Abstract
Atypical N-methyl-d-aspartate (NMDA) receptors are expressed in podocytes. Sustained (≥24 h) application of 50 to 100 μM NMDA to immortalized mouse podocytes evoked a marked increase in the production of reactive oxygen species (ROS) such as H2O2. This effect of NMDA was associated with increased cell-surface expression of p47(phox), a cytosolic regulatory subunit of the NADPH oxidase NOX2. NMDA-evoked generation of ROS drove an increase in steady-state surface expression of transient receptor potential canonical (TRPC) 6 channels, which was blocked by the NMDA antagonist dizocilpine (MK-801) and by a membrane-permeable scavenger of ROS. The effect of NMDA on TRPC6 was observed using cell surface biotinylation assays and also with whole-cell recordings made under conditions designed to facilitate detection of current through TRPC6. NMDA mobilization of TRPC6 channels was blocked by concurrent treatment with the NMDA antagonist MK-801 and by a membrane-permeable scavenger of ROS. Mobilization of TRPC6 was also evoked by l-homocysteic acid. NMDA treatment also increased nuclear localization of endogenous nuclear factor of activated T cells, which could be blocked by MK-801, by scavenging ROS, by the calcineurin inhibitor cyclosporine, and by the TRPC channel inhibitor 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl]imidazole (SKF-96365). NMDA treatment also evoked robust activation of Rho but not Rac, consistent with previous studies of downstream effectors of TRPC6 activation. Exposing cells to NMDA for 24 h reduced total and cell surface expression of the podocyte markers nephrin and podocin, but there was no loss of cells. With longer NMDA exposure (72 h), we observed loss of cells associated with nuclear fragmentation and increased expression of caspase-3, caspase-6, and Bax, suggesting an apoptotic process.
Introduction
Podocytes comprise one of several essential components of the glomerular filtration barrier (Pavenstädt et al., 2003). Primary processes extending from the podocyte cell body ramify and wrap around the glomerular capillary. A series of small foot processes extend from the major processes of podocytes and attach to the external face of the basement membrane of the glomerular capillary. Specialized junctions between adjacent foot processes, known as slit diaphragms, provide a permselective pathway that allows convective movement and diffusion of water and small solutes into the urinary space, while excluding macromolecules.
Structural alterations in podocyte foot processes occur in many glomerular diseases, leading to loss or displacement of slit diaphragms (Shirato, 2002). Sustained elevation of Ca2+ influx into podocytes may represent a common pathway driving these changes (Lavin and Winn, 2011). A landmark observation was the discovery that gain-of-function mutations in TRPC6 channels can cause familial forms of focal and segmental glomerulosclerosis (Reiser et al., 2005; Winn et al., 2005). A similar pathology is observed in mice overexpressing wild-type or mutant TRPC6 channels selectively in podocytes (Krall et al., 2010).
TRPC6 channels are not the only possible pathway for Ca2+ influx into podocytes. In addition to other members of the TRPC family (Tian et al., 2010), ionotropic NMDA receptors provide a potential pathway for Ca2+ influx into these cells (Rastaldi et al., 2006; Anderson et al., 2011). NMDA receptors were originally identified in neurons, where they are normally activated by synaptic release of glutamate. Activated NMDA receptors show varying degrees of permeability to Ca2+ (Jahr and Stevens, 1993), which plays an important role in driving the downstream consequences of NMDA receptor activation.
NMDA receptors have been intensively studied in neuronal cells for two reasons. First, their activation induces several forms of synaptic plasticity in adult (Bliss and Collingridge, 1993) and developing (Cline and Constantine-Paton, 1989) nervous systems. Second, excessive NMDA receptor activation initiates a process known as excitotoxicity, resulting in death of neurons and glia driven in part by Ca2+ overload (Choi, 1992; Hardingham, 2009). Excitotoxicity is also associated with increased production of reactive oxygen species (ROS) (Kishida et al., 2005; Girouard et al., 2009). Excitotoxicity can be produced by several endogenously occurring amino acids that activate NMDA receptors, including dicarboxylic acids, sulfur-containing amino acids derived from methionine metabolism, and products of the kynurenine pathways of tryptophan metabolism (Kim et al., 1987; Olney et al., 1987).
It is now known that NMDA receptors are expressed in many different peripheral tissues (Patton et al., 1998; Parisi et al., 2009; Makhro et al., 2010; Mashkina et al., 2010; Tyagi et al., 2010), including podocytes (Rastaldi et al., 2006; Anderson et al., 2011), although their normal physiological functions in these tissues are not well understood. We have recently characterized the functional properties of NMDA receptors of podocytes (Anderson et al., 2011). These receptors are strongly activated by NMDA and are blocked by d-aminophosphonovaleric acid and dizocilpine (MK-801). Responses to NMDA are potentiated by d-serine and are nearly eliminated by an inhibitor of glycine and d-serine binding sites on NR1 subunits. As with neuronal NMDA receptors, the podocyte receptors are permeable to Ca2+ and subjected to voltage-dependent inhibition by Mg2+. However, podocyte NMDA receptors also exhibit several unusual features. The most significant is the fact that they cannot be activated by l-glutamate or l-aspartate (Anderson et al., 2011). Thus, although there is evidence that l-glutamate can be locally secreted by podocytes (Giardino et al., 2009), it seems likely that it acts primarily on metabotropic glutamate receptors (Puliti et al., 2011; Gu et al., 2012). However, podocyte NMDA receptors are robustly activated by l-homocysteic acid (l-HCA), an endogenously occurring product of methionine metabolism (Anderson et al., 2011). These metabolites are elevated in hypertension, diabetes, and chronic kidney diseases (Jager et al., 2001; Francis et al., 2004) and can reach levels sufficient to activate NMDA receptors in vivo (Tyagi et al., 2010), especially in chronic kidney disease (Heinz et al., 2009).
In the present study we have characterized pathways whereby activation of podocyte NMDA receptors could lead to glomerular dysfunction. We have observed that podocyte NMDA receptors lead to increased NOX-dependent production of ROS, which stimulates increased surface expression of functional TRPC6 channels. This in turn evokes a cyclosporine (CsA)-sensitive movement of nuclear factor of activated T cells (NFAT) into the nucleus. NMDA receptor activation also caused a fall in the expression of nephrin and podocin and, after 72 h, can evoke apoptotic cell death.
