Abstract
Cashew nut shell liquid (CNSL) has been used in traditional medicine for the treatment of a wide variety of pathophysiological conditions. To further define the mechanism of CNSL action, we investigated the effect of cashew nut shell extract (CNSE) on two matrix metalloproteinases, MMP-2/gelatinase A and MMP-9/gelatinase B, which are known to have critical roles in several disease states. We observed that the major constituent of CNSE, anacardic acid, markedly inhibited the gelatinase activity of 3T3-L1 cells. Our gelatin zymography studies on these two secreted gelatinases, present in the conditioned media from 3T3-L1 cells, established that anacardic acid directly inhibited the catalytic activities of both MMP-2 and MMP-9. Our docking studies suggested that anacardic acid binds into the MMP-2/9 active site, with the carboxylate group of anacardic acid chelating the catalytic zinc ion and forming a hydrogen bond to a key catalytic glutamate side chain and the C15 aliphatic group being accommodated within the relatively large S1′ pocket of these gelatinases. In agreement with the docking results, our fluorescence-based studies on the recombinant MMP-2 catalytic core domain demonstrated that anacardic acid directly inhibits substrate peptide cleavage in a dose-dependent manner, with an IC50 of 11.11 μM. In addition, our gelatinase zymography and fluorescence data confirmed that the cardol-cardanol mixture, salicylic acid, and aspirin, all of which lack key functional groups present in anacardic acid, are much weaker MMP-2/MMP-9 inhibitors. Our results provide the first evidence for inhibition of gelatinase catalytic activity by anacardic acid, providing a novel template for drug discovery and a molecular mechanism potentially involved in CNSL therapeutic action.
Introduction
Cashew nut shell liquid (CNSL), a by-product of processing the cashew (Anacardium occidentale), is a rich source of long-chain nonisoprenoid phenolics that have been used in traditional medicine, which includes use as an anesthetic in leprosy and psoriasis, promotion of wound healing, and treatment of conditions such as ulcers and tooth abscesses (Himejima and Kubo, 1991). The major constituent of CNSL is anacardic acid (alkenyl salicylic acid), present in a few forms, all containing a C15 alkenyl side chain but differing in the number of double bonds from zero to three (Paramashivappa et al., 2001), in addition to cardanols (3-alkenyl phenols) and cardols (5-alkenyl resorcinols). Anacardic acid is a phytochemical of interest because of its wide-ranging bioactivities that comprise microbicidal, insecticidal, and mulloscicidal properties (Gellerman et al., 1969; Mendes et al., 1990; Begum et al., 2002; Kubo et al., 2003).
The bactericidal properties of anacardic acid are more effective against Gram-positive bacteria, which include the medically relevant Streptococcus mutans, a causative agent in tooth decay, the acne-causing Propionibacterium acnes, the stomach ulcer-forming Helicobacter pylori, and the infectious methicillin-resistant Staphylococcus aureus (Muroi and Kubo, 1993, 1996; Kubo et al., 1994b, 1999). Anacardic acid also has a potent antioxidant effect (Trevisan et al., 2006) and, thus, is capable of protecting human cells from oxidative stress and providing a gastroprotective effect against ethanol-induced damage (Morais et al., 2010). Because of these antioxidant functions, anacardic acid has been proposed to be a useful chemoprotectant (Trevisan et al., 2006) and to have a role in skin care (Kubo et al., 2006).
Because of the interesting chemical properties of anacardic acid, studies are beginning to define its effects on distinct classes of enzymes. These include enzymatic inhibition to various degrees by anacardic acid on xanthine oxidase, tyrosinase, and lipoxygenase (Grazzini et al., 1991; Kubo et al., 1994a; Masuoka and Kubo, 2004; Ha and Kubo, 2005). Effects on the post-translational cellular machinery, in which anacardic acid mediates the activation of aurora kinase (Kishore et al., 2008), have also been observed, whereas it inhibits small ubiquitin-like modifier E1 (SUMO) ligase activity and thus perturbs protein SUMOylation (Fukuda et al., 2009). It is noteworthy that potential anticancer-related functions have been attributed to anacardic acid, including the inhibition of prostaglandin synthesis by cyclooxygenases (Grazzini et al., 1991), which are known to have roles in carcinogenesis (Langenbach et al., 1999; Arun and Goss, 2004). Other potential anticancer functions occur through the inhibition of estrogen receptor α DNA binding, diminishing both gene transcription and breast cancer cell proliferation (Schultz et al., 2010). Anacardic acid also inhibits the histone acetyltransferase (HAT) activity of transcription coactivators (Balasubramanyam et al., 2003). Furthermore, anacardic acid has been reported to suppress expression of nuclear factor-κB-regulated gene products that are involved in proliferation and in invasion, leading to potentiation of apoptosis (Sung et al., 2008).
However, despite these important recent advances, the key molecular mechanisms behind the traditional use of CNSL in wound healing and in treating several pathophysiological conditions, which are probably mediated by anacardic acid, have not been clearly defined. In this regard, possible protein targets for anacardic acid include the matrix metalloproteinases (MMPs), because this family of proteins is known to have critical roles in both extracellular matrix remodeling (Nagase and Woessner, 1999; Vu and Werb, 2000) and inflammatory responses, in addition to pathological conditions that include cancer metastasis (Stamenkovic, 2003). Hence, we focused our efforts on two MMPs, MMP-2 and MMP-9, the gelatinases secreted by cells that display highly impaired regulation and elevated protein levels in both chronic wounds and in certain tumors (Jezierska and Motyl, 2009). Our studies with 3T3-L1 mouse embryonic fibroblast cells clearly demonstrate that anacardic acid directly inhibits gelatinase enzymatic activity. Computational-based docking results indicate that anacardic acid readily binds to the MMP-2 or the MMP-9 active site. Our fluorescence studies reveal that anacardic acid inhibits peptide substrate cleavage by the MMP-2 catalytic core domain in a dose-dependent manner. Moreover, our combined fluorescence and gelatinase zymography studies agree with the docking-predicted binding mode of anacardic acid to MMP-2 or MMP-9, because similar compounds lacking key functional groups compared with anacardic acid such as aspirin, salicylic acid, and the cardol-cardanol mixture from cashew nut shell extract all inhibited gelatinase activity to a lesser extent. Thus, our results provide a novel molecular mechanism of action of anacardic acid, providing a new template for MMP-2/MMP-9 drug discovery and a potential link to the therapeutic functions of CNSL.
Materials and Methods
Materials.
3T3-L1 mouse fibroblast cells were obtained from American Type Culture Collection (Manassas, VA) through the National Center for Cell Sciences (Pune, Maharashtra, India). Saturated anacardic acid was obtained commercially from Calbiochem (San Diego, CA). Cell culture media and supplements and the other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Extraction of Anacardic Acid from Cashew Nut Shell Liquid.
