Abstract
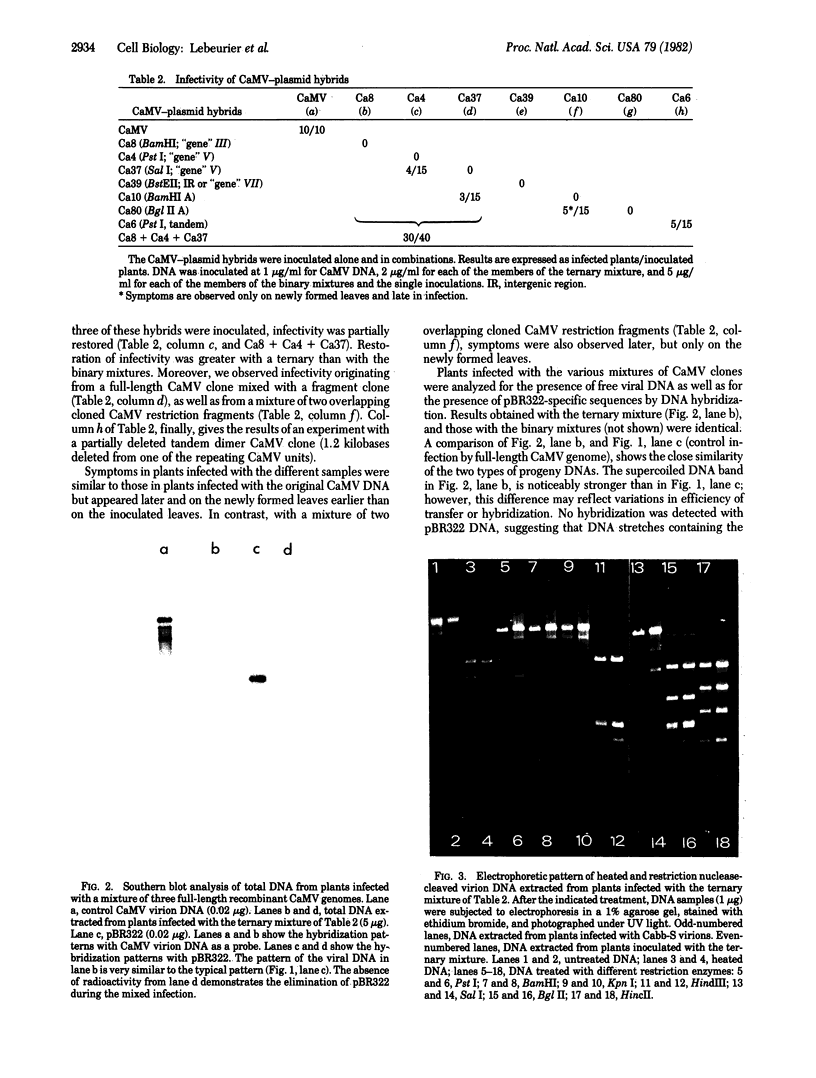
Ligation and recombination of the DNA of cauliflower mosaic virus (CaMV) is demonstrated by the following experiments: (i) Ligation: Different noninfectious fragments of the CaMV genome (obtained after insertion into plasmid pBR322 followed by enzymatic excision) regained infectivity when mixtures of them were used to inoculate their host. The symptom appearance was delayed by comparison with a typical CaMV infection, and only the newly formed leaves were affected. (ii) Recombination: Pairs of noninfectious recombinant full-length CaMV genomes (integrated into pBR322 at different restriction endonuclease sites) regained infectivity upon simultaneous inoculation of a sensitive host. The symptomatology of the resulting infection was indistinguishable from that of a typical CaMV infection. We show that progeny DNA had the same characteristics (size, structure, restriction endonuclease digestion pattern) as bona fide CaMV DNA, and that the vector pBR322 had been completely eliminated. A cloned tandem dimer of CaMV DNA with a partial deletion similarly was infectious in the plant assays. This system should be useful to study the expression of mutant genomes, thus allowing characterization of the CaMV genes.
Keywords: infective DNA, DNA ligation, DNA cloning
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Killos L., Sanders-Haigh L., Kretschmer P. J., Diacumakos E. G. Replication and expression of thymidine kinase and human globin genes microinjected into mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5399–5403. doi: 10.1073/pnas.77.9.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Fried M., Klein B., Murray K., Greenaway P., Tooze J., Boll W., Weissmann C. Infectivity in mouse fibroblasts of polyoma DNA integrated into plasmid pBR322 or lambdoid phage DNA. Nature. 1979 Jun 28;279(5716):811–816. doi: 10.1038/279811a0. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Hohn T., Hohn B., Lesot A., Lebeurier G. Restriction map of native and cloned cauliflower mosaic virus DNA. Gene. 1980 Oct;11(1-2):21–31. doi: 10.1016/0378-1119(80)90083-9. [DOI] [PubMed] [Google Scholar]
- Hohn T., Richards K., Geneviève-Lebeurier Cauliflower mosaic virus on its way to becoming a useful plant vector. Curr Top Microbiol Immunol. 1982;96:194–236. [PubMed] [Google Scholar]
- Honigman A., Oppenheim A. B., Hohn B., Hohn T. Plasmid vectors for positive selection of DNA inserts controlled by the lambda pL promoter, repressor and antitermination function. Gene. 1981 Apr;13(3):289–298. doi: 10.1016/0378-1119(81)90033-0. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Hull R. Replication of cauliflower mosaic virus and transcription of its genome in turnip leaf protoplasts. Virology. 1978 May 15;86(2):468–481. doi: 10.1016/0042-6822(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Walker L. L., Dudley R. K. Cloned Cauliflower Mosaic Virus DNA Infects Turnips (Brassica rapa). Science. 1980 Jun 13;208(4449):1265–1267. doi: 10.1126/science.208.4449.1265. [DOI] [PubMed] [Google Scholar]
- Lebeurier G., Hirth L., Hohn T., Hohn B. Infectivities of native and cloned DNA of cauliflower mosaic virus. Gene. 1980 Dec;12(1-2):139–146. doi: 10.1016/0378-1119(80)90024-4. [DOI] [PubMed] [Google Scholar]
- Lebeurier G., Whitechurch O., Lesot A., Hirth L. Physical map of DNA from a new cauliflower mosaic virus strain. Gene. 1978 Nov;4(3):213–226. doi: 10.1016/0378-1119(78)90019-7. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Pipas J. M., Pearson-White S., Nathans D. Isolation of mutants of an animal virus in bacteria. Science. 1980 Sep 19;209(4463):1392–1396. doi: 10.1126/science.6251547. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Volovitch M., Drugeon C., Yot P. Studies on the single-stranded discontinuities of the cauliflower mosaic virus genome. Nucleic Acids Res. 1978 Aug;5(8):2913–2925. doi: 10.1093/nar/5.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]







