Summary
Background and objectives
Many patients with ESRD, particularly minorities and women, face barriers in completing the steps required to obtain a transplant. These eight sequential steps are as follows: medical suitability, interest in transplant, referral to a transplant center, first visit to center, transplant workup, successful candidate, waiting list or identify living donor, and receive transplant. This study sought to determine the effect of navigators on completion of steps.
Design, setting, participants, & measurements
Cluster randomized, controlled trial at 23 Ohio hemodialysis facilities. One hundred sixty-seven patients were recruited between January 2009 and August 2009 and were followed for up to 24 months or until study end in February 2011. Trained kidney transplant recipients met monthly with intervention participants (n=92), determined their step in the transplant process, and provided tailored information and assistance in completing the step. Control participants (n=75) continued to receive usual care. The primary outcome was the number of transplant process steps completed.
Results
Starting step did not significantly differ between the two groups. By the end of the trial, intervention participants completed more than twice as many steps as control participants (3.5 versus 1.6 steps; difference, 1.9 steps; 95% confidence interval, 1.3–2.5 steps). The effect of the intervention on step completion was similar across race and sex subgroups.
Conclusions
Use of trained transplant recipients as navigators resulted in increased completion of transplant process steps.
Introduction
Compared with long-term dialysis treatment, kidney transplantation generally offers a longer life span and better quality of life (1,2). Obtaining a kidney transplant requires patients to complete a series of steps (Table 1): medical suitability, interest in transplant, referral to a transplant center, first visit to center, transplant workup, successful candidate, waiting list or identify living donor, and receive transplant (3). Medical suitability refers to the absence of absolute contraindications to transplantation (e.g., a systemic infection or recent malignancy) (4). The transplant center workup typically requires several visits and involves a medical history and physical examination, psychosocial assessment, evaluation and treatment of medical conditions, and laboratory studies (5). Patients who complete this workup and are found to be successful transplant candidates may be placed on a deceased-donor waiting list or may obtain a kidney transplant from a living donor.
Table 1.
Steps involved in obtaining a deceased-donor or living-donor kidney transplant
| Step | Step Completion |
|---|---|
| 1. Suitability for referral to transplant center | Dialysis facility records or nephrologist indicates that patient has no absolute contraindications to kidney transplantation. |
| 2. Interest in transplantation | Patient expresses an interest in considering a deceased- or living-donor transplant. |
| 3. Referral call to transplant center | Transplant center records indicate that referral was made by patient, nephrologist, or dialysis facility. |
| 4. First visit to transplant center | Transplant center records indicate that patient made an initial visit to transplant center. |
| 5. Transplant center workup | Transplant center records indicate that patient completed workup. |
| 6. Successful transplant candidate | Transplant center records indicate that patient is a successful transplant candidate. |
| 7. On waiting list or evaluate potential living donor | Transplant center records indicate that patient is on a deceased-donor waiting list or a potential living donor is being evaluated. |
| 8. Receive transplant | Transplant center records indicate that patient received a deceased- or living-donor kidney transplant. |
Many patients, particularly minorities and women, face barriers in completing the steps required to obtain a transplant (3,6–10). These barriers include inadequate assessment of medical suitability, lack of information about transplantation, reliance on nephrologists to make referrals to transplant centers, and difficulty completing the transplant center workup (3,6–11). We hypothesized that navigators might help patients complete transplant process steps in a more efficient and equitable manner. Navigators are individuals who educate patients and help them navigate through the medical system (12). We reasoned that kidney transplant recipients may be ideal navigators for other patients because of a shared experience with ESRD (13). Similar peer-mediated interventions have been successful in educational settings (14).
Materials and Methods
Participants and Facilities
All 23 hemodialysis facilities that belong to the three largest chains in Cuyahoga County, Ohio, participated. A data manager used a random-number generator to assign facilities to an intervention or control group. To minimize the possibility of contamination by nephrologists who work at both intervention and control facilities, we first determined whether each nephrologist had more patients at intervention facilities or at control facilities. We then included in the study only the larger group of patients.