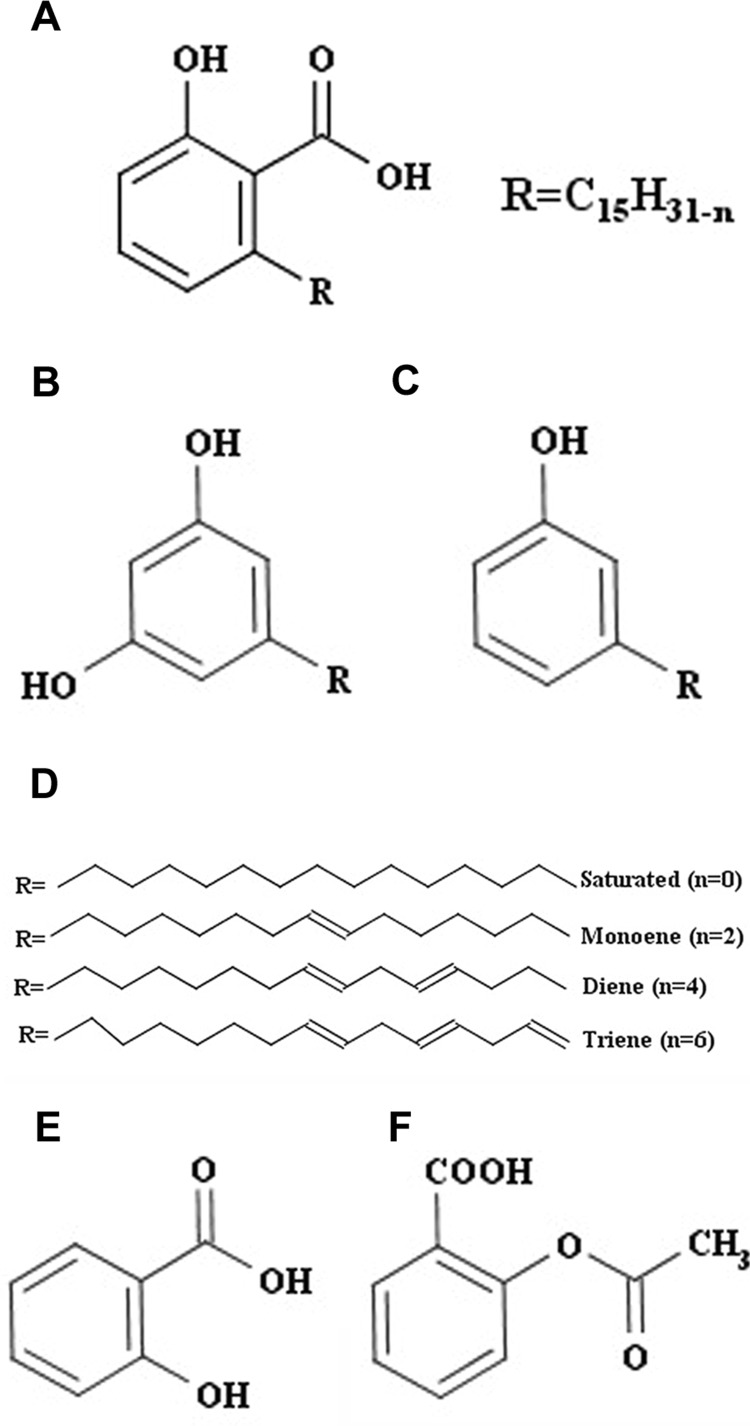
Locally available cashew shells from Kollam, Kerala, India, were crushed, and the cashew nut shell was subjected to extraction by shaking with petroleum ether (PE) in a rotary shaker for 24 h at ambient temperature. The solvent was removed by rotary evaporation below 40°C, obtaining a brown-colored oily residue henceforth referred to as cashew nut shell extract (CNSE). CNSE contains a mixture of at least three analogous compounds, anacardic acid (Fig. 1A), cardol (Fig. 1B), and cardanol (Fig. 1C), having C15 side chains that are either fully saturated or containing one, two, or three double bonds (Fig. 1D). Thin-layer chromatography analysis of this extract was conducted using a solvent system containing PE (70%), ethyl acetate (28%), and formic acid (2%) with visualization by spraying a mixture of 1) equal volumes of aqueous solution of ferric chloride (1%) and potassium ferricyanide (1%) and 2) methanolic ferric chloride (1%). Usually separation of individual components of CNSE is performed by precipitation of anacardic acid as calcium salt (Paramashivappa et al., 2001). This procedure is useful for large-scale separation. However, we found that anacardic acid could be conveniently separated from the other constituents by column chromatography on SiO2 and eluting with PE containing increasing proportions of chloroform, different from methods described previously (Paramashivappa et al., 2001). Anacardic acid (450 mg) was obtained from 2.0 g of PE extract from CNSE. The identity of anacardic acid was established by the following procedure: 1) HPLC, with a Shimadzu LC-20, a Phenomenex C-18 reverse-phase Luna column with a prominence diode array detector, a mobile phase of acetonitrile (72%), H2O (18%), and acetic acid (10%) and absorbance monitored at 245 nm, revealed that our anacardic acid extract contained 56.2% triene, 18.3% diene, and 24.2% monoene forms and 1.3% of the fully saturated C15 aliphatic chain (Supplemental Fig. 1). 2) The HPLC/mass spectrometry data were generated by an Agilent 1290 series ultra-high-performance liquid chromatograph coupled to an Agilent ion trap mass spectrometer (6340 series), with electrospray interface. The masses of the three major molecular peaks (MH+) corresponded to 343 m/e for the triene, 345 m/e for the diene, and 347 m/e for the monoene forms of anacardic acid (Supplemental Fig. 2). 3) 1H NMR spectra (Bruker AV II 500 spectrometer) were in agreement with those reported in the literature (Philip et al., 2008; Silva et al., 2008) (Supplemental Fig. 3). A 20 mg/ml (60 mM) solution of isolated anacardic acid mixture and cardol-cardanol extract was prepared in 100% dimethyl sulfoxide (DMSO), stored at −20°C, and then diluted as needed in cell culture medium. Reconstitution of all the stocks were completed in such a way that the working concentration of the DMSO was kept to 0.5% or lower.
Fig. 1.
Structure of anacardic acid and related compounds. The main components of CNSE are anacardic acid (A), cardol (B), and cardanol (C). Anacardic acid consists of a salicylic acid group, with a substituted alkyl chain of 15 carbon atoms, which occurs as saturated, monoene, diene, and triene (n indicates the number of H atoms removed) (D). Acetylation of the hydroxyl group of salicylic acid (E) generates aspirin (F).
Cell Culture.
3T3-L1 mouse fibroblast cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (v/v), 1% penicillin, 1% streptomycin, and 0.1% amphotericin B.
Cytotoxicity Assay.
With use of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), cytotoxicity assays were performed for cells treated with and without anacardic acid in serum-free DMEM. 3T3-L1 cells were seeded at a density of 7500 cells/well in a 96-well microtiter plate and incubated overnight. Cells were treated with and without anacardic acid (at a concentration range of 0.5–12.5 μM) in both serum-free and serum-containing DMEM for 24 h. Then 20 μl of 5 mg/ml MTT was added to each well and incubated for 3 h at 37°C. The media were removed after incubation and 150 μl of MTT solvent (4 mM HCl and 0.1% Nonidet P-40 in isopropanol) was added for solubilization. After shaking briefly for 5 min, the absorbance was read at 590 nm with a reference filter of 620 nm using a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT).
Gelatin Zymography.
The zymography assay (Ratnikov et al., 2002) used gelatin as a substrate for MMP-2 and MMP-9. Gelatin at a concentration of 0.1% was incorporated into 10% polyacrylamide gel containing 0.4% SDS. Electrophoresis under nonreducing conditions was performed using a Bio-Rad mini-gel system at 125 V for 90 to 120 min. After electrophoresis, the gels were washed twice for 30 min in 2.5% Triton X-100 (v/v) to remove the SDS and then incubated overnight in the developing buffer [50 mM Tris-HCl, pH 7.6, 200 mM NaCl, 5 mM CaCl2, 0.2% (v/v) Brij-35] at 37°C. Digestion bands were quantitated by Quantity One (Bio-Rad Laboratories, Hercules, CA).
Cellular Studies.
The 3T3-L1 cells seeded in a 12-well plate on reaching confluence, were washed twice with phosphate-buffered saline and then treated with or without quercetin (50 μg/ml), commercial anacardic acid (1 μg/ml), a mixture of cardol-cardanol (1 μg/ml), CNSE (1 μg/ml), and anacardic acid isolated from CNSE (1 μg/ml). After 24 h, the conditioned media were collected, centrifuged to avoid cellular debris, mixed with 4× sample buffer containing 62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 1% SDS, and 0.00625% (w/v) bromphenol blue, and then loaded for electrophoresis on a 10% SDS-polyacrylamide gel electrophoresis gel for zymography studies. All the experiments were performed in triplicate.
Conditioned Media Studies.
3T3-L1 cells were trypsinized and seeded on a 10-cm plate. After reaching confluence, cells were washed twice with phosphate-buffered saline and treated with serum-free DMEM and incubated at 37°C for 24 h. The media were collected after 24 h and centrifuged to avoid cell debris, and aliquots were stored at −20°C before subsequent experimentation. This was referred to as conditioned media. To study the dose-dependent inhibition of anacardic acid, the conditioned media aliquots were incubated with or without anacardic acid, at a concentration range of 10 to 100 μM for 1 h at 37°C. To ensure that the long-chain of anacardic acid plays an important role in MMP inhibition, conditioned media were treated with different concentrations (10–100 μM) of salicylic acid (SA), and aspirin (50 μM). To study the effect of anacardic acid on MMP-9 inhibition, conditioned media were treated with anacardic acid isolated from CNSE and SA (10–100 μM). The samples were mixed with 4× sample buffer containing 62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 1% SDS, and 0.00625% (w/v) bromphenol blue and loaded on a 10% SDS-polyacrylamide gel electrophoresis gel for zymography analysis. Control experiments confirming the presence of MMP-2 included the addition of 20 mM EDTA and 5 mM dithiothreitol to the incubation buffer and the sample buffer, respectively.
Purification of MMP-2 Catalytic Domain.