Materials and Methods
Cell Culture Protocols, Transfection, and Drugs.
Cell culture protocols have been described previously (Kim et al., 2008, 2009, 2010, 2012). In brief, the MPC-5 mouse podocyte cell line (obtained from Dr. Peter Mundel, Harvard Medical School, Cambridge, MA) was propagated at 33°C in RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 U/ml penicillin-streptomycin, with recombinant mouse γ-interferon (Sigma-Aldrich), in humidified 5% CO2 incubators. Podocyte differentiation and expression of podocyte markers were induced by removal of γ-interferon and a temperature switch to 37°C for 14 days. NMDA, l-HCA, MK-801, 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl]imidazole (SKF-96365), and CsA were obtained from Sigma-Aldrich (St. Louis, MO). Manganese(III) tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP) was obtained from Oxis International (Beverly Hills, CA).
Assay of ROS Generation.
Generation of H2O2 by podocytes was measured using the OxiSelect fluorometric assay (Cell Biolabs, Inc., San Diego, CA) according to the manufacturer's instructions, as described previously in detail (Kim et al., 2012). In this assay, H2O2 in the presence of horseradish peroxidase causes oxidation of 10-acetyl-3,7-dihydroxyphenoxazine to resorufin, a fluorescent product with a high extinction coefficient, which could be measured using a fluorescence microplate reader.
Immunoblot Analysis, Cell-Surface Biotinylation Assays, and Nuclear Localization of NFAT.
Immunoblot analysis and cell-surface biotinylation were performed using standard methods as described in detail previously (Kim et al., 2008, 2009, 2010, 2012). The primary antibodies were rabbit anti-TRPC6 (Alomone Labs, Jerusalem, Israel), rabbit anti-p47(phox) and rabbit anti-NOX2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-NOX4 (Epitomics, Burlingame, CA), rabbit anti-podocin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-nephrin (Abcam, Cambridge, MA), and mouse monoclonal anti-β-actin (Millipore Corporation, Billerica, MA). For cell surface biotinylation assays, intact podocytes were treated with a membrane-impermeable biotinylation reagent, sulfo-N-hydroxy-succinimidobiotin (Thermo Fisher Scientific, Waltham, MA) (1 mg/ml) for 1 h. The reaction was stopped, cells were lysed, and biotinylated proteins from the cell surface were recovered by incubation with immobilized streptavidin-agarose beads. A sample of the initial cell lysate was retained for analysis of total proteins and in some experiments of β-actin. These samples were quantified by immunoblot analysis followed by densitometric analysis using ImageJ software. Bar graphs describing these data were constructed from three to five repetitions of each experiment. NFAT localization in podocyte nuclei was measured using a commercial assay (Active Motif Inc., Carlsbad, CA) and a nuclear extract kit purchased from the same manufacturer. In this assay we also used a mouse monoclonal anti-histone and a mouse monoclonal anti-β-actin (Millipore Corporation). In separate experiments on NFAT localization, podocytes were subjected to various treatments and fixed in 4% paraformaldehyde at room temperature for 10 min. Fixed cells were permeablized, blocked, and incubated with rabbit anti-NFATc1 (Active Motif Inc.), and nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) for 1 h at 37°C. After washing, cells were treated with Alexa Fluor 488-conjugated anti-rabbit IgG (Invitrogen, Carlsbad, CA) and then were rinsed and mounted, and images were collected on an Olympus FV-1000 inverted stage confocal microscope using a Plan Apo N 60X 1.42NA oil-immersion objective and processed by FluoView software.
Electrophysiology.
Whole-cell recordings were made as described previously (Kim et al., 2012) from control cells and from cells that had been exposed for 24 h to NMDA or a combination of NMDA and inhibitors. All cells were treated with 100 μM 1-oleoyl-2-acetyl-sn-glycerol (OAG) for 15 min before whole-cell recordings. This diacylglycerol analog does not activate TRPC5 (Hofmann et al., 1999), and we have previously shown that responses to OAG in podocytes are abolished after TRPC6 knockdown (Kim et al., 2012). In all recordings, the bath solution contained 150 mM NaCl, 5.4 mM CsCl, 0.8 mM MgCl2, 5.4 mM CaCl2, and 10 mM HEPES, pH 7.4. Pipette solutions contained 10 mM NaCl, 125 mM CsCl, 6.2 mM MgCl2, 10 mM HEPES, and 10 mM EGTA, pH 7.2. Currents were evoked by a ramp voltage commands (−80 to +80 mV over 2.5 s) from a holding potential of −40 mV. In each cell, currents were measured before and after superfusion of 50 μM La3+, which blocks TRPC6 channels but not TRPC5, in podocytes (Tian et al., 2010) or before and after 50 μM SKF-96365. Data were digitized using a Digidata interface (Molecular Devices, Sunnyvale, CA), and stored for off-line analysis using PClamp software (Molecular Devices). La3+-sensitive currents were obtained by digital subtraction, and the peak measured at +80 mV was used for quantitative analysis of the effects of previous NMDA and drug treatments.
Analysis of Podocyte Cell Death and Small GTPases.
Rho and Rac activation were measured using commercial glutathione transferase pull-down assays from Cytoskeleton Inc. (Denver, CO) according to the manufacturer's instructions. Analyses of apoptosis markers were performed using rabbit antibodies against caspase-3, caspase 6, Bax, and Bcl-XL (Cell Signaling Technology, Danvers, MA). Nuclear fragmentation was detected using a commercial immunofluorescence-based APO-BrdU terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (Invitrogen). In addition, overall cell viability was determined using the CytoSelect assay (Cell Biolabs Inc.) and by measuring release of lactate dehydrogenase into media using an assay from Roche Applied Science (Indianapolis, IN).
