Abstract
Mutational modification of distinct muscarinic receptor subtypes has yielded novel designer G protein-coupled receptors (GPCRs) that are unable to bind acetylcholine (ACh), the endogenous muscarinic receptor ligand, but can be efficiently activated by clozapine-N-oxide (CNO), an otherwise pharmacologically inert compound. These CNO-sensitive designer GPCRs [alternative name: designer receptors exclusively activated by designer drug (DREADDs)] have emerged as powerful new tools to dissect the in vivo roles of distinct G protein signaling pathways in specific cell types or tissues. As is the case with other GPCRs, CNO-activated DREADDs not only couple to heterotrimeric G proteins but can also recruit proteins of the arrestin family (arrestin-2 and -3). Accumulating evidence suggests that arrestins can act as scaffolding proteins to promote signaling through G protein-independent signaling pathways. To explore the physiological relevance of these arrestin-dependent signaling pathways, the availability of an arrestin-biased DREADD would be highly desirable. In this study, we describe the development of an M3 muscarinic receptor-based DREADD [Rq(R165L)] that is no longer able to couple to G proteins but can recruit arrestins and promote extracellular signal-regulated kinase-1/2 phosphorylation in an arrestin- and CNO-dependent fashion. Moreover, CNO treatment of mouse insulinoma (MIN6) cells expressing the Rq(R165L) construct resulted in a robust, arrestin-dependent stimulation of insulin release, directly implicating arrestin signaling in the regulation of insulin secretion. This newly developed arrestin-biased DREADD represents an excellent novel tool to explore the physiological relevance of arrestin signaling pathways in distinct tissues and cell types.
Introduction
G protein-coupled receptors (GPCRs) represent a superfamily of cell surface receptors that regulate the activity of virtually all physiological functions. Characteristically, GPCRs are activated via binding of extracellular ligands, which trigger conformational changes that allow the receptors to interact with and activate specific classes of heterotrimeric G proteins. The activated GPCRs are rapidly phosphorylated by G protein-coupled receptor kinases (GRKs) (Pierce et al., 2002). In most cases, the phosphorylated receptors bind to members of the arrestin protein family (arrestin-2 and -3; also known as β-arrestin-1 and -2), a process that interferes with receptor/G protein coupling and promotes GPCR internalization by targeting the receptors to clathrin-coated pits (Pierce et al., 2002).
However, during the past decade, it has become increasingly clear that arrestin-2 and -3 can serve as adaptor proteins that transduce signals to multiple effector pathways (Rajagopal et al., 2010; Shukla et al., 2011). Although some progress has been made in delineating the physiological functions of arrestin signaling pathways in various tissues, much remains to be learned about the in vivo physiological relevance of arrestin-mediated signaling. Such information is not only of theoretical interest but also could be exploited for the development of novel classes of clinically useful drugs, including arrestin-biased agonists (Rajagopal et al., 2010; Shukla et al., 2011).
To facilitate studies aimed at elucidating the physiological roles of arrestin-2/-3-dependent signaling, we set out to develop a designer GPCR that can recruit arrestins after binding of an exogenous ligand but that is no longer able to activate heterotrimeric G proteins. During the past few years, clozapine-N-oxide (CNO)-sensitive designer GPCRs have emerged as highly useful tools to dissect the in vivo roles of distinct G protein signaling pathways in specific cell types or tissues (Armbruster et al., 2007; Alexander et al., 2009; Guettier et al., 2009). Structurally, these novel receptors [alternative names: designer receptors exclusively activated by designer drug (DREADDs) or second-generation receptors activated solely by synthetic ligand (RASSLs)] are muscarinic acetylcholine (ACh) receptors that contain two point mutations within the orthosteric binding pocket (Armbruster et al., 2007; Guettier et al., 2009). As a result of these mutations, these engineered receptors are unable to bind ACh, the endogenous muscarinic receptor ligand, but can be efficiently activated by CNO, an otherwise pharmacologically inert compound (Armbruster et al., 2007; Guettier et al., 2009). CNO stimulation of DREADDs leads to the activation of distinct classes of heterotrimeric G proteins as well as the recruitment of arrestin-2 and -3 (Alvarez-Curto et al., 2011; K. Nakajima and J. Wess, unpublished data), in a fashion similar to that observed with endogenous GPCRs.
In the present study, we describe the generation and functional characterization of a novel M3 muscarinic receptor-based DREADD containing a point mutation within the highly conserved DRY motif [Rq(R165L)] that lacks the ability to activate heterotrimeric G proteins (CNO treatment has no effect on the levels of conventional second messengers) but still retains the ability to recruit arrestin-2 and -3 in a CNO-dependent fashion. Rq(R165L)-dependent arrestin recruitment was functionally relevant, as demonstrated in extracellular signal-regulated kinase (ERK) 1/2 phosphorylation assays performed with cultured HEK293T cells. Moreover, we found that CNO treatment of Rq(R165L)-expressing MIN6 cells stimulated insulin release in an arrestin-dependent fashion. This latter finding strongly supports the notion that this newly developed arrestin-biased DREADD represents a powerful new tool to study the physiological relevance of arrestin-dependent signal cascades in distinct tissues and cell types.