The MMP-2 catalytic domain (amino acid sequence as in PDB code 1QIB, residues 88–250), was cloned into a plasmid (DNA 2.0). Protein expression was induced by 0.4 mM isopropyl β-d-thiogalactoside being added to the media of Escherichia coli BL21 (DE3) cells grown at 37°C at OD600 nm of 0.5, and cells were grown for additional 5 h. The expressed MMP-2 protein was then purified as described previously (Dhanaraj et al., 1999).
Fluorescence Assay.
A fluorogenic substrate, Mca-Pro-Leu-Ala-Nva-Dap (Dnp)-Ala-Arg-NH2 (Enzo Life Sciences, Inc., Farmingdale, NY), was used for fluorescence studies (Knight et al., 1992), and 30 μM substrate and 20 ng of purified MMP-2 catalytic domain were used for the assay. The working concentration of substrate, enzyme, anacardic acid, cardol-cardanol, and CNSE was prepared in assay buffer (50 mM HEPES, 10 mM CaCl2, and 0.05% Brij-35, pH 7.5). The MMP-2 catalytic domain was incubated with anacardic acid for 30 min before the fluorescent substrate was added. The experiment was performed in a 96-well black plate, and the plate was read at excitation/emission of 360/460 nm (using a Synergy HT Multi-Mode Microplate Reader) 10 min after the substrate was added. Percent inhibition was calculated using the following formula: Inhibition % = 1 − (F − Fmin)/(Fmax − Fmin) × 100, where Fmin is the negative control, which contains only the fluorescence-labeled ligand or fluorescence-labeled ligand and anacardic acid as required, and Fmax is the positive control, which is a mixture of fluorescence-labeled ligand and the enzyme.
Computational Docking.
The AutoDockTools (http://mgltools.scripps.edu) package was used to generate input files for the docking runs and ligand site characterization, using our previously defined approach (Perry et al., 2009). In brief, the size and characterization of the optimal ligand binding site of the catalytic MMP-2 domain (PDB code 1QIB) and the MMP-9 catalytic domain (PDB code 2OVX), were performed using AutoLigand (Harris et al., 2008). A grid box of 40 × 44 × 44 points on a 1-Å grid was used to enclose the entire MMP-2 catalytic domain structure to generate affinity maps for use by AutoLigand. For the MMP-9 catalytic domain, a grid box of 44 × 44 × 44 points on a 1-Å grid was used to generate affinity maps for use by AutoLigand. For docking studies, a grid box of 44 × 46 × 60 points on a 0.375-Å grid spacing, centered on the ligand-binding sites for MMP-2 or MMP-9 identified by AutoLigand, were used to generate affinity maps for use by AutoDock4 (Morris et al., 1998). Anacardic acid was docked to either MMP-2 or MMP-9 catalytic domains with a Lamarckian genetic algorithm starting with an initial population of 500 randomly positioned inputs because of the large number of active torsions. The maximum number of energy evaluations was set to 2.5 × 107 and used a mutation rate of 0.02 with a crossover rate of 0.8. Results were clustered at 2.0-Å root mean square deviation.
Statistical Analysis.
Statistical analysis was conducted using Prism (GraphPad Software Inc., San Diego, CA). Statistical comparisons were performed using Student's t test and one-way analysis of variance followed by Dunnett's or Tukey's test. A value of P < 0.05 was considered significant. All values are expressed as the mean ± S.E.M. of triplicate determinations from three independent experiments.
Results
Regulation of MMP-2 Activity by Components of CNSE.
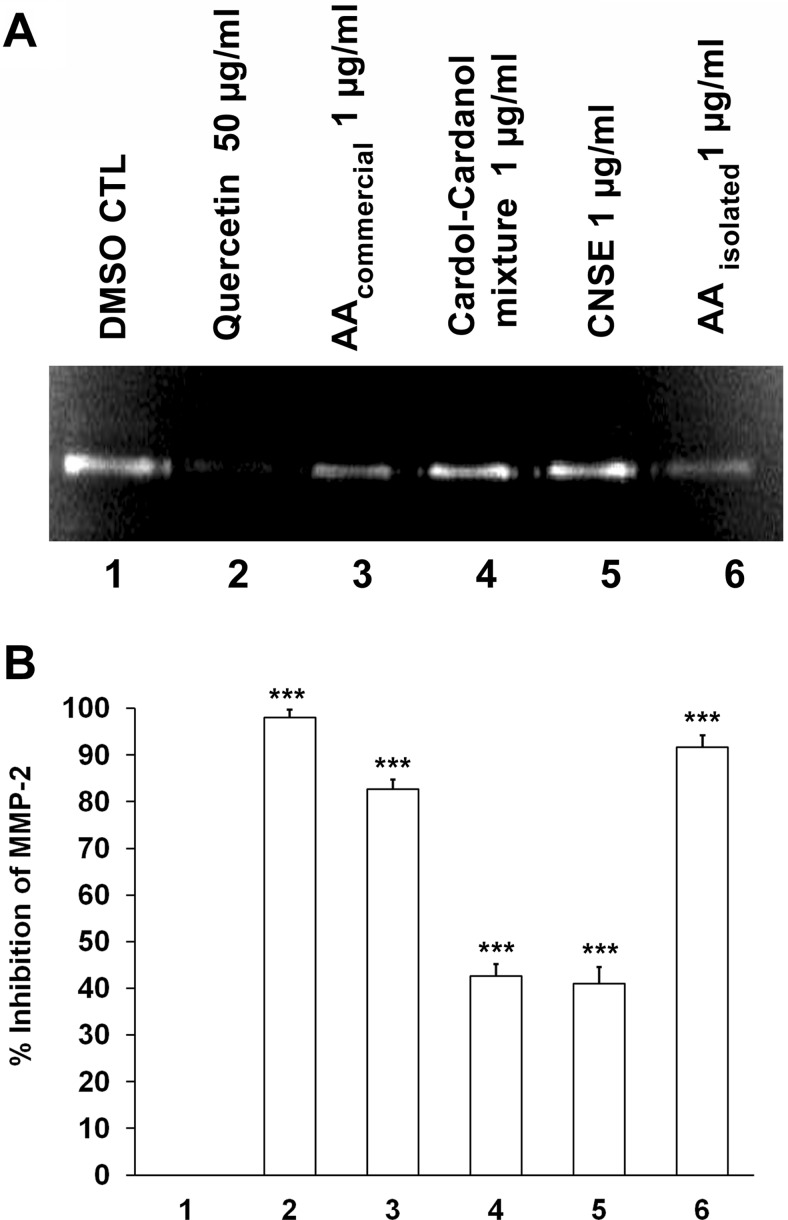
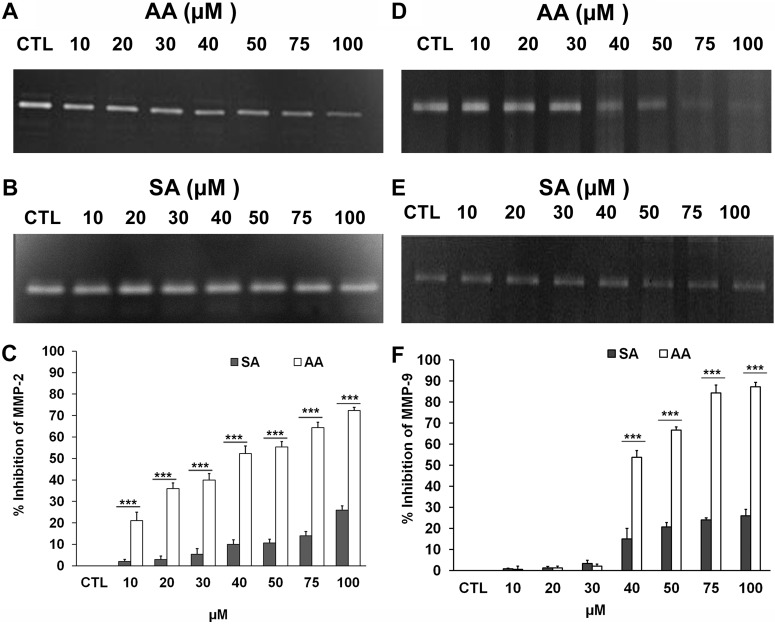
To study the regulation of MMP-2 by the major constituents of CNSE, we incubated 3T3-L1 cells in serum-free DMEM with CNSE extract, the extracted mixture of cardol-cardanol, extracted anacardic acid (AA), and commercially available anacardic acid containing a saturated C15 chain as a control. Quercetin, which is known to inhibit the activity of MMP-2 and MMP-9 in cells (Vijayababu et al., 2006) was also used as an additional control. The gelatin zymography studies (Fig. 2, A and B) determined that the CNSE extract inhibits cellular gelatinase activity and that the anacardic acid component of CNSE is the most active compound in this regard. Both anacardic acid isolated from CNSE and the commercially available compound significantly inhibited secreted MMP-2 gelatinase activity at a concentration of 1 μg/ml (3 μM). These values are significantly greater than the inhibition observed on incubation with 1 μg/ml CNSE extract containing a mixture of anacardic acid or cardol-cardanol or the inhibition by 1 μg/ml cardol-cardanol mixture.
