Abstract
P2X receptors are trimeric membrane proteins. When they bind extracellular ATP, a conformational change occurs that opens a transmembrane ion channel. The ATP-binding pocket is formed in a cleft between two subunits, and a critical amino acid residue for ATP contact is Lys69 (P2X2 numbering). In the present work, we sought to determine whether the binding of fewer than three ATP molecules could open the ion channel. We expressed eight concatenated cDNAs in human embryonic kidney cells, which encoded three serially joined, epitope-tagged, subunits with either Lys or Ala at position 69 (denoted as KKK, KKA, KAK, AKK, KAA, AKA, AAK, and AAA). Western blotting of surface-biotinylated proteins indicated that breakdown of concatemers to individual subunits was minimal. Recording of membrane currents in response to ATP (whole cell and excised outside-out patch) showed that all formed functional channels except AAK, AKA, and AAA. There was no difference in the kinetics of activation and deactivation among KKK, KKA, KAK, and AKK channels, and amplitude of the unitary conductances was in all cases not different from that found after expression of a single wild-type subunit. Currents through KKA and KAK receptors were larger than those observed for AKK receptors. The results indicate that trimeric P2X receptors containing only two intact binding sites can be readily activated by ATP.
Introduction
P2X receptors are trimeric ion channels that are widely distributed in mammalian tissues, where they are involved in a range of processes from inflammation to excitatory synaptic transmission (North, 2002). Each P2X subunit has two membrane-spanning domains, an extracellular domain that contains the ATP-binding sites, and intracellular N and C termini (North, 2002). The binding of ATP to the extracellular domain causes a conformational change in the receptor, which results in the opening of a pore permeable to small cations. Abundant evidence supports the trimeric architecture. This includes the following: 1) concentration-response curves of native receptors on dorsal root ganglion neurons (Bean, 1990); 2) single-channel kinetics of cloned and expressed P2X2 receptors (Ding and Sachs, 1999); 3) recordings of membrane currents in concatenated receptors carrying a reporter mutation (Stoop et al., 1999); 4) biochemical purification on nondenaturing gels (Nicke et al., 1998); and 5) the structure of crystallized truncated zebrafish P2X4 receptors in both closed (Kawate et al., 2009) and open conformations (Hattori and Gouaux, 2012).
ATP binds at the interface between two subunits (Wilkinson et al., 2006; Marquez-Klaka et al., 2007; Kawate et al., 2009; Hattori and Gouaux, 2012). Experimental mutagenesis shows that the key residues involved are Lys69, Lys71, Phe183, and Thr184 from one subunit, and Asn288, Phe289, Arg290, and Lys308 from another subunit (P2X2 numbering; reviewed by Browne et al., 2011). Wilkinson et al. (2006) proposed that Lys69 from one subunit joins with Lys308 from a different subunit within a single ATP-binding site. The recent open-channel structure of the zebrafish P2X4 receptor indicates that several of these residues participate in direct contacts with the ATP molecules. For example, oxygen atoms on the γ-phosphorus contact the side-chain positively charged nitrogens of both Lys69 (P2X2 numbering) on one subunit and Arg290 and Lys308 on another.
Given that the holoprotein presents three ATP-binding sites, the purpose of the present work was to investigate whether P2X receptor channel opening could be evoked by occupancy of one, two, or three such sites. Experiments on heteromeric P2X2/3 receptors suggest that fewer than three binding sites need to be occupied to elicit channel opening (Jiang et al., 2003), but that approach depended on the use of αβ-methylene-5′-ATP for selective activation of the P2X2/3 receptors. There is little evidence with respect to any homomeric receptor. We sought to address this by using a concatenated cDNA that encoded a protein with three conjoined subunits.
We used Lys69 as the key residue involved in ATP binding. Substitution of this lysine by alanine has been shown to prevent ATP activation of P2X1 (Ennion et al., 2000; Wilkinson et al., 2006), P2X2 (Jiang et al., 2000), P2X3 (Wilkinson et al., 2006), P2X4 (Wilkinson et al., 2006), and P2X7 (Wilkinson et al., 2006) receptors. It is now clear from the structure of the ATP-bound P2X receptor that the ammonium nitrogen atom of Lys69 nestles within the “U” of the triphosphate chain, making direct contact with α-, β-, and γ-phosphate groups. For the present experiments, we constructed a series of concatenated cDNAs encoding K69A substitutions. Assuming that a concatenated receptor holoprotein is formed by direct translation of this cDNA, this approach allowed us to study the properties of receptors in which none (i.e., wild type), one, two, or three binding-site Lys69 residues had been replaced by alanine.
