivanova L, Tarantal AF, Matsell DG: Phenotypic transition of the collecting duct epithelium in congenital urinary tract obstruction. J Biomed Biotechnol 2010: 696034, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C [published online ahead of print May 23, 2012]. Kidney Int doi: 10.1038/ki.2012.169 [DOI] [PMC free article] [PubMed]
- 12.Gaspari F, Perico N, Remuzzi G: Measurement of glomerular filtration rate. Kidney Int Suppl 63: S151–S154, 1997 [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation : National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative : National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics 111: 1416–1421, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Barratt J, Topham P: Urine proteomics: The present and future of measuring urinary protein components in disease. CMAJ 177: 361–368, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisitkun T, Shen RF, Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101: 13368–13373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzales P, Pisitkun T, Knepper MA: Urinary exosomes: Is there a future? Nephrol Dial Transplant 23: 1799–1801, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Wynn TA: Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf G: Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int 70: 1914–1919, 2006 [DOI] [PubMed] [Google Scholar]
- 20.El-Sherbiny MT, Mousa OM, Shokeir AA, Ghoneim MA: Role of urinary transforming factor-beta1 in the diagnosis of upper urinary tract obstruction in children. J Urol 168: 1798–1800, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Palmer LS, Maizels M, Kaplan WE, Firlit CF, Cheng EY: Urine levels of transforming growth factor-beta 1 in children with ureteropelvic junction obstruction. Urology 50: 769–773, 1997 [DOI] [PubMed] [Google Scholar]
- 22.MacRae Dell K, Hoffman BB, Leonard MB, Ziyadeh FN, Schulman SL: Increased urinary transforming growth factor-beta(1) excretion in children with posterior urethral valves. Urology 56: 311–314, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Nolte C, Moos M, Schachner M: Immunolocalization of the neural cell adhesion molecule L1 in epithelia of rodents. Cell Tissue Res 298: 261–273, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Allory Y, Audard V, Fontanges P, Ronco P, Debiec H: The L1 cell adhesion molecule is a potential biomarker of human distal nephron injury in acute tubular necrosis. Kidney Int 73: 751–758, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kwon TH, Nielsen J, Møller HB, Fenton RA, Nielsen S, Frøkiaer J: Aquaporins in the kidney. Handb Exp Pharmacol 190: 95–132, 2009 [DOI] [PubMed] [Google Scholar]
- 26.van Roy F, Berx G: The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci 65: 3756–3788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Liu Y: Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 159: 1465–1475, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García-Castro MI, Vielmetter E, Bronner-Fraser M: N-Cadherin, a cell adhesion molecule involved in establishment of embryonic left-right asymmetry. Science 288: 1047–1051, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Nürnberger J, Feldkamp T, Kavapurackal R, Opazo Saez A, Becker J, Hörbelt M, Kribben A: N-cadherin is depleted from proximal tubules in experimental and human acute kidney injury. Histochem Cell Biol 133: 641–649, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Laksmi NK, Khullar M, Kaur B, Ahuja M, Mahajan JK, Mittal BR, Bhattacharya A, Medhi B: Association of angiotensin converting enzyme and angiotensin type 2 receptor gene polymorphisms with renal damage in posterior urethral valves. J Pediatr Urol 6: 560–566, 2010 [DOI] [PubMed] [Google Scholar]
- 31.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]