Fig. 2.
Regulation of MMP-2 activity by components of CNSE. A, zymogram showing MMP-2 activity of conditioned media from 3T3-L1 fibroblast cells treated with 0.5% DMSO (lane 1), quercetin at 50 μg/ml (lane 2), commercial anacardic acid (AA) (Calbiochem) (lane 3), the cardol-cardanol mixture (lane 4), CNSE (lane 5), and anacardic acid isolated form CNSE (lane 6), each at 1 μg/ml. B, a representative plot of percentage inhibition observed in the zymogram. Each bar represents the mean ± S.E. of triplicate determinations from three independent experiments. ***, P < 0.001 (one-way analysis of variance with Dunnett's multiple-comparison post-test). CTL, control.
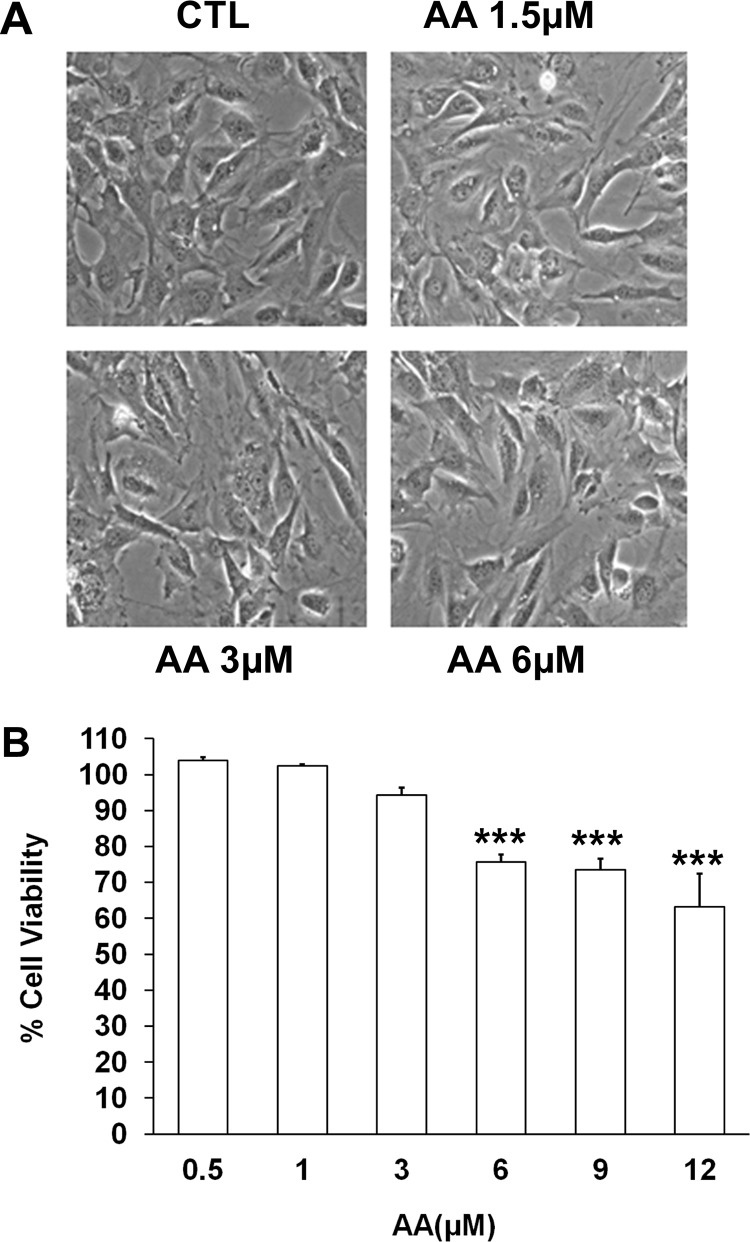
Determining Anacardic Acid Cytotoxicity in 3T3-L1 Cells.
To confirm that the observation of reduced MMP-2 gelatinase activity was not due to cytotoxicity, we conducted studies to determine the concentration at which anacardic acid is toxic to the cells by both analyzing morphological changes upon incubating cells with anacardic acid and by using the classic MTT cell viability assay (Mosmann, 1983). Of importance, we observed that there is no visible morphological alteration of 3T3-L1 cells when treated with 1.5, 3, or 6 μM anacardic acid for 24 h (Fig. 3A) compared with control 3T3-L1 cells that were not exposed to anacardic acid. Results of our MTT analysis (Fig. 3B) showed that no significant cytotoxic effects occur in cells that are incubated with anacardic acid in serum-free DMEM at the 3 μM concentration used in the previous assay. An increase in cytotoxicity is observed in concentrations of anacardic acid that are notably higher than that used in our assay, and the observed cytotoxicity at these concentrations is mitigated when cells are incubated in serum-containing media (Supplemental Fig. 4).
Fig. 3.
Effect of anacardic acid isolated from CNSE on 3T3-L1 cells. A, morphological examination of confluent cells treated with and without anacardic acid (AA) isolated from CNSE (at a concentration range of 1.5 to 6 μM) in serum-free DMEM after 24 h. B, cell viability using a MTT cytotoxicity assay was performed for the cells treated with anacardic acid isolated from CNSE (at a concentration range of 0.5 to 12 μM) in serum-free DMEM. Each bar represents the mean ± S.E. of triplicate determinations from three independent experiments. ***, P < 0.001 (one-way analysis of variance with Dunnett's multiple-comparison post-test).
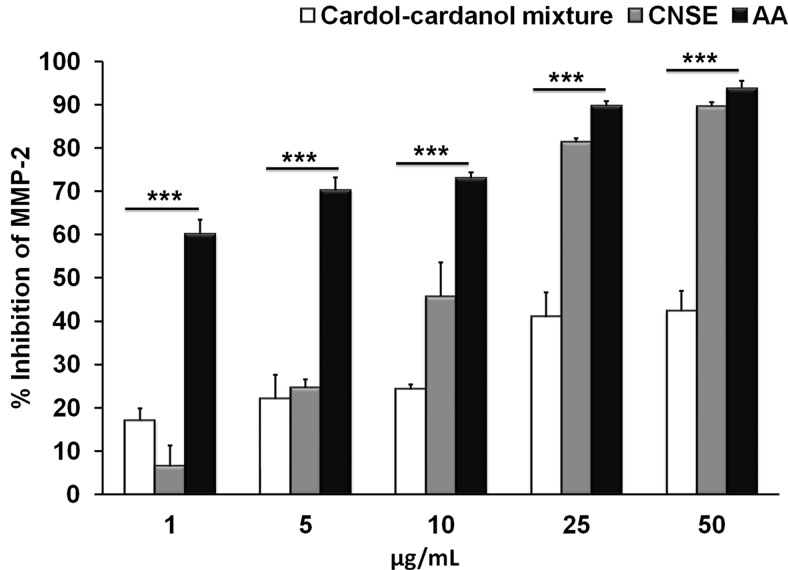
Fluorescence-Based Studies on MMP-2 with CNSE Components.
The relative contribution of MMP-2 inhibitory activities of CNSE, the cardol-cardanol mixture, and anacardic acid were compared by conducting fluorescence-based studies with a direct assay of the MMP-2 activity using the fluorogenic substrate Mca-Pro-Leu-Ala-Nva-Dap (Dnp)-Ala-Arg-NH2. Controls for these studies (Supplemental Fig. 5) show a distinct 6-fold increase in MMP-2 activity (Supplemental Fig. 5B) over basal (Supplemental Fig. 5A) upon incubation of the fluorogenic substrate with the purified recombinant catalytic core domain of MMP-2. We observed that anacardic acid significantly inhibits MMP-2 activity over the entire concentration range tested (1–50 μg/ml). In addition, as a control, the commercially available saturated anacardic acid (Calbiochem, San Diego, CA) demonstrated a concentration-dependent increase in the percentage inhibition of MMP-2 activity, which is very similar to that of CNSE-purified anacardic acid (Supplemental Fig. 6). The cardol-cardanol mixture was inhibitory to a lesser extent, even at the highest concentration (50 μg/ml) (Fig. 4). Upon comparing the structures of cardol (Fig. 1B), cardanol (Fig. 1C), and anacardic acid (Fig. 1A), the major difference in these structures is the presence of the COOH group in anacardic acid, which is absent in both cardol and cardanol, indicating that this group plays a crucial role in the inhibition of MMP-2 activity.