Materials and Methods
Drugs and Plasmids.
CNO was obtained from the National Institutes of Health (Bethesda, MD) as part of the Rapid Access to Investigative Drug Program funded by the National Institute of Neurological Disorders and Stroke. ACh chloride, oxotremorine-M (OXO-M) ammonium iodide, atropine sulfate, and forskolin were from Sigma-Aldrich (St. Louis, MO). [3H]N-methylscopolamine ([3H]NMS; 79–83 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA).
The coding sequence of Rq, an M3 muscarinic receptor-based DREADD (Guettier et al., 2009), including an N-terminal hemagglutinin (HA) epitope tag, was amplified by polymerase chain reaction and inserted into the pcDNA3.1(−) vector (Invitrogen, Carlsbad, CA) using the EcoRI and BamHI sites present in the polylinker. To generate a mutant version of Rq that was unable to couple to G proteins, we generated the Rq(R165L) construct containing the R165L point mutation, using the QuikChange site-directed mutagenesis kit (Invitrogen). For bioluminescence resonance energy transfer (BRET) studies, we generated plasmids [vector backbone, pcDNA3.1(+)] coding for receptors in which the C terminus of Rq or Rq(R165L) was fused to the coding sequence of Renilla reniformis luciferase 8 (Luc), yielding Rq-Luc and Rq(R165L)-Luc, respectively. These plasmids were obtained by using a strategy similar to the one we used to generate M3-Luc (McMillin et al., 2011). The correctness of all coding regions was verified by sequencing. Plasmids coding for rat GRK2 and Venus-tagged versions of arrestin-2 and -3 (V-arr2 and V-arr3, respectively) were kindly provided by Drs. Diaz Gimenez and Vsevolod Gurevich (Vanderbilt University) (Vishnivetskiy et al., 2011). The mammalian expression plasmid coding for the wild-type M3 muscarinic receptor (rat) has been described previously (Schöneberg et al., 1995).
Cell Culture and Transfections.
COS-7 cells and HEK293T cells were obtained from American Type Culture Collection (Manassas, VA) and cultured according to established protocols. MIN6 cells were a kind gift from Dr. Abner Notkins (National Institute of Dental and Craniofacial Research, National Institutes of Health) and were cultured as described previously (Ishihara et al., 1993). COS-7 cells were transfected with plasmid DNA using Lipofectamine and Plus reagent (Invitrogen) according to the manufacturer's protocol. HEK293T and MIN6 cells were transfected as described under Small Interfering RNA-Mediated Arrestin-2/3 Knockdown.
[3H]NMS Radioligand Binding Studies.
[3H]NMS saturation and ACh and CNO inhibition binding assays were performed using membranes prepared from transfected COS-7 cells, as described previously (Li et al., 2005; McMillin et al., 2011). In all inhibition binding studies, a fixed concentration of [3H]NMS (20 nM) was used. ACh and CNO IC50 values were converted to Ki values using the Cheng-Prusoff equation. Data were analyzed by using Prism 4.0 software (GraphPad Software, Inc., San Diego, CA).
Calcium Mobilization Assay.
Approximately 48 h after transfection, cells were incubated with increasing concentrations of CNO, and changes in intracellular calcium levels were determined using FLIPR technology (Molecular Devices, Sunnyvale, CA). All measurements were performed in 96-well plates, as described previously (Li et al., 2007; McMillin et al., 2011). CNO concentration-response curves were analyzed using Prism 4.0 software.
BRET (Arrestin Recruitment) Studies.
To monitor receptor-mediated arrestin-2 and -3 recruitment, we followed a protocol similar to that described previously (Klewe et al., 2008). COS-7 cells were seeded onto six-well plates at a density of ∼3 × 105 cells/well. Approximately 24 h after plating, cells were cotransfected with plasmids coding for Rq-Luc or Rq(R165L)-Luc (200 ng), GRK2 (200 ng), and V-arr2 or V-arr3 (800 ng). In control samples, vector DNA [pcDNA3.1(−), 800 ng] was added instead of the arrestin plasmids. After ∼48 h, cells were trypsinized, transferred to microcentrifuge tubes, and centrifuged at 110g for 5 min at room temperature. Cell pellets were resuspended in 900 μl of phosphate-buffered saline supplemented with glucose (1 mg/ml), ascorbic acid (1 mM), and EDTA-free complete protease inhibitor (Roche Applied Science, Indianapolis, IN). Subsequently, 45-μl aliquots were added to individual wells of a white opaque 96-well plate and were incubated with increasing concentrations of CNO for 45 min at room temperature (PerkinElmer Life and Analytical Sciences). Total fluorescence was first measured via excitation at 485 nm and monitoring emission at 535 nm. Coelenterazine-h (Promega, Madison, WI) was then added to each well at a final concentration of 5 μM, and emissions were measured at 530 and 480 nm. Total luminescence was subsequently measured in the absence of filters. All measurements were performed using the Mithras LB 940 plate reader (Berthold Technologies, Bad Wildbad, Germany). The BRET ratio was defined as the ratio of emission at 530 nm to emission at 480 nm after the addition of coelenterazine-h. BRET signals are given as NetBRET ratios calculated by subtracting baseline BRET ratios obtained in the absence of V-arr2 or V-arr3 from BRET ratios obtained in the presence of V-arr2 or V-arr3 (at any given CNO concentration). CNO BRET50 and BRETmax values were obtained using GraphPad Prism 4.0 software.