Study coordinators abstracted medical records to identify community-dwelling patients age 18–70 years who had no absolute contraindications to kidney transplantation (15). We excluded nursing home residents and patients older than 70 years because few transplantations are performed among such individuals (1). Absolute contraindications to kidney transplantation at the two transplant centers in our region include systemic infections, extreme obesity, and active or recent malignancy (4). As a result, we excluded patients with chronic systemic infections, a body mass index >40 kg/m2, or malignancies within the last 2 years. We also excluded patients who had already made a first visit to a transplant center or received a kidney transplant in the past; this was an exclusion criterion because such patients demonstrated an ability to complete key steps in the transplant process. Finally, we excluded patients who had a communication barrier (e.g., those who were mentally incompetent or did not speak English).
Study coordinators described the study to eligible patients during a dialysis treatment and obtained written informed consent. Each participant was given $15 every 6 months to thank him or her for participation. This study was approved by the institutional review board of MetroHealth Medical Center, Cleveland, Ohio, and was registered at ClinicalTrials.gov (identifier NCT00805038).
Baseline Assessment
Unblinded study coordinators abstracted medical records of intervention and control participants to obtain demographic and medical characteristics. Coordinators also abstracted medical records and interviewed participants to determine their baseline step in the transplant process. The baseline step was defined as the earliest step that was incomplete at the beginning of the study.
Intervention Group
We hired and trained three study coordinators who were kidney transplant recipients to act as navigators for intervention group participants. Their training included instruction on the kidney transplant process, human subjects protection, medical records abstraction, and motivational interviewing. A transplant navigator met monthly with each intervention participant during a dialysis treatment, reviewed his or her medical record, and determined the participant’s current step. On the basis of the current step, the navigator carried out the following tasks for the first seven steps:
Step 1. Suitability for Referral to Transplant Center.
Patients with absolute contraindications to kidney transplantation listed in their medical records were not eligible to participate. However, some patients were told by providers that they were unsuitable for transplantation but had no absolute contraindications listed in their records. In these cases, study staff contacted the participant’s nephrologist for clarification. Participants with absolute contraindications remained at this step. Participants without absolute contraindications moved forward to the next step.
Step 2. Interest in Transplantation.
Participants were educated about the advantages and disadvantages of transplantation, the steps in the transplant process, and what to expect after transplantation. Navigators also shared their personal experiences with dialysis and transplantation.
Step 3. Referral Call to Transplant Center.
Participants were given the phone numbers of local transplant centers, a list of the information that they might be asked to provide, and questions to ask.
Step 4. First Visit to Transplant Center.
Participants were given directions to the transplant center, a list of things to take, and questions to ask. In addition, navigators explored transportation options for getting to the transplant center and reminded participants of upcoming appointments.
Step 5. Transplant Center Workup.
Navigators explained to participants what to expect in a transplant center workup. Navigators also monitored completion of specific aspects of the workup, communicated with transplant center staff about outstanding tasks, and encouraged participants to complete the workup in a timely fashion.
Step 6. Successful Transplant Candidate.
Navigators served as an ongoing source of support and information. Navigators also educated participants about how to discuss living donation with potential donors.
Step 7. On Waiting List or Evaluate Potential Living Donor.
Navigators served as an ongoing source of support and information. Navigators monitored participants’ status on the waiting list and results of living-donor evaluation.
Control Group
Control participants continued to receive care from their nephrologists and dialysis facilities. A study coordinator who was not a transplant recipient assessed control participants every 3 months. This assessment included medical record abstraction and a brief interview to determine participants’ current step.
Follow-up Procedures
Participants were recruited between January 2009 and August 2009 and were followed up for 24 months or until they died, moved, withdrew, or reached the study end in February 2011. Study coordinators abstracted medical records and interviewed participants to determine their final step. Because of the sequential nature of the transplant process, completion of a particular step implied that prior steps had also been completed (see Table 1). To parallel the categorization of baseline step, the final step was defined as the farthest step that was incomplete at the end of the study. If intervention participants were unable to complete specific steps by the end of the study, navigators noted whether this was due to a medical limitation, financial concerns, patient reluctance, or ending the trial early.
Outcomes
The primary outcome measure was the number of steps completed, defined as the difference between final and baseline steps. Secondary outcomes were impediments to step completion among intervention participants.