Materials and Methods
Molecular Biology.
Mutations in rat P2X2 receptors were made as described previously (Cao et al., 2009). The wild-type P2X2 concatenated trimer was created as described previously (Browne et al., 2011), using four restriction sites (NheI, SacII, NotI, and ApaI). The initial methionines of the second and third subunits were removed, leaving a –GRG- linker between the first and second subunits and –GGRR- between the second and third. A C-terminal EE-epitope tag (EYMPME) was also included in each subunit. The final product was ligated into pcDNA3.1(+) vector (Invitrogen, Paisley, UK).
Mutations were introduced into the concatemer by cutting the subunits from the concatemer at the appropriate restriction sites and ligating into a shuttle vector. Site-directed mutagenesis was then performed on the individual P2X2 subunit using the QuikChange method (Agilent Technologies, Santa Clara, CA). The resulting mutation was then polymerase chain reaction-amplified with the appropriate 5′ and 3′ restriction sites on the polymerase chain reaction primer, and the product was then ligated back into the concatemer to give the final product. The constructs were sequenced to confirm the coding region.
Monomeric and concatenated P2X2 subunits were transiently expressed together with green fluorescent protein cDNA in human embryonic kidney (HEK) 293 cells by Lipofectamine 2000 (Invitrogen), using 25 ng/ml monomer and 50 ng/ml concatemer cDNA and 50 ng/ml green fluorescent protein cDNA. Transfected cells were seeded on glass coverslips coated with poly-l-lysine.
Plasma Membrane Protein Biotinylation, Cross-Linking, and Western Blotting.
Forty-eight hours after transfection, cells were washed twice in phosphate-buffered saline containing 1 mM calcium and 0.5 mM magnesium. Cell surface proteins were labeled using 1 mg/ml sulfo-N-hydroxysulfosuccinimide-LC-biotin (sulfo-NHS-LC-biotin; Thermo Fisher Scientific, Waltham, MA) for 30 min at 4°C. Free biotin was quenched by addition of 50 mM Tris (pH 8.0), and cells were then scraped and lysed for 30 min at 4°C under agitation in lysis buffer (20 mM HEPES, 100 mM NaCl, 5 mM EDTA, 1% nonyl phenoxypolyethoxyethanol, pH 7.4, and protease inhibitor cocktail; Thermo Fisher Scientific). Lysates were centrifuged (16,000g, 10 min, 4°C), and total protein extracts were collected. Biotinylated proteins were precipitated by overnight incubation with NeutrAvidin-agarose beads at 4°C. After extensive washes with lysis buffer, proteins were eluted by mixing with lithium dodecyl sulfate loading buffer (Invitrogen) supplemented with 10% β-mercaptoethanol and heating at 80°C for 5 min. Proteins were separated by electrophoresis on NuPage 4 to 12% Bis-Tris gels (Invitrogen), transferred to nitrocellulose membranes, and visualized using an anti-EE antibody (1/5000; Bethyl Laboratories, Montgomery, TX).
Cross-linking was performed as described above for plasma membrane protein biotinylation except that N-hydroxysulfosuccinimide diazirine (sulfo-NHS-SDA) and its uncharged analog N-hydroxysuccinimide diazirine (NHS-SDA) were used at a final concentration of 2 mM, and cells were exposed to UV (365 nm) light for 15 min after quenching. Cells were then lysed, and proteins were separated as described above.
Electrophysiological Recording.
Recordings were made at room temperature 24 to 48 h after transfection, using whole-cell and outside-out configurations of the patch-clamp technique. Patch pipettes were pulled from borosilicate glass (World Precision Instruments, Stevenage, UK) and had final resistances of 2 to 4 MΩ for whole-cell recording (thin-walled glass) and 10 to 20 MΩ for single-channel recording (thick-walled glass). The usual holding potential was −60 and −120 mV for whole-cell and single-channel recordings, respectively. The basic extracellular solution contained the following: 147 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 13 mM d-glucose. ATP stock solutions were made in the extracellular solution, and the pH was adjusted to 7.3 using NaOH. The intracellular (pipette) solution contained the following: 145 mM NaCl, 10 mM HEPES, and 10 mM EGTA. For single-channel recordings, NaF was used in place of NaCl (Ding and Sachs, 1999). All solutions were maintained at pH 7.3 and 300 to 315 mOsM. Chemicals were purchased from Sigma-Aldrich (Poole, Dorset, UK).