Results
All of these experiments were performed on the differentiated cells of an immortalized mouse podocyte cell line, which express functional NMDA receptors (Anderson et al., 2011) along with many other podocyte markers (Kim et al., 2008, 2009, 2010). Initial experiments were motivated by previous reports that activation of neuronal NMDA receptors leads to increased production of ROS (Kishida et al., 2005; Girouard et al., 2009). We observed that exposure to NMDA increased production of ROS in podocytes using a fluorometric assay based on production of a highly fluorescent product in the presence of horseradish peroxidase (Fig. 1A). This assay primarily detects production of H2O2, and we observed that 24 h of exposure to 100 μM NMDA caused a marked increase in H2O2 production that was maintained with longer exposures up to 72 h. However, a 6-h exposure to NMDA did not produce a detectable increase in bulk cytosolic H2O2 above that seen in untreated cells. The NMDA-evoked increase in H2O2 was not seen in cells concurrently treated with 100 μM MnTBAP, a membrane-permeable mimetic of superoxide dismutase (Batinić-Haberle, 2002) and catalase (Day et al., 1997) that we have used previously in studies of mouse podocytes (Kim et al., 2012).
Fig. 1.
NMDA treatment increases generation of ROS and mobilization of p47(phox) in a podocyte cell line. A, increase in H2O2 generation in podocytes treated with 100 μM NMDA for 24 to 72 h is quenched by concurrent exposure to 100 μM MnTBAP, a scavenger of ROS. NMDA treatment for 6 h was not sufficient to produce an increase in bulk cytosolic H2O2 large enough to be detected by this fluorometric assay. In this and subsequent figures, bar graphs denote means ± S.E.M. B, representative cell surface biotinylation assay showing the increase in steady-state surface expression of p47(phox) after a 24-h exposure to 100 μM NMDA. This effect was completely blocked by concurrent exposure to 10 μM MK-801. C, densitometric analysis of three repetitions of the experiment illustrated in B. Con, control; AU, arbitrary units.
ROS are produced as a product of various NADPH oxidases, which have been implicated in NMDA receptor-mediated excitotoxic responses in neurons (Kishida et al., 2005; Girouard et al., 2009). The NADPH oxidase NOX2 is normally regulated by formation of complexes at the cell surface with cytosolic regulatory subunits, including one known as p47(phox) (Bedard and Krause, 2007). Using cell-surface biotinylation assays, we observed that 24-h treatment with NMDA resulted in a marked increase in the steady-state surface expression of p47(phox) that was blocked by concurrent treatment with the NMDA antagonist MK-801 (Fig. 1, B and C). In contrast, NMDA had no consistent effect on the steady-state surface expression of NOX2 or NOX4 (data not shown). Taken together, these data indicate that, as in neurons, NMDA receptor activation in podocytes results in oxidative stress associated with increased production of ROS that is mediated at least in part by modulation of an NADPH oxidase isoform.
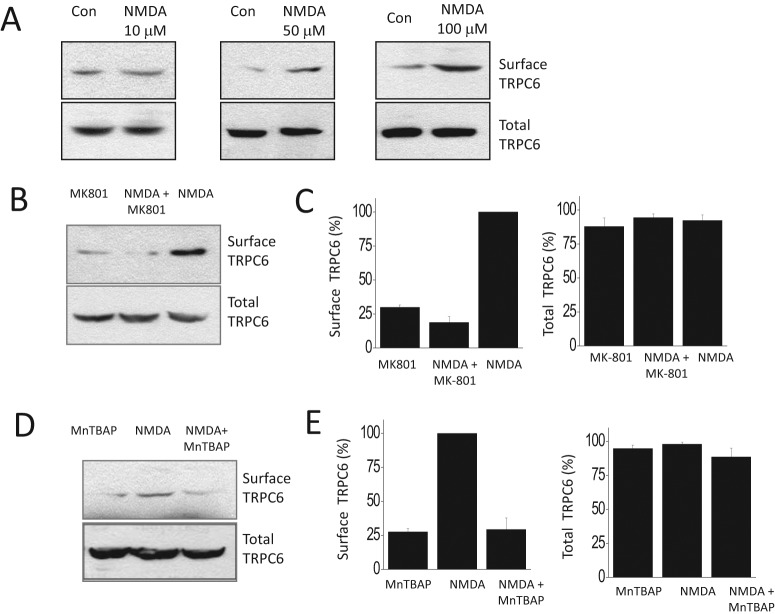
Previous studies have shown that TRPC6 channels are redox-sensitive proteins (Wang et al., 2009; Graham et al., 2010), and we have shown that exogenous application of H2O2 and other treatments that increase generation of ROS result in increased surface expression of podocyte TRPC6 channels (Kim et al., 2012). In the present study, we have observed that 24-h treatment with 50 or 100 μM NMDA caused a robust increase in the steady-state surface expression of podocyte TRPC6 channels (Fig. 2A). Moreover, this effect was blocked by concurrent application of MK-801 (Fig. 2B) or MnTBAP (Fig. 2C).
Fig. 2.
NMDA treatment evokes an increase in steady-state surface expression of podocyte TRPC6 channels. A, representative cell surface biotinylation assays showing surface expression of TRPC6 after 24 h of NMDA exposure at the concentrations indicated. Note the robust effects of NMDA at 50 and 100 μM. B, the effect of 24-h exposure to 100 μM NMDA is completely inhibited by concurrent exposure to 10 μM MK-801. C, densitometric analysis of three repetitions of the experiment shown in B. D, the effect of 100 μM NMDA is also inhibited by concurrent exposure to a 100 μM concentration of the ROS scavenger MnTBAP. E, densitometric analysis of three repetitions of the experiment shown in D. Con, control.
We also observed an increase in functional macroscopic currents with pharmacological properties of TRPC6 (Fig. 3). In these experiments, podocytes were treated with NMDA receptor agonists and antagonists for 24 h. Cells were then treated with 100 μM OAG for 15 min before whole-cell recordings. OAG is a membrane-permeable analog of diacylglycerol that can activate TRPC6, TRPC3, and TRPC7 channels (Hofmann et al., 1999). We have previously shown that the OAG-evoked macroscopic currents in podocytes are eliminated by TRPC6 knockdown (Kim et al., 2012). Whole-cell currents were evoked by 2.5-s duration ramp voltage commands (−80 to +80 mV) before and after application of 50 μM La3+ (Fig. 3A), which blocks TRPC6 channels but not TRPC5 channels in podocytes (Tian et al., 2010). The La3+-sensitive components of the currents were obtained by digital subtraction in each cell and then quantified (Fig. 3B). We observed that La3+-sensitive currents (measured at +80 mV) in cells previously exposed to NMDA were nearly 3-fold larger than those observed in untreated cells, and the effects of NMDA were blocked by concurrent treatment with either 10 μM MK-801 or 100 μM MnTBAP (Fig. 3, A and B). In addition, we observed that the currents recorded using these protocols were almost completely inhibited by 50 μM SKF-96365 (Fig. 3C), an agent that blocks cationic channels in the TRPC family and that nearly eliminates TRPC6 currents in podocytes at this concentration (Kim et al., 2012). It is important to note that in these experiments, NMDA treatments were completed before electrophysiology, and NMDA was not present at the time the recordings were made. Taken together, these results indicate that NMDA-evoked generation of ROS results in mobilization of functional TRPC6 channels. The electrophysiological data are therefore consistent with the results of cell surface biotinylation assays.