Statistical Analyses
We used mixed-effects models for continuous variables and generalized estimating equation (GEE) models for categorical variables to compare the baseline characteristics of intervention and control participants while accounting for clustering within facilities (16). Intervention effects were assessed using an F-test (in mixed-effects models) or generalized score test (in GEE analyses). We used GEEs to assess the mean baseline and final number of steps for each group, the difference (total number of steps completed) for each group, and the difference in total steps between the two groups (assuming a Poisson distribution and using a linear link). The identity link for the latter analysis was used to directly assess mean differences. The intervention effect was estimated for the overall sample and within each level for sex, race, and baseline step. GEE models for within-group analyses included only the intercept, while models for between-group analyses included the intercept and a group indicator. We used an exchangeable working covariance matrix for all GEE models (except for the analysis by baseline step, for which an independence covariance matrix was used to avoid occasional nonconvergence), and we obtained empirical (robust) variance estimates to account for clustering within facilities.
We carried out multiple imputation to account for missing data. Specifically, we imputed the final step for 12 intervention and 9 control patients who moved or withdrew (detailed in Figure 1) and for 51 intervention and 35 control patients who did not complete 24 months before the study end in February 2011. To account for information on the steps achieved at the time of dropout, each imputed value was obtained, in the manner of empirical Bayes/shrinkage estimation, as an optimal (minimum variance) linear combination of the predicted value based on the baseline covariates (i.e., from a regression model fit to the nondropout sample) and a predicted value based on the individual responses (baseline step and final step at dropout) under a linear model. The covariate-predicted component of the imputations was obtained by predicted mean matching, using five as the number of closest observations for the draws and including all baseline characteristics of participants as covariates (listed in Table 1) (17). In addition, imputed total steps were constrained so that the final number of steps was no more than eight (Table 1). Twenty completed datasets were obtained by imputing total steps in this manner. We did not impute endpoints for patients who died but used the number of steps they completed before death as their endpoints. The multiple imputed (completed) datasets were each analyzed using the relevant GEE model, and t tests accounting for the within- and between-dataset variability were used to assess intervention effects (18).
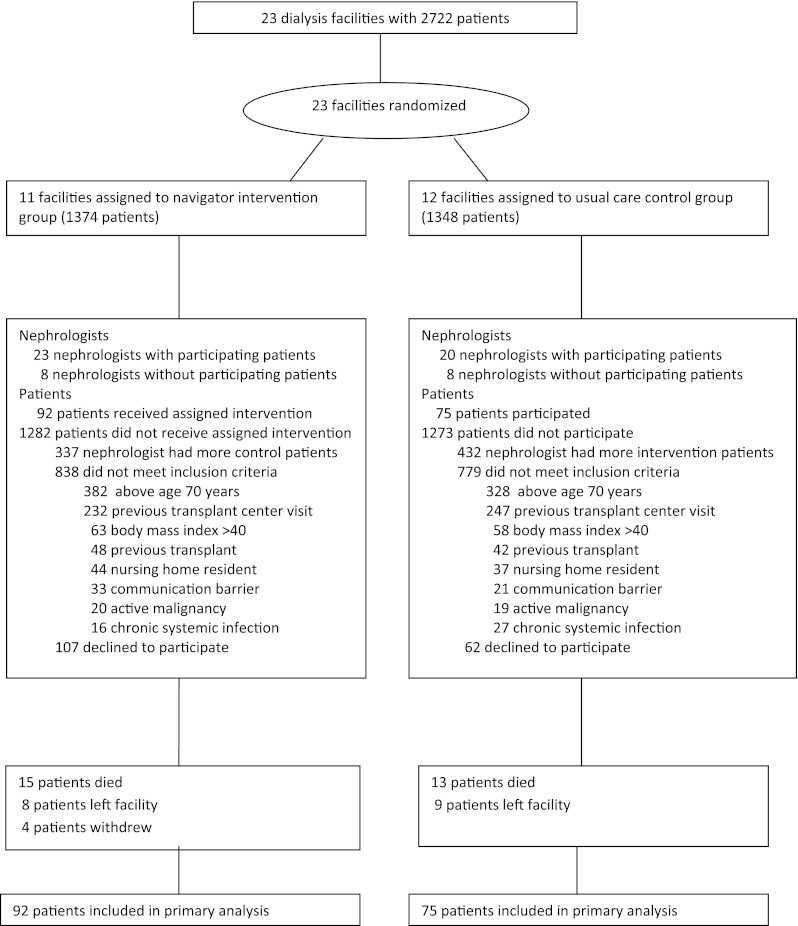
Figure 1.