Currents were recorded with a patch-clamp amplifier (Axopatch 200B) using pClamp 9 software (Molecular Devices, Sunnyvale, CA). The data were low-pass-filtered at 2 kHz and digitized at 10 (single channel) or 5 kHz (whole cell). Dose-response curves for ATP were constructed by applying different concentrations with a RSC200 rapid perfusion system (Bio-Logic, Claix, France). ATP was applied for 2 s. Current-voltage relations were obtained from the currents measured during a linear voltage ramp (−120 to +120 mV in 500 ms). Between 60 and 80% of the series resistance was electronically compensated to minimize voltage errors in whole-cell recordings. For single-channel recording, low concentrations of ATP were generally used (0.3–3 μM) to obtain single-channel openings. Single-channel conductances were measured as chords from −120 to 0 mV.
For studies of the time course of current activation, we used a pressurized (1.4 bar) RSC200 system (Bio-Logic) and applied ATP to cells that had been lifted free from the surface of the coverslip. Kinetics of activation (up to 400 ms) and deactivation (up to 700 ms) were fitted with single exponentials. The time constant for solution exchange obtained by measuring the current observed when stepping to 50% hypotonic solution was 2 ms; we consider that the limiting resolution for a whole-cell current was approximately 5 to 10 ms.
Data Analysis.
Electrophysiological data were analyzed using Clampfit 9 software (Molecular Devices) and Origin 8.2 (OriginLab, Northampton, MA). Single-channel data were analyzed using QuB (www.qub.buffalo.edu). The mean amplitudes of single-channel currents were measured from all-points amplitude histograms that were fit to a sum of two Gaussian distributions. Pooled data are presented as the mean ± S.E.M. Tests for differences among groups were made using nonparametric analysis of variance and post hoc Kruskal-Wallis tests. Concentration-response curves were fit to the equation I = Imax/[1 + (EC50/[A])nH].
Results
Cells expressing the concatenated wild-type receptor (denoted KKK) had a macroscopic sensitivity to ATP similar to that of cells expressing the wild-type monomeric receptor (denoted K). In both cases, ATP (2 s) evoked sustained inward currents that declined by less than 10% during the application and deactivated fully within 1 s when the application was discontinued (Fig. 1A). The concentrations evoking half-maximal currents (EC50) values were 16 ± 2.0 (n = 9) and 19 ± 1.2 (n = 14) μM for K and KKK, respectively (Table 1). However, the peak current amplitude in KKK receptors (341 ± 39 pA/pF, n = 23) was approximately 50% that in wild-type monomeric receptors (796 ± 149 pA/pF, n = 4). This may be due to lower expression of the concatemer (see Surface Expression of Concatenated P2X2 Receptors).
Fig. 1.
Concatenated P2X2 receptors are functional with fewer than three Lys69 residues. A, representative currents evoked by ATP (2 s; 300 μM for KKK, KKA, and KAK, and 1 mM for AKK, KAA, AKA, and AAA) in HEK293 cells transfected with the eight concatenated constructs. Whole-cell recordings were made at a holding potential of −60 mV. Receptors containing a single domain with a K69A substitution (KKA, KAK, and AKK) showed robust responses to ATP, whereas smaller currents were observed with two of the constructs containing two K69A substitutions (KAA and AAK). B, concentration-response curves for the concatenated constructs. Data points and error bars show mean ± S.E.M.
TABLE 1.