Fig. 4.
Fluorescence-based studies on MMP-2 inhibition by CNSE components. Plot show percentage inhibition of the MMP-2 catalytic core domain in the presence of different concentrations of CNSE, the cardol-cardanol mixture, and anacardic acid isolated from CNSE (at a concentration range of 1 to 50 μg/ml). ***P < 0.001 (one-way analysis of variance).
In Silico Docking Studies.
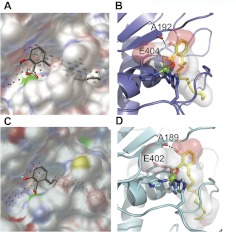
The observed cellular inhibition of gelatinase activity by anacardic acid could be mediated through either perturbation of catalytic activity, inhibition of secretion, or more indirect effects, such as inhibition of transcription or stabilization of the inactive proform of each enzyme. Hence, we used in silico docking methods to help define whether anacardic acid was capable of directly inhibiting catalytic activity, through binding of the active site of MMP-2 and of MMP-9. AutoDock has been defined previously as the most reliable method for studying MMP-inhibitor complexes, in a comparative study of fully automated docking programs (Hanessian et al., 2001). Therefore, we used AutoDock-based molecular docking to characterize the binding site and to predict the binding mode of anacardic acid to previously determined MMP-2 and MMP-9 catalytic domain structures (Dhanaraj et al., 1999; Tochowicz et al., 2007). First, the AutoLigand code (Harris et al., 2008) was used to characterize the ligand-binding sites. AutoLigand works by finding a set of contiguous points that make up the best total affinity for a given volume. By searching the space encompassing the entire target protein, the code was used to find the optimal binding site based on total energy per volume. The AutoLigand results (Fig. 5) indicate that the optimal binding site in both MMP-2 and in MMP-9 contains their zinc catalytic centers and their S1′ pockets, which in both gelatinases constitutes an aliphatic tunnel with a few hydrogen bond acceptor sites. In MMP-2 the optimal result was a fill of 100 points with a total volume of 295 Å3 and a total energy per volume of −0.195 kcal/mol Å3. In MMP-9 the optimal result was a fill of 100 points with a total volume of 284 Å3 and a total energy per volume of −0.219 kcal/mol Å3. Next, anacardic acid was docked to the MMP-2 and the MMP-9 AutoLigand target sites, using AutoDock4 (Morris et al., 1998). Because of the large number of free rotations in the C15 aliphatic segment of anacardic acid, AutoDock produced a large number of binding poses. Strikingly, however, in MMP-2, 94 of the 100 dockings placed the head group in the aliphatic pocket, with the carboxylate group functioning as a zinc-binding group and forming a hydrogen bond to the active site Glu404 side chain that functions in hydrolyzing peptide substrate (Fig. 5, A and B). The hydroxyl group of anacardic acid also forms a hydrogen bond to backbone oxygen of Ala192. It is noteworthy that the extensive, lipophilic C15 chain binds in the aliphatic S1′ tunnel. The reasonable docking energies ranged from −7.5 to −9.6 kcal/mol with 37 of 100 in the −9.6 cluster (Supplemental Video). Likewise, for MMP-9, anacardic acid bound in 71 of the 100 dockings with the head group in the aliphatic pocket and the C15 chain in the aliphatic S1′ tunnel (Fig. 5, C and D), with the largest cluster of 28 of 100 having a docking energy of −10.6 kcal/mol. The anacardic acid carboxylate group also functions as a zinc-binding group in MMP-9 and forms a hydrogen bond to the Glu402 side chain. The hydroxyl group of anacardic acid forms a hydrogen bond to backbone oxygen of Ala189 in MMP-9. It is noteworthy that the docked anacardic acid and the AutoLigand fill volume for MMP-2 and MMP-9 are consistent with previous structurally defined inhibitors bound to MMPs, such as the batimastat ligand in the MMP-2 crystal structure (Dhanaraj et al., 1999).
Fig. 5.
Predicted binding mode of anacardic acid to gelatinase. A, surface diagram of the docked structure of anacardic acid into the MMP-2 active site, with the AutoLigand fill points on a 1-Å grid spacing. The gray points represent optimal locations for carbon atoms, and the red points represent optimal locations for hydrogen bond acceptor atoms. B, ribbon structure of MMP-2 with the docked structure of anacardic acid shown in sticks. The large green sphere is the coordinated zinc atom and the transparent surface surrounding anacardic acid represents the AutoLigand optimized binding pocket. Hydrogen bonding is depicted as black dashed lines. C, surface diagram of docked structure of anacardic acid into the MMP-9 active site. D, ribbon structure of MMP-9 with docked structure of anacardic acid shown in sticks. Surface diagram figures were generated using PMV (http://mgltools.scripps.edu); ribbon diagrams were generated by PyMOL (http://www.pymol.org/).
Anacardic Acid Inhibits the Catalytic Activity of MMP-2 and MMP-9.
To confirm and further characterize the in silico results suggesting a direct inhibition of gelatinase activity by anacardic acid, conditioned media containing the secreted MMP-2 enzyme was obtained from 3T3-L1 cells that were not previously treated with anacardic acid. The presence of gelatinolytic activity in our zymography studies corresponding to MMP-2 (Supplemental Fig. 7A), loss of this activity as a result of a reduction of the disulfide bond in the presence of 5 mM dithiothreitol (Supplemental Fig. 7B), and a loss of activity in the presence of 20 mM EDTA (Supplemental Fig. 7C), most likely resulting from the chelation of the essential catalytic Zn+2 ion, confirm the presence of MMP-2 in the conditioned media. Anacardic acid exhibited a clear dose-dependent inhibition of secreted MMP-2 gelatinolytic activity in the conditioned media (Fig. 6A). Salicylic acid contains the same head group of anacardic acid but lacks the C15 aliphatic chain, indicated to be important in MMP-2/MMP-9 binding as inferred from our in silico studies. Compared with anacardic acid, treatment with salicylic acid results in significantly lower levels of inhibition throughout the concentration range tested (Fig. 6B). We observed that although anacardic acid at a concentration of 100 μM inhibited MMP-2 activity at 72%, salicylic acid at the same concentration exhibited merely 26% inhibition (Fig. 6C). Acetylation of the hydroxyl group of salicylic acid generates aspirin (acetylsalicyclic acid). Our docking studies predict that this hydroxyl group in anacardic acid has a role in binding to the MMP-2 active site and that acetylation of this group is likely to produce a steric hindrance in the binding. In addition, aspirin also lacks the long C15 side chain that is predicted to bind within the S1′ tunnel. In concurrence with these observations, our studies demonstrated that aspirin at 50 μM had no significant inhibitory effect on MMP-2 (Supplemental Fig. 8). It is noteworthy that the active site of the related MMP-9 is structurally highly conserved with MMP-2, and, in keeping with this conservation, we also clearly observed by gelatin zymography that anacardic acid significantly inhibits MMP-9 activity from the conditioned media (Fig. 6D). As observed in the case of MMP-2, salicylic acid was not found to inhibit MMP-9 as effectively as anacardic acid (Fig. 6, E and F).
Fig. 6.
Effect of different modulators on MMP-2 and MMP-9 enzymatic activity. Zymogram showing MMP-2 activity of conditioned media from 3T3-L1 cells (not previously exposed to either anacardic acid or SA. A, treated with 0.5% DMSO (CTL) (lane 1) and 10, 20, 30, 40, 50, 75, and 100 μM anacardic acid (AA) isolated from CNSE (lanes 2–8, respectively). B, treated with 0.5% DMSO (lane 1) and 10, 20, 30, 40, 50, 75, and 100 μM salicylic acid (lanes 2–8, respectively). C, representative plot of the zymogram showing percentage MMP-2 inhibition at different concentrations of anacardic acid and salicylic acid. Zymogram showing MMP-9 activity of conditioned media from 3T3-L1 cells (not previously exposed to anacardic acid and salicylic acid). Each bar represents the mean ± S.E. of triplicate determinations from three independent experiments. ***, P < 0.001 (Student's t test). D, treated with 0.5% DMSO (lane 1) and 10, 20, 30, 40, 50, 75, and 100 μM anacardic acid isolated from CNSE (lanes 2–8, respectively). E, treated with 0.5% DMSO (lane 1) and 10, 20, 30, 40, 50, 75, and 100 μM salicylic acid (lanes 2–8, respectively). F, representative plot of the zymogram showing percentage MMP+ inhibition by anacardic acid and salicylic acid. Each bar represents the mean ± S.E. of triplicate determinations from three independent experiments. ***, P < 0.001 (Student's t test).