Flow of participants through the trial.
As secondary analyses, GEE/multiple imputation methods were used within prespecified subgroups defined by key baseline covariates (sex, race, and baseline step). We also verified the analyses of the primary outcome with a GEE model assuming that the number of completed steps (out of the potential number of steps given the baseline number) follows a binomial distribution and using a logit link (with the model otherwise the same as before). This analysis gave similar results, which are not presented. All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC).
On the basis of prior work, we anticipated that the SD of the number of steps completed would be approximately 2.0 steps. To detect a clinically important effect size of 1.0 step (or standardized effect size of 0.5) would require 126 total participants with a two-tailed α level of 0.05 and 80% power (19). However, these estimates must be increased to account for the possible nonindependence of patients clustered within facilities. We found a small amount of clustering of outcomes by facility in our previous work (intraclass cluster coefficient, ρ < 0.025). This gives an inflation factor of 1 + (m−1) ρ, where m is the average number of patients at each facility (in this study, 126 patients divided by 23 facilities). This gives an inflation factor of 1.11 and a total sample size requirement of 140 patients for our primary outcome.
Results
Participant Characteristics
Figure 1 shows the flow of participants through the trial. One hundred sixty-seven patients began the trial: 92 intervention participants and 75 control participants. We excluded 377 patients in intervention facilities because their nephrologists had more patients in control facilities; we therefore assigned these patients to the control group. Similarly, we excluded 432 patients in control facilities because their nephrologists had more patients in intervention facilities. A total of 1617 patients did not meet eligibility criteria. One hundred sixty-nine eligible patients declined to participate. Compared with the 167 participants, these 169 nonparticipants were somewhat older (58 versus 55 years; P=0.01) and had been undergoing hemodialysis longer (4.0 versus 3.0 years; P=0.0001) but did not differ in other demographic characteristics. Intervention and control participants had generally similar baseline characteristics (Table 2).
Table 2.
Baseline characteristics of intervention and control groups
| Characteristic | Intervention Group (n=92) | Control Group (n=75) | P Value |
|---|---|---|---|
| Age | |||
| 18–44 yr | 14 (15) | 9 (12) | |
| 45–54 yr | 31 (34) | 18 (24) | 0.10 |
| 55–64 yr | 39 (42) | 30 (40) | |
| 65–70 yr | 8 (9) | 18 (24) | |
| Male | 47 (51) | 47 (63) | 0.07 |
| Race | |||
| black | 65 (71) | 49 (65) | |
| white | 17 (18) | 21 (28) | 0.50 |
| other | 10 (11) | 5 (7) | |
| Cause of renal failure | |||
| hypertension | 37 (40) | 36 (48) | |
| diabetes | 35 (38) | 23 (31) | 0.82 |
| GN | 9 (10) | 8 (11) | |
| other | 11 (12) | 8 (11) | |
| Time receiving dialysis | . | ||
| <18 mo | 45 (49) | 24 (32) | |
| 18–36 mo | 18 (20) | 26 (35) | 0.06 |
| >36 mo | 29 (32) | 25 (33) | |
| Education | |||
| less than high school | 11 (12) | 13 (17) | |
| high school graduate | 37 (40) | 34 (45) | 0.12 |
| some college | 31 (34) | 21 (28) | |
| college graduate | 13 (14) | 7 (9) | |
| Mean comorbid conditions ± SD (n)a | 1.6±1.2 | 1.8±1.3 | 0.17 |
| Baseline step | |||
| suitability | 39 (42) | 35 (47) | |
| interest | 3 (3) | 8 (11) | 0.63 |
| referral call | 46(50) | 26 (35) | |
| first visit | 4 (4) | 6 (8) |
Data are expressed as number (percentage) of participants unless otherwise indicated.
On the basis of the presence of the following 10 disease categories: coronary artery disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, depression or psychosis, previous solid tumor or hematologic malignancy, connective tissue disease, asthma or chronic obstructive pulmonary disease, diabetes mellitus, and drug or alcohol abuse.