Small Interfering RNA-Mediated Arrestin-2/3 Knockdown.
Knockdown of arrestin-2 or -3 expression was achieved by using the following small interfering RNAs (siRNAs) (Ahn et al., 2003): arrestin-2, 5′-AAAGCCUUCUGCGCGGAGAAU; arrestin-3, 5′-AAGGACCGCAAAGUGUUUGUG (duplexes; Ambion, Austin, TX). The negative control siRNA used in these experiments was identical to the one used previously (Ahn et al., 2003). For arrestin knockdown studies, HEK293T or MIN6 cells (∼1 × 106 cells) were seeded into six-well plates. Approximately 24 (HEK293T cells) or 48 h later (MIN6 cells), cells were cotransfected with 0.8 μg of receptor DNA [Rq or Rq(R165L)] and 120 pmol of arrestin-2, arrestin-3, or control siRNA. HEK293T cells were cotransfected using GeneSilencer transfection reagent (Gene Therapy Systems, Inc., San Diego, CA), as described previously (Ahn et al., 2003). MIN6 cells were cotransfected by using Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen). Approximately 48 h after transfection, cells were subjected to various functional assays. We used the same arrestin siRNAs for HEK293T and MIN6 cells (human sequences described by Ahn et al., 2003), because the corresponding mouse sequences differ at only one or two positions from the human siRNA sequences used.
Western Blotting (ERK1/2 Phosphorylation) Studies.
HEK293T cells (∼3 × 105 cells) were seeded into 12-well plates. Approximately 24 h later, cells were transfected (see previous paragraph) and incubated for another 24 h. After a 5- to 6-h starvation period in serum-free medium (Wei et al., 2003), cells were treated with increasing concentrations of CNO for 2 min at 37°C. Subsequently, the medium was removed, and 1× Laemmli sample buffer was added to each well. Whole-cell lysates were sonicated, resolved on Novex 10% Tris-glycine polyacrylamide gels (Invitrogen), and transferred to nitrocellulose membranes for immunoblotting. Phosphorylated ERK1/2 (pERK1/2), total ERK1/2, and arrestin-2 and -3 were detected via immunoblotting using the following primary antibodies: rabbit monoclonal anti-phospho-p44/42 (pERK, 1:2000; Cell Signaling Technology, Danvers, MA), rabbit polyclonal anti-p44/42 (total ERK1/2, 1:2000; Cell Signaling Technology), and mouse monoclonal anti-arrestin-2 and -3 (1:2000; BD Biosciences, San Jose, CA). Horseradish peroxidase-labeled secondary antibodies (Cell Signaling Technology) were used to detect the primary antibodies via chemiluminescence. Chemiluminescent detection was performed using the SuperSignal Western Pico reagent or SuperSignal Extended Dura reagent (Thermo Fisher Scientific, Waltham, MA). Immunoreactive bands were quantified by densitometry using ImageJ software (http://rsbweb.nih.gov/ij/).
cAMP Assay.
MIN6 cells were transfected with the Rq(R165L) construct as described under Small Interfering RNA-Mediated Arrestin-2/3 Knockdown. Approximately 48 h later, cells were trypsinized, collected by centrifugation, and resuspended in phosphate-buffered saline containing glucose (1 mg/ml) and EDTA-free complete protease inhibitor (Roche Applied Science) at a density of 1 × 106 cells/ml. Subsequently, 10-μl aliquots were added to 200 μl polymerase chain reaction tubes and incubated with the same volume (10 μl) of increasing concentrations of CNO or forskolin for 25 min at 37°C. The incubation mixtures were then transferred into white-bottom 384-well plates (∼5000 cells/well), and cells were lysed to determine drug-dependent changes in cAMP levels using a fluorescence resonance energy transfer-based cAMP detection technique (cAMP dynamic 2 kit; Cisbio Bioassays, Bedford, MA) according to the manufacturer's protocol.
Insulin Release Assay.
MIN6 cells that had been cotransfected with different combinations of plasmid DNAs and siRNAs (see Small Interfering RNA-Mediated Arrestin-2/3 Knockdown) were seeded into 96-well plates (∼1 × 105 cells/well). Approximately 48 h later, the cells were incubated with increasing concentrations of CNO at 37°C for 1 h in Krebs-Ringer bicarbonate/HEPES buffer containing 16.7 mM glucose. Insulin release was determined by measuring insulin concentrations in the incubation medium using an insulin enzyme-linked immunosorbent assay kit (Crystal Chem, Inc., Downers Grove, IL). CNO concentration-response curves were analyzed using Prism 4.0 software.
Statistics.
Data are expressed as means ± S.E.M. for the indicated number of observations. For comparisons between two groups, the paired or unpaired Student's t test (two-tailed) was used, as appropriate. For multiple comparisons, the one-way analysis of variance was applied. A p value of <0.05 was considered statistically significant.
Results and Discussion
Identification of an M3 Receptor-Based DREADD Unable to Couple to Gq.