Properties of concatenated P2X2 receptors
| Construct | Peak Current (n)a | EC50 (n) | nH (n)b | g (n)c | ton (n)d | toff (n)e | Membrane Expression (n)f |
|---|---|---|---|---|---|---|---|
| pA/pF | μM | pS | ms | ms | % | ||
| K | 796 ± 149 (4) | 16 ± 2.0 (9) | 2.1 ± 0.2 (9) | 22 ± 1.8 (4) | 23 ± 1.7 (5) | 88 ± 21 (7) | N.D. |
| KKK | 341 ± 39 (23) | 19 ± 1.2 (14) | 2.8 ± 0.2 (14) | 24 ± 1.0 (12) | 63 ± 8.0 (16)d | 114 ± 19 (10) | 100 (3) |
| AKK | 62 ± 11 (18) | 92 ± 28 (14) | 1.3 ± 0.2 (14) | 20 ± 5.9 (4) | 88 ± 9.7 (7)d | 171 ± 31 (7) | 93 ± 27 (3) |
| KAK | 163 ± 39 (11) | 24 ± 5.0 (11) | 2.1 ± 0.2 (11) | 24 ± 1.7 (4) | 58 ± 12 (7)d | 92 ± 9.4 (8) | 104 ± 20 (3) |
| KKA | 146 ± 33 (4) | 25 ± 4.4 (13) | 1.7 ± 0.1 (13) | 21 ± 1.1 (7) | 87 ± 6.0 (9)d | 171 ± 11 (10) | 112 ± 15 (3) |
| KAA | 55 ± 13 (13) | 22 ± 1.9 (12) | 1.9 ± 0.2 (12) | No events (8) | 91 ± 11 (11)d | 132 ± 5.1 (11) | 90 ± 12 (3) |
| AKA | 0.8 ± 0.2 (13) | N.D. | N.D. | No events (5) | N.D. | N.D. | 88 ± 18 (3) |
| AAK | 24 ± 10 (7) | 21 ± 2.3 (11) | 1.9 ± 0.2 (11) | No events (7) | N.D. | N.D. | 114 ± 36 (3) |
| AAA | 0.5 ± 0.1 (11) | N.D. | N.D. | No events (5) | N.D. | N.D. | 113 ± 23 (3) |
N.D., not determined.
K is different from all others (p < 0.05), and AKK is different from KAK and KKA (p < 0.05).
No differences (p > 0.05).
Measured at −120 mV.
Measured at 10 μM ATP, K is different from KKA, AKK, and KAA (p < 0.01).
Measured at 10 μM ATP, K is different from KKA (p < 0.05), and KKA is different from KAK (p < 0.01).
No differences (p > 0.05).
The time courses of activation and deactivation of the current were well fitted by single exponentials, for concentrations 3, 10 (Table 1), 30, and 100 μM. There were no significant differences in kinetics between K and KKK receptors.
Concatenated Receptors with Two, One, or No Lys69 Residues.
Concatenated subunits in which only two of the three domains contained lysine at position 69 provided robust membrane currents in response to ATP (Fig. 1). On average, these had approximately half the amplitude of current observed for the KKK construct (Table 1). The currents evoked by 100 μM ATP were smaller when the alanine was in the first position (AKK) than when it was in the second (KAK) or third (KKA) position (p < 0.05) (Fig. 1B). The whole-cell currents showed inward rectification typical of P2X2 receptors (Evans et al., 1996), and this was not obviously different among the forms tested.
The unitary conductance was determined by outside-out patch recording, using application of 0.3 to 3 μM ATP. ATP readily evoked unitary currents in KKK, AKK, KAK, and KKA constructs, and there was no difference in their unitary conductances (p > 0.05) (Fig. 2A; Table 1).
Fig. 2.
Unitary conductance and kinetics are unchanged in P2X2 concatemers with K69A substitutions. A, ATP (3 μM) activates single channels in outside-out patches from cells expressing the wild-type P2X2 receptor either as a monomer (K) or as a concatemer (KKK). Openings were also observed when one K69A substitution was present (KAK shown as illustration); however, no activity was seen when two or three K69A substitutions were present. All-points histograms fit to a sum of two Gaussian distributions are shown on the right. Data were low-pass-filtered at 3 kHz and digitized at 10 kHz; holding potential was −120 mV. B, kinetics of activation and deactivation in whole cell do not differ significantly between KKK, AKK, KAK, and KKA.
At the whole-cell level, the time course of activation of ATP-induced currents became markedly faster with higher agonist concentrations (Fig. 2B), but there was no difference between wild-type channels and KKK concatemers (measured at 3, 10, 30, and 100 μM; p > 0.05). There was also no significant difference in the kinetics of activation among KKK, AKK, KAK, and KKA receptors for 3, 10, and 30 μM (p > 0.05), although KKK activated more rapidly than the other forms when tested at 100 μM (p < 0.05). The deactivation of currents evoked by ATP was independent of agonist concentration, and there were no consistent significant differences among the four concatenated forms studied (KKK, AKK, KAK, and KKA) (p > 0.05).