Completion of Steps in Transplant Process
Intervention patients completed more than twice as many steps as control patients (3.5 versus 1.6 steps; difference, 1.9 steps; 95% confidence interval, 1.3–2.5 steps; Table 3). The effect of the intervention on step completion was similar across race and sex subgroups.
Table 3.
Number of steps completed among 92 intervention and 75 control participants
| Variable | Intervention | Control | Difference in Differences | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | Mean Baseline Step ± SEM | Mean Final Step ± SEM | Difference (95% CI) | Patients (n) | Mean Baseline Step ± SEM | Mean Final Step ± SEM | Difference (95% CI) | Difference (95% CI) | P Value | |
| All participants | 92 | 2.1±0.2 | 5.6±0.1 | 3.5 (3.1–4.0) | 75 | 2.0±0.2 | 3.6±0.3 | 1.6 (1.2–2.0) | 1.9 (1.3–2.5) | <0.001 |
| Sex | ||||||||||
| male | 47 | 2.0±0.3 | 5.6±0.1 | 3.8 (3.2–4.5) | 47 | 2.1±0.2 | 3.7±0.3 | 1.6 (1.1–2.3) | 2.1 (1.2–2.9) | <0.001 |
| female | 45 | 2.2±0.2 | 5.6±0.2 | 3.4 (3.1–3.8) | 28 | 2.0±0.2 | 3.5±0.5 | 1.5 (1.0–2.3) | 1.9 (1.1–2.7) | <0.001 |
| Race | ||||||||||
| black | 65 | 2.1±0.2 | 5.6±0.1 | 3.5 (3.1–3.9) | 49 | 2.2±0.2 | 3.8±0.4 | 1.6 (1.2–2.3) | 1.9 (1.1–2.6) | <0.001 |
| white | 17 | 2.4±0.3 | 5.8±0.4 | 3.4 (2.5–4.6) | 21 | 1.8±0.3 | 3.8±0.4 | 1.8 (1.4–2.3) | 1.7 (0.6–2.8) | 0.003 |
| other | 10 | 1.9±0.1 | 5.1±0.4 | 3.5 (2.4–5.2) | 5 | 1.8±0.4 | 2.5±0.6 | 0.6 (0.1–2.7) | 2.8 (1.2–4.4) | <0.001 |
| Baseline step | ||||||||||
| suitability | 39 | 1.0±0.0 | 5.2±0.2 | 4.3 (4.0–4.7) | 35 | 1.0±0.0 | 2.5±0.4 | 1.4 (0.8–2.3) | 2.9 (2.1–3.7) | <0.001 |
| interest | 3 | 2.0±0.0 | 4.6±0.7 | 2.6 (1.5–4.4) | 8 | 2.0±0.0 | 3.1±0.4 | 1.2 (0.6–2.2) | 1.4 (-0.2–3.0) | 0.09 |
| referral call | 46 | 3.0±0.0 | 5.8±0.2 | 2.8 (2.4–3.3) | 26 | 3.0±0.0 | 4.9±0.3 | 1.9 (1.3–2.7) | 1.0 (0.2–1.8) | 0.02 |
| first visit | 4 | 4.0±0.0 | 6.9±0.3 | 3.1 (2.5–4.0) | 6 | 4.0±0.0 | 5.9±0.6 | 2.0 (1.3–3.1) | 1.2 (0.02–2.3) | 0.05 |
CI, confidence interval.
By the end of the trial, 17 (18%) intervention participants and 6 (8%) control participants were on a deceased-donor transplant waiting list (P=0.07). In addition, potential living donors were identified for 3 (3%) intervention participants and 0 (0%) control participants (P=0.06). However, no deceased- or living-donor transplants occurred by the end of the trial. No adverse events or adverse effects were associated with the intervention.
Reasons for Failing to Complete Steps
Even though our intervention was an overall success, many intervention participants failed to become successful transplant candidates. Table 4 lists the specific reasons that intervention participants were unable to complete their final step. For example, 24 intervention participants did not make a referral call to a transplant center by the end of the trial. Sixteen participants had medical limitations, such as acute or chronic conditions, that they wanted to address before calling. Three participants had concerns about the cost of transplantation or immunosuppressive medications. Ten participants were reluctant to call because of fears regarding surgery and rejection or because they felt fine on dialysis. Twelve participants died or left the dialysis facility before the trial ended.
Table 4.