We generated and characterized a rat M3 muscarinic receptor-based DREADD that selectively couples to G proteins of the Gq family (Rq) (Guettier et al., 2009). For the sake of simplicity, we refer to this receptor as “Rq” throughout this article.
Many studies have shown that mutational modification of an arginine residue that is highly conserved among class A GPCRs (R3.50 according to the Ballesteros-Weinstein GPCR numbering system; part of the highly conserved DRY motif) abolishes or drastically reduces agonist-dependent GPCR activation (http://www.gpcr.org/7tm/). Consistent with this observation, the solved crystal structure of an agonist-β2-adrenergic receptor-Gs complex indicates that R3.50 contacts key regions of the G protein α-subunit that are predicted to be critical for productive receptor/G protein coupling (Rasmussen et al., 2011).
We previously reported that a mutant M3 receptor (rat) containing the R165L3.50 point mutation virtually lost its ability to stimulate muscarinic agonist-induced inositol phosphate production in transfected COS-7 cells (Li et al., 2005). In contrast, this mutation did not interfere with agonist and antagonist binding affinities and receptor expression levels (Li et al., 2005). To examine whether this point mutation also disrupted signaling mediated by the Rq designer receptor, we generated the Rq(R165L) mutant receptor. In agreement with the data reported previously (Li et al., 2005), CNO treatment of Rq(R165L)-expressing COS-7 cells had no significant effect on intracellular calcium levels ([Ca2+]i), as studied via FLIPR technology (Fig. 1A). In contrast, CNO induced robust, concentration-dependent increases in [Ca2+]i in Rq-expressing COS-7 cells (Fig. 1A). The Emax value for this response amounted to ∼75% of the Emax value observed with COS-7 cells expressing the wild-type M3 muscarinic receptor (rat) (Schöneberg et al., 1995) after stimulation with the full muscarinic agonist, OXO-M (Fig. 1B).
Fig. 1.
Biochemical studies performed with transfected COS-7 cells. A, B, calcium assays. COS-7 cells transiently expressing the Rq or Rq(R165L) receptors (A) or the wild-type rat M3 muscarinic receptor (B) were incubated with increasing concentrations of CNO or OXO-M, respectively (vector = pcDNA). Drug-induced changes in [Ca2+]i were measured via FLIPR. A, data from a representative experiment (two additional experiments gave similar results). B, pooled data from three independent experiments (means ± S.E.M.). All assays were performed in duplicate. The CNO EC50 value at the Rq receptor was 28.2 ± 7.7 nM, and the OXO-M EC50 value at the wild-type M3 receptor was 2.33 ± 0.31 nM (means ± S.E.M., n = 3). AU, arbitrary units. C, BRET (arrestin recruitment) studies. COS cells coexpressing Rq-Luc or Rq(R165L)-Luc (BRET donor), V-arr2 or V-arr3 (BRET acceptor), and GRK2 were incubated with increasing concentrations of CNO. BRET measurements were performed as described under Materials and Methods. BRET signals are expressed as NetBRET ratios obtained by subtracting baseline BRET ratios obtained in the absence of BRET acceptor (V-arr2 or V-arr3) from BRET ratios measured in the presence of V-arr2 or V-arr3. The panel shows data from a representative experiment performed in duplicate. Two additional experiments gave similar results. CNO BRET50 values (in μM) were as follows (means ± S.E.M., n = 3): Rq-Luc + V-arr2, 0.15 ± 0.03; Rq-Luc + V-arr3, 1.45 ± 1.05; Rq(R165L)-Luc + V-arr2, 0.37 ± 0.07; and Rq(R165L)-Luc + V-arr3, 1.24 ± 0.45.
Radioligand binding studies performed with membranes from transfected COS-7 cells confirmed that the R165L point mutation had little effect on [3H]NMS and CNO binding affinities and [3H]NMS Bmax values (Table 1). This observation indicates that the inability of Rq(R165L) to stimulate increases in [Ca2+]i was not due to receptor misfolding and/or lack of CNO binding. Moreover, like Rq, the Rq(R165L) receptor failed to bind ACh, the endogenous muscarinic receptor agonist (Table 1). Because Rq selectively couples to G proteins of the Gq family (Armbruster et al., 2007; Guettier et al., 2009; Alvarez-Curto et al., 2011) and activation of this class of G proteins triggers pronounced increases in [Ca2+]i, our data indicate that the Rq(R165L) construct lacks the ability to activate Gq.
TABLE 1.
Comparison of the ligand-binding properties of the Rq and Rq(R165L) constructs
The indicated receptors were transiently expressed in COS-7 cells. [3H]NMS saturation and [3H]NMS/CNO or [3H]NMS/ACh competition binding experiments were performed using membrane homogenates prepared from transfected COS-7 cells, as described under Materials and Methods. Data are presented as means ± S.E.M. of at least three independent experiments, each performed in duplicate.
| Receptor | [3H]NMS Binding |
Ki |
||
|---|---|---|---|---|
| KD | Bmax | CNO Binding | ACh Binding | |
| nM | pmol/mg | μM | ||
| Rq | 16.2 ± 3.0 | 16.7 ± 1.1 | 1.36 ± 0.08 | N.D. |
| Rq(R165L) | 14.4 ± 1.6 | 12.7 ± 0.1 | 1.47 ± 0.14 | N.D. |
N.D., no detectable inhibition of [3H]NMS binding (20 nM) at ACh concentrations up to 0.1 mM.