P2X2 constructs containing two K69A substitutions showed small ATP-evoked currents in the cases of KAA and AAK (Fig. 1A; Table 1). ATP never evoked currents in the case of the AKA construct (Fig. 1A; Table 1). In outside-out patch recordings, we failed to observe any unitary events for KAA, AKA, or AAK receptors, when tested with a range of ATP concentrations (0.3–10 μM) in five to eight patches. Concatenated receptors containing no lysine residues at position 69 (AAA) also never responded to ATP in whole-cell (1 mM, n = 5) or outside-out patch recordings (100 μM, n = 5). Likewise, in confirmation of previous work (Jiang et al., 2000; Wilkinson et al., 2006), we did not observe currents in the monomeric K69A construct with ATP concentrations up to 10 mM (n = 14).
Surface Expression of Concatenated P2X2 Receptors.
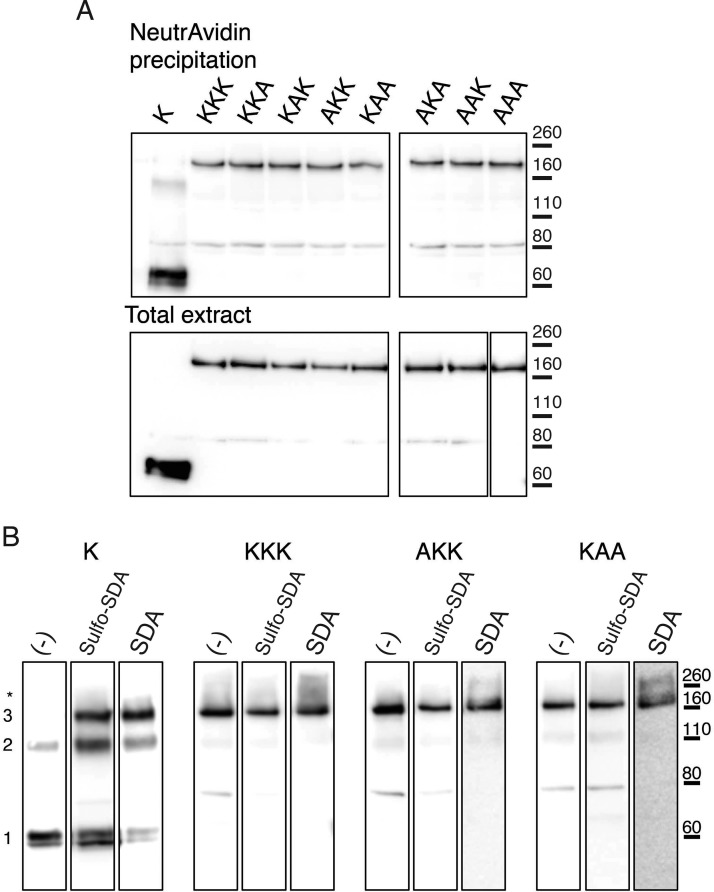
Concatenated receptors might be degraded, with resultant expression of single subunits. They might also form channels in unnatural assemblies: for example, the N-terminal domains of three concatemers might form a channel without participation of the second and third domains. We examined these possibilities by Western blotting of membrane proteins, using the EE epitope inserted into each domain of the concatemer. Figure 3A and Table 1 show that all the receptors with K69A-containing subunits had surface expression that was not significantly different from that seen for the KKK concatemer.
Fig. 3.
Membrane expression and oligomerization of concatenated subunits. A, membrane expression. HEK293 cells transfected with each of the eight concatenated constructs were labeled with sulfo-NHS-LC-biotin, and membrane proteins were precipitated with NeutrAvidin beads. Detection was with anti-EE antibody, from cell surface receptors (top) or total extract (bottom). Results are representative of three independent experiments. B, assembly of concatenated subunits was studied by Western blot after protein cross-linking on living HEK cells with either the membrane-impermeable cross-linker sulfo-SDA or the membrane-permeable cross-linker SDA. After transfection with monomer P2X2 subunits (K), cross-linking indicates formation of monomers (note two bands corresponding to glycosylated and nonglycosylated subunits), dimers, and trimers. Concatenated subunits migrate mainly as trimers after cross-linking. Note that a small proportion of highest molecular weight complex (indicated by an asterisk) can be detected for KKK and KAA concatemers when cross-linked with SDA. Numbers on the left indicate molecular weights expected for monomer, dimer, and trimer. Results are representative of three independent experiments.