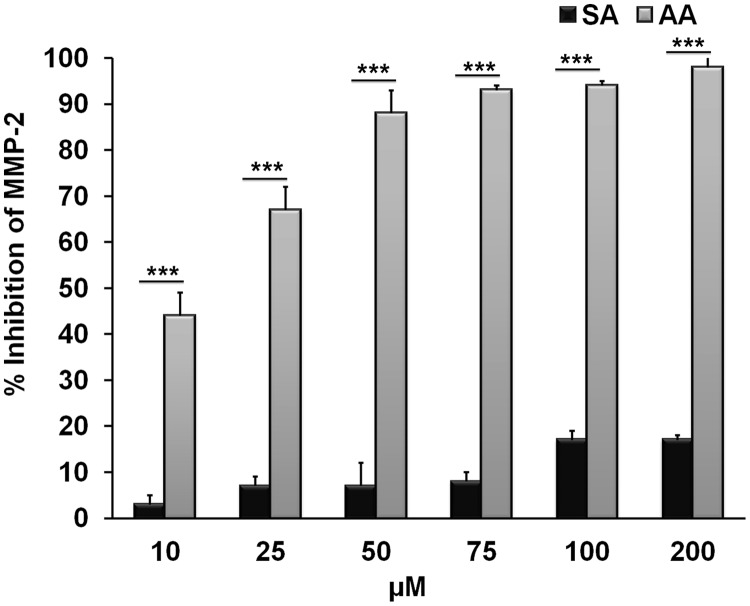
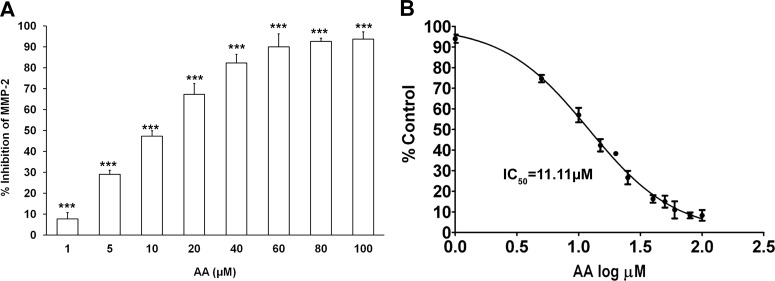
These results were further confirmed with the fluorescence-based assay using the catalytic core domain of MMP-2 and comparing the inhibitory activities of anacardic acid isolated from CNSE and salicylic acid. Whereas anacardic acid significantly inhibited (98%) MMP-2 activity, salicylic acid exhibited only 17% inhibition even at the highest concentration tested (200 μM) (Fig. 7), underscoring the importance of the aliphatic side chain of anacardic acid in binding and inhibition of gelatinolytic activity. Of interest, the extent of inhibition demonstrated by commercially available saturated anacardic acid (Calbiochem) was similar to that from CNSE, indicating that the partial unsaturation of the C15 chain is not critical to the inhibitory activity (Supplemental Fig. 6). In addition, to further establish the role of the carboxylic group in the inhibition of MMP-2 and MMP-9 by anacardic acid, we isolated, purified, and characterized cardanol from CNSE (Supplemental Fig. 9, A–C). Cardanol is also identical to anacardic acid, but the one important difference is that it lacks the carboxylate group. Cardanol also shows minimal inhibition of MMP-2 catalytic activity compared with anacardic acid under the same conditions, thus supporting our in silico docking studies, which emphasizes the importance of the carboxylate group of anacardic acid in binding the zinc present in the catalytic site of MMP-2 (Supplemental Fig. 10). Finally, we further characterized this direct inhibition of the MMP-2 catalytic core domain by CNSE-isolated anacardic acid, using the fluorogenic substrate described earlier. We observed that anacardic acid exhibits a distinct dose-dependent inhibition of the MMP-2 activity with an IC50 of 11.11 μM (Fig. 8, A and B).
Fig. 7.
Comparison of MMP-2 inhibition by anacardic acid or salicylic acid using fluorescence-based studies. Plot showing percentage inhibition of the MMP-2 catalytic core domain in the presence of different concentrations of anacardic acid isolated from CNSE or salicylic acid (10–200 μM). ***, P < 0.001 (Student's t test).
Fig. 8.
Dose response of MMP-2 inhibition by anacardic acid (AA). A, plot showing percentage inhibition of the MMP-2 catalytic core domain in the presence of different concentrations of anacardic acid isolated from CNSE (1–100 μM). ***, P < 0.001 (one-way analysis of variance with Dunnett's multiple-comparison post-test). B, dose-response curve and determination of IC50 of anacardic acid-mediated inhibition of MMP-2 activity. Each bar represents the mean ± S.E. of triplicate determinations from three independent experiments.
Discussion
Selective inhibition of MMPs could have substantial benefits in treating a number of disease states and in promoting wound healing. However, despite major efforts toward development of MMP inhibitors, only doxycycline (Periostat), a tetracycline used for treating periodontal disease, and glucosamine sulfate, used for treating osteoarthritis, are commercially available. Natural products form one source of potential MMP inhibitors and, interestingly, CNSL has been noted in traditional medicine for its use in promoting wound healing. In addition, the major constituent of CNSL is anacardic acid, and some indirect links for anacardic acid modulating MMP function have been observed previously. Anacardic acid can down-regulate the expression of MMP-1 through inhibition of p300 HAT activity (Kim et al., 2009) and can also reduce expression of MMP-9 through the effects on nuclear factor-κB-regulated gene products (Sung et al., 2008). Therefore, we analyzed whether anacardic acid and the other major extracted components of CNSE could have any direct effects on two secreted gelatinases, MMP-2 and MMP-9, which are known to play key roles in several pathological conditions. Indeed, CNSE components inhibit gelatinase activity of 3T3-L1 cells, with the anacardic acid component having the greatest effect.
Regulation of MMP activity can occur at different levels including transcriptional control, altered processing of the inactive zymogenic form, or catalytic inhibition by a group of tissue inhibitors of matrix metalloproteinases that interact with the MMP active site. Our computational docking analyses suggested that MMP-2 inhibition occurs through anacardic acid directly binding to the MMP-2 active site, involving interactions similar to those observed during structure-based inhibitor design studies on the MMPs. Because carboxylate groups are the second most common zinc-binding group in MMP inhibitors developed so far (reviewed in Lia et al., 2009), it is noteworthy that the interaction of anacardic acid with MMP-2 also probably involves its carboxylate group functioning as a zinc-binding moiety, whereas cardanol, which lacks the carboxylate group shows minimal inhibition compared with anacardic acid. Different members of the MMP family all contain a similarly folded enzymatic domain that uses a zinc ion for catalysis. Thus, in case of both natural substrates and newly developed MMP inhibitors, the nearby sites, and, in particular, the S1′ pocket, are observed to form their basis for substrate selectivity (reviewed in Maskos and Bode, 2003). Our docking studies suggest that the large C15 aliphatic chain of anacardic acid readily binds into the relatively deep S1′ pocket of MMP-2 and MMP-9 (Lovejoy et al., 1999) used in recognition of gelatin substrate. It is conceivable that anacardic acid may preferentially inhibit MMPs that have a deeper S1′ pocket compared with MMPs with a shallow pocket. Therefore, the potential use of anacardic acid as a natural “bio-drug” suggests that it now joins a small list of previously defined natural product compounds that have interesting activities against MMPs (Mannello, 2006; reviewed in Lia et al., 2009). This list includes the long-chain fatty acid molecules, such as oleic acid and elaidic acid, which are micromolar inhibitors of MMP-2 (Berton et al., 2001). Of interest, our docking results indicate a molecular mechanism for these fatty acid compounds, in which their carboxylate groups bind to the active site zinc ion and their fatty acid chains are incorporated into the large S1′ site pocket of MMP-2 and MMP-9.