Reasons that patients in intervention group did not become successful transplant candidates
| Final Step | Total Patients (n) | Patients with Specific Reasons (n) | |||
|---|---|---|---|---|---|
| Medical Limitation | Financial Concern | Patient Reluctance | Died or Left Facility | ||
| Interest | 2 | 1 | 0 | 2 | 0 |
| Referral call | 24 | 16 | 3 | 10 | 12 |
| First visit | 15 | 11 | 1 | 6 | 5 |
| First workup | 23 | 11 | 3 | 11 | 6 |
| Successful candidate | 8 | 8 | 0 | 0 | 0 |
Some patients had more than one reason.
Discussion
We found that using trained transplant recipients as navigators resulted in increased completion of steps in the kidney transplant process. Moreover, the effect of the intervention on step completion was similar across race and sex subgroups. Although our trial was not designed to determine the effect of navigators on transplantation, completion of sequential steps in the transplant process is necessary for transplantation to occur. Moreover, we found promising results related to the number of intervention participants who reached the penultimate step in the transplant process (deceased-donor waiting list or evaluate potential living donor).
Previous randomized, controlled trials of navigators have generally focused on cancer screening or treatment but have not used patients as navigators (20–27). Our intervention has the advantage of being simple but also easily tailored to the circumstances of individual patients. By engaging the participation of almost all dialysis facilities in a large geographic area, we enhanced the generalizability of our findings. With the exception of race, participant characteristics were similar to those of dialysis patients nationally (1). The large number of black participants reflects the inner-city location of many of the participating dialysis facilities.
Our results have important implications for patients, providers, and health policy makers. Patients with ESRD may benefit from learning more about kidney transplantation and obtaining help in completing steps in the transplant process. In the absence of formal navigators, patients may need to actively obtain this knowledge and assistance from their physicians and dialysis facilities, local transplant centers and kidney transplant recipients, and family and friends. Providers should realize that usual care is insufficient to adequately educate patients and help them achieve access to kidney transplantation. In particular, we found that many patients are inappropriately categorized as unsuitable for referral. Even though we restricted our study sample to patients without absolute contraindications to transplantation, 89 participants were categorized by their providers as medically unsuitable for referral at baseline (Table 2). However, our intervention was very successful among this subgroup, with intervention participants completing an average of 4.2 steps (95% confidence interval, 3.9–4.5 steps; Table 2). Policy makers may consider funding navigators to work at each of the more than 5000 dialysis facilities nationwide (1). This would not only help patients complete steps in the transplant process but also create meaningful job opportunities for the many kidney transplant recipients who are currently unemployed (28).
Several limitations must be considered in interpreting our results. A much larger sample size and follow-up duration would be necessary to determine the effect of navigation on actual transplantation. As a result, future navigator programs should carefully evaluate the effect of navigation on transplantation. The transplant process experiences of our 167 study participants may not be representative of the experiences of all patients at the 23 participating facilities (see Figure 1). The results rely on a large amount of imputed data. Because of funding limitations, the study end date could not be extended beyond February 2011. As a result, participants who were recruited after March 2009 were unable to complete 24 months of follow-up. Our navigators were paid the same salaries as study coordinators and spent much of their time collecting research data. Such data collection would be unnecessary if navigators were used in clinical settings. We were unable to determine whether cost would impede setting up navigator programs. Increasing the number of individuals receiving living donor transplants will increase the total number of transplants performed. However, increasing the number of individuals on the deceased-donor waiting list will not increase the total number of transplants performed. As a result, continued efforts to increase organ donation are necessary. Although our intervention was an overall success, many intervention patients did not become transplant candidates (Table 4). Note that navigation is unlikely to influence medical limitations or patients who die or have moved. However, further refinements to our approach may increase its potency to influence financial concerns and patient reluctance.
In conclusion, we found that navigators increase completion of steps in the transplant process among all patients as well as among race and sex subgroups. Further work is needed to determine the effect of navigators on actual transplantation and on race and sex disparities. We recommend that nephrologists, dialysis facilities, and regulatory agencies use the transplant process steps we identified (Table 1) to develop clinical performance measures to monitor and improve access to transplantation. Using peer navigators may also be useful in other settings and for other conditions as a way to both improve care and reduce disparities.