BRET (Receptor-Arrestin Recruitment) Assays Performed with Transfected COS-7 Cells.
We next used BRET technology to examine whether Rq(R165L) was still able to recruit arrestins in a CNO-dependent fashion. BRET techniques are widely used to monitor GPCR/arrestin interactions with high sensitivity in live cells (Hamdan et al., 2005; Klewe et al., 2008; Alvarez-Curto et al., 2011; Kocan and Pfleger, 2011; Vishnivetskiy et al., 2011).
Following a protocol similar to that described previously (Klewe et al., 2008), we cotransfected COS-7 cells with a receptor construct containing a C-terminal R. reniformis luciferase 8 sequence [Rq-Luc or Rq(R165L)-Luc; BRET donor], a modified version of arrestin-2 or -3 containing an N-terminal Venus tag (V-arr2 or V-arr3, respectively; BRET acceptor), and a GRK2 expression construct. Consistent with data published previously (Alvarez-Curto et al., 2011), CNO treatment of cells coexpressing Rq-Luc and V-arr2 or V-arr3 resulted in concentration-dependent BRET signals, indicative of CNO-dependent arrestin recruitment (Fig. 1C). We noted that CNO-dependent maximal BRET responses (BRETmax) were ∼2.5-fold higher for the Rq-Luc/V-arr3 combination, compared with the Rq-Luc/V-arr2 pair (BRETmax values: Rq-Luc/V-arr2, 0.025 ± 0.003; Rq-Luc/V-arr3, 0.063 ± 0.007; p < 0.01; n = 3).
Rq(R165L)-Luc-expressing cells yielded CNO-dependent BRET signals that were qualitatively similar to those observed with Rq-Luc (Fig. 1C). However, compared with Rq-Luc, the interaction of Rq(R165L)-Luc with V-arr3 was characterized by a ∼30% reduction in BRETmax (0.045 ± 0.007; n = 3; p < 0.05, compared with Rq/V-arr3) (Fig. 1C). The Rq(R165L)-Luc/V-arr2 combination yielded a BRETmax value (0.026 ± 0.005; n = 3) that was similar to the one observed with the Rq-Luc/V-arr2 pair (Fig. 1C). CNO BRET50 values are given in the legend to Fig. 1C.
Taken together, these data indicate that the Rq(R165L) receptor fails to activate Gq but retains the ability to recruit arrestin-2 and -3 in a CNO-dependent fashion. Thus, Rq(R165L) represents the first arrestin-biased DREADD.
Calcium Mobilization and ERK1/2 Phosphorylation Studies Performed with HEK293T Cells.
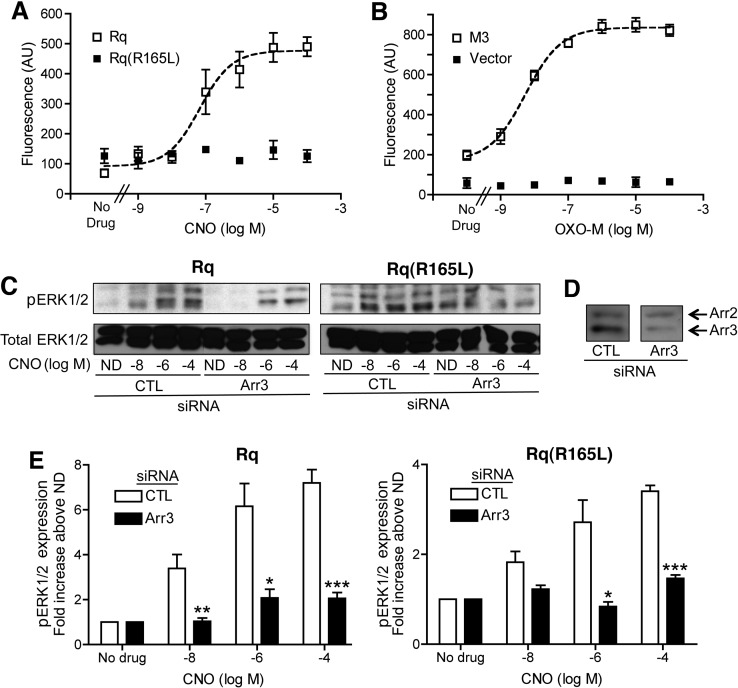
Many studies have shown that GPCR-dependent phosphorylation of ERK1/2 in HEK293T cells is mediated, at least in part, by arrestin-3-mediated signaling (see, for example, Wei et al., 2003). In agreement with the data obtained with COS-7 cells, CNO treatment of Rq(R165L)-expressing HEK293T cells had no significant effect on [Ca2+]i but resulted in robust, concentration-dependent increases in [Ca2+]i in Rq-expressing HEK293T cells (Fig. 2A). The Emax value for this latter response amounted to ∼65% of the Emax value observed with OXO-M-stimulated HEK293T cells expressing the wild-type M3 receptor (Fig. 2B).
Fig. 2.