These docking results agreed with our gelatin zymography studies performed on these two secreted gelatinases present in conditioned media that were isolated from 3T3-L1 cells, which had not previously been exposed to any CNSE components. Inhibition of both MMP-2 and MMP-9 occurred in a dose-dependent manner, suggesting a direct interaction of anacardic acid with the catalytic activity of the enzyme. We also analyzed the effects of salicylic acid and aspirin, two compounds sharing similarities to anacardic acid in the ring structure but lacking a C15 chain, and, in the case of aspirin, an additional acetylation of the ring hydroxyl group. Salicylic acid shows significantly lower inhibition than anacardic acid, suggesting that the long C15 chain of anacardic acid plays an important role in gelatinase binding and inhibition. Furthermore, aspirin had no significant effect in inhibiting the activity of MMP-2 from conditioned media, which is most likely due to steric hindrance resulting from acetylation of the hydroxyl group that seems to play a key role in the binding of anacardic acid to MMP-2/MMP-9.
Anacardic acid is already being used as a template for initial drug discovery research against a number of interesting targets, including the use of derivatives of anacardic acid as inhibitors of glyceraldehyde-3-phosphate dehydrogenase from the Trypanosoma cruzi pathogen that causes Chagas disease (Pereira et al., 2008). Several studies have focused on analogs of anacardic acid being used as antibacterial agents against methicillin-resistant S. aureus (Green et al., 2007), against Mycobacterium smegmatis and Mycobacterium tuberculosis (Swamy et al., 2007), and against Streptococcus mutans (Green et al., 2008). Another approach is the use of anacardic acid analogs for targeting bacterial histidine protein kinase-mediated two-component regulatory systems (Kanojia et al., 1999). In addition, analogs of anacardic acid are being developed for HAT inhibition (Eliseeva et al., 2007), which could provide a new avenue for the treatment of cancer. It is noteworthy that our analyses provide a novel molecular mechanism for anacardic acid that involves inhibition of MMP-2 and MMP-9 function. Our fluorescence-based study on the recombinant MMP-2 catalytic core domain clearly demonstrated that anacardic acid directly inhibits the catalytic activity in a dose-dependent manner with an IC50 of 11.11 μM. This result suggests that anacardic acid could be used as a novel template for design and synthesis of analogs that have drug-like properties and improved binding characteristics to selectively inhibit the gelatinases MMP-2 and MMP-9. This finding is significant because MMP-2 and MMP-9 are most strongly correlated to metastatic potential, with metastatic tumor cell lines expressing higher levels of these MMPs than nonmetastatic varieties (Liotta et al., 1980). In addition, the MMP-2 gene was observed to be one of the key genes mediating aggressive metastasis of breast cancer to the lungs (Minn et al., 2005). Furthermore, MMP-2 and MMP-9 are produced by the nonmalignant cells present in a tumor (Wilhelm et al., 1989) and have key roles in angiogenesis (Brooks et al., 1996). Thus, the development of new gelatinase inhibitors is likely to be of importance in combating a wide range of diseases such as arthritis, cancer, and inflammatory disease states including chronic wounds, the latter being of particular relevance to individuals with diabetes. In conclusion, these studies provide a molecular basis for the regulation of MMP-2 and MMP-9 by anacardic acid and give a strong impetus for the natural products drug discovery paradigm. Furthermore, these studies also provide the basis for exploring cost-effective, novel therapeutic applications for CNSL components and future synthetic derivatives.
Supplementary Material
Acknowledgments
We thank Dr. Anna Travesa, Dr. Rajesh K. Grover (Scripps), and Dr. Ayyappan Ramesh Nair (Amrita) for critical reading of the article, and Dr. Walter Schrenk (Amrita) for helping with the mass spectrometry analysis. We acknowledge that the original idea to pursue this approach of natural product lead identification from cashew nut shell oil came through regular discussions with and constant guidance from Mata Amritanandamayi Devi, Chancellor, Amrita Vishwa Vidyapeetham University.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported in part by Amrita University Research, the National Institutes of Health National Cancer Institute [Grant CA92584]; the National Institutes of Heath National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant AR059968]; and the Council of Scientific and Industrial Research and University Grants Commission (junior research fellowships to A.O. and J.N., respectively).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- CNSL
- cashew nut shell liquid
- HAT
- histone acetyltransferase
- MMP
- matrix metalloproteinase
- PE
- petroleum ether
- CNSE
- cashew nut shell extract
- HPLC
- high-performance liquid chromatography
- DMSO
- dimethyl sulfoxide
- DMEM
- Dulbecco's modified Eagle's medium
- MTT
- 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- SA
- salicylic acid
- PDB
- Protein Data Bank
- Mca
- (7-methoxycoumarinyl) a cetyl
- Dap
- [3(2-dinitrophenyl 2,3-diaminopropionyl].
Authorship Contributions
Participated in research design: Omanakuttan, Kumar, Tainer, Perry, and Nair.
Conducted experiments: Omanakuttan, Nambiar, Varghese, and Banerji.
Contributed new reagents or analytic tools: Bose, Pandurangan, Banerji, and Perry.
Performed data analysis: Omanakuttan, Nambiar, Harris, Kumar, Banerji, Perry, and Nair.
Wrote or contributed to the writing of the manuscript: Omanakuttan, Nambiar, Kumar, Perry, and Nair.
References
- Arun B, Goss P. (2004) The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol 31:22–29 [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. (2003) Small molecule modulators of histone acetyltransferase p300. J Biol Chem 278:19134–19140 [DOI] [PubMed] [Google Scholar]
- Begum P, Hashidoko Y, Islam MT, Ogawa Y, Tahara S. (2002) Zoosporicidal activities of anacardic acids against Aphanomyces cochlioides. Z Naturforsch C 57:874–882 [DOI] [PubMed] [Google Scholar]
- Berton A, Rigot V, Huet E, Decarme M, Eeckhout Y, Patthy L, Godeau G, Hornebeck W, Bellon G, Emonard H. (2001) Involvement of fibronectin type II repeats in the efficient inhibition of gelatinases A and B by long-chain unsaturated fatty acids. J Biol Chem 276:20458–20465 [DOI] [PubMed] [Google Scholar]
- Brooks PC, Strömblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 85:683–693 [DOI] [PubMed] [Google Scholar]
- Dhanaraj V, Williams MG, Ye QZ, Molina F, Johnson LL, Ortwine DF, Pavlovsky A, Rubin JR, Skeean RW, White AD, et al. (1999) X-ray structure of gelatinase A catalytic domain complexed with a hydroxamate inhibitor. Croat Chem Acta 72:zpg575–591 [Google Scholar]
- Eliseeva ED, Valkov V, Jung M, Jung MO. (2007) Characterization of novel inhibitors of histone acetyltransferases. Mol Cancer Ther 6:2391–2398 [DOI] [PubMed] [Google Scholar]
- Fukuda I, Ito A, Hirai G, Nishimura S, Kawasaki H, Saitoh H, Kimura K, Sodeoka M, Yoshida M. (2009) Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem Biol 16:133–140 [DOI] [PubMed] [Google Scholar]
- Gellerman JL, Walsh NJ, Werner NK, Schlenk H. (1969) Antimicrobial effects of anacardiac acids. Can J Microbiol 15:1219–1223 [DOI] [PubMed] [Google Scholar]
- Grazzini R, Hesk D, Heininger E, Hildenbrandt G, Reddy CC, Cox-Foster D, Medford J, Craig R, Mumma RO. (1991) Inhibition of lipoxygenase and prostaglandin endoperoxide synthase by anacardic acids. Biochem Biophys Res Commun 176:775–780 [DOI] [PubMed] [Google Scholar]
- Green IR, Tocoli FE, Lee SH, Nihei K, Kubo I. (2007) Molecular design of anti-MRSA agents based on the anacardic acid scaffold. Bioorg Med Chem 15:6236–6241 [DOI] [PubMed] [Google Scholar]
- Green IR, Tocoli FE, Lee SH, Nihei K, Kubo I. (2008) Design and evaluation of anacardic acid derivatives as anticavity agents. Eur J Med Chem 43:1315–1320 [DOI] [PubMed] [Google Scholar]
- Ha TJ, Kubo I. (2005) Lipoxygenase inhibitory activity of anacardic acids. J Agric Food Chem 53:4350–4354 [DOI] [PubMed] [Google Scholar]