Fig. 3.
NMDA treatment increases TRPC6-like cationic currents in podocytes. All cells were treated with 100 μM OAG for 15 min before recordings were made. A, examples of whole-cell currents evoked by ramp voltage commands (−80 mV to +80 mV in 2.5 s) made from a holding potential of −40 mV. Traces on the left show currents before and after bath superfusion of 50 μM La3+, and traces on the right are digital subtractions [control (Con) − La3+]. Examples shown are from control cells and from cells treated for the previous 24 h with 100 μM NMDA in presence and absence of 10 μM MK-801 or 100 μM MnTBAP, as indicated. NMDA was not present at the time recordings were made. Note the increase in La3+-sensitive currents in NMDA-treated cells and inhibition of this effect by MK-801 and MnTBAP. B, summary of results of recordings with n = 10 cells in each group. The ordinate represents the La3+-sensitive current measured at +80 mV. *, P < 0.05 as determined by one-way analysis of variance followed by Tukey's post hoc test. C, as in A except that traces show currents evoked before and after application of 50 μM SKF-96365.
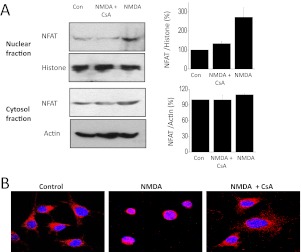
These observations raised the possibility that sustained activation of NMDA receptors in podocytes can engage functionally significant pathways that are downstream of TRPC6. One such pathway entails activation of NFAT (Schlöndorff et al., 2009). Highly phosphorylated NFAT normally resides in the cytosol. However, TRPC6-mediated activation of calcineurin results in dephosphorylation of NFAT, causing some of it to translocate to the nucleus where it can regulate gene expression (Scott et al., 1997; Kuwahara et al., 2006). Using an assay based on cell fractionation, we observed that 100 μM NMDA treatment for 24 h caused a marked increase in the amount of NFAT that is located in nuclei (a cell fraction enriched in histone) but did not produce a substantial effect on cytosolic NFAT (a much larger pool in a cell fraction in which actin is abundant) (Fig. 4A). This effect of NMDA was blocked by concurrent exposure to 10 μM MK-801. It was also blocked by 50 μM SKF-96365. It is noteworthy that SKF-96365 does not block NMDA receptors in neurons (Zhu et al., 2005), and this result suggests that mobilization of TRPC6 underlies this response to NMDA. Stimulatory effects of NMDA could also be seen using confocal microscopy to examine localization of NFAT after drug treatments (Fig. 4B). These experiments showed that NMDA treatment increased the nuclear localization of NFAT and that this effect was blocked by concurrent treatment with MK-801 and SKF-96365. Using the same methods, we also observed that the effects of NMDA on nuclear localization of NFAT were also blocked by concurrent treatment with 10 μM CsA, an inhibitor of the phosphatase calcineurin (Fig. 5).
Fig. 4.
NMDA increases nuclear localization of NFAT in podocytes. A, NMDA treatment for 24 h increases the amount of NFAT detected by immunoblot in a nuclear extract of podocytes. Histone expression was used to monitor loading. There was no change in NFAT expression in the cytosolic fraction in which actin expression was used to monitor loading. The effects of NMDA were blocked by either 10 μM MK-801 or 10 μM SKF-96365, an inhibitor of TRPC6 channels. B, densitometric analysis of three repetitions of the experiment shown in A. C, confocal images of podocytes showing expression of NFAT (red) and DAPI-stained nuclei (blue). Note the concentration of NFAT in nuclei after a 24-h NMDA treatment and inhibition of this effect by MK-801 and SKF-96365. Con, control.
Fig. 5.
NMDA stimulation of nuclear NFAT expression in podocytes is blocked by cyclosporine. Using the methods described in the previous figure, we observed that NMDA effects were blocked by concurrent exposure to 10 μM CsA, as assessed by immunoblot (A) and confocal (B) detection of nuclear NFAT. Con, control.
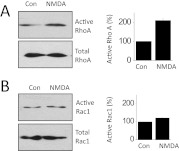
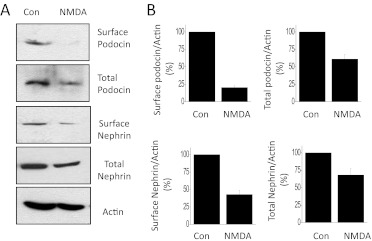
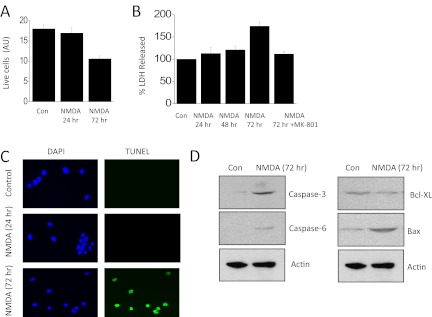
One significant consequence of activation of TRPC6-calcineurin signaling cascades in podocytes is modulation of small GTPases (Greka and Mundel, 2012). We have previously shown that NMDA can cause activation of Rho, which we have repeated in the present studies (Fig. 6A). We have extended these observations to show that NMDA treatment for 24 h produces much less Rac activation (Fig. 6B). Rac activation in podocytes is reported to be preferentially coupled to TRPC5 signaling cascades (Tian et al., 2010). Another consequence of NMDA treatment for 24 h is an apparent dedifferentiation or loss of cell function as evidenced by a marked fall in total expression of the podocyte markers nephrin and podocin relative to that of actin and an even larger relative decrease in the steady-state surface expression of these membrane proteins (Fig. 7). This result cannot be explained by a loss of viable cells, at least at 24 h. Thus, we observed that normal numbers of cells are still present after 24 h of NMDA treatment (Fig. 8A), which indicates that these cells are remarkably resistant to excitotoxic effects of NMDA compared with neurons (Choi, 1992). However, 72 h of continuous exposure to NMDA causes podocytes to die as evidenced by simply counting the number of cells that can be labeled with vital dyes (Fig. 8A) or by monitoring release of lactate dehydrogenase into the medium (Fig. 8B). As in neurons (Hardingham, 2009), the cell death evoked by 72-h NMDA treatment seems to be mediated in part by apoptosis. Thus, NMDA treatment resulted in marked fragmentation of podocyte DNA detected using immunofluorescence TUNEL assays (Fig. 8C). TUNEL signal was not detected in control cells or in cells treated with NMDA for 24 h. In addition, NMDA treatment for 72 h evoked increases in caspase-3, caspase-6, and Bax and a fall in Bcl-XL, as measured by immunoblot (Fig. 8D).