Signaling studies performed with transfected HEK293T cells. A, B, calcium assays. HEK293T cells transiently expressing the Rq or Rq(R165L) receptors (A) or the wild-type rat M3 muscarinic receptor (B) were incubated with increasing concentrations of CNO or OXO-M, respectively (vector = pcDNA). Drug-induced changes in [Ca2+]i were measured by via FLIPR. Data are presented as means ± S.E.M. of four independent experiments, each performed in duplicate. The CNO EC50 value at the Rq receptor was 0.25 ± 0.16 μM, and the OXO-M EC50 value at the wild-type M3 receptor was 5.22 ± 1.48 nM, respectively. AU, arbitrary units. C to E, ERK phosphorylation assay. HEK293T cells were transfected with expression constructs coding for Rq or Rq(R165L), together with either arr3 siRNA or negative control siRNA (CTL). Cells were stimulated with increasing concentrations of CNO for 2 min at 37°C. Subsequently, cell lysates were probed for the expression of total ERK1/2 and pERK1/2 via immunoblotting. C, representative Western blots. ND, no drug. D, representative immunoblotting data indicating that treatment of HEK293T cells with arr3 siRNA led to a strong reduction in arr3 protein expression (note that the arrestin antibody used recognized both arr2 and arr3). E, a summary of pERK1/2 protein levels (pooled data). In each individual experiment, band intensities in the absence of CNO were set equal to 1 (ND, no drug). Each bar represents the mean ± S.E.M. from three or four independent experiments. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus the corresponding control response.
Because the Rq(R165L) receptor retained the ability to recruit arrestins in BRET assays, we next examined whether this receptor was able to stimulate ERK1/2 phosphorylation in HEK293T cells. Specifically, we transfected HEK293T cells with plasmid DNA coding for Rq or Rq(R165L), together with either arrestin-3 siRNA or negative control siRNA. Under these experimental conditions, the use of arrestin-3 siRNA reduced arrestin-3 protein expression by ∼70% (Fig. 2D). After a 2-min incubation at 37°C with increasing concentrations of CNO, cells were lysed and probed for the expression of total ERK1/2 and pERK1/2 via immunoblotting. As shown in Fig. 2, C and E, CNO stimulated ERK1/2 phosphorylation in both Rq- and Rq(R165L)-expressing cells in a concentration-dependent fashion. Consistent with the outcome of the BRET recruitment assays, CNO treatment of Rq-expressing cells resulted in more robust signals at all CNO concentrations used (∼2-fold increase in pERK1/2 accumulation at 1 and 100 μM CNO, compared with Rq(R165L); p < 0.05). After treatment of cells with arrestin-3 siRNA, CNO-induced ERK1/2 phosphorylation was greatly reduced in Rq-expressing cells and virtually abolished in Rq(R165L)-expressing cells (Fig. 2E). These data indicate that Rq(R165L) is not only able to recruit arrestins but can also initiate arrestin-dependent signaling in a CNO-dependent fashion.
Although we observed pronounced arrestin-3-dependent ERK1/2 phosphorylation after only 2 min of CNO treatment, a longer CNO incubation time (45 min) was required to obtain robust BRET signals in the arrestin recruitment assays. The most likely explanation for this phenomenon is that different conformational states of arrestin and/or different arrestin signaling complexes are involved in generating the signals measured in the two different assays.
Second-Messenger and Insulin Release Assays Performed with MIN6 Cells.
To demonstrate the usefulness of the arrestin-biased Rq(R165L) designer receptor to address biologically relevant questions, we expressed the Rq(R165L) construct, as well as Rq (for control purposes), in MIN6 cells (transfection efficiency, ∼10–20%, as judged by the use of a green fluorescent protein reporter plasmid; data not shown). MIN6 cells are derived from mouse pancreatic β-cells and are widely used as an in vitro model system to study the regulation of insulin release and other β-cell functions (Ishihara et al., 1993).
Because increases in [Ca2+]i or intracellular cAMP levels stimulate the release of insulin from β-cells and insulinoma cells (Ahrén, 2009), we first examined whether CNO-dependent activation of Rq(R165L) affected the levels of these second messengers. As expected, CNO treatment of Rq-expressing MIN6 cells resulted in concentration-dependent increases in [Ca2+]i (Fig. 3A). In contrast, CNO had no significant effect on [Ca2+]i in Rq(R165L)-expressing MIN6 cells (Fig. 3A). The Emax value for Rq-mediated increases in [Ca2+]i amounted to ∼15% of the Emax value observed after OXO-M stimulation of M3 receptors endogenously expressed by MIN6 cells (Ruiz de Azua et al., 2010) (Fig. 3B). The relatively low efficacy of the Rq receptor in MIN6 cells (compared with COS-7 and HEK293T cells) is most likely a consequence of the relatively low transfection efficiency that we observed with MIN6 cells (see previous paragraph). Moreover, incubation of Rq(R165L)-expressing MIN6 cells with CNO did not lead to any detectable changes in cAMP levels (Fig. 3C).
Fig. 3.