- Hanessian S, Moitessier N, Therrien E. (2001) A comparative docking study and the design of potentially selective MMP inhibitors. J Comput Aided Mol Des 15:873–881 [DOI] [PubMed] [Google Scholar]
- Harris R, Olson AJ, Goodsell DS. (2008) Automated prediction of ligand-binding sites in proteins. Proteins 70:1506–1517 [DOI] [PubMed] [Google Scholar]
- Himejima M, Kubo IJ. (1991) Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. Agric Food Chem 39:418–421 [Google Scholar]
- Jezierska A, Motyl T. (2009) Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit 15:RA32–RA40 [PubMed] [Google Scholar]
- Kanojia RM, Murray W, Bernstein J, Fernandez J, Foleno BD, Krause H, Lawrence L, Webb G, Barrett JF. (1999) 6-Oxa isosteres of anacardic acids as potent inhibitors of bacterial histidine protein kinase (HPK)-mediated two-component regulatory systems. Bioorg Med Chem Lett 9:2947–2952 [DOI] [PubMed] [Google Scholar]
- Kim MK, Shin JM, Eun HC, Chung JH. (2009) The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PLoS One 4:e4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore AH, Vedamurthy BM, Mantelingu K, Agrawal S, Reddy BA, Roy S, Rangappa KS, Kundu TK. (2008) Specific small-molecule activator of aurora kinase A induces autophosphorylation in a cell-free system. J Med Chem 51:792–797 [DOI] [PubMed] [Google Scholar]
- Knight CG, Willenbrock F, Murphy G. (1992) A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett 296:263–266 [DOI] [PubMed] [Google Scholar]
- Kubo I, Kinst-Hori I, Yokokawa Y. (1994a) Tyrosinase inhibitors from Anacardium occidentale fruits. J Nat Prod 57:545–551 [DOI] [PubMed] [Google Scholar]
- Kubo I, Masuoka N, Ha TJ, Tsujimoto K. (2006) Antioxidant activity of anacardic acids. Food Chem 99:555–562 [Google Scholar]
- Kubo I, Muroi H, Kubo A. (1994b) Naturally occurring antiacne agents. J Nat Prod 57:9–17 [DOI] [PubMed] [Google Scholar]
- Kubo I, Nihei K, Tsujimoto K. (2003) Antibacterial action of anacardic acids against methicillin resistant Staphylococcus aureus (MRSA). J Agric Food Chem 51:7624–7628 [DOI] [PubMed] [Google Scholar]
- Kubo J, Lee JR, Kubo I. (1999) Anti-Helicobacter pylori agents from the cashew apple. J Agric Food Chem 47:533–537 [DOI] [PubMed] [Google Scholar]
- Langenbach R, Loftin CD, Lee C, Tiano H. (1999) Cyclooxygenase-deficient mice. A summary of their characteristics and susceptibilities to inflammation and carcinogenesis. Ann NY Acad Sci 889:52–61 [DOI] [PubMed] [Google Scholar]
- Lia NG, Shib ZH, Tang YP, Duan JA. (2009) Selective matrix metalloproteinase inhibitors for cancer. Curr Med Chem 16:3805–3827 [DOI] [PubMed] [Google Scholar]
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284:67–68 [DOI] [PubMed] [Google Scholar]
- Lovejoy B, Welch AR, Carr S, Luong C, Broka C, Hendricks RT, Campbell JA, Walker KA, Martin R, Van Wart H, et al. (1999) Crystal structures of MMP-1 and -13 reveal the structural basis for selectivity of collagenase inhibitors. Nat Struct Biol 6:217–221 [DOI] [PubMed] [Google Scholar]
- Mannello F. (2006) Natural bio-drugs as matrix metalloproteinase inhibitors: new perspectives on the horizon? Recent Pat Anticancer Drug Discov 1:91–103 [DOI] [PubMed] [Google Scholar]
- Maskos K, Bode W. (2003) Structural basis of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Mol Biotechnol 25:241–266 [DOI] [PubMed] [Google Scholar]
- Masuoka N, Kubo I. (2004) Characterization of xanthine oxidase inhibition by anacardic acids. Biochim Biophys Acta 1688:245–249 [DOI] [PubMed] [Google Scholar]
- Mendes NM, de Oliveira AB, Guimarães JE, Pereira JP, Katz N. (1990) Molluscacide activity of a mixture of 6-n-alkyl salicylic acids (anacardic acid) and 2 of its complexes with copper (II) and lead (II). Rev Soc Bras Med Trop 23:217–224 [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. (2005) Genes that mediate breast cancer metastasis to lung. Nature 436:518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais TC, Pinto NB, Carvalho KM, Rios JB, Ricardo NM, Trevisan MT, Rao VS, Santos FA. (2010) Protective effect of anacardic acids from cashew (Anacardium occidentale) on ethanol-induced gastric damage in mice. Chem Biol Interact 183:264–269 [DOI] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662 [Google Scholar]
- Mosmann T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63 [DOI] [PubMed] [Google Scholar]
- Muroi H, Kubo I. (1993) Structure-antibacterial activity relationships of anacardic acids. J Agric Food Chem 41:1780–1783 [Google Scholar]
- Muroi H, Kubo I. (1996) Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus. J Appl Bacteriol 80:387–394 [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494 [DOI] [PubMed] [Google Scholar]
- Paramashivappa R, Kumar PP, Vithayathil PJ, Rao AS. (2001) Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale L.) nut shell liquid. J Agric Food Chem 49:2548–2551 [DOI] [PubMed] [Google Scholar]
- Pereira JM, Severino RP, Vieira PC, Fernandes JB, da Silva MF, Zottis A, Andricopulo AD, Oliva G, Corrêa AG. (2008) Anacardic acid derivatives as inhibitors of glyceraldehyde-3-phosphate dehydrogenase from Trypanosoma cruzi. Bioorg Med Chem 16:8889–8895 [DOI] [PubMed] [Google Scholar]
- Perry JJ, Harris RM, Moiani D, Olson AJ, Tainer JA. (2009) p38α MAP kinase C-terminal domain binding pocket characterized by crystallographic and computational analyses. J Mol Biol 391:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip JY, Da Cruz Francisco J, Dey ES, Buchweishaija J, Mkayula LL, Ye L. (2008) Isolation of anacardic acid from natural cashew nut shell liquid (CNSL) using supercritical carbon dioxide. J Agric Food Chem 56:9350–9354 [DOI] [PubMed] [Google Scholar]
- Ratnikov BI, Deryugina EI, Strongin AY. (2002) Gelatin zymography and substrate cleavage assays of matrix metalloproteinase-2 in breast carcinoma cells overexpressing membrane type-1 matrix metalloproteinase. Lab Invest 82:1583–1590 [DOI] [PubMed] [Google Scholar]
- Schultz DJ, Wickramasinghe NS, Ivanova MM, Isaacs SM, Dougherty SM, Imbert-Fernandez Y, Cunningham AR, Chen C, Klinge CM. (2010) Anacardic acid inhibits estrogen receptor α-DNA binding and reduces target gene transcription and breast cancer cell proliferation. Mol Cancer Ther 9:594–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MS, De Lima SG, Oliveira EH, Lopes JA, Chaves MH, Reis FA, Citó AM. (2008) Anacardic acid derivatives from Brazilian propolis and their antibacterial activity. Elect Quim 33:53–58 [Google Scholar]
- Stamenkovic I. (2003) Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200:448–464 [DOI] [PubMed] [Google Scholar]
- Sung B, Pandey MK, Ahn KS, Yi T, Chaturvedi MM, Liu M, Aggarwal BB. (2008) Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-κB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-κBα kinase, leading to potentiation of apoptosis. Blood 111:4880–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BN, Suma TK, Rao GV, Reddy GC. (2007) Synthesis of isonicotinoylhydrazones from anacardic acid and their in vitro activity against Mycobacterium smegmatis. Eur J Med Chem 42:420–424 [DOI] [PubMed] [Google Scholar]
- Tochowicz A, Maskos K, Huber R, Oltenfreiter R, Dive V, Yiotakis A, Zanda M, Pourmotabbed T, Bode W, Goettig P. (2007) Crystal structures of MMP-9 complexes with five inhibitors: contribution of the flexible Arg424 side-chain to selectivity. J Mol Biol 371:989–1006 [DOI] [PubMed] [Google Scholar]
- Trevisan MT, Pfundstein B, Haubner R, Würtele G, Spiegelhalder B, Bartsch H, Owen RW. (2006) Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity.. Food Chem Toxicol 44:188–197 [DOI] [PubMed] [Google Scholar]
- Vijayababu MR, Arunkumar A, Kanagaraj P, Venkataraman P, Krishnamoorthy G, Arunakaran J. (2006) Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol Cell Biochem 287:109–116 [DOI] [PubMed] [Google Scholar]
- Vu TH, Werb Z. (2000) Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 14:2123–2133 [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Collier IE, Marmer BL, Eisen AZ, Grant GA, Goldberg GI. (1989) SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem 264:17213–17221 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.