Fig. 6.
Effects of NMDA on activation of small GTPases in podocytes. A, representative glutathione transferase pull-down assay (left) and densitometric analysis of three repetitions of this assay (right) showing that 24-h exposure to 100 μM NMDA evokes activation of RhoA but does not affect total RhoA. B, NMDA treatment does not change levels of activated or total Rac1. Con, control.
Fig. 7.
NMDA treatment reduces total and surface expression of podocyte markers. Cells were treated with 100 μM NMDA for 24 h, and total and surface expression of nephrin and podocin was analyzed using cell-surface biotinylation assays. Actin was used as a separate loading control. NMDA treatment caused a reduction in total expression of both nephrin and podocin relative to actin. This treatment caused even larger reductions in the amount of these proteins that could be detected at the cell surface. A, a representative example of this experiment. B, densitometric analysis of three repetitions of this experiment. All signals in the ordinate are normalized to total actin. Con, control.
Fig. 8.
More prolonged NMDA treatment causes loss of viable podocytes. A, a 24-h treatment with 100 μM NMDA did not affect podocyte viability as assessed by dye-exclusion procedures, but 72-h treatments reduced cell viability. B, a similar pattern was observed by monitoring release of lactate dehydrogenase (LDH) into the medium, and the effect of a 72-h treatment with NMDA was blocked by concurrent exposure to MK-801. C, treatment with NMDA for 72 h evoked DNA fragmentation as assessed by TUNEL staining of nuclei (green fluorescence). This was not seen in control cells or in cells exposed to NMDA for 24 h. In these images, nuclei are counterstained using DAPI (blue fluorescence). D, a 72-h exposure to 100 μM NMDA increased expression of apoptotic markers caspase-3, caspase-6 and Bax and caused a modest decrease in Bcl-XL. AU, arbitrary units; Con, control.
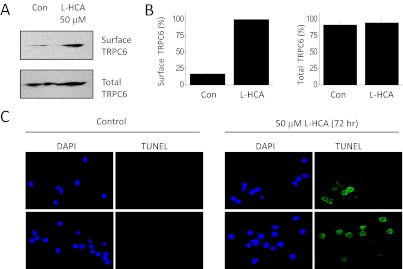
NMDA is an artificial ligand, albeit one that is a highly selective agonist for NMDA receptors. The question arises as to whether an endogenously occurring NMDA receptor agonist can evoke similar effects. We observed that 24-h exposure to 50 μM l-HCA evoked a robust increase in surface expression of TRPC6 channels as assessed by cell surface biotinylation assays, a response that was indistinguishable from that evoked by NMDA (Fig. 9, A and B), as well as an increase in amplitudes of OAG-evoked TRPC6 currents (data not shown). We also observed that a 72-h exposure to 50 μM l-HCA evoked nuclear fragmentation similar to that evoked by NMDA (Fig. 9C), suggesting that this NMDA agonist can also evoke apoptotic cell death.
Fig. 9.
An endogenous agonist of NMDA receptors also mobilizes podocyte TRPC6 channels and evokes nuclear fragmentation. A, representative cell surface biotinylation assay showing an increase in steady-state surface expression of podocyte TRPC6 channels after a 24-h exposure to 50 μM l-HCA. B, densitometric analysis of three repetitions of the experiment shown in A. C, TUNEL assays of nuclear fragmentation in control podocytes and in podocytes treated with 50 μM l-HCA for 72 h. Con, control.
Discussion
In this study we have demonstrated a functional link between sustained activation of podocyte NMDA receptors and the mobilization of TRPC6 channels to the cell. Mobilization of TRPC6 channels seems to be driven by generation of ROS and leads to engagement of associated downstream signaling cascades, including calcineurin, NFAT, and Rho. Sustained activation of NMDA receptors also leads to reduced surface expression of the essential slit diaphragm proteins nephrin and podocin and eventually induces apoptotic cell death. The pathways activated by NMDA may not be entirely in series. For example, it is likely that continuous production of ROS will exert deleterious effects on podocytes independent of TRPC6 mobilization, and calcineurin effects on Rho do not require activation of NFAT (Faul et al., 2008). However, coupling to TRPC6 is likely to amplify the adverse effects of sustained NMDA receptor activation.