In the absence of any cross-linkers, P2X2 receptors formed by expression of single subunits were observed mainly as monomers, with two bands representing the glycosylated and unglycosylated forms of the protein (Fig. 3B). After treatment with the either membrane-permeant SDA or membrane-impermeant sulfo-SDA cross-linker, P2X2 subunits were observed mainly as trimers (Fig. 3B). In contrast, the three concatenated constructs tested were predominantly observed as trimers when tested in the absence of cross-linkers. No bands corresponding to monomers were seen with any of the concatenated receptors, although some very faint bands were present that corresponded in size to that expected for a dimer. There was some evidence for the formation of higher molecular weight assemblies, but these were mainly observed for KAA (asterisk in Fig. 3B). Taken together, these experiments indicate that degradation or higher order assemblies, if they occur, represent only a small fraction of forms of P2X2 at the plasma membrane.
Discussion
There are certain caveats concerning the use of concatenated subunits, and it is important to have confidence that they form P2X receptors in which each of the concatenated domains functions as might an independent subunit of the normal trimeric channel. It is possible that the larger concatenated protein may traffic less readily to the plasma membrane, and this could contribute to the observation that maximal currents evoked at KKK concatemers were only approximately half the amplitude of currents observed after monomer expression. Furthermore, the concatenated construct in the membrane might be subject to conformational constraints because two of the three N and C terminals are “tethered.” This may alter intrinsic gating and may also impair interactions with key partner proteins. Indeed, the “linker” sequence may itself have unwanted conformational effects. Finally, it is also possible that the concatemer degrades into monomeric products before insertion into the membrane.
Previous reports of concatenated P2X2 subunits have suggested that the protein traffics to the plasma membrane in a largely intact state. In the first such paper, Stoop et al. (1999) used the reporter mutation T336C to test the effectiveness of a methanethiosulfonate to inhibit ATP-evoked currents. For concatemers of three subunits, the degree of inhibition was related to number of T336C substitutions introduced, consistent with each playing a similar role in blocking the permeation pathway. Nagaya et al. (2005) exploited concatemers to demonstrate that two cysteines involved in forming a disulfide bond came from different subunits. They reported no evidence for monomer or dimer formation or receptor aggregation. On the other hand, Nicke et al. (2003) found a considerable amount of monomeric and dimeric byproduct after expression of concatenated P2X1 receptors. A possible explanation for the difference might be related to the fact that the P2X2 C terminus is 73 amino acids longer than that of the P2X1 receptor, which perhaps offers greater conformational freedom. Another difference concerns the linker used. Nicke et al. (2003) used a poly-glutamine sequence, which might tend to aggregate the proteins. In the current work, the sequence at the first junction site was …GLAQLDPGLNEYMPMEGRGGRRLA…, and at the second junction site, it was …GLAQLDPGLNEYMPMEGGRRGRRLA… (where the underline indicates the residues that replace the initial M of the second and third subunits).
In the present experiments, we found little evidence that significant breakdown was occurring. Quantification of blots from four independent experiments indicated that <2% of the protein was in monomeric form. Furthermore, the cross-linking experiments indicated that, except perhaps in the case of KAA (Fig. 3B), there was no significant hexamer or nonamer expression, suggesting that channels were not being formed by assemblies comprising two or three concatemers. However, even a very small proportion of breakdown might release sufficient monomeric subunits to form channels and produce detectable currents. Therefore, we cannot exclude the possibility that such a small amount of breakdown, though not detected by Western blotting, might account for the small currents after expression of KAA and AAK forms. For this reason, we focus the subsequent discussion on the results obtained with KKA, KAK, and AKK channels and with the assumption that the currents observed after expression of these constructs result from channels formed by the intact concatenated proteins.
Receptors that had two intact ATP-binding sites (KKA, KAK, and AKK) gave robust responses to ATP, and the current properties were similar to those of wild-type receptors. Single-channel recordings showed that there was no change in the unitary conductance of the receptors containing K69A substitutions, and there were no striking changes in the macroscopic kinetics of channel opening and closing. Given the critical role of the ammonium nitrogen of Lys69 in contacts with the α-, β-, and γ-phosphate moieties of the ATP molecule (Hattori and Gouaux, 2012) and the prior evidence that even Arg substitutes only poorly for Lys at this position (Jiang et al., 2000), it seems reasonable to conclude that any binding site lacking this residue will have a greatly reduced microscopic affinity for ATP. However, there is no evidence to suggest that it opens to a conducting state that is distinct from that observed with three Lys residues at position 69. In other words, the binding of two rather than three molecules of ATP can activate the P2X2 receptor.