Second-messenger studies performed with MIN6 cells. A, B, calcium assays. MIN6 cells transfected with the Rq or Rq(R165L) constructs (A) or vector DNA (pcDNA) (B) were incubated with increasing concentrations of CNO or OXO-M, respectively. Drug-induced changes in [Ca2+]i were measured by via FLIPR. A, data from a representative experiment (two additional experiments gave similar results). B, pooled data from three independent experiments. All assays were performed in duplicate. The CNO EC50 value at the Rq receptor was 28.2 ± 7.7 nM, and the OXO-M EC50 value at the wild-type M3 receptor was 1.00 ± 0.12 μM, respectively (means ± S.E.M., n = 3). AU, arbitrary units. B, cAMP assay. MIN6 cells transiently expressing the Rq(R165L) construct were incubated with increasing concentrations of forskolin or CNO. cAMP concentrations were measured in cell lysates by using a fluorescence resonance energy transfer-based cAMP kit (for details, see Materials and Methods). Data are given as means ± S.E.M. of three independent experiments, each performed in duplicate.
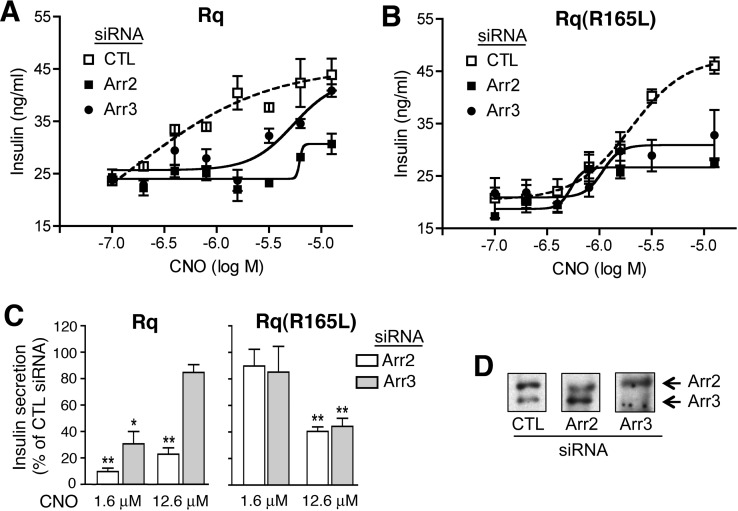
To study the potential role of arrestin signaling in regulating insulin secretion, we transfected MIN6 cells with plasmid DNA coding for Rq or Rq(R165L), together with either arrestin-2 or -3 siRNA or negative control siRNA. In both Rq- and Rq(R165L)-expressing cells treated with control siRNA, CNO stimulated insulin release in a concentration-dependent fashion (Fig. 4, A and B). However, treatment of both Rq- and Rq(R165L)-expressing cells with arrestin-2 or -3 siRNA led to significant reductions in insulin secretion (Fig. 4, A and C). Arrestin-2 or -3 knockdown reduced but did not completely prevent insulin secretion after CNO treatment of Rq(R165L)-expressing cells. One possible explanation for this observation is that treatment of MIN6 cells with arrestin-2/3 siRNA lowered but did not abolish arrestin-2/3 protein expression (∼50–70% reduction in arrestin-2/3 expression) (Fig. 4D). In agreement with our findings, several studies suggest that activation of arrestin-dependent signaling pathways can promote insulin release from pancreatic β-cells or insulinoma cells (Sonoda et al., 2008; Kong et al., 2010).
Fig. 4.
Insulin release studies. MIN6 cells were transfected with expression plasmids coding for either Rq (A) or Rq(R165L) (B), together with either arrestin-2 or -3 siRNA (arr2 or arr3 siRNA) or negative control siRNA (CTL). Representative concentration-response curves are shown. Three independent experiments gave similar results. C, pooled insulin release data obtained in three independent experiments. The panel shows relative insulin responses at two different CNO concentrations (CTL siRNA = 100%). *, p < 0.05; **, p < 0.01, versus the corresponding control response. D, arrestin levels were monitored via Western blotting using an anti-arrestin antibody that recognized both arr2 and arr3. Cells were incubated with increasing concentrations of CNO for 60 min at 37°C in the presence of 16.7 mM glucose, and insulin secretin into the medium was determined as described under Materials and Methods. CNO EC50 (in μM) and Emax values (in ng insulin/ml) were as follows (means ± S.E.M., n = 3): Rq + CTL siRNA, EC50 = 0.55 ± 0.33 and Emax = 43.5 ± 3.5; Rq + arr2 siRNA, EC50 = 4.60 ± 0.96 and Emax = 28.1 ± 1.5; Rq + arr3 siRNA, EC50 = 2.98 ± 0.84 and Emax = 39.9 ± 2.1; Rq(R165L) + CTL siRNA, EC50 = 2.14 ± 0.40 and Emax = 40.3 ± 3.82; Rq(R165L) + arr2 siRNA, EC50 = 1.16 ± 0.88 and Emax = 27.2 ± 0.80; and Rq(R165L) + arr3 siRNA, EC50 = 1.33 ± 0.32 and Emax = 26.9 ± 2.93.