In neurons, the NMDA-evoked generation of ROS is mediated by activation of the NADPH oxidase NOX2 (Kishida et al., 2005; Girouard et al., 2009). Our results suggest that a similar mechanism occurs in podocytes, because we observed increased surface expression of p47(phox) after 24 h of NMDA treatment and inhibition of NMDA effects by a ROS scavenger. In contrast, we did not see marked increases in steady-state surface expression of NO2 or NOX4. Recall that p47(phox) is one of two cytosolic auxiliary subunits of NOX2, and its translocation to the cell surface is an essential step leading to activation of NOX2 catalytic activity (Bedard and Krause, 2007). Previous studies have shown that TRPC6 are redox-sensitive channels and that ROS affects both the gating and steady-state expression of these channels on the cell surface (Wang et al., 2009; Graham et al., 2010; Kim et al., 2012). We have previously shown that generation of ROS via mobilization of the NADPH oxidase NOX4 contributes to insulin-evoked mobilization of TRPC6 channels, and we have proposed that this is a normal feature of insulin signaling in podocytes (Kim et al., 2012). Therefore, it is interesting that NMDA seems to mobilize podocyte TRPC6 channels through its actions on a different NADPH oxidase (NOX2). The mechanisms whereby NMDA receptor activation leads to activation of NOX2 are not clear. However, it is possible that there are multiple independently regulated populations of TRPC6 channels. In this regard, TRPC6 channels have been detected in the cell body and major processes of podocytes as well as at the slit diaphragm (Reiser et al., 2005; Huber et al., 2006), and TRPC6 channels are likely to reside in molecularly distinct complexes, depending on their location in the cell (Roselli et al., 2002; Huber et al., 2006). It is also possible that transient elevations of ROS may play an important role in normal signaling cascades, but continuous elevation of these species may induce pathophysiological responses, as with Ca2+ signaling (Lavin and Winn, 2011).
The NMDA-evoked increase in surface expression of podocyte TRPC6 channels is associated with an increase in OAG-activated cationic currents in podocytes that are blocked by La3+ or SKF-96365. This effect of NMDA was abolished by concurrent exposure to the NMDA antagonist MK-801 and by quenching ROS. NMDA-evoked mobilization of TRPC6 channels also increased NFAT localization in podocyte nuclei. NFAT is a transcription factor that is regulated by the phosphatase calcineurin, which dephosphorylates NFAT and thereby allows its translocation to the nucleus (Scott et al., 1997). A contribution of TRPC6 channels to this pathway was initially established in cardiac cells, where it is thought to play a role in angiotensin-mediated cardiac hypertrophy (Kuwahara et al., 2006). A role for NFAT in podocyte signaling was implicated by studies in cultured cells using a luminescent NFAT promoter (Schlöndorff et al., 2009; Nijenhuis et al., 2011), and it has been observed more recently that podocyte-specific overexpression of NFAT leads to albuminuria and glomerulosclerosis, along with reduced expression of nephrin and synaptopodin (Wang et al., 2010).
In the present study we observed that NMDA-evoked movement of endogenous NFAT to the nucleus was completely blocked by MK-801 and was also blocked by the TRPC inhibitor SKF-96365. We should note that SKF-96365 does not inhibit NMDA receptors (Zhu et al., 2005), and these data therefore suggest that the effects of NMDA on NFAT require amplification by TRPC channels. In this regard, we also observed partial inhibition of NMDA-evoked NFAT activation after TRPC6 knockdown using small interfering RNA (data not shown), although we should also note that the TRPC6 knockdown in those experiments was not complete. We also blocked NMDA-evoked NFAT mobilization using CsA, confirming that calcineurin activation is an essential intermediate step in this cascade, as described previously (Schlöndorff et al., 2009; Wang et al., 2011). Calcineurin can also affect the podocyte cytoskeleton, in part through dephosphorylation of synaptopodin, which in turn affects Rho signaling (Faul et al., 2008). A previous study has suggested that TRPC6 is preferentially coupled to RhoA signaling in podocytes, whereas TRPC5 is preferentially coupled to Rac1 (Tian et al., 2010). In the present study, we confirmed that NMDA causes robust activation of Rho, consistent with the observed mobilization of TRPC6. In a separate set of experiments we have observed that NMDA causes at least some mobilization of TRPC5 channels to the cell surface (data not shown), but for whatever reason this was not sufficient to cause activation of Rac. It is possible that Rac activation also requires activation or overactivation of angiotensin signaling systems in these cells (Tian et al., 2010). In any case, a role for TRPC5 in podocyte physiology has not as yet been established by in vivo experiments.
Treatment of podocytes with NMDA for 24 h evoked a fall in total expression of both nephrin and podocin relative to that of actin. It is noteworthy that this treatment caused an even more striking decrease in the steady-state surface expression of these membrane proteins. A reduction in podocin and nephrin expression at the protein level is observed in several acquired glomerular diseases (Horinouchi et al., 2003; Koop et al., 2003) and may be a consequence of NFAT activation (Wang et al., 2010). With even longer exposure to NMDA (72 h), we observed apoptotic cell death on the basis of several criteria. In this regard, sustained activation of calcineurin in podocytes can also lead to apoptosis (Wang et al., 2011). Likewise, ROS-mediated activation of TRPC6 has been implicated in podocyte pathology evoked by puromycin aminonucleoside, and this effect is sensitive to inhibition of NOX2 (Wang et al., 2009), suggesting that the combination of oxidative stress and Ca2+ overload is highly deleterious for the normal function of podocytes and their foot processes (Lavin and Winn, 2011). Nevertheless, it is notable that podocytes are much less sensitive to toxic effects of NMDA than neurons, which generally die within 6 h of the onset of treatment.