There were differences among the P2X2 concatemers with one K69A substitution that depended on the position of the subunit carrying the mutation. Currents formed by KKA and KAK receptors were significantly larger than those observed for AKK. In a trimeric concatemer, two of the three N termini of the channel will be constrained by the junction to the adjoining C terminus. This observation might, therefore, suggest that movement of a “free” N terminus, together with an intact binding site containing Lys69, might facilitate the first stages of conformational rearrangement when ATP binds in order to reduce the energy barrier to the first movement. In this respect, we note that Stoop et al. (1999) also found that a T336C substitution in the first of the three concatenated subunits had a disproportionate impact on the whole-cell current. We now know that there is substantial movement of the first transmembrane domain during channel opening (Hattori and Gouaux, 2012), and our results suggest that this occurs more readily for a subunit with a free N terminus than for a subunit in which the N terminus is tethered to the C terminus of the preceding subunit.
Three main approaches have been used to dissect the contribution of agonist binding to the separate subunits of homomultimeric ion channels in heterologous expression systems. The first is the estimation of the number of distinct conducting states by maximal-likelihood fitting to one or more models. This can be done at the level of macroscopic currents or from single-channel openings in a range of agonist concentrations. The former method indicated that homopentameric α7 nicotinic (Papke et al., 2000) and 5-hydroxytryptamine 3A (Mott et al., 2001) receptors were activated by the binding of three agonist molecules. The latter method indicated that the homopentameric glycine receptor can open with fewer than five glycine molecules bound, although the amplitude of the predominant unitary conductance is the same in all cases (Beato et al., 2002; Colquhoun and Sivilotti, 2004). The second approach has been to use varying amounts of UV irradiation to covalently link an agonist molecule to one, two, three, or four subunits of the tetrameric cyclic nucleotide-gated channel. In this case, the agonist must be slightly modified to introduce a photo-affinity label. Those results show that the unitary conductance increases stepwise with the number of tethered agonist molecules (Karpen and Ruiz, 2002). The third approach, which was initially used for tetrameric voltage-gated channels (e.g., Hurst et al., 1992), has been to concatenate subunits where one or more have a mutation known to impair agonist binding. This method was used by Janssens and Voets (2011) for the activation by extracellular menthol of transient receptor potential melastatin-8 channels. In that case, the main effect of menthol is to shift the channel voltage dependence of channel activation; its ability to do this was linearly dependent on the number of subunits carrying a high-affinity menthol-binding site. Unfortunately, the concatenation approach is less readily applied to pentameric or tetrameric channels of the nicotinic and glutamate families, because the N and C termini of each subunit lie on opposite sides of the membrane. [Although Schorge and Colquhoun (2003) achieved a similar result by coexpressing a cDNA encoding the first part of the N-methyl-d-aspartate protein, including transmembrane domains 1 and 2, with a cDNA that encoded transmembrane domain 3 and the C-terminal tail.]
The present experiments using concatemers take advantage of the fact that one amino acid residue (Lys69) is quite critical for ATP binding to the P2X2 receptor. The crystal structure of an ATP-bound form of the receptor (Hattori and Gouaux, 2012) confirms our premise that a binding pocket lacking this residue would have very much reduced microscopic affinity for ATP. Therefore, our results show that a P2X2 receptor with only two molecules of ATP bound can readily open to a full conducting state, with a unitary conductance not different from that of wild-type receptors. Furthermore, we cannot exclude the possibility that binding of a single ATP molecule may also be effective. On the other hand, the positional dependence of the effect of the lysine-to-alanine substitution serves as a reminder of the caution that is required when interpreting channel function from experiments based on subunit concatenation.
Acknowledgments
We thank Helen Broomhead and Rosemary Gaskell for technical support with molecular and cell biology.
This work was supported by the Wellcome Trust [Grant 093140/Z/10/Z].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- HEK
- human embryonic kidney
- sulfo-NHS-LC-biotin
- sulfo-N-hydroxysulfosuccinimide-LC-biotin
- sulfo-NHS-SDA
- N-hydroxysulfosuccinimide diazirine
- NHS-SDA
- N-hydroxysuccinimide diazirine
- KKK
- P2X2 receptor formed from three concatenated wild-type subunits
- AKK
- KAK, and KKA, P2X2 receptors formed from three concatenated subunits in which the first, second, or third such subunits contained K69A substitution
- KAA
- AKA, and AAK, P2X2 receptors formed from three concatenated subunits in which the second and third, first and third, or first and second such subunits contained K69A substitution
- AAA
- P2X2 receptor formed from three concatenated subunits in which all three subunits contained K69A substitution.