In conclusion, we developed a new CNO-sensitive designer GPCR [Rq(R165L)] that fails to activate G proteins but is able to recruit arrestins and promote arrestin-dependent signaling. When expressed in a β-cell line, activation of Rq(R165L) resulted in arrestin-dependent insulin release, highlighting the potential usefulness of this newly developed, arrestin-biased DREADD to study the physiological roles of arrestin signaling pathways. It should be noted that arrestin-biased GPCRs have been described previously (Wei et al., 2003; Shenoy et al., 2006). However, the Rq(R165L) construct offers the great advantage that it can only be activated by administration of an exogenous ligand (CNO) that is otherwise pharmacologically inert. Studies with mice (or other experimental animals) expressing this arrestin-biased DREADD in a tissue-specific fashion should provide detailed novel information about the potential physiological relevance of arrestin-dependent signal pathways.
Acknowledgments
We thank Sara McMillin (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) and Diaz Gimenez and Vsevolod Gurevich (Vanderbilt University) for help setting up the BRET assays, for providing various plasmids, and for helpful comments on the manuscript. We also thank Inigo Ruiz de Azua and Yoskaly Lazo Fernandez and Tong Liu (National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases) for technical advice and helpful discussions.
This work was supported by the Intramural Research program of the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- GPCR
- G protein-coupled receptor
- GRK
- G protein-coupled receptor kinases
- CNO
- clozapine-N-oxide
- DREADD
- designer receptor exclusively activated by designer drug
- MIN6
- mouse insulinoma
- ACh
- acetylcholine
- OXO-M
- oxotremorine-M
- [3H]NMS
- [3H]N-methylscopolamine
- HA
- hemagglutinin
- BRET
- bioluminescence resonance energy transfer
- Luc
- R. reniformis luciferase 8
- V-arr2
- Venus-tagged version of arrestin-2
- V-arr3
- Venus-tagged version of arrestin-3
- siRNA
- small interfering RNA
- pERK1/2
- phosphorylated ERK1/2.
Authorship Contributions
Participated in research design: Nakajima and Wess.
Conducted experiments: Nakajima.
Contributed new reagents or analytic tools: Nakajima.
Performed data analysis: Nakajima.
Wrote or contributed to the writing of the manuscript: Nakajima and Wess.
References
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. (2003) Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA 100:1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrén B. (2009) Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 8:369–385 [DOI] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63:27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Curto E, Prihandoko R, Tautermann CS, Zwier JM, Pediani JD, Lohse MJ, Hoffmann C, Tobin AB, Milligan G. (2011) Developing chemical genetic approaches to explore G protein-coupled receptor function: validation of the use of a receptor activated solely by synthetic ligand (RASSL). Mol Pharmacol 80:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104:5163–5168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, et al. (2009) A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA 106:19197–19202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Audet M, Garneau P, Pelletier J, Bouvier M. (2005) High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based beta-arrestin2 recruitment assay. J Biomol Screen 10:463–475 [DOI] [PubMed] [Google Scholar]
- Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. (1993) Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36:1139–1145 [DOI] [PubMed] [Google Scholar]
- Klewe IV, Nielsen SM, Tarpø L, Urizar E, Dipace C, Javitch JA, Gether U, Egebjerg J, Christensen KV. (2008) Recruitment of beta-arrestin2 to the dopamine D2 receptor: insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology 54:1215–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan M, Pfleger KD. (2011) Study of GPCR-protein interactions by BRET. Methods Mol Biol 746:357–371 [DOI] [PubMed] [Google Scholar]
- Kong KC, Butcher AJ, McWilliams P, Jones D, Wess J, Hamdan FF, Werry T, Rosethorne EM, Charlton SJ, Munson SE, et al. (2010) M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc Natl Acad Sci USA 107:21181–21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Nowak NM, Kim SK, Jacobson KA, Bagheri A, Schmidt C, Wess J. (2005) Random mutagenesis of the M3 muscarinic acetylcholine receptor expressed in yeast: identification of second-site mutations that restore function to a coupling-deficient mutant M3 receptor. J Biol Chem 280:5664–5675 [DOI] [PubMed] [Google Scholar]
- Li B, Scarselli M, Knudsen CD, Kim SK, Jacobson KA, McMillin SM, Wess J. (2007) Rapid identification of functionally critical amino acids in a G protein-coupled receptor. Nat Methods 4:169–174 [DOI] [PubMed] [Google Scholar]
- McMillin SM, Heusel M, Liu T, Costanzi S, Wess J. (2011) Structural basis of M3 muscarinic receptor dimer/oligomer formation. J Biol Chem 286:28584–28598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650 [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. (2010) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 9:373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, et al. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz de Azua I, Scarselli M, Rosemond E, Gautam D, Jou W, Gavrilova O, Ebert PJ, Levitt P, Wess J. (2010) RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc Natl Acad Sci USA 107:7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneberg T, Liu J, Wess J. (1995) Plasma membrane localization and functional rescue of truncated forms of a G protein-coupled receptor. J Biol Chem 270:18000–18006 [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. (2006) β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem 281:1261–1273 [DOI] [PubMed] [Google Scholar]
- Shukla AK, Xiao K, Lefkowitz RJ. (2011) Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci 36:457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. (2008) Beta-arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc Natl Acad Sci USA 105:6614–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Gimenez LE, Francis DJ, Hanson SM, Hubbell WL, Klug CS, Gurevich VV. (2011) Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J Biol Chem 286:24288–24299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. (2003) Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100:10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]