Given all this findings, the question arises as to what physiological conditions might lead to sustained activation of podocyte NMDA receptors and the initiation of these cascades? The NMDA receptors of podocytes are not activated by l-glutamate or l-aspartate, in very marked contrast to neuronal NMDA receptors (Anderson et al., 2011). The molecular basis for this anomalous feature is not understood, but it suggests that NMDA receptors are not part of the local glutamate signaling pathway recently identified in podocytes (Rastaldi et al., 2006; Giardino et al., 2009). That pathway instead seems to be mediated by metabotropic glutamate receptors (Puliti et al., 2011; Gu et al., 2012). Podocyte (Anderson et al., 2011) and neuronal (Kim et al., 1987; Olney et al., 1987) NMDA receptors are robustly activated by sulfur-containing derivatives of methionine metabolism, such as l-HCA. These metabolites are markedly elevated in chronic kidney disease (Massy, 2006; Potter et al., 2008) and can achieve circulating concentrations sufficient to activate NMDA receptors (Heinz et al., 2009). In this regard, the deleterious effects of hyperhomocysteinemia on cardiac tissue are markedly reduced in mice with heart-specific knockdown of NMDA receptor NR1 subunits (Tyagi et al., 2010). In addition, a variety of treatments that lead to chronically elevated serum l-homocysteine levels in rodents result in glomerulosclerosis and effacement of podocyte foot processes (Yi et al., 2007; Sen et al., 2010; Zhang et al., 2010a,b, 2011). Moreover, the glomerulosclerosis and proteinuria evoked by hyperhomocysteinemia can be reduced by in vivo administration of MK-801 (Zhang et al., 2010b) and suppressed by knockdown of auxiliary subunits of NOX2 (Zhang et al., 2011). We have previously demonstrated that podocyte NMDA receptors are activated by l-HCA (Anderson et al., 2011), and in the present study we observed that l-HCA could also induce robust mobilization of TRPC6, as was seen with NMDA. As with NMDA, exposure to l-HCA for 72 h also evoked nuclear fragmentation. These results raise the possibility of a pathophysiological positive feedback loop in which elevated circulating l-homocysteine metabolites lead to NMDA receptor- and TRPC6-mediated glomerular damage and kidney disease. This could result in additional elevations in l-homocysteine and acceleration of nephropathy. It is possible that intervening in this vicious cycle through inhibition of either NMDA receptors or TRPC6 channels could slow the loss of nephrons and may represent a plausible strategy for treatment of chronic glomerulodegenerative diseases.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK8259]; and by an American Society for Nephrology James M. Scherbenske Award.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- TRPC
- transient receptor potential canonical
- NMDA
- N-methyl-d-aspartic acid
- ROS
- reactive oxygen species
- MK-801
- dizocilpine maleate
- l-HCA
- l-homocysteic acid
- NOX
- NADPH oxidase
- CsA
- cyclosporine
- NFAT
- nuclear factor of activated T cells
- SKF-96365
- 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl]imidazole
- MnTBAP
- manganese(III) tetrakis(4-benzoic acid)porphyrin chloride
- DAPI
- 4′-6-diamidino-2-phenylindole
- OAG
- 1-oleoyl-2-acetyl-sn-glycerol
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Authorship Contributions
Participated in research design: Kim, Anderson, and Dryer.
Conducted experiments: Kim and Anderson.
Performed data analysis: Kim and Anderson.
Wrote or contributed to the writing of the manuscript: Kim and Dryer.
References
- Anderson M, Suh JM, Kim EY, Dryer SE. (2011) Functional NMDA receptors with atypical properties are expressed in podocytes. Am J Physiol Cell Physiol 300:C22–C32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batinić-Haberle I. (2002) Manganese porphyrins and related compounds as mimics of superoxide dismutase. Methods Enzymol 349:223–233 [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39 [DOI] [PubMed] [Google Scholar]
- Choi DW. (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276 [DOI] [PubMed] [Google Scholar]
- Cline HT, Constantine-Paton M. (1989) NMDA receptor antagonists disrupt the retinotectal topographic map. Neuron 3:413–426 [DOI] [PubMed] [Google Scholar]
- Day BJ, Fridovich I, Crapo JD. (1997) Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys 347:256–262 [DOI] [PubMed] [Google Scholar]
- Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. (2008) The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14:931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis ME, Eggers PW, Hostetter TH, Briggs JP. (2004) Association between serum homocysteine and markers of impaired kidney function in adults in the United States. Kidney Int 66:303–312 [DOI] [PubMed] [Google Scholar]
- Giardino L, Armelloni S, Corbelli A, Mattinzoli D, Zennaro C, Guerrot D, Tourrel F, Ikehata M, Li M, Berra S, Carraro M, Messa P, Rastaldi MP. (2009) Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J Am Soc Nephrol 20:1929–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, Iadecola C. (2009) NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci 29:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, Yorio T, Ma H, Ma R. (2010) Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem 285:23466–23476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka A, Mundel P. (2012) Cell biology and pathology of podocytes. Annu Rev Physiol 74:299–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Liu W, Wei J, Yan Z. (2012) Regulation of N-methyl-d-aspartic acid (NMDA) receptors by metabotropic glutamate receptor 7. J Biol Chem 287:10265–10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE. (2009) Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem Soc Trans 37:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz J, Kropf S, Luley C, Dierkes J. (2009) Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: a meta-analysis. Am J Kidney Dis 54:478–489 [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397:259–263 [DOI] [PubMed] [Google Scholar]
- Horinouchi I, Nakazato H, Kawano T, Iyama K, Furuse A, Arizono K, Machida J, Sakamoto T, Endo F, Hattori S. (2003) In situ evaluation of podocin in normal and glomerular diseases. Kidney Int 64:2092–2099 [DOI] [PubMed] [Google Scholar]
- Huber TB, Kwoh C, Wu H, Asanuma K, Gödel M, Hartleben B, Blumer KJ, Miner JH, Mundel P, Shaw AS. (2006) Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest 116:1337–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager A, Kostense PJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Donker AJ, Stehouwer CD. (2001) Serum homocysteine levels are associated with the development of (micro)albuminuria: the Hoorn study. Arterioscler Thromb Vasc Biol 21:74–81 [DOI] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. (1993) Calcium permeability of the N-methyl-d-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci USA 90:11573–11577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Anderson M, Dryer SE. (2012) Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol 302:F298–F307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Choi KJ, Dryer SE. (2008) Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface. Am J Physiol Renal Physiol 295:F235–F246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Chiu YH, Dryer SE. (2009) Neph1 regulates steady-state surface expression of Slo1 Ca2+-activated K+ channels: different effects in embryonic neurons and podocytes. Am J Physiol Cell Physiol 297:C1379–C13888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Suh JM, Chiu YH, Dryer SE. (2010) Regulation of podocyte BKCa channels by synaptopodin, Rho, and actin microfilaments. Am J Physiol Renal Physiol 299:F594–F604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JP, Koh JY, Choi DW. (1987) l-Homocysteate is a potent neurotoxin on cultured cortical neurons. Brain Res 437:103–110 [DOI] [PubMed] [Google Scholar]