Authorship Contributions
Participated in research design: Stelmashenko, Lalo, North, and Compan.
Conducted experiments: Stelmashenko, Lalo, Yang, Bragg, and Compan.
Performed data analysis: Stelmashenko, Lalo, North, and Compan.
Wrote or contributed to the writing of the manuscript: Stelmashenko, Lalo, North, and Compan.
References
- Bean BP. (1990) ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci 10:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. (2002) Openings of the rat recombinant alpha 1 homomeric glycine receptor as a function of the number of agonist molecules bound. J Gen Physiol 119:443–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne LE, Cao L, Broomhead HE, Bragg L, Wilkinson WJ, North RA. (2011) P2X receptor channels show threefold symmetry in ionic charge selectivity and unitary conductance. Nat Neurosci 14:17–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Broomhead HE, Young MT, North RA. (2009) Polar residues in the second transmembrane domain of the rat P2X2 receptor that affect spontaneous gating, unitary conductance, and rectification. J Neurosci 29:14257–14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sivilotti LG. (2004) Function and structure in glycine receptors and some of their relatives. Trends Neurosci 27:337–344 [DOI] [PubMed] [Google Scholar]
- Ding S, Sachs F. (1999) Single channel properties of P2X2 purinoceptors. J Gen Physiol 113:695–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennion S, Hagan S, Evans RJ. (2000) The role of positively charged amino acids in ATP recognition by human P2X(1) receptors. J Biol Chem 275:29361–29367 [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. (1996) Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol 497:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Kavanaugh MP, Yakel J, Adelman JP, North RA. (1992) Cooperative interactions among subunits of a voltage-dependent potassium channel. Evidence from expression of concatenated cDNAs. J Biol Chem 267:23742–23745 [PubMed] [Google Scholar]
- Janssens A, Voets T. (2011) Ligand stoichiometry of the cold- and menthol-activated channel TRPM8. J Physiol 589:4827–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, North RA. (2003) Subunit arrangement in P2X receptors. J Neurosci 23:8903–8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Surprenant A, North RA. (2000) Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J Biol Chem 275:34190–34196 [DOI] [PubMed] [Google Scholar]
- Karpen JW, Ruiz M. (2002) Ion channels: does each subunit do something on its own? Trends Biochem Sci 27:402–409 [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. (2009) Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Gouaux E. (2012) Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Klaka B, Rettinger J, Bhargava Y, Eisele T, Nicke A. (2007) Identification of an intersubunit cross-link between substituted cysteine residues located in the putative ATP binding site of the P2X1 receptor. J Neurosci 27:1456–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Erreger K, Banke TG, Traynelis SF. (2001) Open probability of homomeric murine 5-HT3A serotonin receptors depends on subunit occupancy. J Physiol 535:427–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Tittle RK, Saar N, Dellal SS, Hume RI. (2005) An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem 280:25982–25983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A, Bäumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. (1998) P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 17:3016–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A, Rettinger J, Schmalzing G. (2003) Monomeric and dimeric byproducts are the principal functional elements of higher order P2X1 concatamers. Mol Pharmacol 63:243–252 [DOI] [PubMed] [Google Scholar]
- North RA. (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed] [Google Scholar]
- Papke RL, Meyer E, Nutter T, Uteshev VV. (2000) alpha7 receptor-selective agonists and modes of alpha7 receptor activation. Eur J Pharmacol 393:179–195 [DOI] [PubMed] [Google Scholar]
- Schorge S, Colquhoun D. (2003) Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci 23:1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, North RA. (1999) Contribution of individual subunits to the multimeric P2X(2) receptor: estimates based on methanethiosulfonate block at T336C. Mol Pharmacol 56:973–981 [DOI] [PubMed] [Google Scholar]
- Wilkinson WJ, Jiang LH, Surprenant A, North RA. (2006) Role of ectodomain lysines in the subunits of the heteromeric P2X2/3 receptor. Mol Pharmacol 70:1159–1163 [DOI] [PubMed] [Google Scholar]