Disclosures
S.D.N. reports receiving grant support from Genzyme. D.E.H. reports receiving payments for lectures from Novartis and Genentech.
Acknowledgments
We are grateful to the patients and health providers who participated in this project.
This work was supported by grants DK51472002265 and RR024989 from the National Institutes of Health, Bethesda, Maryland. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Initiating and Completing the Kidney Transplant Evaluation Process: The Red Queen’s Race,” on pages 1551–1552.
References
- 1.US Renal Data System : USRDS 2010 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Cangro CB, Hariharan S, Hricik DE, Kerman RH, Roth D, Rush DN, Vazquez MA, Weir MR, American Society of Transplantation : The evaluation of renal transplantation candidates: Clinical practice guidelines. Am J Transplant 1[Suppl 2]: 3–95, 2001 [PubMed] [Google Scholar]
- 5.Gallon LG, Leventhal JR, Kaufman DB: Pretransplant evaluation of renal transplant candidates. Semin Nephrol 22: 515–525, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM: Racial disparities in access to renal transplantation—clinically appropriate or due to underuse or overuse? N Engl J Med 343: 1537–1544, 2, 1537, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM: The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med 341: 1661–1669, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Ladin K, Rodrigue JR, Hanto DW: Framing disparities along the continuum of care from chronic kidney disease to transplantation: barriers and interventions. Am J Transplant 9: 669–674, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navaneethan SD, Singh S: A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant 20: 769–775, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU: Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol 6: 1760–1767, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Coorey GM, Paykin C, Singleton-Driscoll LC, Gaston RS: Barriers to preemptive kidney transplantation. Am J Nurs 109: 28–37, quiz 38, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Freeman HP, Muth BJ, Kerner JF: Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract 3: 19–30, 1995 [PubMed] [Google Scholar]
- 13.Ramirez AG, Turner BJ: The role of peer patients in chronic disease management. Ann Intern Med 153: 544–545, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Mohan M: Peer tutoring as a technique for teaching the unmotivated. Child Study J 1: 217–225, 1971 [Google Scholar]
- 15.Shapiro R, Sarwal MM: Pediatric kidney transplantation. Pediatr Clin North Am 57: 393–400, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Donner A, Klar N: Design and Analysis of Cluster Randomization Trials in Health Research, New York, Oxford University Press, 2000 [Google Scholar]
- 17.Heitjan F, Little RJ: Multiple imputation for the fatal accident reporting system. Appl Stat 40: 13–29, 1991 [Google Scholar]
- 18.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, John Wiley & Sons, 1987 [Google Scholar]
- 19.Hulley SB, Cummings SR: Designing Clinical Research, Baltimore, Williams & Wilkins, 1988 [Google Scholar]
- 20.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L: A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc 100: 278–284, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Ell K, Vourlekis B, Xie B, Nedjat-Haiem FR, Lee PJ, Muderspach L, Russell C, Palinkas LA: Cancer treatment adherence among low-income women with breast or gynecologic cancer: A randomized controlled trial of patient navigation. Cancer 115: 4606–4615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrante JM, Chen PH, Kim S: The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: A randomized controlled trial. J Urban Health 85: 114–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH: Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health 82: 216–224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maxwell AE, Jo AM, Crespi CM, Sudan M, Bastani R: Peer navigation improves diagnostic follow-up after breast cancer screening among Korean American women: Results of a randomized trial. Cancer Causes Control 21: 1931–1940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Percac-Lima S, Grant RW, Green AR, Ashburner JM, Gamba G, Oo S, Richter JM, Atlas SJ: A culturally tailored navigator program for colorectal cancer screening in a community health center: A randomized, controlled trial. J Gen Intern Med 24: 211–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasser KE, Murillo J, Lisboa S, Casimir AN, Valley-Shah L, Emmons KM, Fletcher RH, Ayanian JZ: Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med 171: 906–912, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Phillips CE, Rothstein JD, Beaver K, Sherman BJ, Freund KM, Battaglia TA: Patient navigation to increase mammography screening among inner city women. J Gen Intern Med 26: 123–129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Mei SF, Krol B, van Son WJ, de Jong PE, Groothoff JW, van den Heuvel WJ: Social participation and employment status after kidney transplantation: A systematic review. Qual Life Res 15: 979–994, 2006 [DOI] [PubMed] [Google Scholar]