- Kishida KT, Pao M, Holland SM, Klann E. (2005) NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem 94:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, Bruijn JA. (2003) Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol 14:2063–2071 [DOI] [PubMed] [Google Scholar]
- Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, Carpio JD, Ruiz P, Mezzano SA, Li J, Wei C, Reiser J, Young JI, Walz K. (2010) Podocyte-specific overexpression of wild type or mutant trpkc6 in mice is sufficient to cause glomerular disease. PLoS One 5:e12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. (2006) TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116:3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin PJ, Winn MP. (2011) TORCing up the importance of calcium signaling. J Am Soc Nephrol 22:1391–1393 [DOI] [PubMed] [Google Scholar]
- Makhro A, Wang J, Vogel J, Boldyrev AA, Gassmann M, Kaestner L, Bogdanova A. (2010) Functional NMDA receptors in rat erythrocytes. Am J Physiol Cell Physiol 298:C1315–C1325 [DOI] [PubMed] [Google Scholar]
- Mashkina AP, Cizkova D, Vanicky I, Boldyrev AA. (2010) NMDA receptors are expressed in lymphocytes activated both in vitro and in vivo. Cell Mol Neurobiol 30:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massy ZA. (2006) Therapy of hyperhomocysteinemia in chronic kidney disease. Semin Nephrol 26:24–27 [DOI] [PubMed] [Google Scholar]
- Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Möller CC, Hamming I, Navis G, Wetzels JF, Berden JH, Reiser J, Faul C, van der Vlag J. (2011) Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179:1719–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Price MT, Salles KS, Labruyere J, Ryerson R, Mahan K, Frierdich G, Samson L. (1987) l-Homocysteic acid: an endogenous excitotoxic ligand of the NMDA receptor. Brain Res Bull 19:597–602 [DOI] [PubMed] [Google Scholar]
- Parisi E, Almadén Y, Ibarz M, Panizo S, Cardús A, Rodriguez M, Fernandez E, Valdivielso JM. (2009) N-Methyl-d-aspartate receptors are expressed in rat parathyroid gland and regulate PTH secretion. Am J Physiol Renal Physiol 296:F1291–F1296 [DOI] [PubMed] [Google Scholar]
- Patton AJ, Genever PG, Birch MA, Suva LJ, Skerry TM. (1998) Expression of an N-methyl-d-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone 22:645–649 [DOI] [PubMed] [Google Scholar]
- Pavenstädt H, Kriz W, Kretzler M. (2003) Cell biology of the glomerular podocyte. Physiol Rev 83:253–307 [DOI] [PubMed] [Google Scholar]
- Potter K, Hankey GJ, Green DJ, Eikelboom JW, Arnolda LF. (2008) Homocysteine or renal impairment: which is the real cardiovascular risk factor? Arterioscler Thromb Vasc Biol 28:1158–1164 [DOI] [PubMed] [Google Scholar]
- Puliti A, Rossi PI, Caridi G, Corbelli A, Ikehata M, Armelloni S, Li M, Zennaro C, Conti V, Vaccari CM, Cassanello M, Calevo MG, Emionite L, Ravazzolo R, Rastaldi MP. (2011) Albuminuria and glomerular damage in mice lacking the metabotropic glutamate receptor 1. Am J Pathol 178:1257–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastaldi MP, Armelloni S, Berra S, Calvaresi N, Corbelli A, Giardino LA, Li M, Wang GQ, Fornasieri A, Villa A, Heikkila E, Soliymani R, Boucherot A, Cohen CD, Kretzler M, Nitsche A, Ripamonti M, Malgaroli A, Pesaresi M, Forloni GL, Schlöndorff D, Holthofer H, D'Amico G. (2006) Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J 20:976–978 [DOI] [PubMed] [Google Scholar]
- Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. (2005) TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attié T, Gubler MC, Antignac C. (2002) Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol 160:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlöndorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR. (2009) TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol 296:C558–C569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE, Ruff VA, Leach KL. (1997) Dynamic equilibrium between calcineurin and kinase activities regulates the phosphorylation state and localization of the nuclear factor of activated T-cells. Biochem J 324:597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen U, Munjal C, Qipshidze N, Abe O, Gargoum R, Tyagi SC. (2010) Hydrogen sulfide regulates homocysteine-mediated glomerulosclerosis. Am J Nephrol 31:442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato I. (2002) Podocyte process effacement in vivo. Microsc Res Tech 57:241–246 [DOI] [PubMed] [Google Scholar]
- Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstädt H, Pavenstaedt H, Hsu HH, Schlondorff J, Ramos A, Greka A. (2010) Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 3:ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Vacek JC, Givvimani S, Sen U, Tyagi SC. (2010) Cardiac specific deletion of N-methyl-d-aspartate receptor 1 ameliorates mtMMP-9 mediated autophagy/mitophagy in hyperhomocysteinemia. J Recept Signal Transduct Res 30:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chang JH, Paik SY, Tang Y, Eisner W, Spurney RF. (2011) Calcineurin (CN) activation promotes apoptosis of glomerular podocytes both in vitro and in vivo. Mol Endocrinol 25:1376–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, Liapis H, Miner JH, Chen F. (2010) Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol 21:1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wei X, Zhang Y, Ma X, Li B, Zhang S, Du P, Zhang X, Yi F. (2009) NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell Physiol Biochem 24:619–626 [DOI] [PubMed] [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. (2005) A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308:1801–1804 [DOI] [PubMed] [Google Scholar]
- Yi F, dos Santos EA, Xia M, Chen QZ, Li PL, Li N. (2007) Podocyte injury and glomerulosclerosis in hyperhomocysteinemic rats. Am J Nephrol 27:262–268 [DOI] [PubMed] [Google Scholar]
- Zhang C, Hu JJ, Xia M, Boini KM, Brimson C, Li PL. (2010a) Redox signaling via lipid raft clustering in homocysteine-induced injury of podocytes. Biochim Biophys Acta 1803:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Xia M, Boini KM, Li CX, Abais JM, Li XX, Laperle LA, Li PL. (2011) Epithelial-to-mesenchymal transition in podocytes mediated by activation of NADPH oxidase in hyperhomocysteinemia. Pflugers Arch 462:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yi F, Xia M, Boini KM, Zhu Q, Laperle LA, Abais JM, Brimson CA, Li PL. (2010b) NMDA receptor-mediated activation of NADPH oxidase and glomerulosclerosis in hyperhomocysteinemic rats. Antioxid Redox Signal 13:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZT, Munhall A, Shen KZ, Johnson SW. (2005) NMDA enhances a depolarization-activated inward current in subthalamic neurons. Neuropharmacology 49:317–327 [DOI] [PubMed] [Google Scholar]